Abstract
In cattle, conceptus-maternal interactions are critical for the establishment and maintenance of pregnancy. A major component of this early interaction involves the transport of nutrients and secretion of key molecules by uterine epithelial cells to help support conceptus development during the peri-implantation period of pregnancy. Objectives were to: 1) analyze temporal changes in the amino acid (AA) content of uterine luminal fluid (ULF) during the bovine estrous cycle; 2) understand conceptus-induced alterations in AA content; 3) determine expression of AA transporters in the endometrium and conceptus; and 4) determine how these transporters are modulated by (Progesterone) P4. Concentrations of aspartic acid, arginine, glutamine, histidine, lysine, isoleucine, leucine, phenylalanine and tyrosine decreased on Day 16 of the estrous cycle but increased on Day 19 in pregnant heifers (P<0.05). Glutamic acid only increased in pregnant heifers on Day 19 (P<0.001). Asparagine concentrations were greater in ULF of cyclic compared to pregnant heifers on Day 7 (P<0.05) while valine concentrations were higher in pregnant heifers on Day 16 (P<0.05). Temporal changes in expression of the cationic AA transporters SLC7A1 SLC7A4 and SLC7A6 occurred in the endometrium during the estrous cycle/early pregnancy coordinate with changes in conceptus expression of SLC7A4, SLC7A2 and SLC7A1 (P<0.05). Only one acidic AA transporter (SLC1A5) increased in the endometrium while conceptus expression of SLC1A4 increased (P<0.05). The neutral AA transporters SLC38A2 and SLC7A5 increased in the endometrium in a temporal manner while conceptus expression of SLC38A7, SLC43A2, SLC38A11 and SLC7A8 also increased (P<0.05). P4 modified the expression of SLC1A1, -1A4, -1A5, -38A2, -38A4, -38A7, -43A2, -6A14, -7A1, -7A5 and -7A7 in the endometrium. Results demonstrate that temporal changes in AA in the ULF reflect changes in transporter expression in the endometrium and conceptus during early pregnancy in cattle, some of which are modified by P4.
Introduction
A major cause of infertility in cattle is embryo mortality due to conceptus and/or uterine dysfunction during the pre-implantation period of pregnancy. In cattle, the majority of embryonic loss occurs prior to maternal recognition of pregnancy [1] which occurs on approximately Day 16 of pregnancy [2], [3]. Thus, conceptus-maternal interactions are critical to the establishment and maintenance of pregnancy. Major components of this early conceptus-maternal interaction involve transporters for nutrients and secretions of key molecules by uterine epithelial cells that support conceptus development during the peri-implantation period of pregnancy [4], [5], [6], [7]. These secretions and transported molecules make up histotroph that represents maternal contributions to uterine luminal fluid (ULF). The requirement for these uterine-derived secretions and transported nutrients for successful pregnancy is well established in the ovine uterine gland knockout model in which the conceptus fails to elongate [8]. In cattle, ULF is a prerequisite for development of the embryo beyond the hatched blastocyst stage in vivo, and attempts to artificially induce elongation of bovine conceptuses in vitro have been unsuccessful [9], [10]. The ULF of ruminants is composed of both secreted and transported molecules that include numerous secreted proteins [11], [12], glucose [13], ions [13], fatty acids [14] and amino acids [13], [15]. In sheep, a significant increase in total amino acid content occurs between Days 10 and 16 of pregnancy [13]. Moreover, the expression of cationic [16], acidic and neutral amino acid transporters is altered in a spatial and temporal manner in both the endometrium and conceptus of sheep during early pregnancy [13] and is modulated by ovarian progesterone (P4) and/or conceptus interferon tau (IFNT), prostaglandins and cortisol in vivo [17], [18], [19].
In spite of the importance of maternally-derived secretions, including amino acids, there are limited reports pertaining to the requirements for conceptus elongation and successful pregnancy recognition in cattle. What is known, however, is that amino acids are utilized by the early embryo both in vitro and in vivo [20], [21], [22], [23]. In addition, total amounts of amino acids increase in ULF as the estrous cycle progresses [24] and are higher in ULF of pregnant compared to non-pregnant heifers on Day 18 [12]. Furthermore the abundance of individual amino acids (e.g., valine) is regulated by P4 [25].
The hypothesis tested was that the amino acid composition of bovine ULF changes in a temporal manner during the estrous cycle and early pregnancy due to alterations in the expression of their transporters in the endometrium and conceptus. The objectives were to: 1) analyze the temporal changes in the amino acid content of ULF during the bovine estrous cycle; 2) understand conceptus-induced alterations in amino acid content of ULF during critical windows of early pregnancy; 3) determine expression of the transporters in the endometrium and conceptus responsible for shuttling these amino acids into and out of the uterine lumen; and 4) determine how these transporters are modulated in the endometrium by P4.
Materials and Methods
All experimental procedures involving animals were licensed by the Department of Health and Children, Ireland, in accordance with the Cruelty to Animals Act (Ireland 1876) and the European Community Directive 86/609/EC and were sanctioned by the Animal Research Ethics Committee of University College Dublin. Unless otherwise stated, all chemicals were sourced from Sigma (Dublin, Ireland).
Study 1: Analysis of the amino acid content of uterine luminal fluid and expression of amino acid transporters in the endometrium during the estrous cycle and peri-implantation period of early pregnancy
The estrous cycles of 100 cross-bred beef heifers were synchronized as previously described [26] by insertion of a controlled internal drug release (CIDR) device (1.38 g progesterone; InterAg, Hamilton, New Zealand) into the vagina for 8 days. A 2 ml intramuscular injection of a prostaglandin F2α (PG) analogue (Estrumate, Shering-Plough Animal Health, Hertfordshire, UK: equivalent to 0.5 mg cloprostenol) was administered one day before CIDR removal. Heifers were checked for estrus and only those detected in standing heat (estrus = Day 0) were utilized further. In order to generate pregnant (P) and cyclic (C) tissues, heifers were assigned randomly to either an inseminated group (n = 59) or a non-inseminated cyclic control group (n = 24). Cyclic heifers were slaughtered on Day 7, 10, 13, or 16 of the estrous cycle and inseminated heifers were slaughtered on Day 7, 10, 13, 16 or Day 19 of pregnancy. These stages correspond in pregnant animals to the times of blastocyst formation, blastocyst hatching, initiation of elongation, maternal recognition of pregnancy and initiation of implantation, respectively. Cyclic heifers were not included for the Day 19 time-point as they had undergone luteolysis at this stage. At slaughter, the uterine horn ipsilateral to the corpus luteum (CL) was flushed with 20 ml of 10 mM Tris (pH 7.2) and in the inseminated group, only those flushings that contained an appropriately developed conceptus (i.e., correct stage for age) were processed further. Recovered ULF was centrifuged at 1,000 g for 15 min, supernatant decanted and immediately snap frozen in 1 ml aliquots in liquid nitrogen for subsequent amino acid analysis. Intercaruncular and caruncular endometrial tissues were dissected out separately from the uterine horn ipsilateral to the CL and snap-frozen in liquid nitrogen for RNA extraction and quantitative real-time PCR (qRT-PCR).
Study 2: Expression of amino acid transporters in the conceptus during key developmental stages
Analysis of the expression of amino acid transporters in the embryo/conceptus was carried out by screening RNA sequencing data generated as previously described [27]. Briefly, estrus synchronization of cross-bred beef heifers (n = 72) was performed as described for Study 1. Heifers observed in standing heat were inseminated. Animals were assigned randomly for slaughter on Day 7, 10, 13, 16 or 19 of pregnancy. At slaughter, each uterine horn was flushed with 20 ml phosphate buffered saline (PBS) containing 3% fetal calf serum (FCS), the embryo/conceptus recovered and snap frozen in liquid nitrogen. Only those uterine flushings with conceptuses at the correct morphological stage of development for their age were analysed. RNA was extracted from whole conceptuses, cDNA libraries were prepared and cluster generation and sequencing were carried out using standard procedures for the Illumina genome analyzer sequencer (www.illumina.com). The RNAseq samples were processed through the standard software pipeline for the Genome Analyzer (http://bioinfo.cgrb.oregonstate.edu/docs/solexa/SCS2_01_IPAR1_01_Release_Notes.pdf). The CASAVA module from Illumina software was used to process RNAseq data. All data were aligned against the BosTau4 genome and a pseudochromosome containing potential splice junction sequences was generated. This gene expression data set was then screened for expression of members of the solute-like carrier (SLC) gene family for transport of amino acids.
Study 3: Identification of changes in endometrial expression of amino acid transporters by manipulation of P4 concentrations in vivo
The estrous cycles of cross-bred beef heifers were synchronized as described in Study 1 and only those observed in standing estrus (n = 52) were used. Heifers were assigned randomly to one of three treatments, (i) high P4 (n = 12), (ii) normal P4 (n = 12) and (iii) low P4 (n = 28). Heifers in the high P4 group had a progesterone-releasing intravaginal device (PRID, CEVA, Libourne, France) inserted on Day 3 of the estrous cycle to elevate P4 concentrations [28], while heifers in the control group received no P4 manipulation. Heifers assigned to the low P4 group received three intramuscular injections of PG (Estrumate, Shering-Plough Animal Health, Hertfordshire, UK) on Day 3, 3.5 and 4 of the estrous cycle to reduce P4 output from the CL, as previously described [29]. Daily blood samples were obtained from all heifers up to day of slaughter on either Day 7 or Day 13 of the estrous cycle. Following slaughter, intercaruncular endometrial tissue from the tip of the uterine horn ipsilateral to the CL was recovered, snap frozen in liquid nitrogen for subsequent RNA extraction and qRT-PCR was performed for selected amino acid transporters.
Analysis of uterine luminal fluid amino acids
The amino acid content of ULF was measured by High Performance Liquid Chromatography (HPLC) as previously described [23]. In summary, the amino acids present in ULF were derivatised with O-Phthaldialdehyde reagent, supplemented with 1 mg/ml 2-mercaptoethanol. Reverse phase chromatography was subsequently performed on an Agilent 1100 Series HPLC system coupled with a Phenomenex HyperClone 5 mm C-18 ODS 250×4.6 mm column (Phenomenex, Macclesfield, UK). A gradient elution with two buffers comprised of (A) 80% 83 mM sodium acetate, 19.5% methanol, 0.5% tetrahydrofuran, and (B) 80% methanol and 20% 83 mM sodium acetate was used to separate OPA-amino acid derivatives at 30°C for 60 min at a flow rate of 1.3 ml/min. Concentrations (µM) were determined by comparing the area under the curve for each peak to those given from certified standards.
Quantitative real-time PCR analysis (qRT-PCR)
For both Studies 1 and 3 total RNA was extracted from 100 mg of endometrial tissue using Trizol reagent as per manufacturer's instructions. RNA clean-up and on-column DNase treatment were performed (Qiagen, Crawley, Sussex, UK). Both the quality and quantity of extracted RNA was determined using the Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and Nanodrop 1000 (Thermo Fischer Scientific, DE, USA), respectively. One microgram of total RNA was converted to complementary DNA (cDNA) using Superscript III (Applied Biosystems, Foster City, CA, USA) and random hexamers as per manufacturer's instructions. All primers were designed using Primer-BLAST software (www.ncbi.nlm.nih.gov/tools/primer-blast/) to span exon-exon boundaries where possible. Each qRT-PCR reaction was carried out on the 7500 Fast Real-Time PCR System (Applied Biosystems) with 50 ng cDNA, optimized primer concentrations (Table S1), and 7.5 µl FAST Sybrgreen mastermix (Applied Biosystems) in a final reaction volume of 15 µl. Cycling conditions were as follows: 2 min at 50°C, 10 min at 95°C, and 40 cycles of 95°C for 15 sec and 60°C for 1 min and were carried out with the inclusion of a dissociation curve to ensure specificity of amplification. A standard curve was included for each gene of interest as well as for the normalizer gene to obtain primer efficiencies. All raw cycle threshold values were then imported into qbaseplus software (Biogazelle, Zwijnaarde, Belgium) where data were calibrated, normalized and expression values for each gene were determined in arbitrary units (CNRQ).
Data analysis
All data were analysed using the SAS statistical package (SAS Institute Inc., Cary, NC, version 9.1.3). For gene expression analysis, the log of the calibrated, normalized, relative expression values (CNRQ) in arbitrary units from the endometrium or transcripts per million from the embryo/conceptus were inputted into the SAS program while total concentrations of amino acids in the ULF were used for amino acid analysis. Data were checked for normality and homogeneity of variance by histograms, qqplots, and formal statistical tests as part of the UNIVARIATE procedure of SAS. Data that were not normally distributed were transformed by raising the variable to the power of lambda. The appropriate lambda value was obtained by conducting a Box-Cox transformation analysis using the TRANSREG procedure of SAS. The transformed data were used to calculate P values. Gene expression values were analyzed using the general linear model procedures (PROC GLM) with day, pregnancy status, P4 concentration and/or tissue type (i.e., caruncular vs intercaruncular) where appropriate as the main effects. Treatment effects on gene expression were separated by Tukey's test and a p value of ≤0.05 was considered significant. Concentrations of amino acids were analyzed with the MIXED procedure of SAS. Fixed effects included experimental treatment (cyclic and pregnant), day, and their interaction. The interaction term, if not statistically significant (P>0.10), was subsequently excluded from the final model. Heifer within treatment was included as a random effect. The type of variance-covariance structure used was chosen depending on the magnitude of the Akaike information criterion for models run under compound symmetry, unstructured, autoregressive, or Toeplitz variance-covariance structures. Differences between treatments were determined by F-tests using Type III sums of squares. The PDIFF command incorporating the Tukey test was applied to evaluate pairwise comparisons between treatment means.
Results
Amino acid content of ULF during the peri-implantation period of pregnancy in cattle
Analysis of the ULF detected the presence of 18 amino acids of which threonine and glycine were most abundant on all days examined. The acidic amino acids, aspartic and glutamic acids, were detected in the ULF throughout the estrous cycle and early pregnancy. Aspartic acid concentrations decreased on Day 16 in both pregnant and cyclic heifers compared to all other days; however, on Day 19 of pregnancy, concentrations were higher (P<0.05) than for Day 16 (Table 1). Glutamic acid concentrations did not change between Days 7 and 16 (P>0.05), but increased from Day 16 to Day 19 in pregnant heifers (P<0.001).
Table 1. The effect of day and pregnancy status on the abundance of amino acids (µM) in bovine uterine luminal fluid during the peri-implantation period of pregnancy on Days 7, 10, 13, 16 for cyclic heifers and Days 7, 10, 13, 16 and 19 for pregnant heifers (n = 5 per treatment per time-point).
| Amino acid | Treatment | Day 7 | Day 10 | Day 13 | Day 16 | Day 19 | Day effect | Pregnancy effect | Day x Pregnancy |
| Basic amino acids | |||||||||
| Arginine | Cyclic | 12.34±2.77ax | 8.34±2.48ax | 13.32±2.48ax | 7.31±2.77bx | - | *** | * | ns |
| Pregnant | 7.89±2.26ay | 5.97±2.26ay | 7.32±2.26ay | 7.14±2.77ax | 15.94±7.15b | ||||
| Histidine | Cyclic | 5.67±1.21ab | 4.23±1.21ab | 5.79±1.21a | 2.37±1.21b | - | ns | ns | ns |
| Pregnant | 3.69±0.99a | 4.26±0.99a | 5.01±1.40ab | 4.33±1.21a | 8.54±2.88b | ||||
| Lysine | Cyclic | 16.38±2.92a | 9.78±2.90ab | 13.57±2.61a | 5.58±1.75b | - | ns | ns | ns |
| Pregnant | 10.38±2.95a | 7.23±2.76a | 8.43±1.77a | 9.55±10.70a | 21.47±8.51b | ||||
| Acidic amino acids | |||||||||
| Aspartic acid | Cyclic | 12.52±2.92a | 12.62±2.61a | 12.25±2.61a | 4.50±2.92b | - | * | ns | ns |
| Pregnant | 9.08±2.38a | 9.70±2.38a | 8.93±2.38a | 4.13±2.92b | 9.76±4.37a | ||||
| Glutamic acid | Cyclic | 47.11±10.66a | 43.84±9.54a | 43.54±10.74a | 22.06±16.60a | - | ns | ns | ns |
| Pregnant | 38.58±7.60a | 39.53±4.72a | 40.40±7.74a | 21.76±2.42a | 69.43±27.18b | ||||
| Small neutral amino acids | |||||||||
| Alanine | Cyclic | 35.27±8.09a | 39.35±7.24a | 42.54±7.24a | 23.90±8.09a | - | ns | ns | ns |
| Pregnant | 26.63±6.61a | 27.54±6.61a | 30.28±6.61a | 30.59±8.09a | 51.13±17.99a | ||||
| Asparagine | Cyclic | 3.12±0.35ax | 1.63±0.35b | 1.44±0.31b | 0.80±0.40b | - | *** | ns | 0.1 |
| Pregnant | 2.14±0.29ay | 1.33±0.29b | 1.28±0.40ab | 1.68±0.35ab | 3.41±1.47ab | ||||
| Glycine | Cyclic | 114.12±42.78a | 150.84±92.73a | 65.91±28.07a | 52.35±30.50a | - | ns | ns | ns |
| Pregnant | 129.90±57.83a | 133.98±77.48a | 57.41±36.66a | 30.41±6.00a | 54.78±22.77a | ||||
| Serine | Cyclic | 21.63±3.94a | 20.42±3.52a | 17.27±4.55a | 14.89±3.94a | - | ns | ns | ns |
| Pregnant | 15.78±3.22a | 13.13±3.22a | 22.23±3.52a | 22.90±3.94a | 27.64±9.26a | ||||
| Threonine | Cyclic | 177.03±14.41ax | 268.62±12.89bx | 213.52±12.89ax | 181.33±14.41ax | - | *** | 0.06 | *** |
| Pregnant | 208.61±11.77ax | 195.70±12.89ay | 212.91±11.77ax | 151.07±14.41bx | 124.53±5.96b | ||||
| Large neutral amino acids | |||||||||
| Glutamine | Cyclic | 24.37±4.25ax | 17.08±4.45abx | 24.18±4.17ax | 12.74±3.74bx | - | *** | *** | *** |
| Pregnant | 14.60±7.07ax | 16.47±7.45ax | 21.97±8.20ax | 18.17±1.62by | 36.79±11.89c | ||||
| Isoleucine | Cyclic | 8.81±1.58a | 5.87±1.41ab | 7.36±1.41ab | 3.27±1.58b | - | ns | ns | ns |
| Pregnant | 5.79±1.29a | 4.45±1.29a | 4.39±1.29a | 5.15±1.58a | 9.78±3.82b | ||||
| Leucine | Cyclic | 17.60±3.26a | 12.27±2.91ab | 15.73±2.91a | 6.57±3.26b | - | ns | ns | ns |
| Pregnant | 11.78±2.66a | 9.18±2.66a | 9.73±2.66a | 10.45±3.26a | 20.28±9.32b | ||||
| Methionine | Cyclic | 10.98±1.68ax | 11.83±1.94ax | 5.92±1.50bx | 2.85±1.68bx | - | *** | * | ns |
| Pregnant | 7.83±1.37ax | 6.97±1.37aby | 4.48±1.37abx | 3.43±1.68bx | 7.66±3.39a | ||||
| Phenylalanine | Cyclic | 7.46±1.22a | 5.84±1.22ab | 6.14±1.09a | 2.77±1.22b | - | ns | 0.07 | ns |
| Pregnant | 5.14±1.00a | 3.69±1.00a | 3.59±1.00a | 4.59±1.22a | 9.48±3.82b | ||||
| Tryptophan | Cyclic | 3.46±0.83a | 3.42±0.83a | 2.89±0.96a | 1.23±1.18a | - | ns | ns | ns |
| Pregnant | 2.03±0.68a | 2.65±0.68a | 2.03±0.96a | 2.43±0.96a | 5.49±2.64b | ||||
| Tyrosine | Cyclic | 7.55±1.45a | 6.06±1.45ab | 6.15±1.30ab | 2.66±1.45b | - | ns | ns | ns |
| Pregnant | 4.82±1.19a | 5.41±1.19a | 3.66±1.19a | 4.43±1.45a | 8.45±3.14b | ||||
| Valine | Cyclic | 15.57±3.90ax | 10.07±3.81ax | 13.15±2.97ax | 6.33±0.00bx | - | *** | ns | *** |
| Pregnant | 10.88±1.08ax | 8.81±0.66bx | 7.89±0.53bx | 9.31±0.00cy | 17.10±6.31d | ||||
Significant overall effects of day, pregnancy status and their interactions are noted by an asterisk (*). Temporal differences are indicated by different superscript letters a, b, c, i.e. significant differences in amino acid abundance between sequential days of the estrous cycle or early pregnancy when P<0.05. Differences between pregnant and cyclic heifers on a given day are denoted by x,y when P<0.05.
The basic amino acids arginine, histidine and lysine, displayed similar changes in abundance as the concentration of all these amino acids decreased on Day 16 of the estrous cycle compared to other days of the estrous cycle. This decrease did not occur in pregnant heifers; indeed, the concentrations of all three basic amino acids in ULF was greater on Day 19 compared to Day 16 (P<0.05).
Of the small neutral amino acids, concentrations of alanine, glycine and serine in ULF from both pregnant and cyclic heifers were similar for all days examined (P>0.05). Concentrations of asparagine were highest in cyclic heifers on Day 7, declined significantly by Day 10 and remained low thereafter, while in pregnant heifers, despite an initial decline in concentrations on Day 10, concentrations were relatively stable throughout early pregnancy. Concentrations of asparagine were greater in ULF of cyclic compared to pregnant heifers on Day 7 (P<0.05). Concentrations of threonine were elevated on Day 10 compared to all other days in cyclic heifers while concentrations in pregnant heifers were lower (P<0.05) on Day 16 and 19 compared with earlier time-points.
Of the large neutral amino acids in the ULF, glutamine, isoleucine, leucine, phenylalanine, tyrosine and valine exhibited similar trends in cyclic heifers with a decline in concentration on Day 16 of the estrous cycle. In pregnant heifers, concentrations of these amino acids as well as tryptophan were stable from Day 7 to Day 13, but increased (P<0.05) on Day 16 (glutamine) or Day 19 (isoleucine, leucine, phenylalanine, tryptophan, tyrosine, valine) of pregnancy. In contrast, concentrations of methionine decreased (P<0.05) from Day 13 onwards in cyclic heifers, while in pregnant heifers concentrations were lowest on Day 16.
Expression of cationic amino acid transporters in the endometrium and conceptus during the peri-implantation period of pregnancy
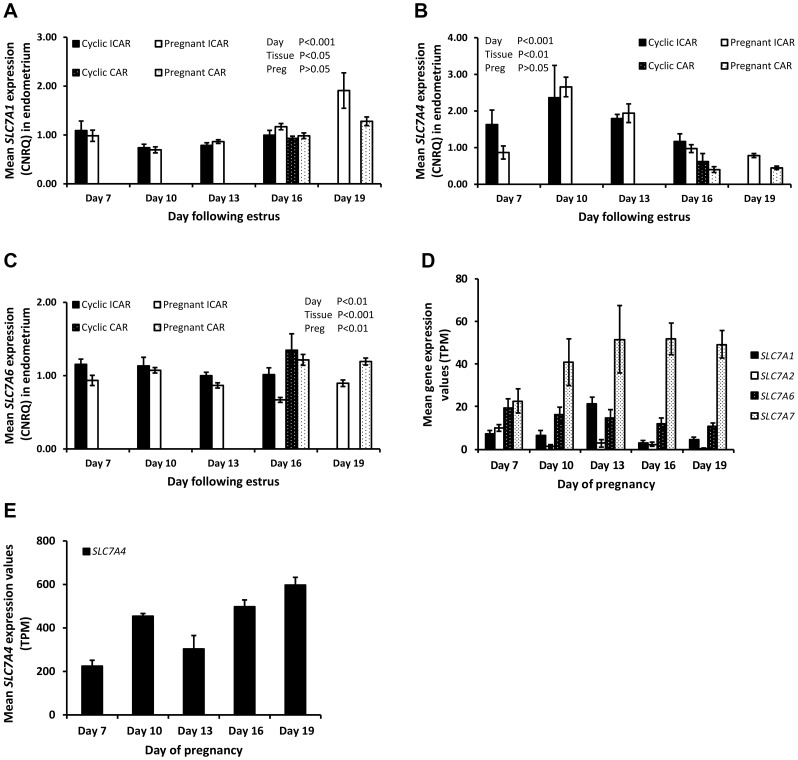
Endometrial expression of SLC7A1 was affected by day with an increase (P<0.01) in expression at the latter stages of the estrous cycle and early pregnancy while all other members of this transport family (SLC7A4 and SLC7A6) decreased (P<0.01) from Day 10 to 16 in the intercaruncular region of the endometrium (Table 2). In addition, expression of SLC7A1 and SLC7A4 mRNAs was less and SLC7A6 expression was greater (P<0.05) in caruncular compared to intercaruncular regions of the endometrium (Figures 1A–C). Pregnancy significantly affected SLC7A6 expression which was less abundant in intercaruncular regions of pregnant compared to cyclic endometria by Day 16. In the conceptus, SLC7A4 was approximately 10-fold more abundant than the other cationic transporters. SLC7A2 expression decreased with increasing conceptus age while SLC7A6 and SLC7A7 remained unchanged. The transporter SLC7A1, increased (P<0.01) as the conceptus developed from Day 7 to Day 13, but declined on Day 16 while SLC7A4 expression was lower (P<0.01) on Day 10 compared to Day 7 (Figures 1 D&E).
Table 2. The effect of day, pregnancy status, progesterone concentration and/or caruncular v intercaruncular endometrial tissue on expression of solute like carrier family member genes for amino acid transporters in the embryo/conceptus and endometrium as determined by RNAseq and quantitative real-time PCR (qRT-PCR) analysis respectively.
| Gene | Embryo/Conceptus | Endometrium | |||
| Day | Day | Caruncular v Intercaruncular | Pregnancy Status | P4 Concentration | |
| SLC1A1 | ** | - | - | - | * |
| SLC1A2 | NS | - | - | - | NS |
| SLC1A3 | * | NS | *** | NS | NS |
| SLC1A4 | *** | NS | *** | NS | * |
| SLC1A5 | *** | *** | NS | P = 0.06 | * |
| SLC38A11 | *** | ** | NS | NS | NS |
| SLC38A2 | ** | *** | ** | ** | * |
| SLC38A4 | - | *** | NS | * | * |
| SLC38A7 | * | *** | *** | NS | * |
| SLC43A2 | *** | *** | *** | NS | * |
| SLC6A14 | NS | ** | *** | P = 0.06 | * |
| SLC7A1 | *** | *** | * | NS | * |
| SLC7A2 | *** | - | - | - | NS |
| SLC7A4 | *** | *** | ** | NS | NS |
| SLC7A5 | ** | ** | NS | NS | * |
| SLC7A6 | NS | ** | *** | ** | NS |
| SLC7A7 | NS | *** | NS | NS | * |
| SLC7A8 | P = 0.06 | ** | NS | NS | NS |
Significant differences are noted by an astrisk (*). Significance set at P<0.05 (*), P<0.01 (**) or P<0.001 (***). A dashed line indicates that gene was not detectable in the specific tissue type.
Figure 1. Gene expression values in the endometrium for cationic amino acid transporters as determined by qRT-PCR analysis.
Data are displayed as mean calibrated, normalized, relative expression values in arbitrary units (CNRQ ± SEM) in the intercaruncular region of the endometrium from cyclic (solid bars) and pregnant (open bars) heifers and caruncular regions of cyclic (black bars white stipple) and pregnant heifers (while bars black stipple) (n = 5 per treatment per time-point). (A) SLC7A1 CNRQ expression with a significant effect of day and tissue type (B) SLC7A4 expression was significantly affected by day and tissue type and (C) SLC7A6 whose expression was significantly affected by day, tissue type and pregnancy status. Significance was set at P<0.05. (D&E) Gene expression values for cationic amino acid transporters in the bovine conceptus during distinct developmental stages. Values were determined by RNA sequencing and are given as mean transcripts per million (TPM ± SEM) with n = 5 per time-point. A significant effect of stage of conceptus development was observed for all genes (P<0.05).
Expression of acidic amino acid transporters in the endometrium and conceptus during the pre-implantation period of pregnancy
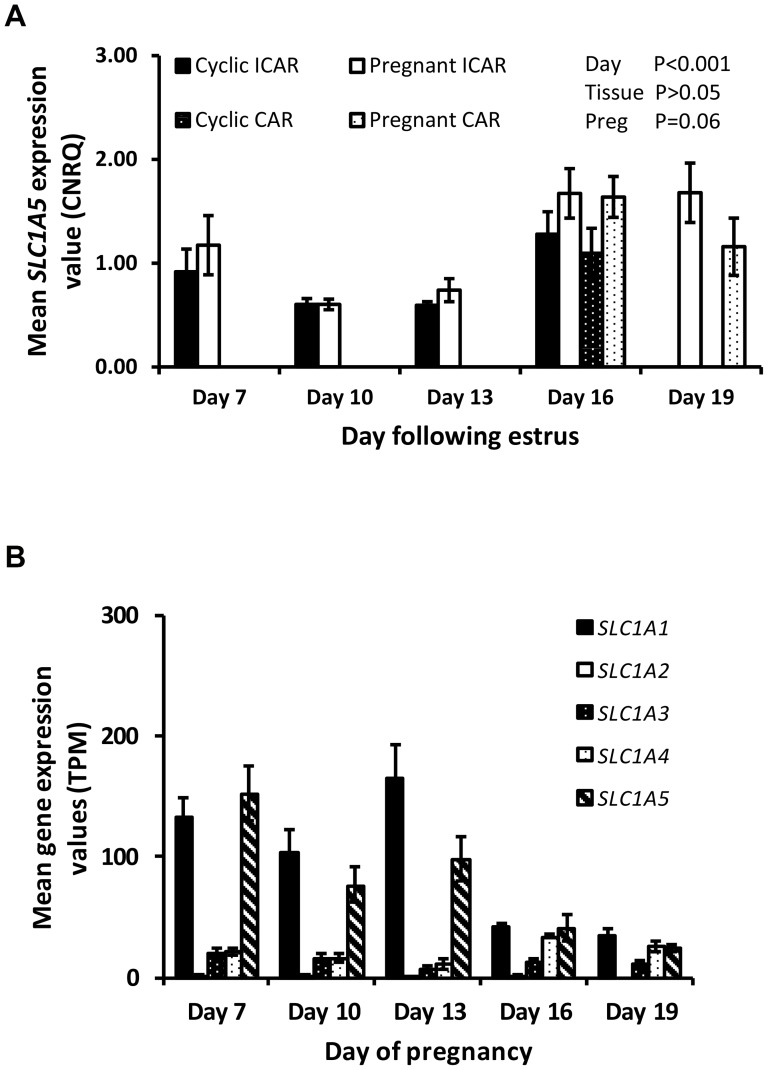
In the endometrium, the expression of SLC1A1, SLC1A2 SLC1A3 and SLC1A4 mRNAs was not affected by day. Expression of SLC1A3 and SLC1A4 mRNAs was lower (P<0.05) in caruncular compared to intercaruncular regions during the latter stages of the estrous cycle and early pregnancy (Table 2). The expression of SLC1A5 in the endometrium increased (P<0.0001) as the estrous cycle and early pregnancy progressed and was affected by pregnancy status (P = 0.06: Figure 2A). In contrast, SLC1A1, SLC1A3 and SLC1A5 in the conceptus decreased with increasing conceptus age. The expression of SLC1A4 increased (P<0.05) as the conceptus elongated while SLC1A2 was not affected by stage of conceptus development (Figure 2B).
Figure 2. Gene expression values in the endometrium for acidic amino acid transporters as determined by qRT-PCR analysis.
Data are displayed as mean calibrated, normalized, relative expression values in arbitrary units (CNRQ ± SEM) in the intercaruncular region of the endometrium from cyclic (solid bars) and pregnant (open bars) heifers and caruncular regions of cyclic (black bars white stipple) and pregnant heifers (while bars black stipple) (n = 5 per treatment per time-point). (A) SLC1A5CNRQ expression with a significant effect of day (P<0.001) and pregnancy status (P = 0.06). (B) Gene expression values for acidic amino acid transporters in the bovine embryo during distinct developmental stages. Values were determined by RNA sequencing and are given as mean transcripts per million (TPM ± SEM) with n = 5 per time-point. A significant effect of stage of embryo development was observed for all genes (P<0.05).
Expression of neutral amino acid transporters in the endometrium and conceptus during the peri-implantation period of pregnancy
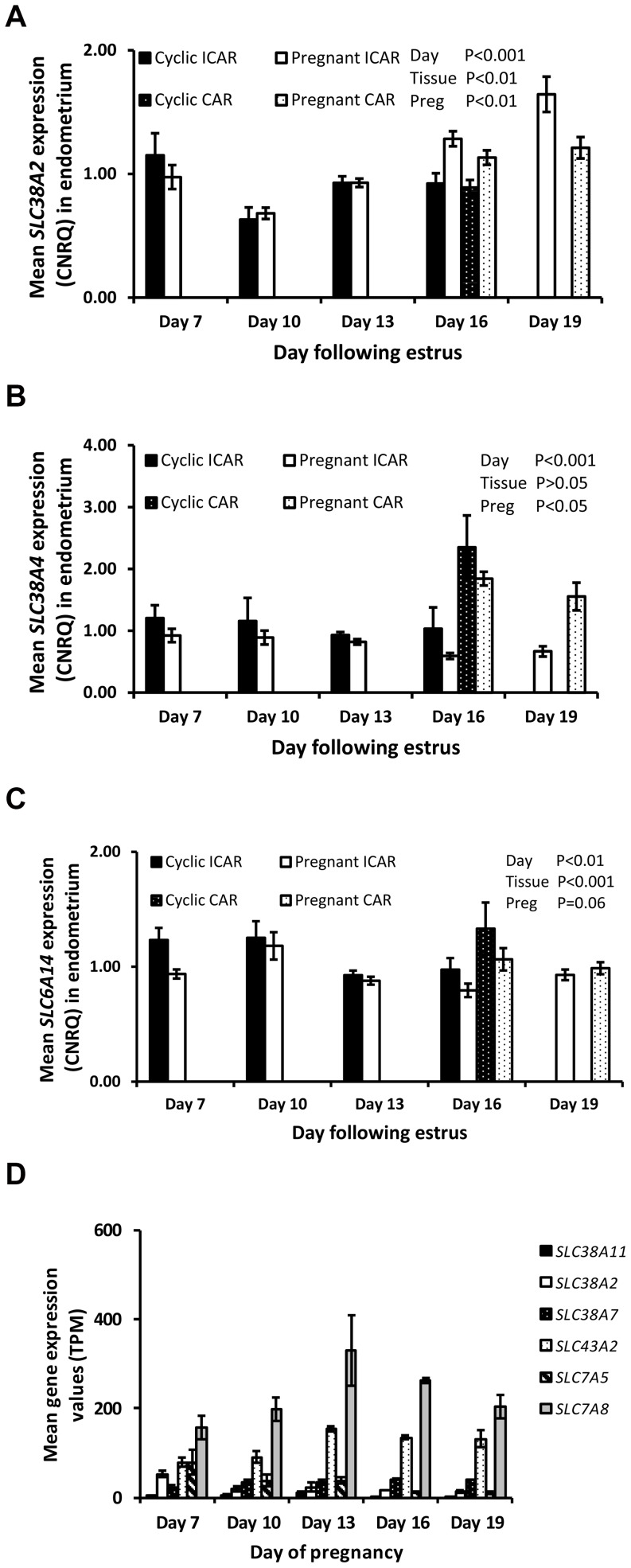
In the endometrium, there was an overall increase in expression of SLC38A2 and SLC43A2, while SLC38A7 and SLC6A14 expression decreased as the estrous cycle and early pregnancy progressed (P<0.05). Expression of SLC38A2, SLC38A7, SLC43A2, and SLC38A11 mRNAs was lower while expression of SLC38A4 and SLC6A14 mRNAs was greater (P<0.05) in caruncular compared to intercaruncular regions of the endometrium on both Days 16 and 19 of pregnancy. In pregnant heifers, SLC38A2 expression increased from Day 10 to Day 19 while SLC38A4 and SLC6A14 expression was lower (P<0.05) in pregnant heifers on Day 16 compared to cyclic controls (Figure 3A–C). In contrast to the endometrium, expression of SLC38A2 decreased, while SLC38A7, SLC43A2, SLC38A11 increased (P<0.05) as conceptus development progressed (Figure 3D). Expression of SLC6A14 was less than one transcript per million at all stages of conceptus development (data not shown).
Figure 3. Gene expression values in the endometrium for neutral amino acid transporters as determined by qRT-PCR analysis.
Data are displayed as mean calibrated, normalized, relative expression values in arbitrary units (CNRQ ± SEM) in the intercaruncular region of the endometrium from cyclic (solid bars) and pregnant (open bars) heifers and caruncular regions of cyclic (black bars white stipple) and pregnant heifers (while bars black stipple) (n = 5 per treatment per time-point). (A) SLC38A2 expression with a significant effect of day, tissue type and pregnancy status (B) SLC38A4 expression was significantly affected by tissue type and pregnancy status and (C) SLC6A14 whose expression was significantly affected by day, tissue type and pregnancy status. Significance was set at P<0.05. (D) Gene expression values for neutral amino acid transporters in the bovine conceptus during distinct developmental stages. Values were determined by RNA sequencing and are given as mean transcripts per million (TPM ± SEM) with n = 5 per time-point. A significant effect of stage of embryo development was observed for all genes (P<0.05).
Expression of the neutral amino acid transporter SLC7A5 increased (P<0.01) in the endometrium as the estrous cycle and early pregnancy progressed with a co-ordinate decrease (P<0.05) in expression as the conceptus developed. Endometrial expression of SLC7A8 decreased to Day 16 in both pregnant and cyclic heifers, but expression increased (P<0.05) on Day 19 in pregnant caruncular and intercaruncular tissue. SLC7A8 in the conceptus was affected by day (P<0.05) with greatest expression on Day 13 of pregnancy.
Modulation of amino acid transporters by progesterone in vivo
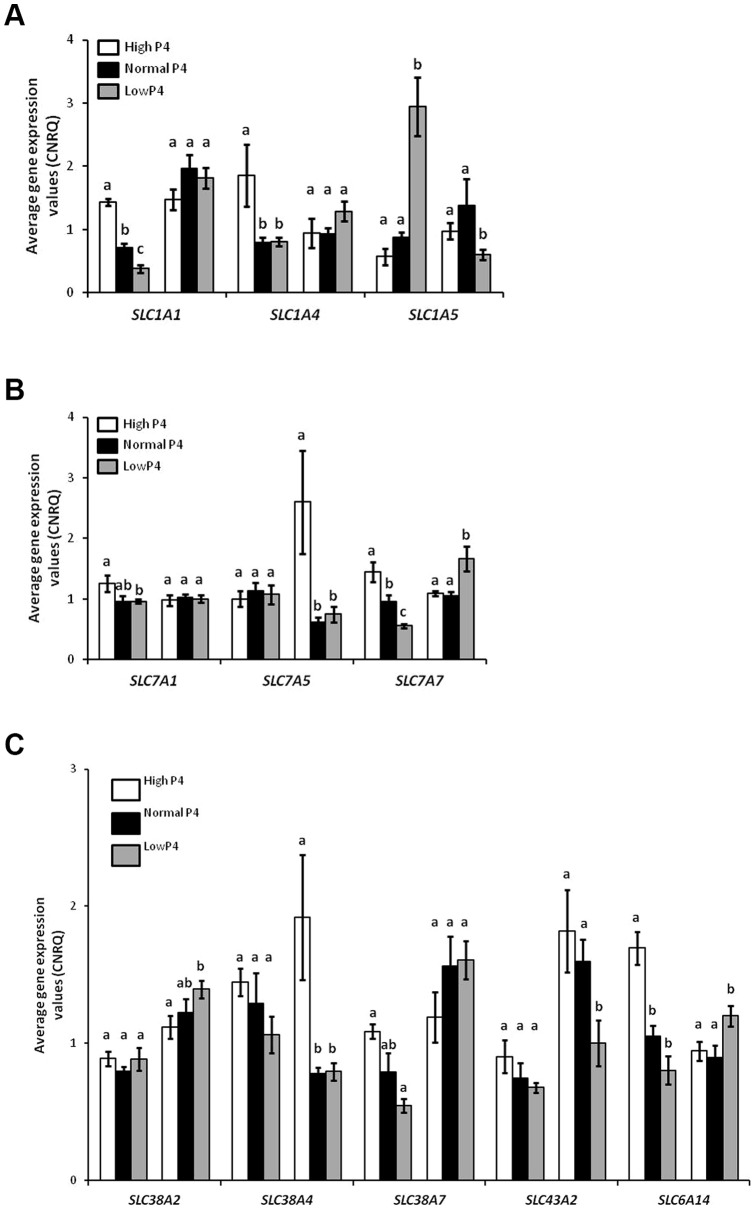
Expression of three acidic amino acid transporters was modulated by concentration of P4 in vivo. On Day 7 of the estrous cycle, expression of SLC1A1 and SLC1A4 was higher (P<0.05) in high P4 heifers compared to control heifers and SLC1A1 expression was lower (P<0.05) in the low P4 group (Figure 4A). In contrast, SLC1A5 expression was higher (P<0.05) in the low P4 group on Day 7 compared to control heifers (Figure 4A). The cationic amino acid transporters SLC7A1 and SLC7A7 were more abundant on Day 7 in the high P4 group, while on Day 13, SLC7A5 and SLC7A7 were higher in the high and low P4 groups, respectively (P<0.05: Figure 4B). Manipulation of P4 concentrations had no effect on the expression of SLC38A2, SLC38A4 or SLC43A2 on Day 7 (P>0.05). However, P4 supplementation increased (P<0.05) SLC38A7 and SLC6A14 expression on Day 7. On Day 13 of the estrous cycle, heifers with low P4 had greater expression of SLC38A2 and SLC6A14, while SLC43A2 expression decreased as compared to values for control heifers (P<0.05: Figure 4C). The expression of SLC38A4 was greater (P<0.05) in high P4 heifers while SLC38A7 expression was similar amongst the three treatment groups.
Figure 4. Gene expression values in the endometrium for heifers with high P4 (black bars: n = 5), normal P4 (white bars: n = 5) and low P4 (gray bars: n = 7) on Days 7 and 13 of the estrous cycle.
All data are displayed as mean calibrated, normalized, relative expression values in arbitrary units (CNRQ ± SEM) for acidic amino acid transporters (A), basic amino acid transporters (B) and neutral amino acid transporter (C). Differences between treatments on a given day are denoted by a, b, c, when P<0.05.
Discussion
This study is the first report on temporal changes in the amino acid content of ULF during the bovine estrous cycle and at key stages of the pre- and peri-implantation periods of pregnancy. Results of this study indicate that the expression of solute-like transporters responsible for the active transport of these amino acids from the endometrium into the ULF is distinct from the expression of transporters that move these nutrients into the conceptus. In addition, expression of certain amino acid transporters in the endometrium was modified by P4 and may therefore play a role in the the capacity of the uterus to support conceptus elongation.
In sheep, glycine was the most abundant amino acid detected in ULF followed by serine [13]. In the current study, threonine and glycine were the most abundant amino acids (10–20 fold greater than most other amino acids). Consistent with our study, data from sheep indicate that most amino acids increase after pregnancy recognition (e.g., arginine, histidine, glycine, glutamic acid, isoleucine, leucine, phenylalanine, tryptophan, tyrosine). In contrast, others do not follow the same pattern in both species; for example, threonine concentrations decreased on Day 16 and 19 of pregnancy in cattle but was reported to increase from Day 12–15 in sheep [13].Whether such discrepancies between cattle and sheep reflect true species differences or are artefacts of different technologies remains to be clarified.
The basic amino acids arginine and lysine are transported by the cationic transporters SLC7A1, SLC7A2, SLC7A3, SLC7A4, SLC7A6, SLC7A7, and SLC3A1 while SLC38A6 transports histidine [16]. Of these transporters expression of SLC7A1 and SLC7A2 increased in response to P4 supplementation and IFNT in vivo in sheep [16], coordinate with increased recoverable amounts of arginine in the ULF [13]. In this study, the overall abundance of arginine in ULF was similar during the estrous cycle and early pregnancy, but increased significantly on Day 19 of pregnancy. This was coordinate with increased expression of its transporter SLC7A1 which also increased in the endometria of pregnant heifers on Day 19. In addition, SLC7A1 expression was greater in heifers with high P4 on Day 7 which is a model associated with advanced conceptus elongation in heifers [30]. The presence of the conceptus was associated with increased expression of transporters for arginine in the endometrium resulting in the increased abundance of arginine in ULF available to the developing conceptus. Arginine was found to stimulate proliferation, protein synthesis and/or migration of sheep and pig trophoblast cells in vitro (Pigs [31]: Sheep [32]). Given that conceptuses of those species also elongate, it is likely that arginine plays a similar role in cattle. What is less clear in cattle, however, is the route of uptake of arginine given the decreased expression of members of the y+ family of transporters (SLC7A1 and -7A2) as the conceptus elongates. It is possible that arginine is transported into the conceptus by other members of the cationic amino acid transporters e.g. SLC7A6 and SLC7A7 whose expression was maintained or increased as the conceptus was undergoing elongation.
The acidic amino acids glutamic and aspartic acid are transported by SLC1A1, -1A2, -1A3 and other acidic amino acids are transported by SLC1A4 and -1A5 from the ASC transport system [33]. Both aspartic and glutamic acid increased between Days 16 and 19 of pregnancy. There was no difference on Day 16, the day of pregnancy recognition [2], [3]; however, endometrial expression of SLC1A5 increased in pregnant heifers on Day 16 and was maintained to Day 19. It is likely that the increase in these amino acids on Day 19 and not before is due to a lag between the increased expression of their transporters on Day 16 and the subsequent detection of an increased abundance of the amino acids. Although concentrations of these acidic amino acids increase in bovine ULF by the initiation of implantation (Day 19), the mechanism of transport of these amino acids seems to differ between the endometrium and conceptus. Given that SLC1A5 increased in the endometrium with a corresponding increase in SLC1A4, we propose that the transport of glutamic and aspartic acid into the uterine lumen is mediated predominantly via SLC1A5; however, uptake of these amino acids by the conceptus is via SLC1A4 in cattle. In addition, the early increase in SLC1A5 expression in P4-supplemented heifers suggests that advanced conceptus elongation in this model is, in part, driven by increased transport of both aspartic and glutamic acids into the uterine lumen via increased expression of SLC1A5 and not other members of the acidic amino acid transporter family.
The most abundant amino acids in bovine ULF in this study were the neutral amino acids. Of the neutral amino acid transporters analysed in the endometrium, increased expression of SLC38A2 in the pregnant endometrium on Days 16 and 19 suggests that this gene is most likely involved in the transport of neutral amino acids into ULF during the pre-implantation period of pregnancy in cattle. Interestingly, asparagine and threonine were less abundant in ULF of pregnant compared to cyclic heifers on Days 7 and 10, respectively. This seems at odds with the fact that pregnancy recognition, has not occurred, and previous studies have shown that Days 15 and 16 of pregnancy are the earliest that differences in gene expression in the endometrium are detectable between cyclic and pregnant heifers [34], [35].
Altering circulating concentrations of P4 in vivo can either advance (in the case of high P4 [36]) or delay (in the case of low P4 [29]) conceptus elongation following transfer of a blastocyst to heifers on Day 7 of the estrous cycle. This study clearly demonstrates that amino acids are an important component of ULF during the estrous cycle and early pregnancy and that the transport of these molecules into ULF from the endometrium occurs throughout this period. An early increase in P4 concentrations increased endometrial expression of SLC1A5, SLC38A7, SLC6A14, SLC7A1 on Day 7 post-estrus, with an early (Day 7) and sustained increase in expression to Day 13 for SLC38A4, SLC7A5 and SLC7A7 compared to control heifers, suggesting that one way in which conceptus elongation is advanced, is through effects on the maternal amino acid transport system. Conversely, in heifers with a delay in the post-ovulatory increase in P4 (associated with smaller conceptuses), expression of SLC43A2, SLC6A14, SLC7A6 and SLC7A7 for amino acid transport into the ULF was sub-optimal. These P4-regulated changes in expression of the transporters in the endometrium are consistent with lower concentrations of histidine and asparagine in ULF of low P4 heifers on Day 13 [37]. Therefore, in vivo manipulation of P4 alters the expression of genes for amino acid transporter from the endometrium into the ULF which has clear consequences for conceptus elongation in vivo which supports findings from studies with ewes [38].
Previous studies have demonstrated that in vitro derived blastocysts have a higher amino acid turnover than their in vivo derived counterparts and, overall, expanded blastocysts deplete more amino acids than those that do not undergo expansion [23]. This is interesting in the context of this study in which the amount of detectable amino acids in ULF increased as development of the blastocyst progressed to an elongated filamentous conceptus. However, once hatched from the zona pellucida the substantial increase in the composition of specific amino acids in the ULF suggests increased requirements for these amino acids to drive conceptus elongation. Moreover, substantial increases in the abundance of amino acids in the gravid as compared to the non-gravid uterine horn on Day 18 of pregnancy [15] indicate that the presence of the conceptus stimulates transport of amino acids into the uterine lumen. Interestingly, the amounts of amino acids in ULF were reduced in heifers with a developmentally compromised conceptus (i.e. cloned embryos) [39] and in sub-fertile animals [40]. The fact that we demonstrated a significant increase in the amount of amino acids in ULF on Day 19 of pregnancy, along with increased expression of their transporters in the endometrium, is consistent with the notion that increased abundance of amino acids in ULF is required for successful pregnancy establishment in cattle. In conclusion, results of this study demonstrated that most amino acids increase in ULF between Days 16 and 19 of pregnancy which is after pregnancy recognition has occurred. The amino acid transporters are temporally regulated in a tissue-specific manner in the endometrium and conceptus during the peri-implantation period of pregnancy and we propose that the transport mechanisms for amino acids into ULF from the endometrium are distinct from those of the conceptus. Moreover, expression of the amino acid transporters in the endometrium in vivo under conditions where conceptus elongation is advanced (elevated P4) or retarded (low P4), may alter the transport of acidic, neutral and cationic amino acids into ULF. We propose that transport of amino acids into the uterine lumen contributes to the capacity of the uterus to stimulate elongation of the conceptus during the peri-implantation period of pregnancy in cattle.
Supporting Information
Primer information used for quantitative real time PCR analysis of candidate genes. All primers were used at a concentration of 300 nM in a final reaction volume of 15 µl. A dissociation curve was included to ensure specificity of each primer pair.
(XLSX)
Acknowledgments
The authors wish to acknowledge the help of all present and previous graduate students, postdoctoral scientists and technical staff at UCD for their assistance in sample collection and Ms. Penny Furney with the progesterone assays.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.
Funding Statement
This work was supported by Science Foundation Ireland under grant number 10/IN.1/B3011 to PL. (www.sfi.ie.) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Diskin MG, Morris DG (2008) Embryonic and early foetal losses in cattle and other ruminants. Reprod Domest Anim 43 Suppl 2 260–267. [DOI] [PubMed] [Google Scholar]
- 2. Northey DL, French LR (1980) Effect of embryo removal and intrauterine infusion of embryonic homogenates on the lifespan of the bovine corpus luteum. J Anim Sci 50: 298–302. [DOI] [PubMed] [Google Scholar]
- 3. Betteridge KJ, Eaglesome MD, Randall GC, Mitchell D (1980) Collection, description and transfer of embryos from cattle 10—16 days after oestrus. J Reprod Fertil 59: 205–216. [DOI] [PubMed] [Google Scholar]
- 4. Forde N, Lonergan P (2012) Transcriptomic Analysis of the Bovine Endometrium: What is Required to Establish Uterine Receptivity to Implantation in Cattle? Journal of Reproduction and Development 58: 189–195. [DOI] [PubMed] [Google Scholar]
- 5.Dorniak P, Bazer FW, Spencer TE (2012) Biological role of interferon tau in endometrial function and conceptus elongation. J Anim Sci. [DOI] [PubMed]
- 6. Bazer FW, Kim J, Ka H, Johnson GA, Wu G, Song G (2012) Select Nutrients in the Uterine Lumen of Sheep and Pigs Affect Conceptus Development. Journal of Reproduction and Development 58: 180–188. [DOI] [PubMed] [Google Scholar]
- 7. Bazer FW (1975) Uterine protein secretions: Relationship to development of the conceptus. J Anim Sci 41: 1376–1382. [DOI] [PubMed] [Google Scholar]
- 8. Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE (2002) Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 124: 289–300. [PubMed] [Google Scholar]
- 9. Alexopoulos NI, Vajta G, Maddox-Hyttel P, French AJ, Trounson AO (2005) Stereomicroscopic and histological examination of bovine embryos following extended in vitro culture. Reprod Fertil Dev 17: 799–808. [DOI] [PubMed] [Google Scholar]
- 10. Brandao DO, Maddox-Hyttel P, Lovendahl P, Rumpf R, Stringfellow D, et al. (2004) Post hatching development: a novel system for extended in vitro culture of bovine embryos. Biol Reprod 71: 2048–2055. [DOI] [PubMed] [Google Scholar]
- 11. Ledgard AM, Meier S, Peterson AJ (2011) Evaluation of the uterine environment early in pregnancy establishment to characterise cows with a potentially superior ability to support conceptus survival. Reprod Fertil Dev 23: 737–747. [DOI] [PubMed] [Google Scholar]
- 12. Berendt FJ, Frohlich T, Schmidt SE, Reichenbach HD, Wolf E, et al. (2005) Holistic differential analysis of embryo-induced alterations in the proteome of bovine endometrium in the preattachment period. Proteomics 5: 2551–2560. [DOI] [PubMed] [Google Scholar]
- 13. Gao H, Wu G, Spencer TE, Johnson GA, Li X, et al. (2009) Select nutrients in the ovine uterine lumen. I. Amino acids, glucose, and ions in uterine lumenal flushings of cyclic and pregnant ewes. Biol Reprod 80: 86–93. [DOI] [PubMed] [Google Scholar]
- 14. Meier S, Walker CG, Mitchell MD, Littlejohn MD, Roche JR (2011) Modification of endometrial fatty acid concentrations by the pre-implantation conceptus in pasture-fed dairy cows. J Dairy Res 78: 263–269. [DOI] [PubMed] [Google Scholar]
- 15. Groebner AE, Rubio-Aliaga I, Schulke K, Reichenbach HD, Daniel H, et al. (2011) Increase of essential amino acids in the bovine uterine lumen during preimplantation development. Reproduction 141: 685–695. [DOI] [PubMed] [Google Scholar]
- 16. Gao H, Wu G, Spencer TE, Johnson GA, Bazer FW (2009) Select nutrients in the ovine uterine lumen. III. Cationic amino acid transporters in the ovine uterus and peri-implantation conceptuses. Biol Reprod 80: 602–609. [DOI] [PubMed] [Google Scholar]
- 17. Dorniak P, Bazer FW, Spencer TE (2011) Prostaglandins regulate conceptus elongation and mediate effects of interferon tau on the ovine uterine endometrium. Biol Reprod 84: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 18. Dorniak P, Welsh TH Jr, Bazer FW, Spencer TE (2011) Endometrial HSD11B1 and cortisol regeneration in the ovine uterus: effects of pregnancy, interferon tau, and prostaglandins. Biol Reprod 86: 124. [DOI] [PubMed] [Google Scholar]
- 19.Dorniak P, Bazer FW, Wu G, Spencer TE (2012) Conceptus-derived prostaglandins regulate endometrial function in sheep. Biol Reprod 87: : 9, 1–7. [DOI] [PubMed] [Google Scholar]
- 20. Leese HJ (2012) Metabolism of the preimplantation embryo: 40 years on. Reproduction 143: 417–427. [DOI] [PubMed] [Google Scholar]
- 21.Wale PL, Gardner DK (2012) Oxygen regulates amino acid turnover and carbohydrate uptake during the preimplantation period of mouse embryo development. Biol Reprod 87: : 24, 21–28. [DOI] [PubMed] [Google Scholar]
- 22. Houghton FD, Hawkhead JA, Humpherson PG, Hogg JE, Balen AH, et al. (2002) Non-invasive amino acid turnover predicts human embryo developmental capacity. Hum Reprod 17: 999–1005. [DOI] [PubMed] [Google Scholar]
- 23. Sturmey RG, Bermejo-Alvarez P, Gutierrez-Adan A, Rizos D, Leese HJ, et al. (2010) Amino acid metabolism of bovine blastocysts: a biomarker of sex and viability. Mol Reprod Dev 77: 285–296. [DOI] [PubMed] [Google Scholar]
- 24. Hugentobler SA, Diskin MG, Leese HJ, Humpherson PG, Watson T, et al. (2007) Amino acids in oviduct and uterine fluid and blood plasma during the estrous cycle in the bovine. Mol Reprod Dev 74: 445–454. [DOI] [PubMed] [Google Scholar]
- 25. Hugentobler SA, Sreenan JM, Humpherson PG, Leese HJ, Diskin MG, et al. (2010) Effects of changes in the concentration of systemic progesterone on ions, amino acids and energy substrates in cattle oviduct and uterine fluid and blood. Reprod Fertil Dev 22: 684–694. [DOI] [PubMed] [Google Scholar]
- 26. Forde N, Mehta JP, McGettigan PA, Mamo S, Bazer FW, et al. (2013) Alterations in expression of endometrial genes coding for proteins secreted into the uterine lumen during conceptus elongation in cattle. BMC Genomics 14: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mamo S, Mehta JP, McGettigan P, Fair T, Spencer TE, et al. (2011) RNA sequencing reveals novel gene clusters in bovine conceptuses associated with maternal recognition of pregnancy and implantation. Biol Reprod 85: 1143–1151. [DOI] [PubMed] [Google Scholar]
- 28. Carter F, Forde N, Duffy P, Wade M, Fair T, et al. (2008) Effect of increasing progesterone concentration from Day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reprod Fertil Dev 20: 368–375. [DOI] [PubMed] [Google Scholar]
- 29. Forde N, Beltman ME, Duffy GB, Duffy P, Mehta JP, et al. (2011) Changes in the endometrial transcriptome during the bovine estrous cycle: effect of low circulating progesterone and consequences for conceptus elongation. Biol Reprod 84: 266–278. [DOI] [PubMed] [Google Scholar]
- 30. Forde N, Carter F, Fair T, Crowe MA, Evans AC, et al. (2009) Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol Reprod 81: 784–794. [DOI] [PubMed] [Google Scholar]
- 31. Kim J, Song G, Wu G, Gao H, Johnson GA, et al. (2013) Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF4EBP1 signal transduction pathway. Biol Reprod 88: 113. [DOI] [PubMed] [Google Scholar]
- 32. Kim JY, Burghardt RC, Wu G, Johnson GA, Spencer TE, et al. (2010) Select nutrients in the ovine uterine lumen. VIII. Arginine stimulates proliferation of ovine trophectoderm cells through MTOR-RPS6K-RPS6 signaling cascade and synthesis of nitric oxide and polyamines. Biol Reprod 84: 70–78. [DOI] [PubMed] [Google Scholar]
- 33. Gao H, Wu G, Spencer TE, Johnson GA, Bazer FW (2009) Select nutrients in the ovine uterine lumen. IV. Expression of neutral and acidic amino acid transporters in ovine uteri and peri-implantation conceptuses. Biol Reprod 80: 1196–1208. [DOI] [PubMed] [Google Scholar]
- 34. Bauersachs S, Ulbrich SE, Reichenbach HD, Reichenbach M, Buttner M, et al. (2012) Comparison of the effects of early pregnancy with human interferon, alpha 2 (IFNA2), on gene expression in bovine endometrium. Biol Reprod 86: 46. [DOI] [PubMed] [Google Scholar]
- 35. Forde N, Carter F, Spencer TE, Bazer FW, Sandra O, et al. (2011) Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant? Biol Reprod 85: 144–156. [DOI] [PubMed] [Google Scholar]
- 36. Clemente M, de La Fuente J, Fair T, Al Naib A, Gutierrez-Adan A, et al. (2009) Progesterone and conceptus elongation in cattle: a direct effect on the embryo or an indirect effect via the endometrium? Reproduction 138: 507–517. [DOI] [PubMed] [Google Scholar]
- 37. Mullen MP, Bazer FW, Wu G, Parr MH, Evans AC, et al. (2014) Effects of systemic progesterone during the early luteal phase on the availabilities of amino acids and glucose in the bovine uterine lumen. Reprod Fertil Dev 26: 282–292. [DOI] [PubMed] [Google Scholar]
- 38. Satterfield MC, Gao H, Li X, Wu G, Johnson GA, et al. (2009) Select nutrients and their associated transporters are increased in the ovine uterus following early progesterone administration. Biol Reprod 82: 224–231. [DOI] [PubMed] [Google Scholar]
- 39. Groebner AE, Zakhartchenko V, Bauersachs S, Rubio-Aliaga I, Daniel H, et al. (2011) Reduced amino acids in the bovine uterine lumen of cloned versus in vitro fertilized pregnancies prior to implantation. Cell Reprogram 13: 403–410. [DOI] [PubMed] [Google Scholar]
- 40. Meier S, Mitchell MD, Walker CG, Roche JR, Verkerk GA (2014) Amino acid concentrations in uterine fluid during early pregnancy differ in fertile and subfertile dairy cow strains. Journal of Dairy Science 97: 1364–1376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer information used for quantitative real time PCR analysis of candidate genes. All primers were used at a concentration of 300 nM in a final reaction volume of 15 µl. A dissociation curve was included to ensure specificity of each primer pair.
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.