Abstract
Background & Aims
Alterations in central corticotropin-releasing factor (CRF) signaling pathways have been implicated in the pathophysiology of anxiety disorders and irritable bowel syndrome (IBS). We aimed to characterize the effects of the CRF receptor 1 (CRF-R1) antagonist, GW876008, on brain and skin conductance responses (SCR) during acquisition and extinction of conditioned fear to the threat of abdominal pain in subjects with IBS and healthy individuals (controls).
Methods
We performed a single center, randomized, double-blind, 3-period crossover study of 11 women with IBS (35.50 ± 12.48 y old) and 15 healthy women (controls) given a single oral dose (20 or 200 mg) of the CRF-R1 antagonist or placebo. Blood-oxygen-level-dependent (BOLD) responses were analyzed using functional magnetic resonance imaging in a tertiary care setting.
Results
Controls had greater SCR during acquisition than extinction, validating the fear conditioning paradigm. In contrast, during extinction, the women with IBS had greater SCR than controls—an effect normalized by administration of a CRF-R1 antagonist. Although the antagonist significantly reduced activity in the thalamus in patients with IBS and controls during acquisition, the drug produced greater suppression of BOLD activity in a wide range of brain regions in IBS patients during extinction, including the medial prefrontal cortex, pons, hippocampus, and anterior insula.
Conclusion
Although CRF signaling via CRF-R1 is involved in fear acquisition and extinction learning related to expected abdominal pain in patients with IBS and controls, this system appears to be upregulated in patients with IBS. This upregulation might contribute to the previously reported abnormal brain responses to expected abdominal pain.
Keywords: corticotropin-releasing factor receptor 1 (CRF-R1) antagonist, irritable bowel syndrome, fear conditioning and extinction
Introduction
IBS is a common gastrointestinal disorder characterized by chronically recurring abdominal pain and discomfort, altered bowel habits, and increased anxiety and hypervigilance to symptom-related stimuli.1 We have previously demonstrated that group differences in brain responses to aversive rectal distension are almost completely accounted for by differences in brain responses to the expectation of such a stimulus, suggesting an important role of conditioned responses to chronic abdominal pain.2 Consistent with this concept, hypervigilance to and arousal by symptom-related stressors (interoceptive and contextual cues) previously associated with distressing gastrointestinal sensations/symptoms may be viewed in the context of Pavlovian fear conditioning, where neutral stimuli (e.g., a light signal) are paired with aversive sensations (e.g., abdominal pain).3 Further evidence to support an important role for aversive visceral learning and memory processes in visceral pain comes from a recent report demonstrating fear conditioning (learned anticipatory fear response) to rectal pain stimuli in healthy control subjects (HCs).4 From this perspective, persistent hypervigilance to and arousal by symptom-related stressors in IBS may result from deficits in the ability to extinguish conditioned fear responses, resulting in symptom persistence even in the absence of abnormal visceral input.5 Similar impairments in fear conditioning and extinction learning have been implicated in several other stress-related disorders, including PTSD.6–8 Extinction is not a process of “unlearning” but rather a process by which new learning of fear inhibition occurs and is superimposed over the initially acquired learned response.9, 10 Such impaired learning processes could play a role in post infectious IBS, where failure to extinguish associations between GI activity and abdominal pain acquired during the acute infection, results in persistent hypervigilance towards gut related signals.
Corticotropin-releasing factor (CRF) is considered the principal coordinator of the vertebrate stress response via widespread actions on peripheral and central targets, all of which serve to orchestrate a host of autonomic, neurochemical, and behavioral responses to stress.11, 12 Perturbations to this system in humans have been linked to a variety of psychiatric disorders and stress-sensitive syndromes, including anxiety disorders13 and irritable bowel syndrome (IBS).14–16 For example, we have recently demonstrated that exaggerated brain responses to a pain threat in IBS patients were attenuated by acute administration of a CRF receptor 1 (CRF-R1) antagonist.17
It remains unknown if IBS patients show similar alterations in the acquisition and/or extinction of fear conditioning as has been reported for patients with certain anxiety disorders, and which brain regions and neuromodulatory systems might be involved. Indirect evidence from clinical trials with cognitive behavioral therapies in IBS suggests that a significant component of the success of these therapies is related to the effort to extinguish persistent conditioned fear responses towards GI related signals and sensations. Given the high comorbidity of stress-related anxiety disorders with IBS,18, 19 coupled with the link between disturbances in fear conditioning and extinction and dysregulation of the CRF/CRF-R1 signaling system,20 we hypothesized that a similar emotional learning process might underlie the chronicity of IBS symptoms. We used functional magnetic resonance imaging (fMRI) and skin conductance response (SCR) measurement in female IBS patients and HCs to characterize the effects of a selective CRF-R1 antagonist, GW876008, on brain responses during acquisition and extinction of conditioned fear to an abdominal pain stimulus. By using a pain stimulus to the body site most often reported by IBS patients as the site of their abdominal pain (e.g., left lower quadrant) as the unconditioned stimulus, and a visual cue as the conditioned stimulus, we aimed to test the following hypotheses: 1) IBS patients compared to HCs show impaired fear conditioning and extinction learning, associated with increased sympathetic nervous system (SNS) responses (as indexed by SCR) and altered activity in fear-related brain circuits. 2) Acute administration of GW876008 modulates activity in these networks in IBS patients compared to HCs during acquisition and extinction, normalizing the exaggerated response in IBS, and this drug effect may be dose dependent.
Materials and Methods
Characterization of the sample
An age-matched sample of right-handed females recruited from the greater Los Angeles community, 14 of which were diagnosed with IBS (mean age, 35.50 ± 12.48 years) and 17 non-IBS HCs (mean age, 33.65 ± 15.87 years), participated in this study. All study participants were recruited by advertisement from the greater Los Angeles population or a database review from participation in one of our studies previously. IBS was diagnosed based on ROME II criteria and assessment by a gastroenterologist or a nurse practitioner trained in the diagnosis of functional bowel disease. Patients included all bowel habits, 43% constipation-predominant, 21% diarrhea predominant, and 36% alternating symptoms of constipation and diarrhea. Participants had a negative urine test for drugs of abuse, lacked any significant medical problems other than IBS, were free of past or present psychiatric illness as determined by the Mini International Neuropsychiatric Interview,21 and were not currently taking any centrally acting medications. All participants were tested in the follicular phase of their menstrual cycle defined as day 3–14 post-menses. The University of California, Los Angeles Medical Institutional Review Board approved all procedures, and each subject provided informed consent. Of the 31 subjects in our sample, 5 individuals (3 IBS, 2 HC) were excluded from the analysis as a result of BOLD signal loss in brain regions of interest across all or at least one of the three functional Magnetic Resonance Imaging (fMRI) study treatment visits.
Study design
This was a single center, randomized, double-blind, PLA-controlled, three-period crossover study of two single oral doses (20 or 200 mg) of the CRF-R1 antagonist, GW876008, versus PLA. The study consisted of an initial screening and familiarization visit, followed by three study treatment visits, each separated by approximately one month. Details regarding study design have been published previously in Hubbard et al., 2011.17
Drug, dosage, and administration
GW876008 (GlaxoSmithKline) is a highly selective and potent antagonist for the G-protein-coupled CRF receptor 1 subtype.22 Based on phase II clinical trials in patients with IBS, 20 and 200 mg doses of GW876008 were chosen in an attempt to provide a sufficient therapeutic range.23, 24 PLA tablets were identical to the active GW876008 tablets in all respects with the exception of omission of the active ingredient. Subjects were assigned to study treatment in accordance with the randomization schedule provided by GlaxoSmithKline.
Experimental design
Conditioned fear learning and extinction were examined using a simple fear conditioning paradigm comprised of three phases, using a pain stimulus applied to the left lower abdomen as an unconditioned stimulus, and a visual cue (red light) as the conditioned stimulus: Acquisition, five trials of the visual cue presentation always followed by an aversive abdominal stimulus (750 ms); Test phase, 10 trials in which the cue was followed by the aversive stimulus on only 50% of the trials; and Extinction, 5 trials in which none of the cues were followed by an aversive stimulus. Each cue presentation lasted for 9 s in each phase and the inter-trial interval was 20.75 s. Supplemental Figure 1A illustrates the experimental design.
To deliver the unconditioned stimulus, two electrode stimulation pads were placed 6 cm apart over each subject’s lower left abdomen in the region overlaying the sigmoid colon. The threat of a pain experience in this region would be expected to generate anticipatory anxiety and hypervigilance since many IBS patients observe pain and show tenderness on physical exams to the left lower abdomen. A Digitimer constant-current stimulator (model DS7A; Digitimer) was used to deliver the transcutaneous electrical stimulation to the abdomen. Stimulation consisted of a pulse train lasting 750 ms with a 2 ms pulse width and a frequency of 37 Hz. During visit 2 (familiarization visit) for each subject, individual pain thresholds used in visits 3–5 were determined using method of limits procedure beginning with a current intensity of 1.0 mA, which was increased in 0.5 mA steps until the subject reported the stimulus was “aversive but tolerable”. The maximum output of the Digitimer is 100mA. The pain stimulus was used as the unconditioned stimulus in this fear-conditioning paradigm and not given to assess visceral perception or pain sensitivity, or the effect of the CRF-R1 antagonist on such subjective responses. The expectation of the abdominal pain stimulus has been shown to activate thalamus, MCC and right aINS (See Supplemental Figure 2). This pattern of activation is nearly identical to that previously reported in female IBS patients during expectation of aversive rectal balloon distension.2 Before the start of the fear conditioning and learning task, each subject underwent an emotional faces task (data to be presented in a separate report) followed by an anticipation or threat of abdominal pain task.17 Supplemental Figure 1B illustrates the fear conditioning paradigm.
Skin conductance response (SCR)
Continuous skin conductance was measured from the second and third fingers of the left hand using MRI compatible electrodes. The signal was sampled at 1000 Hz using a Biopac recording system and AcqKnowledge software version 4.0 (Biopac, Inc, Goleta CA) which was used to filter the raw data with a 0.05 Hz high pass filter and then identify and measure amplitude of event related SCR to the cue period using a latency window of 1–3 seconds and a SCR threshold of 0.01 microsiemens (μS).
fMRI methods
The data were acquired in a whole body 3.0 Tesla Siemens Trio unit and processed using Statistical Parametric Mapping 8 (SPM8; Wellcome Trust Centre for the Study of Cognitive Neurology, London, UK). For each subject, one functional BOLD run was acquired (echo planar T2-weighted gradient echo; repetition time, 3000 ms; echo time, 28 ms; flip angle, 90°; matrix size, 64 × 64; 36 axial slices; field of view, 20 cm; 3 mm thick, skip 1 mm), lasting 12 min. A high-resolution T1-weighted magnetization prepared rapid acquisition gradient echo MRI was acquired to aid in the registration of functional images. Functional images were slice-time and motion corrected, spatially normalized to the MNI template, and spatially smoothed with an 8 mm3 Gaussian kernel using SPM8.
Statistical analyses
Subject-level analyses based on changes in BOLD contrasts were performed with the general linear model (GLM) in SPM8. The first level model included the 30 second crosshair presented at the beginning and the end of the experiment, 5 cues presented during Acquisition, 10 cues presented during the Test phase, 5 cues presented during Extinction, 5 shocks received during Acquisition, 5 shocks received during the Test phase, inter-trial interval fixation crosshairs, and motion realignment regressors.
To quantify brain response to the cue, we created first level beta contrast images by subtracting response to the crosshair presented during the inter-trial interval from response to cue for each trial (i.e., cue-crosshair). These first level contrast images were then entered into an independent sample t-test to assess for group differences during PLA administration in brain response to the cue during Acquisition and Extinction. To assess the effects of the drug, first level beta contrast images representing brain response to the cue (compared to crosshair) during PLA, 20, and 200 mg during Acquisition or Extinction were entered as dependent variables in random effect GLMs using the flexible factorial design in SPM5 with subject, group, and drug specified as factors. A priori contrast analysis using the GLM framework was applied to test for group differences in brain response to the cue during 20 and 200 mg of GW876008 compared to PLA.
Region of interest analysis
Anatomically defined region of interest (ROIs) involved in Acquisition, Test and Extinction of conditioned fear were selected based on a literature review 25–30 and included anterior and posterior insula (aINS, pINS), dorsolateral and ventrolateral prefrontal cortex (dlPFC, vlPFC), medial PFC (mPFC), anterior midcingulate cortex (aMCC), pregenual anterior cingulate cortex (pACC), subgenual ACC (sgACC), amygdala (AMYG), hypothalamus (HYPO), hippocampus (HIPP), thalamus (THAL), midbrain, and dorsal pons. Whole brain analysis results were thresholded at p < 0.001 uncorrected and ROIs tested using small volume correction where significance was interpreted at p < 0.05 after implementing family-wise error correction.
SCR analysis
To test for group and condition differences in SCR, we performed linear contrast analysis on estimates from a mixed-effect model for repeated measured data was applied in SAS 9.2, specifying subject as a random effect and group, drug, and condition (Acquisition, Test, Extinction) as factors. Sensitivity analyses performed with randomization order as a covariate did not alter the results, therefore this variable was excluded as a factor in the models.
Results
Clinical sample characteristics
Table 1 provides the descriptive and inferential statistics for clinical characteristics of the two groups (IBS, n = 11; HCs, n = 15), assessed prior to randomization. IBS patients as a group reported slightly higher, but non-pathological levels of trait anxiety compared to HCs (49.4±8.2 vs. 40.5±9.3; p = 0.02), as well as higher state anxiety on all 3 test days.
Table 1. Clinical characteristics.
Descriptive and inferential statistics for clinical characteristics of IBS patients and HCs.
| IBS (N=11)
|
HCs (N=15)
|
Test stats
|
P
|
|||
|---|---|---|---|---|---|---|
| M | SE | M | SE | |||
| Age | 34.0 | 11.6 | 32.9 | 15.0 | −0.21 | 0.84 |
| BMI | 26.0 | 8.0 | 25.3 | 5.1 | −0.27 | 0.79 |
| STAI Trait Anxiety | 49.4 | 8.2 | 40.5 | 9.3 | −2.5 | 0.02 |
| STAI State Anxiety (PLA day) | 45.6 | 7.0 | 40.9 | 4.3 | −2.1 | 0.05 |
| STAI State Anxiety (20 mg day) | 44.3 | 4.7 | 40.3 | 4.6 | −2.2 | 0.04 |
| STAI State Anxiety (200 mg day) | 48.0 | 7.2 | 41.6 | 5.7 | −2.5 | 0.02 |
Skin conductance responses during conditioned fear and learning task
SCR during PLA
As shown in Figure 1 and Table 2 for IBS during PLA, SCR was significantly lower in Acquisition compared to Extinction, t(1161) = −2.83, p = 0.005. Between group tests indicated greater SCR in HCs compared to IBS during Acquisition, t(192) = 2.89, p = 0.004 and greater SCR in IBS compared to HCs during Extinction, t(1910) = −3.17, p = 0.002.
Figure 1.
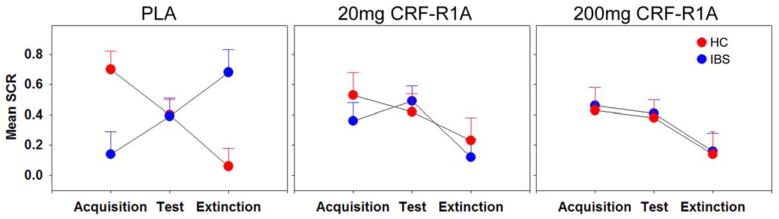
Estimated means for SCR amplitude (μS) and standard errors during Acquisition, Test, and Extinction for PLA, 20 mg and 200 mg doses of the CRF-R1 antagonist (CRF-R1A) for IBS (blue) and HCs (red).
Table 2. Estimated least squares SCR means by conditioning phase and group.
Estimated means (M) and standard errors (SE) for SCR (mean amplitude in μS) for HCs and IBS patients during Acquisition, Test, and Extinction phases of the fear conditioning protocol following administration of PLA, 20 mg of the CRF-R1 antagonist, and 200 mg of the CRF-R1 antagonist.
| HC
|
IBS
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLA
|
20
|
200
|
PLA
|
20
|
200
|
|||||||
| M | SE | M | SE | M | SE | M | SE | M | SE | M | SE | |
| Acquisition | .70 | .12 | .36 | .12 | .46 | .12 | .14 | .15 | .53 | .15 | .43 | .15 |
| Test | .40 | .10 | .49 | .10 | .41 | .09 | .39 | .12 | .42 | .12 | .38 | .12 |
| Extinction | .06 | .12 | .12 | .12 | .16 | .12 | .68 | .15 | .23 | .15 | .14 | .15 |
Effects of drug compared to PLA on SCR
Significant group differences were observed for change in SCR from PLA to 20 mg, t(1181) = −3.06, p = 0.002, and 200 mg, t(1181) = −2.22, p = 0.03. This difference was due to an increase in SCR during drug compared to PLA for IBS, and a decrease for HCs (Table 2, Figure 1).
During Extinction, group differences for SCR were observed between PLA and drug doses of 20 mg, t(1181) = 2.10, p = 0.036, and 200 mg, t(1181) = 2.67, p = 0.008. This difference was due to a decrease in SCR during drug compared to PLA for IBS and no differences for HCs.
Brain activity during conditioned fear and learning task
PLA condition
Extensive group differences in brain activity to the cue were observed between HCs and patients. During Acquisition, patients compared to HCs had greater activity in right ventral aINS extending to the vlPFC (k = 60, p = 0.007, 38, 24, −10, Z = 4.88). On the other hand, greater activity was seen in HCs compared to IBS in bilateral dorsal aINS, pINS, aMCC, THAL, midbrain (including periaqueductal gray [PAG]) and dorsal pons as well as right HIPP, left mPFC, right pACC, right dlPFC, left vlPFC (Supplemental Table 1, Figure 2).
Figure 2.
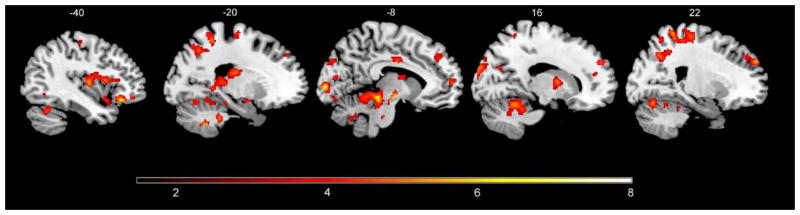
Brain regions showing greater BOLD responses to the cue during Acquisition in HCs compared to IBS patients during PLA condition. Whole-brain maps threshholded at p < 0.001, uncorrected.
During Extinction, IBS had greater activity than HCs in ventral brain regions including right pINS and bilaterally in AMYG, HIPP, HYPO, pACC, sgACC, aINS, vlPFC, mPFC, midbrain, dorsal pons (PAG) and THAL (Supplemental Table 2, Figure 3). Conversely, HCs had greater activity bilaterally in dlPFC (Supplemental Table 3). Of note, significant bilateral THAL deactivation was observed for HCs whereas IBS showed activation of this region during Extinction. No significant deactivations during Extinction were observed for IBS.
Figure 3.
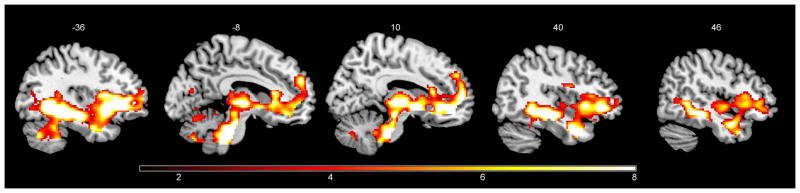
Brain regions showing greater BOLD responses to the cue during Extinction in IBS patients compared to HCs during PLA condition. Whole-brain maps threshholded at p < 0.001, uncorrected.
Effect of CRF-R1 antagonist versus PLA
During Acquisition, no significant group differences were observed following 20 mg of GW876008 compared to PLA. The only within group effect during Acquisition was suppression of bilateral midbrain region in HCs (right midbrain: 2, −36, −10, k = 5; left midbrain: −2, −26, −10, k = 9, p < 0.05, FWE corrected).
During Extinction, the effects of 20 mg of GW876008 on brain activity in response to the cue indicated greater suppression of pons and left midbrain in IBS compared to HCs (left pons: k = 309, −2, −24, −34, z = 4.34; right pons: k = 72, 4, −24, −36, z = 4.38 and 18 −36 −34, k = 18, z = 3.54; left midbrain: k = 10, −2 −28 −20, z = 3.43, p < 0.05, FWE corrected). In addition to suppression in pontine regions, within-group analysis of IBS indicated the midbrain region was also suppressed during Extinction.
Within-group analyses during the Acquisition phase demonstrated that the 200 mg dose of the CRF-R1 antagonist significantly suppressed lateralized clusters of activity in the THAL in both patients (k = 9, p = 0.04, z = 3.83, −20, −30, 6) and HCs (k = 21, p = 0.032, z = 3.68, 14, −28, 4) and additionally suppressed midbrain activity in IBS (k = 13, p = 0.03, z = 3.35, 6, −34, −8). However, no significant group differences were observed during Acquisition. In contrast, during Extinction, 200 mg of the CRF-R1 antagonist compared to PLA produced greater suppression of brain activity in IBS patients compared to HCs for bilateral mPFC, pons, left aINS and right HIPP (Table 3). No brain regions were suppressed by 200 mg of the CRF-R1 antagonist in HCs compared to IBS during Extinction. In fact, within-group analyses indicated that in HCs no regions showed significant suppression during Extinction following CRF-R1 administration at the 200 mg dose compared to PLA. Within-group analysis of IBS patients showed that 200 mg of the antagonist compared to PLA produced suppression bilaterally in the mPFC, midbrain, HIPP, THAL, and pons/brainstem as well as the right aINS (Table 4). No brain regions showed significant differences in activation during CRF-R1 antagonist administration compared to PLA during Acquisition or Extinction in either IBS or HCs.
Table 3. Regions showing more suppression on 200 mg CRF-R1 antagonist during Extinction in IBS compared to HCs.
MNI coordinates (X, Y, Z), cluster- and voxel-level probability values corrected at a family wise error rate of 5%(p), Z equivalent of the t statistic for the peak voxel of the cluster (Zequiv), and the number of voxels comprising the clusters of activation (Cluster k) with in the region of interest (ROI). Abbreviations: aINS-anterior insula, HEMI-Hemisphere, L-left, mPFC-medial prefrontal cortex, R-right.
| ROI | HEMI | cluster P | cluster k | VOXEL p | Zequiv | X | Y | Z |
|---|---|---|---|---|---|---|---|---|
| mPFC | L | 0.037 | 50 | 0.042 | 3.81 | −12 | 48 | −2 |
| mPFC | R | 0.042 | 39 | 0.086 | 3.52 | 16 | 48 | −2 |
| Pons | L | 0.017 | 26 | 0.023 | 3.51 | −10 | −36 | −40 |
| Pons | R | 0.039 | 8 | 0.026 | 3.46 | 2 | −24 | −40 |
| aINS | L | 0.037 | 16 | 0.032 | 3.52 | −36 | 34 | 0 |
| Hippocampus | R | 0.007 | 23 | 0.004 | 3.67 | 32 | −30 | −10 |
Table 4. Regions more activated during PLA compared to 200 mg CRF-R1 antagonist during Extinction (suppression due to drug) in IBS patients.
MNI coordinates (X, Y, Z), cluster- and voxel-level probability values corrected at a family wise error rate of 5%(p), Z equivalent of the t statistic for the peak voxel of the cluster (Zequiv), and the number of voxels comprising the clusters of activation (Cluster K) with in the region of interest (ROI). Abbreviations: aINS-anterior insula, HEMI-Hemisphere, L-left, mPFC-medial prefrontal cortex, pACC-pregenual anterior cingulate cortex, R-right.
| ROI | HEMI | Cluster P | Cluster k | Voxel P | Zequiv | X | Y | Z |
|---|---|---|---|---|---|---|---|---|
| mPFC/pACC | L | 0.018 | 75 | 0.025 | 3.97 | −16 | 48 | 0 |
| mPFC/pACC | R | 0.039 | 42 | 0.099 | 3.47 | 14 | 52 | −2 |
| Hippocampus | L | 0.010 | 15 | 0.004 | 3.74 | −32 | −32 | −8 |
| Hippocampus | R | 0.002 | 70 | 0.000 | 4.62 | 36 | −22 | −10 |
| Midbrain | L | 0.014 | 35 | 0.007 | 3.89 | −14 | −20 | −2 |
| Midbrain | R | 0.014 | 36 | 0.019 | 3.60 | 16 | −22 | −2 |
| Pons | L | 0.001 | 156 | 0.002 | 4.28 | −14 | −42 | −40 |
| Pons | R | 0.011 | 40 | 0.016 | 3.63 | 4 | −26 | −38 |
| Pons | R | 0.057 | 2 | 0.057 | 3.19 | 16 | −40 | −38 |
| Thalamus | L | 0.004 | 76 | 0.005 | 3.97 | −14 | −20 | 0 |
| Thalamus | R | 0.003 | 86 | 0.010 | 3.74 | 18 | −20 | 0 |
| aINS | R | 0.010 | 51 | 0.029 | 3.50 | 34 | 16 | −4 |
Discussion
The aims of this study were to identify differences in the involvement of the CRF/CRF-R1 signaling system in brain responses during acquisition and extinction of conditioned fear to a pain stimulus delivered to the sigmoid region between IBS patients and HCs. Patients and HCs differed in their central and autonomic responses to the conditioning paradigm. In IBS, the CRF-R1 antagonist produced greater suppression of BOLD activity in a wide range of brain regions during extinction, and this inhibition was associated with a reduction of SCR. During acquisition, it had similar inhibitory effects in both groups on thalamic activity, while SCR was reduced in HCs, and normalized in patients. Despite the relatively small sample size both the observed effects of the antagonist on autonomic and brain responses were large (Z>3.0). These data suggest that while CRF/CRF-R1 signaling plays a role in fear acquisition and extinction learning in both IBS and HCs, its relative involvement in both phases of fear conditioning differs significantly between groups. The greater involvement in IBS patients during extinction suggests that observed impairments in emotional learning may be related to an up-regulation of the CRF/CRF-R1 signaling system.
Brain and skin conductance responses during acquisition and extinction under placebo
In the current study, we used a somatic pain stimulus aimed at the sigmoid region of the abdomen as the unconditioned stimulus. The brain regions activated by this paradigm were nearly identical to those found to be activated by the expectation of aversive rectal distension in a recent study by our group,2 confirming the validity of the paradigm to probe emotional learning mechanisms related to abdominal pain expectation. Greater activation of neural circuits known to mediate acquisition and expression of conditioned fear25, 26 was seen in HCs compared to IBS patients, and this was associated with greater SCRs. Brain regions showing greater activation included the mPFC, AMYG, bilateral dorsal INS, THAL, HIPP, midbrain and dorsal pons. One possible explanation for these group differences may be the fact that IBS patients have existing conditioned fear responses resulting from previous learned associations involving distressing abdominal symptoms, engage fewer brain regions, and exhibit reduced autonomic responses to such familiar sensations during acquisition. As we did not use an unpaired control stimulus, we cannot rule out that the observed responses during acquisition were due to sensitization, rather than associative learning. However, the fact that increases in brain responses was reversed during extinction argues against such a possibility.
In contrast to the generally reduced brain responses in IBS during acquisition, patients showed significantly greater activation compared to HCs in the right aINS, a brain region found to be activated in association with increased uncertainty and encoding of anticipatory signals in relation to conditioned fear.29, 31 Increased activation in the aINS during anticipation of rectal distension2, 32 and cortical thinning in this subregion have also been reported in IBS patients.33, 34 The finding of greater activity in the aINS during acquisition of fear conditioning in IBS substantiates previous findings, and further indicates that IBS patients are hyper-responsive to anticipatory signals that engender prediction of uncertain outcomes.
In contrast to the findings during acquisition, patients showed greater brain activity compared to HCs during extinction, and this was associated with a greater SCR. Activated brain regions included bilateral AMYG, HIPPO, HYPO, as well as subregions of the mPFC, ACC, and insular cortices. Patients also showed greater BOLD activity in diencephalic, midbrain and brainstem structures. The AMYG is thought to be critically involved in the acquisition, expression and extinction of conditioned fear, whereas subregions of the mPFC and HIPP mediate fear suppression (extinction learning) and extinction retention and recall.10, 35 The fact that we observed greater activation in these regions as well as other neural structures known to mediate extinction learning suggests that disrupted fear extinction mechanisms may contribute to symptom chronicity in some IBS patients. These results partially overlap with a neurocircuitry model of PTSD,8 in that IBS patients, like patients with PTSD, show increased AMYG activity during extinction. However, we also expected concomitant decreases in the HIPP and mPFC activity, two brain regions with inhibitory effects on the AMYG, mediated by GABAergic projections. Instead, IBS patients showed increased BOLD activity in both of these regions. There are several possible explanations for these findings including: 1) The mPFC is composed of several functionally distinct subregions, which based on rodent studies may have inhibitory or excitatory effects on the amygdala36. 2) The findings may be related to the observed reductions in BOLD activity in the dlPFC in IBS compared to HCs during Extinction. The dlPFC has been implicated in higher-order executive functions including inhibitory cognitive control and emotion regulation, selective attention and visuospatial working memory.37–39 Reductions in dlPFC activity have been associated with intense negative emotions such as anxiety and sadness in healthy individuals, and patients with PTSD and major depression.40–43 Moreover, previous neuroimaging studies have reported diminished dlPFC functioning in IBS compared to HCs during painful rectal distension.44, 45 Thus, reduced dlPFC activity despite greater activity of other brain regions that normally inhibit AMYG (HIPP and mPFC) may be sufficient to produce ineffective corticolimibic inhibition in IBS.
Drug effects on brain responses during acquisition and extinction
Compared to PLA, acute administration of the lower dose of the CRF-R1 antagonist produced no significant regional group differences in BOLD activity during acquisition, while higher doses significantly suppressed lateralized clusters of activity in the THAL in both groups.
During extinction, both doses of the CRF-R1 antagonist produced greater suppression of BOLD activity in the dorsal pons for IBS compared to HCs. This finding is important given the dorsal pons contains the locus coeruleus complex (LCC), which sends dense noradrenergic projections to neural substrates known to mediate extinction learning and retrieval, including the AMYG, HIPP and mPFC.46, 47 In addition, in IBS, higher doses of the antagonist produced extensive reductions in BOLD activity bilaterally for the mPFC as well as the right HIPP and left aINS. The major pathway implicated by animal models in the extinction of conditioned fear comprises mPFC inhibitory projections to the AMYG. The HIPP and aINS have also been implicated in a common core network for human aversive conditioning and are known to play a critical role in extinction learning and retrieval.26, 30 Furthermore, non-human primate studies have identified the widespread distribution of CRF and CRF-R1 throughout the PFC, HIPP, INS, and LCC.48, 49 The fact that the CRF-R1 antagonist selectively attenuated activity in these regions in IBS but not HCs is consistent with impaired extinction learning in IBS patients, and suggests hyperactivity of the CRF/CRF-R1 signaling pathway within this network may contribute in part to this abnormality.
Drug effects on SCR during acquisition and extinction
Following PLA administration, HCs showed greater SCR compared to patients during acquisition. In light of evidence suggesting IBS patients may have previously learned associations for threat-related visceral and contextual cues,5 these results could be interpreted as due to priming of already established fear conditioned circuits leading to diminished SCR compared to HCs.
In contrast, during extinction, IBS patients showed significantly greater SCR compared to HCs following PLA, indicating that IBS patients displayed hyper-responsive SNS responses to a previously conditioned fear-related threat, a finding likely due to deficits in the ability to properly extinguish these responses. These results are consistent with previous research demonstrating hypervigilance and enhanced SNS functioning during anticipation of an aversive visceral stimulus in female IBS patients.50–52 More importantly, our results showed that administration of either low or high doses of the antagonist during extinction effectively abolished SNS hyper-responsivity in patients, but had no discernable effect on HCs. Although the exact mechanism by which this occurs cannot be ascertained by these data alone, IBS symptom chronicity may be due, in part, to HPA sensitization following repeated stress exposure, perhaps via up-regulation of this receptor in the hypothalamus and/or dorsal pontine brainstem nuclei (e.g., LCC), both of which are critically involved in maintenance of stress-related behaviors and autonomic arousal. Indeed, CRF-R1 binding and mRNA expression have been identified in the HYPO and LCC, as well as other regions comprising fear extinction circuitry.48, 49, 53, 54
Conclusions and clinical implications
To our knowledge, this is the first evidence obtained in human subjects that implicate engagement of the CRF/CRF-R1 signaling system in the modulation of brain and autonomic responses during an emotional learning paradigm. Even though this study was not designed or powered to evaluate the role of trait and state anxiety on emotional learning in IBS patients, our findings are consistent with an extensive literature on such a relationship.55, 56 On the one hand, conditioned fear to aversive signals from the body and an upregulation of the central CRF/CRF-R1 signaling system are likely to play an important pathophysiological role in the characteristic findings of both non-pathological trait anxiety levels in the majority of IBS patients17 and in the higher frequency of comorbid anxiety disorders.57 On the other hand, increased levels of trait and state anxiety in the IBS group, may have contributed to the observed group differences in emotional learning in the current study, as previously described in patients with anxiety disorders 6, 10 and rodent models of such disorders.58, 59
Our findings demonstrate the possibility that up-regulation of CRF/CRF-R1 signaling in IBS plays a role in the impaired extinction learning in these patients, manifesting as persistent hypervigilance towards gastrointestinal signals and symptoms.2, 60 The fact that alterations in fear learning can be acutely normalized by oral administration of a selective CRF-R1 antagonist may have implications for IBS pathophysiology and future drug development.
Supplementary Material
Acknowledgments
Grant Support: Research supported by GSK, NIH grants R01 DK048351 (EAM), P50 DK064539 (EAM), K23 DK073451 (KT), K08 DK071626 (JSL), and NIH GI Training Grant T32 DK07180-34.
Abbreviations
- AMYG
amygdala
- aINS
anterior insula
- aMCC
anterior midcingulate cortex
- BOLD
blood-oxygen-level-dependent
- CRF-R1
CRF receptor 1
- CRF
corticotropin-releasing factor
- dlPFC
dorsolateral prefrontal cortex
- EPI
echo-planar imaging
- fMRI
functional Magnetic Resonance Imaging
- GLM
general linear model
- HCs
healthy controls
- HIPP
hippocampus
- HYPO
hypothalamus
- IBS
irritable bowel syndrome
- mPFC
medial prefrontal cortex
- MNI
Montreal Neurological Institute
- PAG
periaqueductal gray
- PLA
placebo
- pINS
posterior insula
- pACC
pregenual anterior cingulate cortex
- SCR
skin conductance responses
- sgACC
subgenual ACC
- SNS
sympathetic nervous system
- THAL
thalamus
- vlPFC
ventrolateral prefrontal cortex
Footnotes
Conflict of Interest: The project was an investigator initiated study, funded by GlaxoSmithKline (GSK). E.A. Mayer has been an advisory board member for GSK, and Drs. Dukes and Kelleher are GSK employees. No other coauthors have conflicts of interest to declare.
Author Contributions
Jennifer S Labus: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis
Catherine S. Hubbard: analysis and interpretation of data; drafting of the manuscript
Joshua Bueller: study concept and design; acquisition of data
Bahar Ebrat: acquisition of data, statistical analyses
Kirsten Tillisch: study concept and design, critical revision of the manuscript for important intellectual content
Michelle Chen: statistical analysis, drafting of the manuscript
Jean Stains: study concept and design, acquisition of data
George E. Dukes: study concept and design, provided study drug
Dennis L. Kelleher: study concept and design, provided study drug
Bruce D. Naliboff: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content, obtained funding
Michael Fanselow: study concept and design; critical revision of the manuscript for important intellectual content
Emeran A. Mayer: Procurement of funding; study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content, obtained funding
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Larsson MBO, Tillisch K, Craig AD, et al. Brain Responses to Visceral Stimuli Reflect Visceral Sensitivity Thresholds in Patients With Irritable Bowel Syndrome. Gastroenterology. 2012;142:463–U111. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaguez L, Coen S, Gregory LJ, et al. Brain response to visceral aversive conditioning: A functional magnetic resonance imaging study. Gastroenterology. 2005;128:1819–1829. doi: 10.1053/j.gastro.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 4.Elsenbruch S, Kattoor J, Hofmann S, et al. Fear conditioning in an abdominal pain model: Neural mechanisms of associative learning and extinction in healthy subjects. Neurogastroenterology and Motility. 2012;24:22–23. [Google Scholar]
- 5.Mayer EA, Naliboff BD, Chang L, et al. Stress and the gastrointestinal tract - V. Stress and irritable bowel syndrome. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2001;280:G519–G524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- 6.Shin LM, Liberzon I. The Neurocircuitry of Fear, Stress, and Anxiety Disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milad MR, Quirk GJ. Fear Extinction as a Model for Translational Neuroscience: Ten Years of Progress. Annual Review of Psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research - Past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Phelps EA, Delgado MR, Nearing KI, et al. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 10.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn AJ, Berridge CW. Physiological and Behavioral-Responses to Corticotropin-Releasing Factor Administration - Is Crf a Mediator of Anxiety or Stress Responses. Brain Research Reviews. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 12.Vale W, Spiess J, Rivier C, et al. Characterization of a 41-Residue Ovine Hypothalamic Peptide That Stimulates Secretion of Corticotropin and Beta-Endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 13.Heim C, Nemeroff CB. Neurobiology of Posttraumatic Stress Disorder. Cns Spectrums. 2009;14:13–24. [PubMed] [Google Scholar]
- 14.Stahl SM, Wise DD. The potential role of a corticotropin-releasing factor receptor-1 antagonist in psychiatric disorders. Cns Spectrums. 2008;13:467–472. doi: 10.1017/s1092852900016709. [DOI] [PubMed] [Google Scholar]
- 15.Taché Y, Kiank C, Stengel A. A role for corticotropin-releasing factor in functional gastrointestinal disorders. Current Gastroenterology Reports. 2009;11:270–277. doi: 10.1007/s11894-009-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. Journal of Gastroenterology. 2007;42:48–51. doi: 10.1007/s00535-006-1942-7. [DOI] [PubMed] [Google Scholar]
- 17.Hubbard CS, Labus JS, Bueller J, et al. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci. 2011;31:12491–500. doi: 10.1523/JNEUROSCI.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen H, Jotkowitz A, Buskila D, et al. Post-traumatic stress disorder and other co-morbidities in a sample population of patients with irritable bowel syndrome. European Journal of Internal Medicine. 2006;17:567–571. doi: 10.1016/j.ejim.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Irwin C, Falsetti SA, Lydiard RB, et al. Comorbidity of posttraumatic stress disorder and irritable bowel syndrome. Journal of Clinical Psychiatry. 1996;57:576–578. doi: 10.4088/jcp.v57n1204. [DOI] [PubMed] [Google Scholar]
- 20.Kehne JH, Cain CK. Therapeutic utility of non-peptidic CRF1 receptor antagonists in anxiety, depression, and stress-related disorders: Evidence from animal models. Pharmacology & Therapeutics. 2010;128:460–487. doi: 10.1016/j.pharmthera.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 (Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 22.Di Fabio R, St-Denis Y, Sabbatini FM, et al. Synthesis and Pharmacological Characterization of Novel Druglike Corticotropin-Releasing Factor 1 Antagonists. Journal of Medicinal Chemistry. 2008;51:7370–7379. doi: 10.1021/jm800744m. [DOI] [PubMed] [Google Scholar]
- 23.Dukes G, Mayer EA, Kelleher DL, Hicks KJ, Boardley RL, Alpers DH. A randomised double blind placebo (PLA) controlled crossover study to evaluate the efficacy and safety of the corticotrophin releasing factor 1 (CRF1) receptor antagonist (RA) GW876008 in irritable bowel syndrome (IBS) patients (Pts) Neurogastroenterology and Motility. 2009;21:84. [Google Scholar]
- 24.Thoua N, Hobson AR, Dukes GE, Kelleher D, Hicks K, Boardley R, Raeburn A, Emmanuel A. The selective CRF-1 receptor antagonist GW876008 attenuates stress induced rectal hypersensitivity in patients with irritable bowel syndrome (IBS) Neurogastroenterology and Motility. 2009;21:85. [Google Scholar]
- 25.Buchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Current Opinion in Neurobiology. 2000;10:219–223. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 26.Buchel C, Dolan RJ, Armony JL, et al. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. Journal of Neuroscience. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delgado MR, Nearing KI, LeDoux JE, et al. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May JC, Delgado MR, Dahl RE, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Sarinopoulos I, Grupe DW, Mackiewicz KL, et al. Uncertainty during Anticipation Modulates Neural Responses to Aversion in Human Insula and Amygdala. Cereb Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sehlmeyer C, Schoning S, Zwitserlood P, et al. Human Fear Conditioning and Extinction in Neuroimaging: A Systematic Review. Plos One. 2009:4. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 32.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–59. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis KD, Pope G, Chen J, et al. Cortical thinning in IBS: Implications for homeostatic, attention, and pain processing. Neurology. 2008;70:153–154. doi: 10.1212/01.wnl.0000295509.30630.10. [DOI] [PubMed] [Google Scholar]
- 34.Jiang ZG, Labus JS, Ashe-McNalley C, et al. Cortical Thinning in Female Patients With Irritable Bowel Syndrome. Gastroenterology. 2012;142:S547–S547. [Google Scholar]
- 35.Milad MR, Wright CI, Orr SP, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience and Biobehavioral Reviews. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 38.Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition & Emotion. 1998;12:353–385. [Google Scholar]
- 39.MacDonald AW, Cohen JD, Stenger VA, et al. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 40.Bremner JD, Narayan M, Staib LH, et al. Neural correlates of sexual abuse-related PTSD. Biol Psychiatry. 1999;45:119S–120S. [Google Scholar]
- 41.Bremner JD, Narayan M, Staib LH, et al. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brody A, Barsom MW, Bota RG, Saxena S. Prefrontal-subcortical and limbic circuit mediation of major depressive disorder. Seminal Clinical Neuropsychiatry. 2001;6:102–112. doi: 10.1053/scnp.2001.21837. [DOI] [PubMed] [Google Scholar]
- 43.Liotti M, Mayberg HS, Jones VM, et al. Interactive effects in the anterior cingulate of sadness and selective attention: A pet study. Biol Psychiatry. 2000;47:125S–126S. [Google Scholar]
- 44.Hall JL, Gonzalez R, Sripada C, et al. Investigating the Emotions Behind Investment Decisions: Fmri Evidence That Subliminal Affective Cues Can Empty Your Wallet. Psychophysiology. 2010;47:S79–S79. [Google Scholar]
- 45.Elsenbruch S, Rosenberger C, Bingel U, et al. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010;139:1310–9. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 46.Jones BE, Halaris AE, Mcilhany M, et al. Ascending Projections of Locus Coeruleus in Rat.1. Axonal-Transport in Central Noradrenaline Neurons. Brain Res. 1977;127:1–21. doi: 10.1016/0006-8993(77)90377-8. [DOI] [PubMed] [Google Scholar]
- 47.Loughlin SE, Foote SL, Bloom FE. Efferent Projections of Nucleus Locus-Coeruleus - Topographic Organization of Cells of Origin Demonstrated by 3-Dimensional Reconstruction. Neuroscience. 1986;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- 48.Millan MA, Jacobowitz DM, Hauger RL, et al. Distribution of Corticotropin-Releasing Factor Receptors in Primate Brain. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:1921–1925. doi: 10.1073/pnas.83.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez MM, Young LJ, Plotsky PM, et al. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. Journal of Comparative Neurology. 1999;408:365–377. [PubMed] [Google Scholar]
- 50.Naliboff BD, Waters AM, Labus JS, et al. Increased Acoustic Startle Responses in IBS Patients During Abdominal and Nonabdominal Threat. Psychosomatic Medicine. 2008;70:920–927. doi: 10.1097/PSY.0b013e318186d858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kilpatrick LA, Ornitz E, Ibrahimovic H, et al. Sex-related differences in prepulse inhibition of startle in irritable bowel syndrome (IBS) Biol Psychol. 2010;84:272–8. doi: 10.1016/j.biopsycho.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spetalen S, Sandvik L, Blomhoff S, et al. Autonomic function at rest and in response to emotional and rectal stimuli in women with irritable bowel syndrome. Digestive Diseases and Sciences. 2008;53:1652–1659. doi: 10.1007/s10620-007-0066-0. [DOI] [PubMed] [Google Scholar]
- 53.Sawchenko PE, Swanson LW. Immunohistochemical Identification of Neurons in the Paraventricular Nucleus of the Hypothalamus That Project to the Medulla or to the Spinal-Cord in the Rat. Journal of Comparative Neurology. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- 54.Van Pett K, Viau V, Bittencourt JC, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of bat and mouse. Journal of Comparative Neurology. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 55.Labus J, Gupta A, Gill HK, et al. Randomised clinical trial: symptoms of the irritable bowel syndrome are improved by a psycho-education group intervention. Alimentary Pharmacology & Therapeutics. 2013;37:304–315. doi: 10.1111/apt.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lackner JM, Coad ML, Mertz HR, et al. Cognitive therapy for irritable bowel syndrome is associated with reduced limbic activity, GI symptoms, and anxiety. Behaviour Research and Therapy. 2006;44:621–638. doi: 10.1016/j.brat.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spiegel BMR, Gralnek IM, Bolus R, et al. Clinical determinants of health-related quality of life in patients with irritable bowel syndrome. Archives of Internal Medicine. 2004;164:1773–1780. doi: 10.1001/archinte.164.16.1773. [DOI] [PubMed] [Google Scholar]
- 58.Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Rodgers RJ. Animal models of ‘anxiety’: where next? Behavioural Pharmacology. 1997;8:477–496. doi: 10.1097/00008877-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Larsson M, Tillisch K, Mayer E, et al. Do IBS patients without rectal hypersensitivity adapt to repeated aversive rectal distensions? Neurogastroenterology and Motility. 2012;24:109–110. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


