Abstract
Noninvasive carotid measurements have independent value in the estimation of future cardiovascular (CV) outcomes in systemic lupus erythematosus (SLE). Natural IgM-antibodies to phosphorylcholine (PC) epitopes can enhance apoptotic-cell clearance and induce anti-inflammatory pathways. Herein, we show that subclinical CV disease, as detected by carotid ultrasound, in a cross-sectional SLE cohort was associated with lower levels of IgM anti-PC, as well as lower levels of the ratio of IgM anti-PC/total IgM, compared to patients without plaque (p=0.004 and p=0.02, respectively). The IgM anti-PC/total IgM association remained significant after adjusting for age, cholesterol and hypertension. Adiponectin and sE-selectin were significantly elevated in patients with plaque, and statistical models showed that combining adiponectin, sE-selectin and IgM anti-PC/total IgM was better for predicting plaque than either test alone.
These results support the hypothesis that IgM-natural autoantibodies may inhibit atherogenesis, and confirms the utility of IgM anti-PC levels as a biomarker for subclinical CV disease.
Keywords: IgM, phosphorylcholine, systemic lupus erythematosus, atherosclerosis, adiponectin, E-selectin, IMT
Introduction
Systemic lupus erythematosus (SLE) is associated with a strikingly increased risk for premature cardiovascular (CV) disease that is a major contributor to early mortality [1]. Indeed, women with SLE between 35 to 44 years of age have a 50-fold greater risk of a myocardial infarction [1] than age matched healthy controls, and lupus patients have an overall 17-fold increased risk of a fatal CV event [2] that cannot be fully explained by traditional Framingham risk factors [1]. Ultrasound measurements of carotid intimal thickness (IMT) have become an accepted non-invasive measure of subclinical atherosclerotic plaques and increased risk of cardiovascular events [3]. In women with SLE who were without a history of CV events, plaques by carotid IMT at baseline were shown to be significantly associated with incident CV clinical events during a mean follow up period of 7.9 years [4].
Surrogate markers related to endothelial cell injury and apoptosis may have utility for identifying a CV risk population. In a recent report, the presence of carotid plaque in SLE patients, as assessed by measurement of carotid IMT, correlated with higher levels of soluble E-selectin (sE-selectin) and adiponectin [5]. E-selectin is known to play a role in mediating adhesion between endothelial cells and leukocytes. Increased levels of soluble E-selectin (sE-selectin) may reflect endothelial activation that occurs in inflammatory diseases [6]. Higher sE-selectin levels are associated with increased risk of cardiovascular disease in both lupus and non-autoimmune patients [7, 8]. In contrast, the adipose-derived factor, adiponectin, is generally considered to be anti-inflammatory and athero-protective, yet elevated adiponectin levels are often found in SLE patients, although the mechanistic implications are unclear [9].
A focus of the present study is the use of natural IgM autoantibodies as biomarkers, as the homeostatic and immunomodulatory properties of naturally arising antibodies (NAb) to oxidation-associated neo-determinants have recently been characterized [10–12]. IgM antibodies that recognize phosphorylcholine (PC) and malondialdehyde (MDA) neo-determinants on apoptotic cells (AC) are common components of the immune system, and in murine studies the induction of anti-PC antibodies blocked the progression of atherosclerosis in hyperlipidemic mice [13]. Furthermore, these IgM anti-PC antibodies can also increase the in vitro and in vivo phagocytic clearance of ACs, inhibit inflammatory signaling in innate immune cells [10–12], and suppress disease in models of autoimmunity [10]. Of clinical relevance, in recent cross-sectional studies it was demonstrated that SLE patients with history of a CV event had significantly lower levels of IgM anti-PC antibodies compared to patients who were event free [14, 15]. Furthermore, higher IgM anti-PC levels were also found to correlate with lower overall lupus clinical disease activity [14].
The current study was initiated to address the hypothesis that decreased levels of IgM anti-PC would be predictive of subclinical atherosclerosis. This was approached by evaluation of sera from a cohort of SLE patients who underwent studies of carotid IMT. In addition, previously identified serologic biomarkers, sE-selectin and adiponectin, were fit into the risk model.
Materials and Methods
Patient population
The recruited patients were previously described [5]. All patients fulfilled at least four of the revised ACR classification criteria for SLE [16], provided consent and were enrolled under a protocol approved by the Institutional Review Board of the New York University School of Medicine.
Clinical measurements
105 SLE patients underwent carotid ultrasound and the presence of carotid plaque was assessed as previously described [5]. Briefly, following the recommendations of the American Society of Echocardiography Carotid Intima-Media Thickness Task Force for identification of pre-clinical vascular changes, high resolution images of right and left common carotid arteries, internal carotid arteries and carotid bulbs were obtained by experienced sonographer using a linear array transducer [17]. The presence of plaque was defined as ≥50% increase over background IMT in any arterial segment.
The clinical status of each SLE patient was assessed with the SELENA revision of the SLE disease activity index (SLEDAI) [18]. Complete blood profiles were also performed by the NYU clinical laboratory.
Biomarker assays
Levels of sE-selectin and total adiponectin were measured by commercial assays, according to the manufacturer’s instructions (R&D systems, Linco Research Inc, respectively). IgM anti-PC, IgM anti-MDA, IgG anti-PC, IgG anti-MDA, and total IgM were assessed by ELISA, as previously described [14].
Statistical analysis
The distributions of biomarkers and other quantitative patient and disease characteristics were compared between the two groups using descriptive summary statistics and boxplots and two- sided Mann-Whitney tests. If the variance of variables were skewed between groups, these values were first logarithmically transformed before analyses, as appropriate. Frequency distributions and Fisher’s exact test was used to compare categorical variables. Non-parametric Spearman correlation coefficients were used to examine the pairwise associations between continuous variables. Multivariable logistic regression analyses were performed when adjusting for age, hypertension and cholesterol levels. Furthermore, to calculate odd ratios and identify the best model to predict plaques; IgM anti-PC, adiponecin, sE-selectin and IgM anti-PC/total IgM, were evaluated in logistic regression models using a stepwise selection method. Analyses were performed with SAS 9.3 software (SAS Institute Inc.) and a p-value<0.05 in two-sided analysis was considered significant.
Results
Of the evaluated 105 SLE patients, 44 met the criteria for presence of carotid plaque. With regard to the Framingham traditional risk factors, patients with plaque were older (49±13 years compared to 37±11 years, p<0.0001), had higher total cholesterol levels (186±41 mg/dl compared to 169±31 mg/dl, p=0.02), and more often had hypertension (56% compared to 23%, p=0.0009)(Table I). In addition, SLE patients with plaque also had elevated levels of sE-selectin (80±62 ng/ml compared to 54±27 ng/ml, p=0.005) and adiponectin (18.7±9.0 μg/ml compared to 14.6±9.4 μg/ml, p=0.01) as previously reported [5]. SLE patients with plaque had significantly lower levels of the protective natural IgM anti-PC antibody compared to those without plaque (22±27 RU/ml compared to 39±47 RU/ml, p=0.004). These results were not simply the reflection of total IgM levels since there were no significant differences in total IgM levels between the groups (p= 0.60). Importantly, the ratio of IgM anti-PC/total IgM was significantly lower in the group with plaque (0.47±0.50 compared to 0.74±0.73, p=0.02) (Table 1, Figure 1). While there were similar associations with other types of antibodies to oxidation-associated neo-determinants, including IgG anti-PC and IgM antibodies binding MDA, these differences were not statistical significant (p=0.06 for both comparisons between patients with or without plaque).
Table 1.
Cohort characteristics of lupus patients with and without plaques
| No plaque | Plaque | p-value | Adjusted p-value# | |||
|---|---|---|---|---|---|---|
| n | n | |||||
| Age (year) | 37.8±11 | 61 | 49.3±12.8 | 44 | <0.0001 | - |
| Caucasian (%, No.) | 36%, n= 22 | 61 | 48%, n=21 | 61 | 0.05 | 0.24 |
| Female (%, No.) | 90%, n=55 | 61 | 95%, n=42 | 44 | 0.31 | 0.81 |
| Body-mass index (kg/m2) | 25.4±6.3 | 54 | 27.1±6.2 | 40 | 0.16 | 0.62 |
| Smoking (%, No.) | 10%, n=6 | 59 | 14%, n=5 | 42 | 0.78 | 0.41 |
| Hypertension (%, No.) | 23%, n=12 | 53 | 56%, n=22 | 39 | 0.0009 | - |
| Systolic blood | 112±17 | 54 | 126±21 | 43 | 0.0008 | 0.21 |
| Diastolic blood pressure | 72±11 | 54 | 77±11 | 43 | 0.06 | 0.87 |
| Total cholesterol (mg/dl) | 169±31 | 56 | 186±41 | 43 | 0.02 | - |
| HDL (mg/dl) | 57±15 | 55 | 56±20 | 42 | 0.43 | 0.68 |
| LDL (mg/dl) | 95±23 | 55 | 106±36 | 41 | 0.21 | 0.48 |
| Triglycerides (mg/dl) | 90±49 | 56 | 113±72 | 43 | 0.10 | 0.25 |
| Homocysteine (μM) | 9.8±3.7 | 53 | 10±3.1 | 34 | 0.60 | 0.92 |
| Anti-dsDNA pos. (%, No.) | 49%, n=29 | 59 | 40%, n=17 | 42 | 0.38 | 0.57 |
| SELENA-SLEDAI (mean±SD) | 3.5±3.3 | 56 | 3.0±3.0 | 43 | 0.38 | 0.21 |
| Age at onset of disease (year) | 26±8.8 | 60 | 34±13 | 41 | 0.002 | 0.53 |
| Duration of disease (year) | 11.4±9.5 | 61 | 16.5±10 | 42 | 0.008 | 0.67 |
| Adiponectin (μg/ml) | 14.6±9.4 | 58 | 18.7±9.0 | 43 | 0.01 | 0.02 |
| sE-selectin (ng/ml) | 53.8±27 | 58 | 80.1±62 | 43 | 0.005 | 0.04 |
| Total IgM (RU/ml) | 75±78 | 61 | 78±85 | 44 | 0.60 | 0.07 |
| IgM anti-PC (RU/ml) | 39±47 | 61 | 22±27 | 44 | 0.004 | 0.08 |
| IgM anti-PC/total IgM | 0.74±0.73 | 61 | 0.47±0.50 | 44 | 0.02 | 0.005 |
| IgM anti-MDA (RU/ml) | 37±80 | 61 | 24±31 | 44 | 0.06 | 0.23 |
| IgM anti-MDA/total IgM | 0.49±0.33 | 61 | 0.37±0.30 | 44 | 0.06 | 0.21 |
| IgG anti-PC (RU/ml) | 36±27 | 61 | 29±27 | 44 | 0.06 | 0.10 |
| IgG anti-MDA (RU/ml) | 18±15 | 61 | 19±29 | 44 | 0.07 | 0.45 |
For continuous variables mean ± standard deviation (SD) are presented. P-values were derived from two-sided Mann-Whitney test for univariate analysis of continuous variables and Fisher’s exact test for categorical variables. P-values were derived from logistic regression analysis for multivariate (adjusted) analysis. Variables with significantly skewed variances, adiponectin, sE-selectin, IgM anti-PC and the ratio IgM anti-PC/total IgM, were log transformed in the analysis. Significant p-values < 0.05 are highlighted in bold font.
Multivariable analysis adjusted for age, hypertension and total cholesterol
Figure 1.
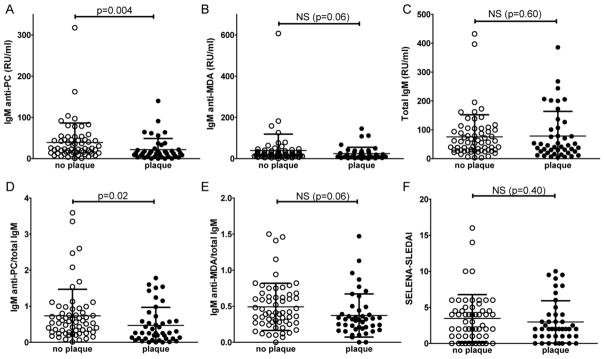
Comparison of SLE patients with and without evidence of carotid plaque by IMT. Serological biomarkers, natural antibody levels and clinical disease activity were compared for 61 SLE patients without plaque and 44 patients with plaque. Graphs are shown for A. IgM anti-PC; B. IgM anti-MDA; C. Total IgM; D. The ratio of IgM anti-PC/total IgM; E. The ratio of IgM anti-MDA/total IgM; F. SELENA-SLEDAI index of lupus disease activity. P-values were derived from Mann-Whitney test.
Multivariable logistic regression analysis showed that the significant correlations of high adiponectin, high sE-selectin and low levels of the ratio IgM PC-IgM/total IgM with the presence of plaque, were each preserved even after adjusting the SLE data set for age, hypertension, and cholesterol (p=0.02, p=0.04, p=0.005, respectively) (Table 1). The levels of adiponectin significantly correlated inversely with IgM anti-PC (p=0.01, Spearman R=−0.26), IgM anti-MDA (p=0.03, Spearman R=−0.22) and total IgM (p=0.01, Spearman R=−0.24). Correlations were also identified between the specific natural IgM antibodies anti-PC and anti-MDA (p<0.0001, R=0.71) with total IgM levels (p<0.0001, Spearman R=0.49; p<0.0001, Spearman R=0.71, respectively) as well as with each other (p<0.0001, Spearman R=0.44). While there was a significant correlation between levels of IgM anti-MDA and IgG anti-MDA (p=0.02, Spearman R=0.22), the correlation of IgM anti-PC with IgG anti-PC was not significant (p=0.06, Spearman R=0.18). Notably, anti-PC IgM levels significantly decreased with age (p=0.03, Spearman R=−0.21) while total IgM did not (p=0.58, Spearman R=−0.06). Hence, the ratio of PC IgM over total IgM levels appeared to be a more robust test than IgM anti-PC alone.
As adiponectin, sE-selectin and IgM anti-PC each independently correlated with carotid plaque in SLE, step-wise logistic regression models were used to identify the best combination of markers for predicting the presence of plaque (Table 2, Supplemental Figure 1). The combination of sE-selectin and adiponectin was superior to either test alone. Most importantly, inclusion of the ratio of IgM anti-PC/total IgM further improved overall sensitivity, as assessed by the area under the curve (AUC) analysis, the best model (AIC=112.4; AUC=0.77; Table 2, Supplemental Figure 1) included sE-selectin (OR=3.8 per 10 unit increase, 95% CI: 1.5–9.6, p=0.004), adiponectin (OR=2.9 per 10 unit increase, 95% CI: 1.3–6.3, p=0.007) and IgM anti-PC over total IgM (OR=0.42 per unit increase, 95% CI: 0.17–1.0, p=0.05).
Table 2.
Logistic regression models
| Model number | Variables # | Odds ratio‡ (95% confidence interval) | p-value | AIC | AUC |
|---|---|---|---|---|---|
| 1 | sE-selectin | 3.2 (1.4–7.3) | 0.005 | 132.7 | 0.67 |
|
| |||||
| 2 | Adiponectin | 2.4 (1.2–4.9) | 0.01 | 134.5 | 0.65 |
|
| |||||
| 3 | IgM anti-PC | 0.62 (0.42–0.92) | 0.02 | 135.1 | 0.67 |
|
| |||||
| 4 | IgM anti-PC/total IgM | 0.46 (0.21–1.0) | 0.05 | 141.5 | 0.66 |
|
| |||||
| 5 | sE-selectin | 3.7 (1.5–9.3) | 0.008 | 123.1 | 0.73 |
| Adiponectin | 2.7 (1.3–5.8) | 0.004 | |||
|
| |||||
| 6 | sE-selectin | 4.2 (1.58–11.2) | 0.004 | 127.8 | 0.75 |
| Adiponectin | 2.4 (1.1–5.2) | 0.03 | |||
| IgM anti-PC | 0.61 (0.39–0.95) | 0.03 | |||
|
| |||||
| 7* | sE-selectin | 3.8 (1.5–9.6) | 0.004 | 112.4 | 0.77 |
| Adiponectin | 2.9 (1.3–6.3) | 0.007 | |||
| IgM anti-PC/total IgM | 0.42 (0.17–1.0) | 0.05 | |||
All models used logarithmically transformed variables for IgM anti-PC, sE-selectin and adiponectin
Odds ratio is for a change of 10 units for the variables IgM anti-PC, sE-selectin and adiponectin
AIC= Akaike information criterion
AUC= Area under the curve of receiver operating characteristic curves (ROC curves) for sensitivity vs. specificity
Best stepwise model
Moreover, evaluation of the potential predictive value of the different biomarkers, using cutoff values based on the highest or lowers quartile (Table 3), demonstrated that having low IgM anti-PC/total IgM (<0.2) was significantly associated with presence of plaque (p=0.003, OR=4.0, sensitivity=41%, specificity=85%). Having any of the three biomarker results: high adiponectin (>21ug/ml), high sE-selectin (>81ug/ml), or low IgM anti-PC/total IgM (<0.2) positive was stronger associated with plaque and had higher sensitivity (p<0.0001, OR=5.8, sensitivity=77%, specificity=64%), although lower specificity.
Table 3.
Predictive value of biomarker assays for presence of plaque in SLE
| Biomarker test# | p-value‡ | Odds Ratio (95% confidence interval) | Sensitivity | Specificity |
|---|---|---|---|---|
| Low IgM anti-PC/total IgM < 0.2 | 0.003 | 4.0 (1.6–10.1) | 41% | 85% |
| High adiponectin > 21 ug/ml | 0.15 | 2.6 (1.0–6.5) | 30% | 83% |
| High sE-selectin > 81 ng/ml | 0.06 | 2.1 (0.8–5.3) | 35% | 82% |
| ≥1 positive biomarker tests Low IgM anti-PC/total IgM < 0.2 High adiponectin > 21 ug/ml High sE-selectin > 81 ng/ml |
<0.0001 | 5.8 (2.4–14.2) | 77% | 64% |
Fisher’s exact test
Cutoff for positive test based on lowest quartile of anti-PC IgM/total IgM, highest quartile adiponectin, and highest quartile sE-selectin.
Discussion
This cross-sectional study of adult SLE patients identified a strong association between subclinical CV disease, as detected by carotid IMT, and low levels of protective IgM anti-PC antibodies and increased levels of adiponectin and sE-selectin. These findings extend previous observations that lower levels of IgM anti-PC are associated with CV disease in other lupus cohorts [14, 15, 19]. The current study also demonstrates that measuring the relative proportion of IgM anti-PC to total IgM may be a better biomarker than levels alone for the development of carotid plaque, which was also found to be independent of the traditional risk factors; hypertension, total cholesterol and age. Nonetheless, as expected age was confirmed to be the strongest independent demographic risk factor for plaque in this cohort. Most importantly, logistic regression modeling showed that combining the previous identified biomarkers, adiponecin and sE-selectin, with IgM anti-PC/total IgM, further improved the identification of the SLE patients with carotid plaques.
Natural IgM that specifically recognize ACs are present in the circulation in all humans from birth and have been postulated to have immunoregulatory functions. Mechanistic studies have shown that anti-AC antibodies, such as IgM anti-PC, can increase the phagocytosis of ACs and inhibit inflammatory pathways, which both play central roles in atherogenesis. Moreover, IgM anti-PC treatment has been shown to induce rapid high levels of the anti-inflammatory phosphatase for the Mitogen Activated Kinase system, MAPK phosphatase-1 (MKP-1, DUSP-1) in innate immune cells [10–12], a pathway that blocks the activation of the primary MAP kinases; p38, JNK and ERK1/2. This pathway has also been implicated as part of the transactivation signaling affected by corticosteroids. Notably, the anti-inflammatory effects on MAPK signaling of anti-PC IgM and corticosteroids have been shown to be additive [12]. However, there are also important differences, as unlike corticosteroids, natural antibodies do not share the adverse metabolic effects for dyslipidemia that can drive atherogenesis. These properties may also contribute to the ability of anti-PC IgM to block atherosclerosis in mouse models [13] and the observed association of higher IgM anti-PC levels with protection from CV disease in clinical surveys [14, 15]. These protective properties are dependent both on the fine binding specificity and the isotype of these antibodies.
In the present investigation we also compared levels of IgM anti-PC to another set of natural IgM antibodies recognizing the oxidation-associated determinant, MDA. While there were some associative trends we did not find the same strong inverse correlation between these antibody levels and the presence of carotid plaque. Similarly, low levels of IgG anti-PC did not reach significance. Hence, the strongest association with protection from subclinical atherosclerosis was found with levels of IgM anti-PC. These data advance the evidence from our previous retrospective report that showed decreased IgM anti-PC in SLE patients with a history of myocardial infarction or stroke [14]. The current report demonstrates that patients in the lowest quartile of IgM anti-PC/total IgM have an odds ratio of 4.0 for carotid plaque. This association is similar to findings previously reported for an independent cohort from Sweden that showed an odds ratio of 2.9 for plaque in SLE patients in the lowest quintile of serum IgM anti-PC levels, although a possible effect of overall lower IgM levels was not considered [19]. Nevertheless, it is not yet clear whether lower levels of these specific types of IgM antibodies precede and/or accelerate the development of this form of vasculopathy, or whether depressed levels reflect an atherosclerosis-associated IgM consumption process.
The pathogenesis of SLE is complex, and the mechanisms responsible for accelerated CV disease remain poorly understood. It has been postulated that dysregulation of type I IFN mediated responses contribute to vascular damage and endothelial repair that may lead to increased development of plaques [20]. No correlation has been seen with overall lupus disease activity and cardiovascular risk [21]. Current therapeutic treatments may affect the progression of CV disease, and corticosteroids especially can increase this risk. Yet, in the current cohort there were no observed differences in the use of immunosuppressive agents between the plaque and no plaque groups, as previously reported [5]. Current guidelines recommend close monitoring of all SLE patients for cardiovascular risk to ensure treatment of any traditional risk factors that can be modified [22]. However, a recent report suggests that a panel of non-traditional biomarkers including piHDL and TWEAK may be valuable for predicting atherosclerosis in SLE [23]. Similarly, we demonstrate that the current set of biomarkers may be combined to better predict disease CV risk. These three biomarkers, adiponectin, sE-selectin and IgM anti-PC levels, connect endothelial stress with the innate as well as the adaptive immune system.
In summary, these data confirm the utility of measuring IgM anti-PC as a biomarker for CV risk, importantly herein as assessed by subclinical disease. In SLE, accelerated atherosclerosis may be a consequence of the loss of protective factors that contribute to the control of inflammation and the efficient clearance of ACs. Therapeutic implications may include strategies for restoring the physiologic balance of these natural autoantibodies that oppose atherogenesis.
Supplementary Material
Highlights.
Natural IgM-antibodies to PC may have protective properties in SLE.
Lower levels of IgM anti-PC were associated with carotid plaque in SLE.
Combining IgM anti-PC, adiponectin, and sE-selectin was better at predicting plaque.
IgM anti-PC may be a valuable biomarker for subclinical atherosclerosis in lupus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, D’Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 2.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, Cote R, Grover SA, Fortin PR, Clarke AE, Senecal JL. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 4.Kao AH, Lertratanakul A, Elliott JR, Sattar A, Santelices L, Shaw P, Birru M, Avram Z, Thompson T, Sutton-Tyrrell K, Ramsey-Goldman R, Manzi S. Relation of carotid intima-media thickness and plaque with incident cardiovascular events in women with systemic lupus erythematosus. Am J Cardiol. 2013;112:1025–1032. doi: 10.1016/j.amjcard.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds HR, Buyon J, Kim M, Rivera TL, Izmirly P, Tunick P, Clancy RM. Association of plasma soluble E-selectin and adiponectin with carotid plaque in patients with systemic lupus erythematosus. Atherosclerosis. 2010;210:569–574. doi: 10.1016/j.atherosclerosis.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egerer K, Feist E, Rohr U, Pruss A, Burmester GR, Dorner T. Increased serum soluble CD14, ICAM-1 and E-selectin correlate with disease activity and prognosis in systemic lupus erythematosus. Lupus. 2000;9:614–621. doi: 10.1191/096120300678828749. [DOI] [PubMed] [Google Scholar]
- 7.Rho YH, Chung CP, Oeser A, Solus J, Raggi P, Gebretsadik T, Shintani A, Stein CM. Novel cardiovascular risk factors in premature coronary atherosclerosis associated with systemic lupus erythematosus. J Rheumatol. 2008;35:1789–1794. [PMC free article] [PubMed] [Google Scholar]
- 8.Rohde LE, Lee RT, Rivero J, Jamacochian M, Arroyo LH, Briggs W, Rifai N, Libby P, Creager MA, Ridker PM. Circulating cell adhesion molecules are correlated with ultrasound-based assessment of carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 1998;18:1765–1770. doi: 10.1161/01.atv.18.11.1765. [DOI] [PubMed] [Google Scholar]
- 9.Rovin BH, Song H, Hebert LA, Nadasdy T, Nadasdy G, Birmingham DJ, Yung Yu C, Nagaraja HN. Plasma, urine, and renal expression of adiponectin in human systemic lupus erythematosus. Kidney Int. 2005;68:1825–1833. doi: 10.1111/j.1523-1755.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Khanna S, Goodyear CS, Park YB, Raz E, Thiel S, Gronwall C, Vas J, Boyle DL, Corr M, Kono DH, Silverman GJ. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol. 2009;183:1346–1359. doi: 10.4049/jimmunol.0900948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182:6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gronwall C, Chen Y, Vas J, Khanna S, Thiel S, Corr M, Kono DH, Silverman GJ. MAPK phosphatase-1 is required for regulatory natural autoantibody-mediated inhibition of TLR responses. Proc Natl Acad Sci U S A. 2012;109:19745–19750. doi: 10.1073/pnas.1211868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 14.Gronwall C, Akhter E, Oh C, Burlingame RW, Petri M, Silverman GJ. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin Immunol. 2012;142:390–398. doi: 10.1016/j.clim.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su J, Hua X, Concha H, Svenungsson E, Cederholm A, Frostegard J. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology (Oxford) 2008;47:1144–1150. doi: 10.1093/rheumatology/ken120. [DOI] [PubMed] [Google Scholar]
- 16.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 17.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 18.Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, Lockshin M, Merrill JT, Belmont HM, Askanase AD, McCune WJ, Hearth-Holmes M, Dooley MA, Von Feldt J, Friedman A, Tan M, Davis J, Cronin M, Diamond B, Mackay M, Sigler L, Fillius M, Rupel A, Licciardi F, Buyon JP. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2550–2558. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 19.Anania C, Gustafsson T, Hua X, Su J, Vikstrom M, de Faire U, Heimburger M, Jogestrand T, Frostegard J. Increased prevalence of vulnerable atherosclerotic plaques and low levels of natural IgM antibodies against phosphorylcholine in patients with systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R214. doi: 10.1186/ar3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahlenberg JM, Kaplan MJ. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu Rev Med. 2013;64:249–263. doi: 10.1146/annurev-med-060911-090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, Crow MK, Schwartz JE, Paget SA, Devereux RB, Salmon JE. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 22.Mosca M, Tani C, Aringer M, Bombardieri S, Boumpas D, Brey R, Cervera R, Doria A, Jayne D, Khamashta MA, Kuhn A, Gordon C, Petri M, Rekvig OP, Schneider M, Sherer Y, Shoenfeld Y, Smolen JS, Talarico R, Tincani A, van Vollenhoven RF, Ward MM, Werth VP, Carmona L. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis. 2010;69:1269–1274. doi: 10.1136/ard.2009.117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon M, Skaggs BJ, Grossman JM, Sahakian L, Fitzgerald J, Wong WK, Lourenco EV, Ragavendra N, Charles-Schoeman C, Gorn A, Karpouzas GA, Taylor MB, Watson KE, Weisman MH, Wallace DJ, Hahn BH. A panel of biomarkers is associated with increased risk of the presence and progression of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:130–139. doi: 10.1002/art.38204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


