Abstract
Patient: Male, 81
Final Diagnosis: Prostate cancer
Symptoms: Anorexia • dark urine • joundice • letargy
Medication: Casodex
Clinical Procedure: —
Specialty: Oncology
Objective:
Adverse events of drug therapy
Background:
Bicalutamide is a nonsteroidal anti-androgen used extensively during the initiation of androgen deprivation therapy with a luteinizing hormone-releasing hormone (LHRH) agonist to reduce the symptoms of tumor flare in patients with metastatic prostate neoplasm. It can cause gynecomastia, hot flashes, fatigue, and decreased libido through competitive androgen receptor blockade. Although not as common, acute drug-induced liver injury is also possible with bicalutamide therapy. Typically, this results in transient derangement of liver function and patients remain asymptomatic. We share our experience with a case of symptomatic acute hepatotoxicity secondary to the use of bicalutamide and use this opportunity to present a brief review of existing literature.
Case Report:
An 81-year-old African American male with metastatic prostate neoplasm presented with nonspecific symptoms along with jaundice of 1-day duration. He was started on a trial of bicalutamide 3 weeks prior to presentation. On physical examination, scleral icterus was noted. Workup revealed acutely elevated liver transaminases (>5 times the upper limit of normal), alkaline phosphatase, conjugated hyperbilirubinemia, and coagulopathy. Other etiologies, including viruses, common toxins, drugs, autoimmune, and copper-induced hepatitis, were considered. Bicalutamide was discontinued and the patient was managed with supportive care. He showed improvement of clinical and laboratory abnormalities within days.
Conclusions:
While rare, clinically significant and potentially life-threatening liver injury can result from use of bicalutamide. Prompt recognition and discontinuation of bicalutamide is necessary to avoid serious complications from this adverse reaction.
MeSH Keywords: Drug-Induced Liver Injury, Nonsteroidal Anti-Androgens, Prostatic Neoplasms
Background
Prostate neoplasm is the leading malignancy of men in the United States and is the second-most common cause of cancer-related death [1]. Androgen deprivation therapy has been the mainstay of treatment for metastatic prostate cancer and of less advanced cancers as neoadjuvant and adjuvant treatment [2]. Treatment modalities encompass conventional surgical bilateral orchiectomy, or use of estrogenic mixes, and, more recently, use of anti-androgen medications with luteinizing hormone-releasing hormone (LHRH) analogues, either in combination or as monotherapy. Due to their relatively better adverse effect profile, nonsteroidal anti-androgens (flutamide, nilutamide, and bicalutamide) have been preferred in this setting. Bicalutamide has been used extensively during the start of androgen deprivation therapy with an LHRH agonist to reduce occurrence of the symptoms of tumor flare in patients with metastatic prostate carcinoma [3].
Case Report
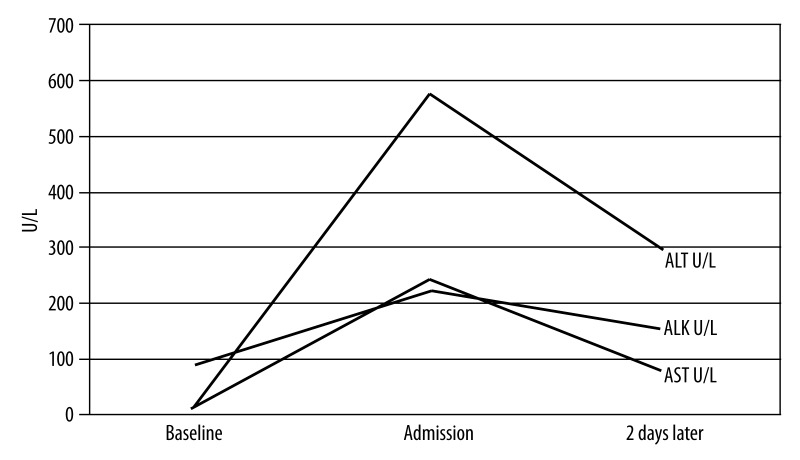
An 81-year-old African American male with metastatic prostate neoplasm (T4N1M1) presented with nonspecific symptoms of anorexia and lethargy of 1-week duration. He also complained of dark discoloration of urine for 1 week and jaundice for 1 day. He had started a trial of bicalutamide 150 mg orally every day 3 weeks prior to presentation. Physical examination result was positive for scleral icterus. Blood work revealed acutely deranged liver function with AST 243 U/L, ALT 576 U/L, and INR of 1.5. Total bilirubin was 3.7 mg/dL, with direct bilirubin of 2.3mg/dL and alkaline phosphatase of 223 U/L, indicating conjugated hyperbilirubinemia. He had normal baseline lab results at the initiation of bicalutamide. Hepatitis A IgM and hepatitis B surface Ag were negative, with hepatitis C RNA levels undetectable. Hepatitis B surface antibody and total hepatitis B core antibody were positive, indicating resolved infection. An abdominal ultrasound did not reveal obstruction. CT of the abdomen ruled out hepatic involvement, although diffuse bony metastatic disease was visualized. Other etiologies, including autoimmune, common toxins, drugs, and iron- and copper-induced insult were considered. However, given the lack of clinical findings, an extensive work up was not undertaken to definitively rule them out.
The patient’s other medical problems included hypertension, hyperlipidemia, coronary artery disease, stroke, and abdominal aortic aneurysm. In addition to bicalutamide, his medications included low-dose aspirin, simvastatin, folic acid, irbesartan, and ranitidine. Bicalutamide was discontinued and the patient was managed with supportive care. He showed clinical and serological improvement within days (Figure 1). The Council for International Organizations of Medical Sciences Scale (CIOMS) score was 7, indicating a probable adverse drug reaction [4] (Table 1).
Figure 1.
Course of laboratory findings from admission to discharge.
ALK – alkaline phosphatase,
ALT – alanine aminotransferase,
AST – aspartate aminotransferase.
Table 1.
CIOMS scale for the cholestatic (± hepatocellular) type of injury in DILI.
| Items for cholestatic ± hepatocellular injury | Possible score | Patient’s score |
|---|---|---|
| Time to onset from the beginning of the drug/herb | +1 to +2 | +2 |
| Course of ALP after cessation of the drug/herb | 0 to +2 | +2 |
| Risk Factors | 0 to +2 | +1 |
| Concomitant drugs(s) or herb(s) | −3 to +0 | 0 |
| Search for non drug/herb causes | −3 to +2 | +1 |
| Previous information on the hepatotoxicity of the drug/herb | 0 to +2 | +1 |
| Response to re-administration | 0 to +3 | 0 |
| Total score for patient | −5 to 13 | 7 |
CIOMS – council for International Organization of Medical Sciences. Total score with resulting causality grading: ≤0, excluded; 1–2, unlikely; 3–5, possible; 6–8, probable; ≥9, highly probable.
Discussion
Bicalutamide is an orally active, nonsteroidal anti-androgen with the chemical formula (2RS)-4’-cyano-3-(4-fluorophenylsulphonyl)-2-hydroxy-2-methyl-3’-(trifluoromethyl)-propionanilide. It competitively antagonizes the actions of androgens of both testicular and adrenal origin at the receptor level, thereby inhibiting the growth of prostate tumors [5]. Unlike steroidal antiandrogens (e.g., cyproterone acetate), nonsteroidal anti-androgens (e.g., flutamide, nilutamide, and bicalutamide) do not suppress testosterone production and therefore offer potential quality of life advantages over castration. The most common adverse effects of bicalutamide are induced by its pharmacologic property of competitive androgen receptor blockade and include gynecomastia, breast pain, hot flashes, fatigue, and decreased libido [3,5]. The available nonsteroidal anti-androgens appear to be similar in terms of the incidence of pharmacologic adverse effects [6]. However, evidence suggests that bicalutamide may have a more favorable profile in terms of nonpharmacologic events. Of the 3 commercially available nonsteroidal anti-androgens, flutamide has the most established clinical evidence of inducing hepatic injury, resulting in nonfatal aminotransferase elevations in 42–62% of patients [6]. A literature search for case reports of nonsteroidal anti-androgens revealed numerous cases of flutamide-induced liver injury, but only 3 cases of bicalutamide toxicity. Our aim is to describe a case and use that to illustrate the wide spectrum of clinical and laboratory abnormalities that patients receiving bicalutamide may present with (Table 2).
Table 2.
Cases reported in literature of bicalutamide-induced liver injury.
| Author | Case* | Abnormal Laboratory* | Outcome | ||
|---|---|---|---|---|---|
| Initial | Later | ||||
| Dawson et al., 1997 [7] | A 60-year-old man with no prior history of liver disease received androgen blockade and localized radiation for prostate cancer (T3cN1M0 Gleason grade 7). CIOMS score not provided |
TB mg/dL | 19.47 | Nor | Hepatic failure improved. Prothrombin time, bilirubin and liver enzymes normalized within 2 months |
| GGT U/L | 121 | Nor | |||
| AST U/L | 1527 | Nor | |||
| ALT U/L | 2690 | Nor | |||
| APhos U/L | 181 | Nor | |||
| INR | 2.21 | Nor | |||
| Beza et al., 2008 [2] | A 61-year-old man with adenocarcinoma of prostate (T3bN0M0 Gleason grade 6) who received CAB with bicalutamide and LHRH agonist. CIOMS score not provided |
TB mg/dl | 11.57 | 49.88 | The clinical course deteriorated, and the patient died |
| DB mg/dl | 8.32 | 29.48 | |||
| AST U/L | 1335 | 216 | |||
| ALT U/L | 1923 | 158 | |||
| APhos U/L | 169 | NR | |||
| GGT U/L | 473 | NR | |||
| O’Bryant, et al., 2008 [8] | A 59-year-old man with metastatic prostate carcinoma (T4N1M1 Gleason grade 9) treated with bicalutamide as part of androgen deprivation therapy prior to chemotherapy. CIOMS score not provided |
AST U/L | 166 | 3206 | The patient died 8 days after bicalutamide therapy was begun secondary to multiorgan failure, as a result of fulminant hepatic failure |
| ALT U/L | 111 | 2050 | |||
| INR | NR | 3.4 | |||
CAB – combined androgen blockade; LHRH – luteinizing hormone receptor agonist; Initial – labs at admission; Subsequent – labs during the course of hospitalization; Nor – normalized but value not reported; NR – not reported; TB – total bilirubin; DT – direct bilirubin; AST – aspartate aminotransferase; ALT – alanine aminotransferase; APhos – alkaline phosphatase; GGT – gamma-glutamyl transpeptidase; INR – international normalized ratio; CIOMS – Council for International Organizations of Medical Sciences.
Dawson et al. [7] reported the first case of bicalutamide-induced fulminant hepatotoxicity in a 60-year-old male with adenocarcinoma of the prostate. He had received cyproterone acetate for 1 month, followed by androgen deprivation therapy with flutamide and goserelin acetate, which was switched to bicalutamide 3 months later. This patient had presented with confusion, jaundice, and encephalopathy after receiving 2 doses of bicalutamide. The reported serology is listed in tabular form (Table 2). The hepatic failure improved after discontinuation of bicalutamide and with supportive care.
In contrast, O’Bryant et al. [8] reported the first case of fatal fulminant bicalutamide-induced liver injury in a 59-year-old male with metastatic prostate neoplasm. On initial diagnosis, the patient was treated with leuprolide acetate, an LHRH agonist, and 1 month of concomitant bicalutamide. He also underwent 4 cycles of combination chemotherapy with cisplatin and etoposide. Subsequently, he received radiation therapy followed by a 3-month period in which he received no treatment. On a follow-up bone scan 2 months later, new widespread metastases were identified. Therefore, bicalutamide was restarted. After receiving 4 doses, he presented to the emergency department with abdominal pain and distension. He developed progressively worsening liver values (Table 2) leading to multiorgan failure and death. Beza et al. described another fatal case of bicalutamide-induced liver injury in a 61-year-old patient [2]. He received 3 months of combined androgen blockade (CAB) with bicalutamide and LHRH agonist for hormonal down-staging therapy of prostate cancer. This was followed by radiation therapy and continued CAB for 2 years. At an unclear time to reaction from therapy onset, the patient developed jaundice, choluria, and acholia, which led to hepatic de-compensation, encephalopathy, and death.
The drug-induced liver injury in our case is supported both by temporal association and the absence of other etiological explanations. Although our patient had been on a statin, this was not a new medication, nor had there been any dose change. In addition, the higher daily dose (150 mg), as opposed to that administered in prior case reports (50 mg), may be associated with a dose-response effect. While several mechanisms have been proposed, none can uniformly explain the occurrence of this adverse reaction in all the cases described.
It is noteworthy that analysis of phases I, II, and III clinical trials evaluating bicalutamide revealed no cases of symptomatic hepatotoxicity; however, in 5 cases of jaundice, an association with bicalutamide could not be ruled out [9,10]. Subsequent studies reported bicalutamide-induced transaminitis and/or hyperbilirubinemia [3,7,11,12]. These elevations are typically transient and patients remain asymptomatic [3]. In 1 study, 6.7% of patients who received bicalutamide-LHRH analog treatment developed abnormal liver enzymes, in comparison to 10.3% for those who received flutamide-LHRH, but there was no significant statistical difference [11].
In 2005, Manso et al. [3] addressed steroidal and non-steroidal anti-androgen-induced hepatoxicity using the Spanish pharmaco-vigilance database. It, however, remained inconclusive for bicalutamide-induced hepatotoxicity due to the scarcity of reported cases. On the other hand, according to research published in 2005 by Guo et al., [13] bicalutamide’s risk of causing liver injury was rated as high by 2 compendia: Facts and Comparisons, and Physicians’ Desk Reference [13].
These cases and pharmacology data represent a varying spectrum of disease, which can be seen as a result of bicalutamide-induced liver injury. Patients are often receiving a complicated, multi-drug regimen, which may make the identification of the culprit drug challenging. The possibility of hepatotoxicity should be borne in mind when administering bicalutamide to facilitate timely intervention.
Conclusions
Bicalutamide is commonly used for treating prostate neoplasm and has a potential hepatotoxicity profile that needs to be considered when prescribing this drug. Once clinical symptoms and abnormal liver function test results are identified, discontinuation of the drug and monitoring of lab results is warranted. Given the potential for hepatotoxicity, initial liver enzyme testing and serial monitoring should be considered. Our case review draws attention to the potential for drug-induced liver injury with this valued therapy, which may range from asymptomatic abnormality of liver values to the rarely reported fatal fulminant hepatotoxicity that may ensue in this setting.
References:
- 1.American Cancer Society. Cancer Facts Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.Beza C, Sánchez Ruiz J, Peracaula Espino FJ, Villanego Beltrán MI. Drug-related hepatotoxicity and hepatic failure following combined androgen blockade. Clin Transl Oncol. 2008;10(9):591–92. doi: 10.1007/s12094-008-0256-5. [DOI] [PubMed] [Google Scholar]
- 3.Manso G, Thole Z, Salgueiro E, et al. A spontaneous reporting of hepatotoxicity associated with antiandrogens: data from the Spanish pharmaco-vigilance system. Pharmacoepidemiol Drug Saf. 2006;15(4):253–59. doi: 10.1002/pds.1168. [DOI] [PubMed] [Google Scholar]
- 4.Teschke R, Eickhoff A, Schulze J. Drug and herb induced liver injury in clinical and translational hepatology: Causality assessment methods, quo vadis? J Clin Translat Hepatol. 2013;1:59–74. doi: 10.14218/JCTH.2013.D002X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fradet Y. Bicalutamide in the treatment of prostate cancer. Expert Rev Anticancer Ther. 2004;4(1):37–48. doi: 10.1586/14737140.4.1.37. [DOI] [PubMed] [Google Scholar]
- 6.McLeod DG. Tolerability of nonsteroidal anti-androgens in the treatment of advanced prostate cancer. Oncologist. 1997;2:18–27. [PubMed] [Google Scholar]
- 7.Dawson L, Chow E, Morton G. Fulminant hepatic failure associated with bicalutamide. Urology. 1997;49:283–84. doi: 10.1016/S0090-4295(96)00355-X. [DOI] [PubMed] [Google Scholar]
- 8.O’Bryant CL, Flaig TW, Utz KJ. Bicalutamide-associated fulminant hepatotoxicity. Pharmacotherapy. 2008;28(8):1071–75. doi: 10.1592/phco.28.8.1071. [DOI] [PubMed] [Google Scholar]
- 9.Kolvenbag G, Blackledge G. Worldwide activity and safety of bicalutamide: a summary review. Urology. 1996;47:70–79. doi: 10.1016/s0090-4295(96)80012-4. [DOI] [PubMed] [Google Scholar]
- 10.McLeod DG, Iversen P, See WA, et al. for the Casodex Early Prostate Cancer Trialists’ Group Bicalutamide 150 mg plus standard care versus standard care alone for early prostate cancer. BJU Int. 2006;97:247–54. doi: 10.1111/j.1464-410X.2005.06051.x. [DOI] [PubMed] [Google Scholar]
- 11.Blackledge GR, Cockshott ID, Furr BJ. Casodex (bicalutamide): overview of a new antiandrogen developed for the treatment of prostate cancer. Eur Urol. 1996;31:30–39. doi: 10.1159/000474547. [DOI] [PubMed] [Google Scholar]
- 12.Kolvenbag GJ, Blackledge GR. Worldwide activity and safety of bicalutamide: a summary review. Urology. 1996;47(1A Suppl):70–79. doi: 10.1016/s0090-4295(96)80012-4. discussion 80–84. [DOI] [PubMed] [Google Scholar]
- 13.Guo JJ, Wigle PR, Lammers K, et al. Comparison of potentially hepatotoxic drugs among major US drug compendia. Res Social Adm Pharm. 2005;1(3):460–79. doi: 10.1016/j.sapharm.2005.06.005. [DOI] [PubMed] [Google Scholar]