Abstract
Background
The aim of this study was to investigate the distribution of different types of primary orbital tumors, histopathological diagnosis, and postoperative complications.
Material/Methods
We analyzed 122 patients (68 women and 54 men) with orbital tumors, hospitalized in the ENT Department of the Medical University of Silesia in Katowice during 1990–2013. The patients were characterized in terms of anatomic, topographical, histopathological, and clinical parameters. The role of diagnostic imagining such as CT, NMR, and fine-needle aspiration (FNB) in preoperative diagnostics is discussed. Results of FNB, cytological, and histopathological examination of the postoperative specimens were compared.
Results
There were 56 (46%) patients with malignant tumors, 42 (34%) with benign tumors, 19 (16%) with inflammatory tumors, and 5 patients (4%) had other tumors. In cases of malignant tumors, local recurrence up to 5 years was found in 36 (64.3%) cases. In the other 20 (35.7%) cases of malignant tumors, the patients remained under close follow-up in the outpatient clinic, without signs of local recurrence (follow-up 1–17 years). According to histopathological examination, malignant tumors were detected in 45.9% of patients and non-malignant tumor in 34.4% of patients. In 19.7% of patients, inflammatory and other types of tumors were diagnosed.
Conclusions
We characterized the occurrence and pathological profiles of orbital tumors. The tumor location, histopathological diagnosis, and postoperative complications give us important information for the diagnosis of tumor prior to biopsy or tumor resection and for the determination of the treatment strategy and possible complications after surgery.
MeSH Keywords: Orbital Diseases - surgery, Orbital Neoplasms - surgery, Orbital Pseudotumor
Background
The classification of orbital tumors is very difficult because tissues of various origins and structures are present in this region [1–3]. The most frequently used classification divides tumors into various categories, including: primary tumors deriving from the orbital tissues, intraocular, corneal, conjunctival tumors, and tumors penetrating the orbital cavity from adjacent areas, as well as metastases from other areas of the body [4].
The topographic anatomy of the eye socket – surrounded by paranasal sinuses and the cranial cavity, and the resulting mutual penetration of disease processes in that region – makes the eye socket an “interdisciplinary” region [6–8]. Therefore, the orbital cavity is an area of interest of many specialists, including ophthalmologists, ENT (ear, nose, and throat) doctors, endocrinologists, neurosurgeons, plastic surgeons, and maxillo-facial surgeons, and the application of many diagnostic methods is needed [7,9–11]. The diagnostics and treatment of the proliferation processes in the eye socket require the close co-operation of the above-mentioned specialists. The main manifestation is the protrusion of the eyeball and limitation of eyeball movement, and dysopsia, swelling, and reddening of conjunctivae, as well as blepharo-edema.
The aim of the study was to perform a histo-clinical analysis of patients with primary orbital tumors, together with the analysis of localization of the tumors within the eye socket, their extensiveness, and relation to the adjacent structures, possible penetration to paranasal sinuses, and estimation of long-term results of treatment.
Material and Methods
The study was conducted on a group of 122 patients with primary orbital tumors, undergoing surgical treatment in the ENT Department of the Medical University of Silesia in Katowice, Poland, in the years 1990–2013, of whom 68 were women (55.7%) aged 23–79 years (average age: 45 years) and 54 were men (44.3%) aged 27–72 years (average age: 40 years). The tumor was located in the left eye socket in 58.2% of patients and in the right in 41.8%. The characteristics of patients, depending upon the histopathological diagnosis, are presented in Tables 1 and 2. In the course of pre-operative diagnostics, most patients had the following examinations performed: X-ray imaging, CT (computed tomography), and/or MRI (magnetic resonance imaging). The locations of tumors were divided into 4 areas: upper-lateral, upper-medial, lower-lateral, and lower-medial on the basis of CT or MRI findings (Table 3). Figure 1A and 1B present results of CT examination of a 65-year-old woman with carcinoma plano epitheliale of the right orbit. Figure 2 presents MRI results of a 29-year-old woman with pleomorphic adenoma in the left orbit.
Table 1.
Characteristics of the patients undergoing surgical treatment with the consideration the histopathological diagnosis.
| Types of orbital tumors | Histo-pathologic diagnosis | Number of cases |
|---|---|---|
| Malignant neoplasms | Tumor mixtus mesenchymalis (fibrosarcoma, osteosarcoma, chondrosarcoma) | 1 |
| Melanoma malignum | 9 | |
| Retinoblastoma | 1 | |
| Haemangioendothelioma | 1 | |
| Lymphoma malignum | 14 | |
| Adenocarcinoma | 4 | |
| Tumor mixtus malignus | 4 | |
| Carcinoma adenoides cysticum | 3 | |
| Carcinoma planoepitheliale | 7 | |
| Carcinoma anaplasticum | 8 | |
| Carcinoma basocellulare | 4 | |
| Benign neoplasms | Tumor mixtus | 8 |
| Haemangioma | 9 | |
| Meningioma meningioendotheliale | 6 | |
| Neurinoma n. optici | 2 | |
| Glioma n. optici | 2 | |
| Neurofibromata | 2 | |
| Lipoma | 2 | |
| Fibroma | 2 | |
| Osteoma | 1 | |
| Congenital defects | Cystis epidermalis | 2 |
| Inflamatory neoplasms | Pseudotumor inflammatorius | 18 |
| Sarcoidosis | 1 | |
| Others | Haematoma | 2 |
| Amyloidosis | 2 | |
| Mikulicz’s disease | 1 | |
| Total | 122 |
Table 2.
Assessment of the types of orbital tumors on the basis of histopathological examination (n=122).
| Type of tumor | Number of cases | [%] |
|---|---|---|
| Malignant tumor | 56 | 45.9 |
| Non-malignant tumor | 42 | 34.4 |
| Inflammatory tumor | 19 | 15.6 |
| Others | 5 | 4.1 |
Table 3.
Assessment of specific quadrants of the eye socket (n=122).
| Quadrant of the eye socket | Number of cases | [%] |
|---|---|---|
| Upper-lateral | 19 | 15.57 |
| Upper-lateral and upper-medial | 14 | 11.47 |
| Upper-medial | 24 | 19.67 |
| Upper-lateral and lower-lateral | 31 | 25.42 |
| Lower-medial | 34 | 27.87 |
Figure 1.
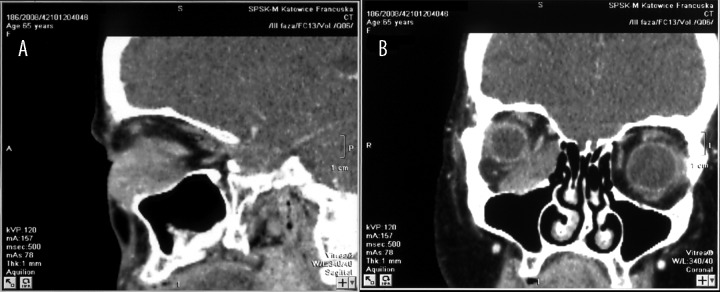
(A, B) Results of CT examination in 65-year-old woman with carcinoma planoepitheliale of the right orbit.
Figure 2.
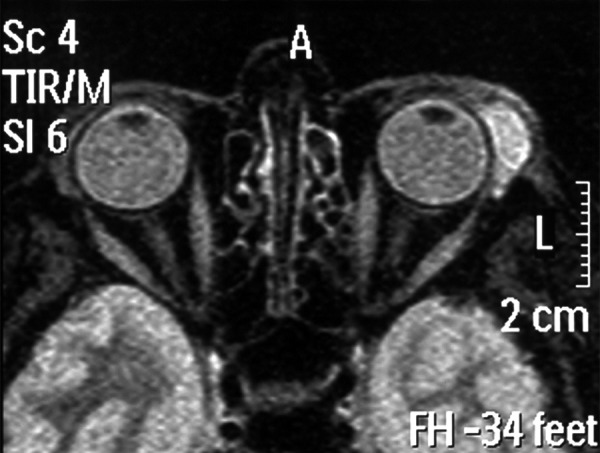
MRI examination of 29-year-old woman with pleomorphic adenoma in the left orbit.
Each patient with the diagnosis of orbital tumor underwent a full ophthalmological examination, before and after the surgery. The examination comprised assessment of vision acuity, assessment of the protrusion degree, assessment of the field of vision, examination of the fundus of the eye, and assessment of eyeball mobility.
In some patients, fine-needle aspiration (FNA) biopsy of the orbital tumor was performed before the operation. However, because of the specific topographic relations in the eye socket, FNA is difficult and has a high percentage of complications and false-negative results, especially in case of intra-conus location of the tumor.
All patients analyzed in this study underwent surgical treatment. In a case of malignant tumors (with the exclusion of lymphomas) the orbital exenteration was performed, followed by complementary radio- and chemotherapy. In cases in which the post-surgical histopathological examination revealed a malignant lymphoma texture, in-depth diagnostics was performed, and after it was confirmed that the focus in the eye socket was the only one in the body, patients underwent hospital hematological treatment.
In patients for whom histopathologic diagnosis of benign tumor was confirmed, depending on the tumor location, 1 of the 2 types of orbitotomy was performed. Lateral orbitotomy according to Krönlein-Reese-Berke was performed each time in a case of non-malignant tumors located laterally in relation to the optical nerve, whereas medial orbitotomy according to Sewall was performed in case the non-malignant tumors located in medial quadrants of the eye socket.
The post-operative material was assessed each time by a specialist in the field of histopathology. In a case of malignant tumors, patients were treated by means of complementary radio- and chemotherapy. All patients were strictly followed up in the laryngological outpatient clinic that is connected with the ENT Department.
The study protocol was approved by the local Research Ethics Committee of the Medical University of Silesia in Katowice.
Descriptive statistics and chi-square test (χ2) were used for data analysis, using SPSS 17.0 software.
Results
Among 122 patients with primary orbital tumors, 58.2% had the tumor located in the left eye socket, while 41.8% were in the right one (Figure 3). The most frequent manifestations in patients with primary tumors of the orbital cavity were as follows: protrusion of the eyeball (displaced at least 2 mm) occurred in 100% of patients, limitation of eyeball movement was observed in 45%, dysopsia (diplopia – in 16%) visual acuity attenuation occurred in 43%, pain in the eyeball in 30%, swelling and reddening of conjunctivae and eyelids in 54%, headaches in 26%, blindness in 6%, and blepharophimosis in 51%.
Figure 3.
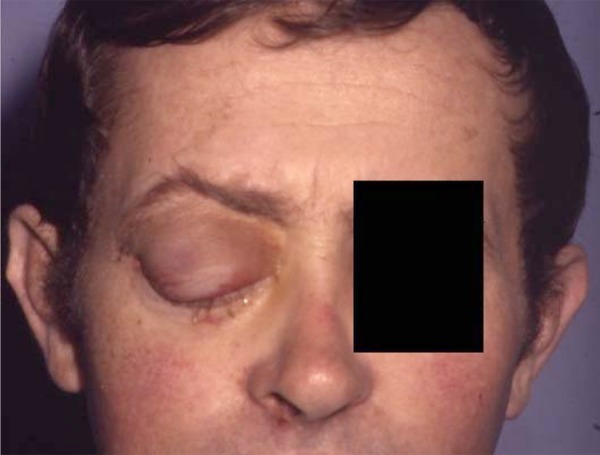
Protrusion of the eyeball in 48-year-old patient with haemangioma.
Table 1. Histopathological diagnosis of the primary tumor. The most common orbital tumor was pseudotumor inflammatorius (14.75% of patients). Lymphoma malignum was detected in 11.47% of patients. Regarding the type of orbital tumors, malignant tumors were observed in 45.9% of patients and non-malignant tumors were diagnosed in 34.4% of patients. Inflammatory tumors occurred in 15.6% of patients. Sarcoidosis, amyloidosis, or Mikulicz’s disease tumors were very rare (Table 2). In analyzing the frequency of occurrence of various histopathological types of orbital tumors, malignant ones were found to be statistically significantly more frequent than benign neoplasmatic (p<0.001) or inflammatory tumors (p=0.006).
Among 56 patients with malignant tumors operated on in our ENT Department, 13 cases had stage T1, 27 had T2, 12 had T3, and 4 cases of tumors were operated on in stage T4. Metastases to lymph nodes of the neck region (stage N1) were diagnosed in 4 patients.
Every time the location of the tumor in the particular quadrant of the eye socket was assessed, the estimation also determined eventual penetration of the tumor outside the eye socket area (Table 3). The analysis of tumor localization inside the orbital cavity revealed that 27.87% of all tumors were situated in the lower-medial part of orbital cavity, 25.4% in upper-lateral and lower-lateral, and 19.67% cases were localized in the upper-medial part (Table 3). Statistical analysis indicated that tumors simultaneously occupying 2 localizations – upper-lateral and upper-medial – are statistically significantly rarer than others. There were no statistically significant differences between the following localizations: lower-medial, upper-lateral, and upper-medial (p=0.331). Tumors were observed statistically significantly in lower-medial in comparison to upper medial localization (p<0.001), upper-lateral and upper-medial (p=0.006), and upper-lateral (p=0.09).
Extension to paranasal sinuses was found in 23 patients (frontal sinus – 8 cases, ethmoidal sinus – 6, maxillary sinus – 9). Figure 4 presents CT examination (3D reconstruction) with malignant tumor (carcinoma anaplasticum) of the upper-medial part of the right orbit penetrating to the cranial cavity.
Figure 4.
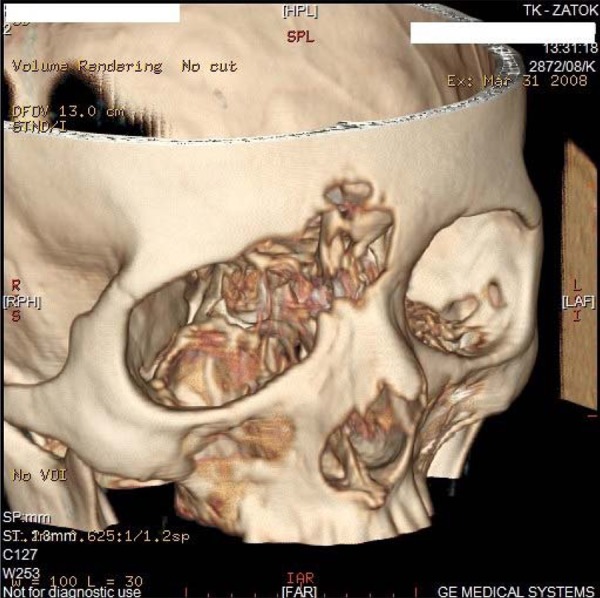
CT examination (3D reconstruction) with malignant tumor (carcinoma anaplasticum) of the upper-medial part of the right orbit penetrating to cranial cavity.
Surgical procedures in the eye socket are not free from complications. The complications after surgical treatment of benign and malignant orbital tumors are divided into vascular, neuro-muscular, inflammatory, and others (Table 4). The most frequent complication after lateral orbitomy was paralysis of muscles responsible for eyeball movement (8.8%). The most frequent complication following medial orbitomy was paralysis of muscles responsible for eyeball movement (15.6%). After orbital exenteration, the most frequent consequence was hemorrhage (8.9%) (Table 4).
Table 4.
Assessment of early and distant complications after orbitotomy and orbital exenteration.
| Complications after orbitotomy and orbital exenteration | ||
|---|---|---|
| Lateral orbitotomy (n=34) | Medial orbitotomy (n=32) | Orbital exenteration (n=56) |
|
|
|
Among 122 patients with orbital tumor, biopsy was performed in 59 cases (48.4%). In 23 patients (38.9%) the results of histopathologic examination and FNA biopsy differed from each other. In 20 cases (16.9%) that discrepancy concerned the pathomorphological texture, which did not require reoperation of patients. In the other 3 cases (5.1%) the discrepancy of examination results concerned the type of tumor (benign/malignant). In those patients, the histopathological examination confirmed the diagnosis of malignant tumor, which was not demonstrated by FNA before the surgery, and for that reason reoperation was necessary for the procedure to be radical.
Vision acuity after specific orbitotomy procedures was checked by an ophthalmologist in each patient at 1 and 3 months and 1 year after the operation.
The operation performed in patients with benign tumors did not cause a deterioration of visual acuity in a comparison to before the operation. Each patient had an obligatory ophthalmological examination after the operation.
In patients with inflammatory tumors of the orbital cavity (19 cases), a temporary deterioration of visual acuity, lasting up to 3 months after the operation, was observed in 4 individuals, whereas a permanent deterioration of visual acuity of 1 diopter was diagnosed in 6 patients. Eyeball mobility was fully maintained in all patients.
When cosmetic defects were involved, a linear scarf after a lateral orbitotomy performed by Krönlein-Reese-Berke method reaching a lateral eye angle was not a serious problem for men, but 9 women treated it as a cosmetic defect despite the fact that it could be partially covered by either by glasses or by an eyebrow in the case of horizontal orbitotomy.
Of the total number of 56 cases of malignant tumors, local recurrence up to 5 years was found in 36 (64.3%) cases. All those patients died. In the other 20 (35.7%) cases of malignant tumors, the patients remained under close follow-up in the outpatient clinic, without signs of local recurrence (follow-up from 1 to 17 years).
Discussion
Primary orbital tumors are very rare. According to the American Cancer Society, frequency of incidence of orbital tumors is less than 1 for 100 000 persons. The general symptomatology of developing orbital tumors is initially quite poor, because surrounding tissues adjust to the slowly growing tumor, delaying definitive diagnosis [5,7,12]. Only after it reaches about 1 cm does the tumor begin to push out the eyeball, and the patient has the vague sensation of tension and bursting in the eye socket [8]. As the tumor enlarges, protrusion of the eyeball occurs, along with limitation of eyeball movement, and dysopsia. Tumors located in the orbital conus cause a relatively quickly increasing protrusion of eyeballs, with deteriorated mobility of the eyeball and early changes at the fundus of the eye and dysopsia [4,7,13].
The selection of surgical method for the treatment of primary orbital tumors always depends upon the type of pathological texture of the tumor, its location within the eye socket (in relation to the eyeball and the optic nerve), and directions of its possible penetration [14]. In malignant tumors, the method of choice is orbital exenteration, possibly followed by radio- and chemotherapy [15,16].
In non-malignant tumors, on the other hand, depending upon the primary location of the tumor, the approach is performed through the medial wall (Sewall’s approach), through the upper wall that is the ceiling of the orbital cavity (Dandy’s and Naffziger’s approach), through the lower wall (Hirsch’s method, with approach through the maxillary sinus), via the eye socket (Knapp’s approach), and via the lateral wall (Kronlein’s approach). The lateral subtemporal approach, developed in 1888 by Kronlein, consists of temporary osteoplastic resection of the eye socket lateral wall [10,16]. All orbitotomies can be divided into simple ones without mobilization of bones and those with engagement of bones (with mobilization) [11].
In our material, on postoperative histopathological examination, the most frequently occurring tumors of the eye socket were pseudotumor inflammatorius (14.75%) and lymphoma malignum (11.47%). In the material analyzed by Pieńkowski et al., malignant neoplasms of the eye socket occurred in 24% of the patients, whereas inflammatory lesions occurred in 4% [17]. According to other authors, the most frequent eye socket neoplasms are inflammatory pseudotumors. Lateral orbitotomy has been the most frequent method of tumor resection [11]. In Caucasian populations, the percentage of people with malignant lymphoma is 5–15% [3,18]. In the elderly population (over 60 years of age), that percentage goes up to 24% [18]. Adenoid cystic carcinoma is another neoplasm frequently occurring in Caucasians [3]. In our material, it occurred less frequently, in only 3 patients. In the studies of Li et al. and Shilds et al. concerning orbital tumors, non-malignant tumors occurred most frequently in that area of the facial skeleton, whereas malignant tumors occurred considerably less frequently [3,12]. On the other hand, Shinder et al. demonstrated that in patients with orbital tumors, primary orbital tumors were the most frequently occurring ones (64%) [4]. A substantial percentage of orbital tumors comprised malignant neoplasms. Assessing the tumor location in our material, we detected such tumors most frequently in the inferior medial quadrant. In the studies of other authors, frequent locations of orbital lesions included he superior half of the orbit, the anterior orbit, and the extraconal space [18].
Many studies of primary orbital tumors have demonstrated a relationship between the types of orbital tumor patients and their age at diagnosis, but these relationships have not yet been clarified [18–20]. Our research confirms the observation that the complex nature of orbital pathologies may be the cause of diagnostic and therapeutic difficulties.
Conclusions
The basic method of treatment of malignant orbital tumors is surgery combined with irradiation and/or chemotherapy.
Late diagnosis is decisive for radical surgical procedures to be performed, which drastically reduce the quality of life and chances for complete recovery.
Fine-needle aspiration (FNA) of the orbital tumor is an important diagnostic examination, and in case there are not contraindications it should be performed each time an orbital tumor is diagnosed.
The results of treatment of malignant orbital tumors are not satisfactory. In the analyzed group of 56 patients with malignant tumors, the 5-year survival rate was only 36%.
The tumor location, histopathological diagnosis, and postoperative complications provide us with important information for the diagnosis of type of tumor prior to biopsy or tumor resection and for the determination of the treatment strategy.
Footnotes
Conflict of interest
The authors confirm that they have no conflicts of interest.
Source of support: Departmental sources
References
- 1.Mendoza-Santiesteban E, Mendoza-Santiesteban CE, Berazaín AR, et al. Diagnosis and surgical treatment of orbital tumors. Semin Ophthalmol. 2010;25(4):123–29. doi: 10.3109/08820538.2010.500188. [DOI] [PubMed] [Google Scholar]
- 2.Tailor TD, Gupta D, Dalley RW, et al. Orbital neoplasms in adults: clinical, radiologic, and pathologic review. Radiographics. 2013;33(6):1739–58. doi: 10.1148/rg.336135502. [DOI] [PubMed] [Google Scholar]
- 3.Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: The 2002 Montgomery Lecture, part 1. Ophthalmology. 2004;111(5):997–1008. doi: 10.1016/j.ophtha.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Shinder R, Al-Zubidi N, Esmaeli B. Survey of orbital tumors at a comprehensive cancer center in the United States. Head Neck. 2011;33(5):610–14. doi: 10.1002/hed.21498. [DOI] [PubMed] [Google Scholar]
- 5.Gierek T, Markowski J, Majzel K, et al. [Primary orbital tumors treated surgically in ENT department of Silesian Medical Academy in Katowice]. Otolaryngol Pol. 1999;53(1):13–18. [in Polish] [PubMed] [Google Scholar]
- 6.Schwartz RM, Coupland SE, Finger PT. Cancer of the orbit and adnexa. Am J Clin Oncol. 2013;36(2):197–205. doi: 10.1097/COC.0b013e31820dbf28. [DOI] [PubMed] [Google Scholar]
- 7.Chipczyńska B, Grałek M, Hautz W, et al. Orbital tumor as an initial manifestation of Wegener’s granulomatosis in children: a series of four cases. Med Sci Monit. 2009;15(8):CS135–38. [PubMed] [Google Scholar]
- 8.Gerbino G, Boffano P, Benech R, et al. Orbital lymphomas: Clinical and radiological features. J Craniomaxillofac Surg. 2013 doi: 10.1016/j.jcms.2013.07.017. pii: S1010-5182(13)00215-1. [DOI] [PubMed] [Google Scholar]
- 9.Khan SN, Sepahdari AR. Orbital masses: CT and MRI of common vascular lesions, benign tumors, and malignancies. Saudi J Ophthalmol. 2012;26(4):373–83. doi: 10.1016/j.sjopt.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toniato G, Skrap M. Orbital lesions: surgical management within a multidisciplinary treatment. J Neurosurg Sci. 2009;53(3):77–91. [PubMed] [Google Scholar]
- 11.Wróbel A, Składzień J, Gawlik J, et al. Orbital tumors treated at the University Hospital Otolaryngology Clinic in Kraków between 1997 and 2011. Przegl Lek. 2013;70(7):417–20. [in Polish] [PubMed] [Google Scholar]
- 12.Liu Y, Ma JR, Xu XL. Transcranial surgery through pterional approach for removal of cranio-orbital tumors by an interdisciplinary team of nurosurgeons and ophthalmologists. Int J Ophthalmol. 2012;5(2):212–16. doi: 10.3980/j.issn.2222-3959.2012.02.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seregard S, Tani E. Fine needle aspiration cytology in orbital tumors. Monogr Clin Cytol. 2012;21:82–89. doi: 10.1159/000331039. [DOI] [PubMed] [Google Scholar]
- 14.Debnam JM, Mayer RR, Esmaeli B, et al. Three-Dimensional Multidetector CT for Anatomic Evaluation of Orbital Tumors. J Ophthalmol. 2013;2013:674230. doi: 10.1155/2013/674230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halli RC, Mishra S, Kini YK, et al. Modified lateral orbitotomy approach: a novel technique in the management of lacrimal gland tumors. J Craniofac Surg. 2011;22(3):1035–38. doi: 10.1097/SCS.0b013e3182107628. [DOI] [PubMed] [Google Scholar]
- 16.Kim JW, Yates BS, Goldberg RA. Total lateral orbitotomy. Orbit. 2009;28(6):320–27. doi: 10.3109/01676830903334028. [DOI] [PubMed] [Google Scholar]
- 17.Pieńkowski P, Wieloch M, Golusiński W, et al. Orbital tumors in material of Department of Head and Neck Surgery and Oncological Laryngology of Greater Poland Cancer Center 2007–2010. Otolaryngol Pol. 2012;66(1):39–42. doi: 10.1016/S0030-6657(12)70747-3. [in Polish] [DOI] [PubMed] [Google Scholar]
- 18.Ohtsuka K, Hashimoto M, Suzuki Y. A review of 244 orbital tumors in Japanese patients during a 21-year period: origins and locations. Jpn J Ophthalmol. 2005;49(1):49–55. doi: 10.1007/s10384-004-0147-y. [DOI] [PubMed] [Google Scholar]
- 19.Demirci H, Shields CL, Shields JA, et al. Orbital tumors in the older adult population. Ophthalmology. 2002;109(2):243–48. doi: 10.1016/s0161-6420(01)00932-0. [DOI] [PubMed] [Google Scholar]
- 20.Nassab RS, Thomas SS, Murray D. Orbital exenteration for advanced periorbital skin cancers: 20 years experience. J Plast Reconstr Aesthet Surg. 2007;60(10):1103–9. doi: 10.1016/j.bjps.2007.02.012. [DOI] [PubMed] [Google Scholar]


