Abstract
The rising incidence of emerging infectious diseases (EID) is mostly linked to biodiversity loss, changes in habitat use and increasing habitat fragmentation. Bats are linked to a growing number of EID but few studies have explored the factors of viral richness in bats. These may have implications for role of bats as potential reservoirs. We investigated the determinants of viral richness in 15 species of African bats (8 Pteropodidae and 7 microchiroptera) in Central and West Africa for which we provide new information on virus infection and bat phylogeny. We performed the first comparative analysis testing the correlation of the fragmented geographical distribution (defined as the perimeter to area ratio) with viral richness in bats. Because of their potential effect, sampling effort, host body weight, ecological and behavioural traits such as roosting behaviour, migration and geographical range, were included into the analysis as variables. The results showed that the geographical distribution size, shape and host body weight have significant effects on viral richness in bats. Viral richness was higher in large-bodied bats which had larger and more fragmented distribution areas. Accumulation of viruses may be related to the historical expansion and contraction of bat species distribution range, with potentially strong effects of distribution edges on virus transmission. Two potential explanations may explain these results. A positive distribution edge effect on the abundance or distribution of some bat species could have facilitated host switches. Alternatively, parasitism could play a direct role in shaping the distribution range of hosts through host local extinction by virulent parasites. This study highlights the importance of considering the fragmentation of bat species geographical distribution in order to understand their role in the circulation of viruses in Africa.
Introduction
Bats are linked to a growing number of emerging infectious diseases (EID) [1], [2] such as Ebola or Marburg Haemorrhagic fevers [3]–[5], SARS Coronavirus [6] and the newish Middle East respiratory syndrome coronavirus (MERS-CoV) [7]. This trend is, inter alia, linked to biodiversity loss, changes in habitat use and increased habitat fragmentation [8].
Few studies have investigated parasite species richness in bats [9]–[11]. However, Turmelle and Olival [12] showed viral richness in bats correlates with IUCN status and population genetic structure. The distribution range of hosts has been often considered as a potential determinant of parasite species richness [13]–[15]. Hosts distributed over large areas are more likely to encounter new parasites that may infect them [14], [16]. However, the shape of the distribution has received little attention [12], [13] but may have implications on the role of bats as pathogen reservoirs. Distribution shape and habitat fragmentation were observed at two different scales and Fahrig [17] suggested that the processes affecting changes in distribution and habitat preference of a species are independent. The shape of the distribution being mostly the products of speciation, extinction and range expansion [18]. Area shape is an important aspect of the distribution of animals and plants, which is strongly linked to population demographics and the subsequent contraction and expansion of their distribution [19], [20]. Therefore, area shape must be taken into account together with phylogenetic information in any comparative analysis of parasite diversity. Two alternative explanations can be proposed on the potential link between host distribution shape and parasite species richness: a longer border, due to fragmentation, may entail higher habitat diversity which would intensify contacts with various sources of parasites leading an overall increase in parasite diversity. Alternatively, a longer border may increase host species vulnerability due to area fragmentation and reduced host population size, hence pathogen transmission.
The first comparative analysis was performed to test the hypothesis that distribution shape and more specifically the fragmentation of the distribution area, correlates with viral richness in bats. We investigate the determinants of viral richness in 15 species of African bats, on which we found new information on virus infection and bat phylogeny. Body weight, roosting behaviour and migration [10], [21] were also included in our analysis because of their potential influences on parasite or viral species richness.
Materials and Methods
Ethic statements
All the capture events, animal handling, euthanasia and transfer of samples across country borders were performed in accordance with the guidelines of the American Society of Mammalogists (http://www.mammalsociety.org/committees/animal-care-and-use) [22]:
Bats were captured following recommendations by Kunz and Parsons [23]. Captured bats were removed carefully from nets as soon as possible to minimize injury, drowning, strangulation, or stress. Safe and humane euthanasia was achieved through the use of inhalant anaesthetic (halothane) prior to autopsy.
All work (capture, euthanasia and autopsy) was carried out with authorization from the respective wildlife authorities of each country. Capture and sacrifice Permit in Gabon: N°0021/MEFE- PA/SG/DGEF/DCF (2009) and N°0031/MEFDD/SG/DGEF/DFC (2010 and 2011), and from the Direction de la Faune et de la Chasse, Ministère des eaux et forêts, de l'environnement et du développement durable, Gabon. Capture and sacrifice permit in Central African Republic (CAR): N°038/MENAESR/D.CAB/DGESR/DRS/SCGPRS. 08, and from the Ministère de l'Education Nationale, de l'Alphabétisation, de l'Enseignement Supérieur et de la Recherche, CAR. Sample collection in Senegal and Republic of Congo: we used samples collected by previous studies on filovirus in bat populations [4], [24], [25].
Study animals
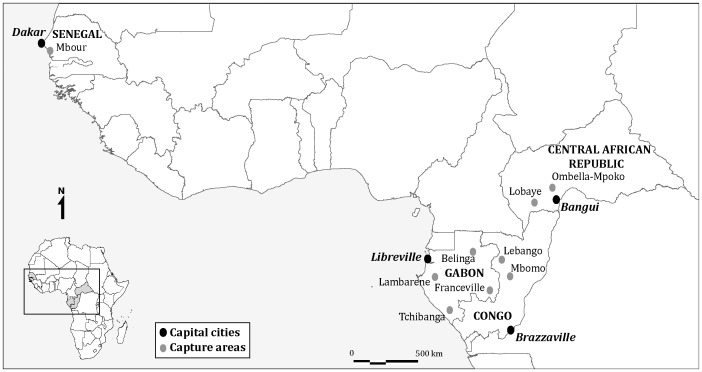
Our study on the correlation of viral richness in bats was conducted using 15 bats species from Central and West Africa. We selected only the species for which we had enough samples and information on viral richness to carry out analysis. Bats were caught in the Republic of Congo, Gabon, Central African Republic (CAR) and Senegal [4]. In the Republic of Congo, bats were caught in 2005 and 2006 at Mbomo (0°25N; 14°41E) and Lebango (0°39′ N; 14°21′ E). In Gabon captures occurred at four sites in 2005, 2006, 2009 and 2010: the first one was located near Franceville (1°37S; 13°36E) the largest town of the Haut-Ogooué province in south-eastern Gabon; the second site was located close to Lambarene (0°41S; 11°01E), the largest town of the Moyen-Ogooué province in western Gabon; the third one was near Tchibanga (2°51S; 11°01E), the main town of the Nyanga province in south-western Gabon; and 3 caves (Faucon Cave: 1°07 N; 13°20 E, Zadié Cave: 0°98 N; 13°19 E and Batouala Cave: 0°82 N; 13°45 E) situated in the Belinga Mountain in Northeastern Gabon. In CAR, samples were collected in 2008 and 2009 at 3 localities: Lobaye (3°46′ S; 18°34′ E), Ombella-Mpoko (4°33′ S; 18°30′ E), and Bangui (4°21 N; 18°33 E), the capital. In Senegal, captures took place at Mbour in 2006 (14°25′ N; 16°57′ E) located about 80 km from Dakar, capital of Senegal (Figure 1).
Figure 1. Geographic location of field sites where bats were captured.
Bats were captured using mist-nets or harp traps. Mist-nets (12×2.4 m) were hoisted either in the tree canopy (defined as “foliage”) or at the entrance of the small roosting caves (defined as “cave”) just before twilight. Harp Traps were used at the entrance of big caves known to harbor large population of bats. Following capture, bats were identified on site by trained field biologists and individually euthanized under sedation in a field laboratory. Bats were weighed using a spring scale prior to autopsy and selected internal organs were collected during autopsy and stored at −80°C for future virological analysis. Data on the ecological traits of the 15 different bat species captured (i.e., roost type, body weight, migratory behaviour and colony size) was gathered from published literature (Table 1, see Annex 1 for references).
Table 1. Factors tested as potential determinants of viral richness (References in Annexe 1).
| Bats species | Viral Richness* | Sample size | Geographical range (km2) | Fragmen-tation | Roost type** | Body weight (g)*** | Migratory | Colony size |
| Coleura afra | 1 | 85 | 3,573,000 | 0.86 | Cave | 9.9 | Yes | 1000 |
| Eidolon helvum | 12 | 1019 | 14,510,000 | 0.23 | Foliage | 177.3 | Yes | 500000 |
| Epomophorus gambianus | 4 | 169 | 4,947,000 | 0.34 | Cave | 87.2 | Yes | 50 |
| Epomops franqueti | 4 | 763 | 4,564,000 | 0.28 | Foliage | 114.7 | Yes | 5 |
| Hipposideros cf. ruber | 4 | 585 | 8,056,000 | 0.57 | Cave | 8.2 | Yes | 500000 |
| Hipposideros gigas | 5 | 230 | 4,357,000 | 0.39 | Cave | 109 | Yes | 300–1000 |
| Hypsignathus monstrosus | 5 | 188 | 3,562,000 | 0.53 | Foliage | 312.5 | Yes | 25–132 |
| Megaloglossus woermanni | 1 | 49 | 3,498,000 | 0.38 | Foliage | 13.3 | Yes | - |
| Micropteropus pusillus | 5 | 706 | 6,704,000 | 0.37 | Foliage | 26.1 | No | 1–10 |
| Miniopterus inflatus | 3 | 275 | 2,423,000 | 0.53 | Cave | 9.5 | Yes | 50 |
| Mops condylurus | 4 | 446 | 9,355,130 | 0.30 | Cave | 22.45 | Yes | 18–200 |
| Myonycteris torquata | 3 | 580 | 4,624,000 | 0.29 | Foliage | 45.7 | Yes | - |
| Neoromicia tenuipinnis | 0 | 35 | 4,279,511 | 0.41 | Cave | 5.3 | Yes | 20 |
| Rousettus aegyptiacus | 13 | 1828 | 4,989,000 | 0.91 | Cave | 120.3 | No | 5000 |
| Taphozous mauritianus | 0 | 9 | 12,436,000 | 0.23 | Foliage | 27.8 | No | 12 |
*Viral richness is obtained from the number of individual bats that we have sampled combined with animals sampled as reported in published papers.
**Foliage includes bats that roost in trees: main bough, under bark, within foliage, hollow branches, under exposed roots, deep in dense foliage and in tree trunks. Cave includes tunnels, cavities or crevices, abandoned mine shafts, roofs and basements of houses.
***Average body weight, both sexes combined.
Bat phylogeny
In order to improve the quality of the comparative analysis, a phylogenetic tree was built using 14 new molecular sequences of the bat mitochondrial cytochrome b gene (Table 2). Total genomic DNA was extracted from ethanol-preserved tissue samples (muscle, liver or spleen) with Genomic DNA Tissue Mini Kit (Geneaid Biotech) according to the manufacturer's protocol. We amplified the mitochondrial gene for cytochrome b (cytb) using primer pairs F1 (modified; 5′- CCACGACCAATGACAYGAAAA-3′) and R1 from Sakai et al. [26] in most microbats, L14724 and H15915 from Irwin et al. [27] in hipposiderids and fruit bats, LGL765F and LGL766R from Bickham et al. [28], [29] in long-fingered bats (Miniopterus inflatus). The volume of PCR reaction was 25 µl, it contained 12.5 µl Combi PPP Master Mix (Top-Bio, Prague, Czech Republic), 200 µM of forward and reverse primers respectively, and 2.5 µl of extracted DNA. PCR protocol consisted in an initial denaturation at 94°C for 3 min, 35 cycles of denaturation for 40 s at 94°C, annealing for 40 s at 50°C, and extension for 90 s at 65°C, and a final extension at 65°C for 5 min. Resulting PCR products were inspected on 1.5% agarose gel and purified with Gel/PCR DNA Fragments Extraction Kit (Geneaid Biotech). If multiple bands appeared, the one of appropriate length was excised and purified from gel using the same purification kit. Purified PCR products were sequenced commercially (Macrogen, Seoul, Korea) with the respective forward primer using BigDye Terminator sequencing chemistry (Applied Biosystems, Foster City, CA, USA) on ABI 3730xl sequencer. Sequences were edited in Sequencher 4.6 (Gene Codes, Ann Arbor, MI, USA), manually checked for correct base reading and protein coding frame, and aligned by eye in BioEdit 7.0 [30]. Sequences of two artiodactyl taxa, Bos taurus (D34635) and Ovis ammon (AJ867276) were added to the alignment as outgroup taxa for rooting the bat phylogeny. Phylogenetic tree including branch lengths was inferred from aligned nucleotide sequences in PAUP*4.0b (Sinauer Associates, Sunderland, Massachusetts, USA) under maximum likelihood (ML) criterion and general time-reversible model of evolution with a portion of invariable sites and gamma distributed variation rates (GTR+I+Γ), which was suggested as the best evolutionary model and whose parameters were estimated in Modeltest 3.7. Topological constraints were set before computation of the ML tree, as corresponding to acknowledged phylogenetic relationships among genera, families and higher taxonomic ranks of bats as referred by Teeling et al. [31] and Almeida et al. [32]. Due to a priori definition of the tree topology, analysis of nodal support was not performed. The constrained ML tree was, however, compared to unconstrained ML tree using a Shimodaira-Hasegawa (SH) test, in order to assess possible significant difference, which might indicate unreliability of the constrained tree. Sequences generated in this study were deposited in the EMBL/DDBJ/Genbank databases under accession number (JQ956436-JQ956449).
Table 2. Characteristic of bats included into phylogenetic analyses in this study and accessions number for all cytB sequences.
| Sample ID | Year of collection | Bat species | Sex | Country | Locality | Tissue source | Source | GenBank accession no. |
| GB2139 | 2005 | Megaloglossus woermanni | M | Congo | Mbomo | Liver | CIRMF | JQ956436 |
| GB2225 | 2005 | Myonycteris torquata | M | Congo | Lebango | Liver | CIRMF | JQ956437 |
| 09/760 | 2009 | Micropteropus pusillus | F | RCA | Ombella-Mpoko | Spleen | IP Bangui | JQ956438 |
| GB2569 | 2006 | Hypsignathus monstrosus | F | Congo | Mbomo | Spleen | CIRMF | JQ956439 |
| GB1961 | 2005 | Epomops franqueti | M | Congo | Lebango | Spleen | CIRMF | JQ956440 |
| GB1661 | 2005 | Eidolon helvum | F | Gabon | Lambaréné | Spleen | CIRMF | JQ956441 |
| GB3320 | 2006 | Epomophorus gambianus | M | Senegal | Mbour | Liver | CIRMF | JQ956442 |
| GB0685 | 2009 | Hipposideros gigas | M | Gabon | Belinga | Spleen | CIRMF | JQ956443 |
| 08/316 | 2008 | Taphozous mauritianus | M | RCA | Lobaye | Spleen | IP Bangui | JQ956444 |
| 08/207 | 2008 | Mops condylurus | M | RCA | Lobaye | Liver | IP Bangui | JQ956445 |
| 08/322 | 2008 | Neoromicia tenuipinnis | M | RCA | Lobaye | Spleen | IP Bangui | JQ956446 |
| GB0332 | 2009 | Miniopterus inflatus | M | Gabon | Belinga | Patagium | CIRMF | JQ956447 |
| GB0675 | 2009 | Hipposideros cf. ruber | F | Gabon | Belinga | Liver | CIRMF | JQ956448 |
| GB0415 | 2009 | Coleura afra | M | Gabon | Belinga | Patagium | CIRMF | JQ956449 |
IP: Institut Pasteur; No data available for the sequence of Rousettus aegyptiacus (Genbank accession number AB085740).
Viral richness
Two methods were used to document viral richness of the studied bat species. First, we tested our bat samples for viruses. We used (i) nested Reverse-Transcription polymerase chain reaction (RT-PCR) assay targeting the RNA-dependent RNA polymerase gene using generic consensus primers for the genus Coronavirus [33]; (ii) hemi-nested RT-PCR targeting the N terminal end of the NS5 gene by using degenerate primers for the genus Flavivirus [34], [35]; and (iii) filoviruses (Marburg virus and Ebola virus) as previously described [4], [36] (Table 3). Then, additional virological data were drawn from literature. In published papers, the methods used to detect viruses directly were mouse inoculation, cell culture, electron microscopy and PCR; indirect methods utilised to detect markers of replication and viral infection in bats from organs, tissues or blood were direct fluorescent antibody, indirect fluorescence antibodies, radio immuno assay, rapid fluorescent focus inhibition test, fluorescent antibody test, and seroneutralization. The serological detection of arbovirus antibodies alone (particularly genus Flavivirus and Alphavirus) was not considered as evidence of a viral association because of some degree of cross-reaction within the virus family, rendering it difficult to differentiate viruses. Viruses forming distinct clusters within the same genus were recorded as a unique viral species. For example, in Rousettus aegyptiacus, bat gammaherpes viruses (Bat GHV) 1, 2, 4, 5, 6 and 7 were recorded as one unique viral species and Bat GHV 3 as another viral species [37]. For Ebola virus, different viral species of this genus were considered as a single virus. For each bat species, we calculated the viral richness as the total number of different viruses described for the given bat species.
Table 3. Samples used for viral screening.
| Coronavirus | Flavivirus | Marburg virus | Ebola virus | |||||||
| Sampling site | Species | Total of samples collected | N° of tested | N° of positive | N° of tested | N° of positive | N° of tested | N° of positive | N° of tested | N° of positive |
| Gabon | Coleura afra | 31 | 23 | 0 | 29 | 0 | - | - | 31 | 0 |
| Eidolon helvum | 60 | 48 | 0 | 32 | 0 | - | - | - | - | |
| Epomops franqueti | 498 | 358 | 0 | 140 | 0 | - | - | - | - | |
| Hipposideros cf.ruber | 540 | 387 | 3 | 498 | 0 | - | - | 521 | 0 | |
| Hipposideros gigas | 234 | 228 | 0 | 227 | 1 | - | - | 233 | 0 | |
| Hypsignathus monstrosus | 43 | 40 | 0 | 14 | 0 | - | - | 1 | 0 | |
| Megaloglossus woermanni | 50 | 47 | 0 | 16 | 0 | - | - | - | - | |
| Micropteropus pusillus | 47 | 43 | 0 | 37 | 0 | - | - | - | - | |
| Miniopterus inflatus | 190 | 52 | 0 | 179 | 0 | - | - | 186 | 0 | |
| Myonycteris torqutata | 243 | 220 | 0 | 98 | 0 | - | - | - | - | |
| Rhinolophus cf. alcyone | 15 | 15 | 0 | 15 | 0 | - | - | 15 | 0 | |
| Rousettus aegyptiacus | 582 | 492 | 0 | 305 | 1 | - | - | 187 | 0 | |
| Congo | Epomops franqueti | 393 | 286 | 0 | 128 | 2* | - | - | - | - |
| Hypsignathus monstrosus | 94 | 42 | 0 | 74 | 0 | - | - | - | - | |
| Megaloglossus woermanni | 20 | 5 | 0 | 20 | 0 | - | - | - | - | |
| Micropteropus pusillus | 273 | 129 | 0 | 100 | 0 | - | - | - | - | |
| Myonycteris torquata | 589 | 286 | 0 | 136 | 0 | - | - | - | - | |
| Rousettus aegyptiacus | 5 | 2 | 0 | 5 | 0 | - | - | - | - | |
| Senegal | Eidolon helvum | 32 | 18 | 0 | - | - | - | - | - | - |
| Epomophorus gambianus | 15 | 15 | 0 | - | - | - | - | - | - | |
| Rousettus aegyptiacus | 58 | - | - | - | - | - | - | - | - | |
| RCA | Eidolon helvum | 295 | 295 | 0 | 295 | 0 | 295 | 0 | 295 | 0 |
| Epomophorus gambianus | 19 | 19 | 0 | 19 | 0 | 19 | 0 | 19 | 0 | |
| Epomops franqueti | 81 | 81 | 0 | 81 | 0 | 81 | 0 | 81 | 0 | |
| Hipposideros gigas | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | |
| Hypsignathus monstrosus | 28 | 28 | 0 | 28 | 0 | 28 | 0 | 28 | 0 | |
| Megaloglossus woermanni | 3 | 3 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | |
| Micropteropus pusillus | 533 | 533 | 2* | 533 | 0 | 533 | 0 | 533 | 0 | |
| Mops condylurus | 160 | 160 | 0 | 160 | 0 | 160 | 0 | 160 | 0 | |
| Myonycteris torquata | 12 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | |
| Neoromicia tenuipinnis | 4 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | |
| Rousettus aegyptiacus | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | |
| Taphozous mauritianus | 8 | 0 | 0 | 8 | 0 | 8 | 0 | 8 | 0 | |
*Pools of ten each.
Geographical distribution size and shape
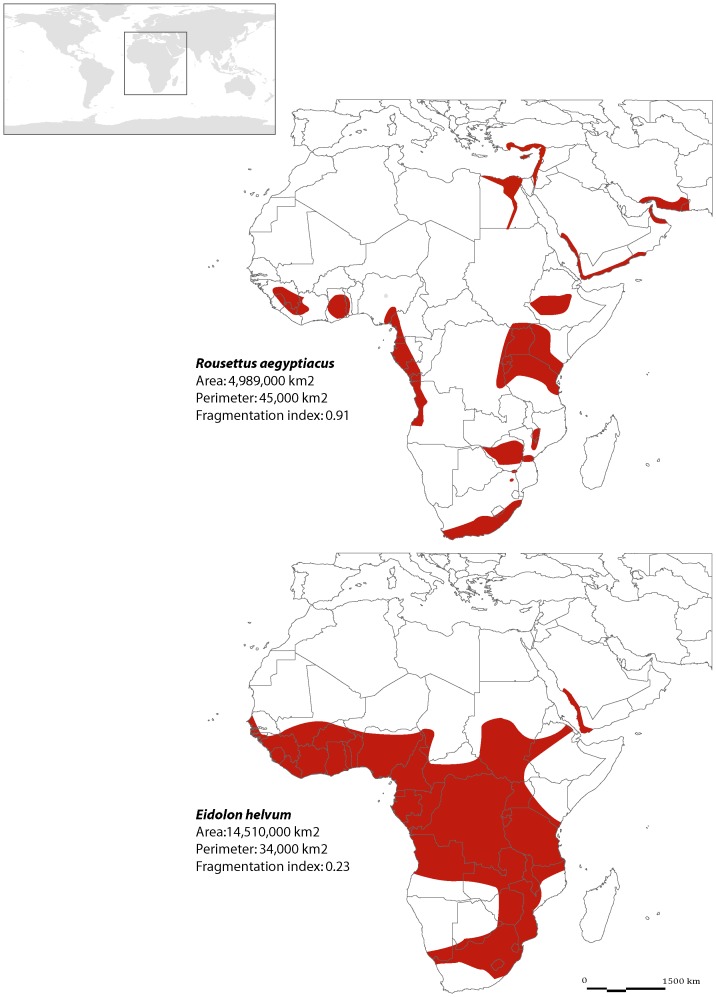
To test the impact of the fragmentation of the distribution area on viral richness in bats, we used the geographic range maps of each studied bat species provided by the ‘IUCN Red List of Threatened Species’ web site, one of the biggest databases available on mammalian distribution, based on international experts' knowledge. The maps were imported in a GIS using MapInfo professional V 5.5. We then drew polygons following species distribution to obtain area and perimeter measures for all drawn polygons. The shape of the geographic range was estimated using the ratio of the total perimeter to the total surface area following the approach used by Kauffman cited in Fortin et al. [38]. The higher the ratio, the greater is the fragmentation of the distribution (Figure 2).
Figure 2. Two examples of bat geographical distribution showing contrasted distribution shape or fragmentation (from [69]).
Comparative analyses of the determinants of viral richness
Using information on bat phylogeny described above, we calculated the independent contrasts for each of the investigated variables with the package APE [39] implemented in R (R Development Core Team 2013). To confirm the proper standardization of contrasts, we regressed the absolute values of standardized contrasts against their standard deviations. Contrasts were then analysed using standard multiple regressions, with all intercepts forced through the origin [40]. We tested the importance of the phylogenetic signal on each variable using the parameter K (which is the ratio of observed phylogenetic covariance divided by the expected covariance under Brownian motion), with the package picante [41] implemented in R (R Development Core Team 2013).
As in previous studies [12], [13], we performed standard multiple regressions using independent contrasts, with the intercept forced at zero and viral richness as the dependent variable. Independent variables were geographical range, fragmentation of the distribution, roost type (foliage vs cave), average body weight and migratory behaviour (yes vs no) (Table 1). We did not include colony size as variable as information was missing for two species. Number of sampled hosts or sampling effort (number of samples we tested added to the number of samples reported in published papers) ware also considered as an independent variable. The analysis was conducted on 14 of the 15 captured species for which sample size was considered sufficient (>30). We then selected the best subset selection of variables using AIC criteria.
Results
Viral richness
We detected coronaviruses from Hipposideros cf. ruber (accession numbers JX174638-JX174640) and Micropteropus pusillus (JX174641 and JX174642). Flaviviruses were detected from Rousettus aegyptiacus (JX174643), Hipposideros gigas (JX174644) and Epomops franqueti (JX174645 and JX174646) (Table 3). We compiled our results with the data found in the literature. We found information on viruses for the 15 selected bat species except for Neoromicia tenuipinnis and Taphozous mauritianus (Table 4).
Table 4. List of viruses found in this study and completed with data from the literature.
| Species | Virus | References |
| Eidolon helvum | Lagos bat virus (LBV), Mokola virus, West Caucasian (WC) virus, Zaire Ebola virus (ZEBOV), Ife virus (Orbivirus), Hendra virus, Nipah virus (NPHV), Rubulavirus, Coronavirus, Rotavirus related, Simplexvirus, Parvovirus | [44]–[56] |
| Micropteropus pusillus | LBV, Coronavirus, ZEBOV, Marburg virus (MBGV), Rift Valley Fever virus (RVF) | This study; [4], [57], [58] |
| Rousettus aegyptiacus | LBV, Bat Gammaherpesvirus (1, 2, 4, 5, 6, 7), Bat Gammaherpesvirus 3, Betaherpesvirus, MBGV, Coronavirus, ZEBOV, Yogue virus, Kasokero virus, Chiropteran Papillomavirus, Henipavirus, Rubulavirus, Flavivirus | This study; [4], [5], [36], |
| Miniopterus inflatus | MBGV, Coronavirus, Rubulavirus | [48], [54], [60], [61] |
| Hipposideros cf. Ruber | RVF, Rubulavirus, Morbillivirus unclassified, Coronavirus, | This study; [54], [58], [64] |
| Hipposideros gigas | Rubulavirus, Morbillivirus unclassified, Flavivirus, Shimoni bat virus, SARS-like CoV | This study; [54], [62], [65] |
| Epomops franqueti | ZEBOV, Reston Ebola virus, MBGV, Flavivirus | This study; [2], [4], [24], [66] |
| Coleura afra | Morbillivirus unclassified | [54] |
| Myonycteris torquata | ZEBOV, Coronavirus (SARS-CoV), Henipavirus | [2], [4], [24], [54], [61], [66] |
| Hypsignathus monstrosus | ZEBOV, Reston Ebola virus, MBGV, Coronavirus (SARS-CoV), NPHV | [2], [4], [24], [45], [46], [54], [61], [66] |
| Megaloglossus woermanni | Rubulavirus | [54] |
| Neoromicia tenuipinnis | No virus found | |
| Taphozous mauritianus | No virus found | |
| Mops condylurus | Bukalassa bat virus, Dakar bat virus, Entebbe bat virus, Coronavirus (SARS-CoV) | [61], [67], [68] |
| Epomophorus gambianus | LBV, NPHV, ZEBOV, Reston Ebola virus | [45], [46], [52], [66] |
West, East and Central Africa, Europe (species from zoo, unspecified origin), South Africa, USA (species from zoo, unspecified origin).
Bat Phylogeny
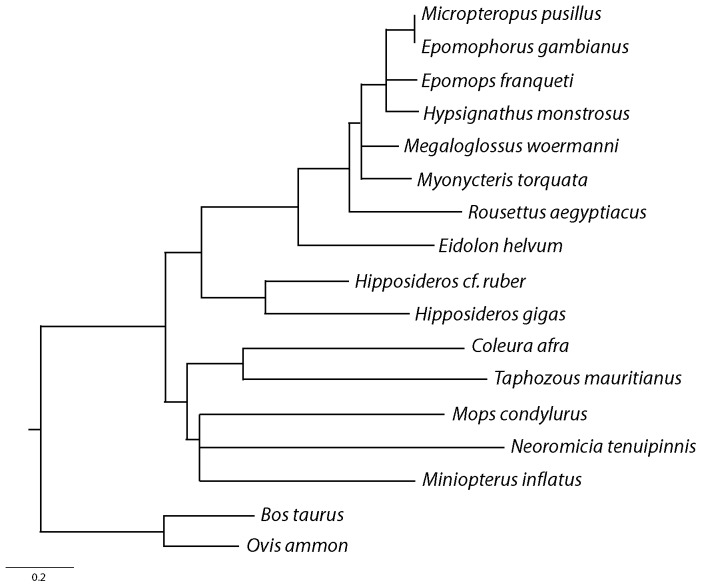
We reconstructed the phylogenetic tree of the bat species investigated in this analysis using 15 sequences under the constraint of acknowledged taxonomic relationships (Figure 3). The constrained tree (−lnL = 6439.91045) did not differ significantly from the unconstrained tree (SH test: diff. lnL = 7.89267, P = 0.126), and was thus considered as a reasonable depiction of bat phylogeny.
Figure 3. Phylogeny of the African bat species investigated in this study.
Determinant of the viral richness
Only viral richness showed statistically significant level of phylogenetic signal using estimates of K among all the traits investigated (Table 5). However, distribution shape showed a level of phylogenetic close to significance (Table 5).
Table 5. Levels of phylogenetic signal in the variables investigated using the parameter K and the parameter lambda.
| Variables | K | P (no signal) |
| Viral richness | 0.519 | 0.044 |
| Host sample size | 0.071 | 0.529 |
| Host weight (body weight) | 0.089 | 0.433 |
| Distribution size | 0.164 | 0.302 |
| Distribution shape | 0.474 | 0.072 |
| Roosting site | 0.023 | 0.478 |
| Migration | 0.014 | 0.732 |
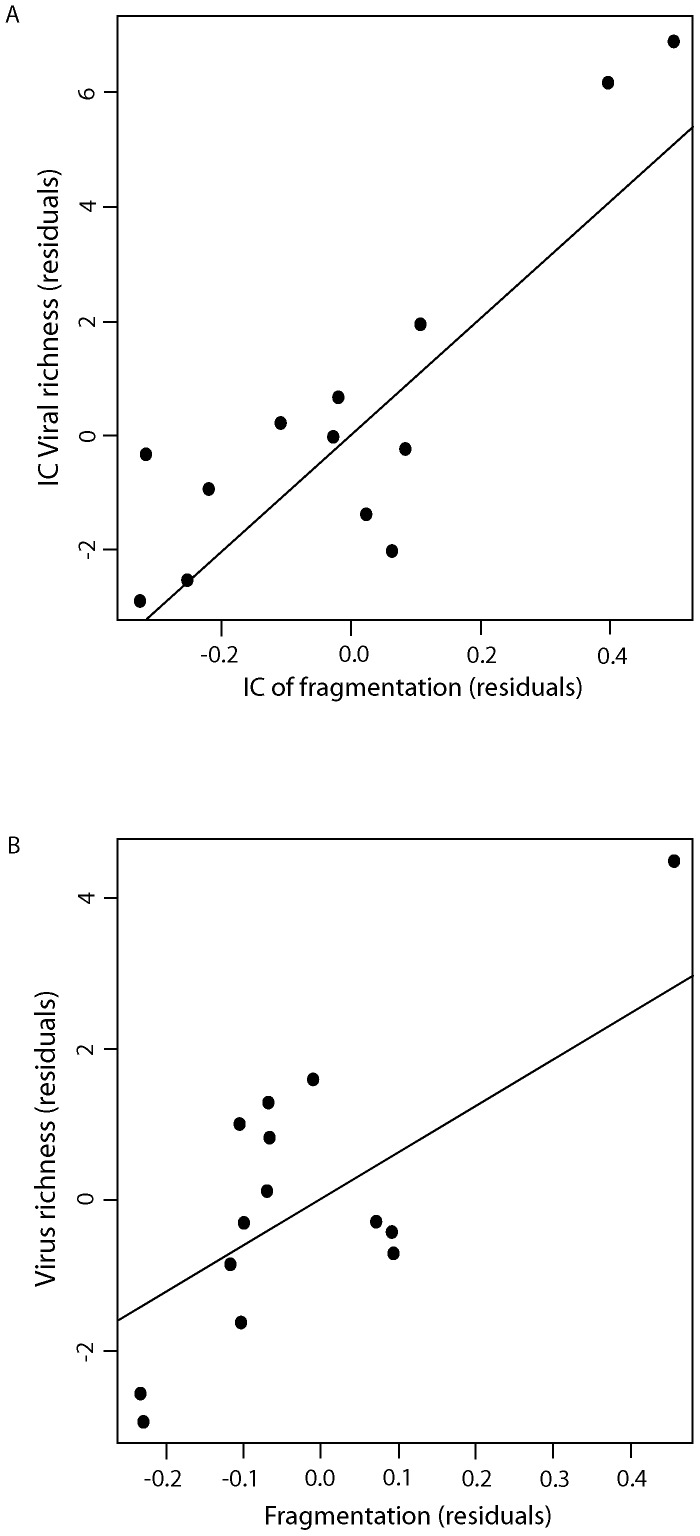
Four variables were retained in the preferred model, which was back-selected, based on the AIC criterion, and using the raw data (non corrected for phylogeny) (Table 6). Using the independent contrasts (variables controlled for phylogeny), the best model had the same four independent variables (Table 6). Taking into account host sampling, we found that viral richness in bats was greater in large-bodied and widely distributed bats and when their geographical distribution was fragmented (Tables 5 & 6). There were no significant relationships between viral richness and migratory behaviour or roosting behaviour. Finally, greater fragmentation of the geographic distribution was highly associated with increased viral richness (Table 7, Figures 4A & 4B).
Table 6. Comparison of models used to test the effects of several independent variables (weight, size and shape of distribution, migration, roosting and sample size) on viral richness of bats (using the independent contrasts), using phylogenetic regression (Independent contrasts) or non-phylogenetic regression (raw values).
| Analysis | Model ranks | AIC |
| Phylogenetic regression (Independent contrasts) | Weight + distribution size + distribution shape + sample size | 19.93 |
| Weight + distribution size + distribution shape + roosting + sample size | 20.67 | |
| Weight + distribution size+ distribution shape + migration + roosting + sample size | 22.66 | |
| Non-phylogenetic | Weight + distribution size + distribution shape + sample size | 17.91 |
| Weight + distribution size + distribution shape + roosting + sample size | 19.51 | |
| Weight + distribution size+ distribution shape + migration + roosting + sample size | 20.87 |
Models are ranked from the least to the most supported according to corrected Akaike information criteria (AIC).
Table 7. Best model explaining viral richness in bats using independent contrasts (initial model is given in Table 6), using the phylogenetic regression (independent contrasts) and non-phylogenetic regression (raw values' and independent variables are ranked according to their contributions to the models using F values).
| Analysis | Independent variables | Slope (SD), P | F-test | P | R2, |
| F-total (P) | |||||
| Phylogenetic regression (Independent contrasts) | Distribution shape | 10.25 (2.18), 0.001 | 35.8 | 0.0002 | |
| Host weight | 3.12 (0.63), 0.0008 | 6.6 | 0.031 | ||
| Host sample | 1.59 (0.65), 0.037 | 5.9 | 0.03 | ||
| R2 = 0.89 | |||||
| F4,9 = 17.9 | |||||
| (0.0003) | |||||
| Non-phylogenetic | Host weight | 2.82 (0.87), 0.009 | 31.95 | 0.0002 | |
| Distribution shape | 6.71 (2.38), 0.02 | 12.66 | 0.005 | ||
| Host sample | 3.17 (0.78),0.002 | 16.51 | 0.002 | ||
| Distribution size | 0.001 (0.0001), 0.01 | 7.16 | 0.02 | ||
| R2 = 0.87 | |||||
| F4,10 = 17.1 (0.0002) |
Figure 4. Partial relationship between viral richness and distribution fragmentation, assessed by a measure of distribution shape using (A) phylogenetic independent contrasts, or (B) raw values (and using residuals from the general regression modelling in Table 7 ).
Discussion
This is the first comparative analysis investigating the effect of distribution shape, i.e. geographical range fragmentation or edge range density, on viral richness in bats. Our first hypothesis was that bats living in caves in sympatry with other species with increased promiscuity and high population density of susceptible individuals, would generate opportunities for cross-species transmission of viruses and their rapid spread. However, our study does not support this hypothesis. Our results showed a significant influence of host body weight, distribution size and shape on viral richness; viral richness increases with larger distribution areas and fragmentation of bat distribution, according to the measure of their distribution shape. Before discussing this correlation, the difference between habitat fragmentation and habitat loss should be considered since Fahrig [17] suggested that the two processes are independent. An ecological explanation of the correlation between viral richness and distribution could be interpreted in the light of the historical biogeography of African bats, which falls within the domain of phylogeny and phylogeographic studies [31]. Range distributions and shapes are the product of speciation, extinction and historical displacements [18]. The accumulation of parasite species, viruses in the present study, could be related to the historical expansion and contraction of bat species' distribution ranges, with potentially strong effects of distribution edges on virus transmission. Indeed, the marginal effect of phylogenetic signal on the distribution shape of the investigated bats (Table 5) suggests that both history and current ecological drivers may have shaped their distribution. For a given distribution area, the most fragmented distributions contain more edges than the less fragmented ones. Positive edge effects could be responsible for the positive effects of distribution shape on either the abundance or distribution of some bat species that may have facilitated virus host switches. However, critical information to explore this issue further is lacking due to the limits of current knowledge on African bats' phylogeography as well as the geographic distribution and phylogeny of their viruses (such as bats and rabies-related viruses [42]). Furthermore, it should be noted that the use of the distribution area obtained from ICUN Red List might not accurately describe the distribution shape of bat species. More accurate and precise distributions would definitively improve the robustness of the study.
An alternative explanation produced by a theoretical study, attributes a direct role of parasitism in limiting the distribution range of hosts through the extinction of local hosts by virulent parasites [43]. However, this hypothesis has not been tested using empirical data.
As previously emphasized, we must differentiate the fragmentation of the distribution from habitat loss, as the consequences on bat species of the habitat loss are likely to be different to the consequences of the range fragmentation. Habitat loss following land use changes has been perceived as a major threat to biological diversity, whereas fragmentation may be positive or negative [42]. Habitat losses may increase species losses and, in turn, induce changes in ecosystem functions, including parasitism. Several studies have shown that parasites suffer more from habitat loss and isolation than their hosts, but other studies emphasize that habitat loss may increase the abundance of some hosts, and consequently their parasite loads, through an increase of host density-dependent transmission [13]. The consequences in terms of surveillance, spill-over and emergence in human populations are then species specific, in relation to their historical biogeography, actual range size and shape, and on-going loss of habitat. As already emphasized by Turmelle and Olival [12], while biogeography can help to identify macro-ecological determinants of pathogen richness, and potentially epidemiological processes, control strategies need to be carried out at local geographic scales.
The number of viruses found in bats in our study added to the viruses described in bats in the literature is certainly an underestimation. Indeed, bats are reservoirs for many viruses and have the peculiarity to maintain viral replication at relatively low levels. Thus, chronicity of viral infections in bats requires the use of highly sensitive detection tools. However, in our study, samples were tested by Reverse-Transcription PCR assay using generic consensus primers, known to decrease sensitivity. The detection of these viruses may be improved by more sensitive methods, such as high-throughput sequencing and viral isolation yet much more expensive than PCR.
Acknowledgments
We thank everyone involved in the collection of samples in the CAR and Gabon, especially Xavier Pourrut, André Delicat, Peggy Motsch, Jérémy Leclercq, Dieudonné Nkoghé, Tabea Binger, and our field assistants Roger Kowé (from CENAREST). We aknowledge Alan Kemp and other anonymous reviewers for their useful comments on the manuscript and George Mapuvire and Hugo Vall for improving the English.
Funding Statement
This work was supported by Global Viral Forecasting, a “Fonds de Solidarité Prioritaire” grant from the Ministère des Affaires Etrangères de la France (FSP n° 2002005700). CIRMF is supported by the government of Gabon, Total-Fina-Elf Gabon, and the Ministère des Affaires Etrangères de la France. T.D. Dallo received a personal scholarship from the BONFOR intramural program at the University of Bonn. This study was also made possible by the generous support of the American people through the United States Agency for International Development (USAID) Emerging Pandemic Threats PREDICT. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wong S, Lau S, Woo P, Yuen K (2007) Bats as a continuing source of emerging infections in humans. Rev Med Virol 67–91 doi:10.1002/rmv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dobson AP (2005) What links bats to emerging infectious diseases? Science 310: 628–629 Available: http://www.ncbi.nlm.nih.gov/pubmed/16254175 [DOI] [PubMed] [Google Scholar]
- 3. Maganga GD, Bourgarel M, Ella GE, Drexler JF, Gonzalez J-P, et al. (2011) Is Marburg virus enzootic in Gabon? J Infect Dis 204 Suppl: S800–3. Available: http://www.ncbi.nlm.nih.gov/pubmed/21987754. Accessed 24 June 2012 [DOI] [PubMed] [Google Scholar]
- 4. Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, et al. (2005) Fruit bats as reservoirs of Ebola virus. Nature 438: 575–576 Available: http://www.ncbi.nlm.nih.gov/pubmed/16319873. Accessed 28 March 2012 [DOI] [PubMed] [Google Scholar]
- 5. Pourrut X, Souris M, Towner JS, Rollin PE, Nichol ST, et al. (2009) Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infect Dis 9: 159 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2761397&tool=pmcentrez&rendertype=abstract. Accessed 25 April 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang L-F, Shi Z, Zhang S, Field H, Daszak P, et al. (2006) Review of bats and SARS. Emerg Infect Dis 12: 1834–1840 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3291347&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Memish ZA, Mishra N, Olival KJ, Fagbo SF, Kapoor V, et al. (2013) Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis 19: 1819–1823 Available: http://wwwnc.cdc.gov/eid/r/article/19/11/pdfs/13-1172.pdf. Accessed 8 November 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keesing F, Belden LK, Daszak P, Dobson AP, Harvell CD, et al. (2010) Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468: 647–652 Available: http://www.ncbi.nlm.nih.gov/pubmed/21124449. Accessed 29 February 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patterson BD, Dick CW, Dittmar K (2008) Parasitism by bat flies (Diptera: Streblidae) on neotropical bats: effects of host body size, distribution, and abundance. Parasitol Res 103: 1091–1100 Available: http://www.ncbi.nlm.nih.gov/pubmed/18633645. Accessed 19 June 2013 [DOI] [PubMed] [Google Scholar]
- 10. Bordes F, Morand S, Ricardo G (2008) Bat fly species richness in Neotropical bats: correlations with host ecology and host brain. Oecologia 158: 109–116 Available: http://www.ncbi.nlm.nih.gov/pubmed/18679724. Accessed 19 June 2013 [DOI] [PubMed] [Google Scholar]
- 11. Luis A, Hayman DTS, O'Shea T, Cryan P, Gilbert A, et al. (2013) A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc R Soc B Biol Sci 280: 20122753 Available: http://rspb.royalsocietypublishing.org/content/280/1756/20122753.short. Accessed 6 May 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turmelle AS, Olival KJ (2009) Correlates of viral richness in bats (order Chiroptera). Ecohealth 6: 522–539 Available: http://www.ncbi.nlm.nih.gov/pubmed/20049506. Accessed 2 July 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bordes F, Morand S (2011) The impact of multiple infections on wild animal hosts: a review. Infect Ecol Epidemiol 1: 1–10 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3426331&tool=pmcentrez&rendertype=abstract. Accessed 26 May 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Torres J, Miquel J, Casanova J-C, Ribas A, Feliu C, et al. (2006) Endoparasite Species Richness of Iberian Carnivores: Influences of Host Density and Range Distribution. Biodivers Conserv 15: 4619–4632 Available: http://link.springer.com/10.1007/s10531-005-5824-8. Accessed 19 June 2013 [Google Scholar]
- 15. Guégan JF, Kennedy CR (1996) Parasite richness/sampling effort/host range: the fancy three-piece jigsaw puzzle. Parasitol Today 12: 367–369 Available: http://www.ncbi.nlm.nih.gov/pubmed/15275176. Accessed 19 June 2013 [DOI] [PubMed] [Google Scholar]
- 16. Lindenfors P, Nunn CL, Jones KE, Cunningham AA, Sechrest W, et al. (2007) Parasite species richness in carnivores: effects of host body mass, latitude, geographical range and population density. Glob Ecol Biogeogr 16: 496–509 Available: http://doi.wiley.com/10.1111/j.1466-8238.2006.00301.x. Accessed 27 May 2013 [Google Scholar]
- 17. Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34: 487–515 Available: http://www.annualreviews.org/doi/abs/10.1146/annurev.ecolsys.34.011802.132419. Accessed 21 May 2013 [Google Scholar]
- 18. Gaston K (1998) Species-range size distributions: products of speciation, extinction and transformation. Philos Trans R Soc B Biol Sci 353: 219–230 Available: http://rstb.royalsocietypublishing.org/content/353/1366/219.short. Accessed 18 June 2013 [Google Scholar]
- 19. Holt R (2003) On the evolutionary ecology of species' ranges. Evol Ecol Res 5: 159–178 Available: http://people.biology.ufl.edu/rdholt/holtpublications/126.pdf. Accessed 19 June 2013 [Google Scholar]
- 20. Cwynar LC, MacDonald GM (1987) Geographical variation of lodgepole pine in relation to population history. Am Nat 129: 463–469. [Google Scholar]
- 21.Messenger SL, Rupprecht CE, Smith JS (2003) Bats, emerging virus infections, and the rabies paradigm. In: Kunz TH, Fenton MB, editors. Bat ecology. Chicago, Illinois: University Press of Chicago. pp. 622–679. [Google Scholar]
- 22. Gannon W, Sikes R (2007) Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal 88: 809–823 Available: http://asmjournals.org/doi/abs/10.1644/06-MAMM-F-185R1.1. Accessed 7 August 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunz TH, Parsons S (2009) Ecological and behavioral methods for the study of bats. 2nd ed. Kunz TH, \Parsons S, editors Baltimore, Maryland: Johns Hopkins University Press. [Google Scholar]
- 24. Pourrut X, Délicat A, Rollin PE, Ksiazek TG, Gonzalez J-P, et al. (2007) Spatial and temporal patterns of Zaire ebolavirus antibody prevalence in the possible reservoir bat species. J Infect Dis 196 Suppl: S176–83 Available: http://www.ncbi.nlm.nih.gov/pubmed/17940947. Accessed 25 May 2012 [DOI] [PubMed] [Google Scholar]
- 25. Towner JS, Pourrut X, Albariño CG, Nkogue CN, Bird BH, et al. (2007) Marburg virus infection detected in a common African bat. PLoS One 2: e764 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1942080&tool=pmcentrez&rendertype=abstract. Accessed 11 July 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakai T, Kikkawa Y, Tsuchiya K, Harada M, Kanoe M, et al. (2003) Molecular phylogeny of Japanese Rhinolophidae based on variations in the complete sequence of the mitochondrial cytochrome b gene. Genes Genet Syst 78: 179–189. [DOI] [PubMed] [Google Scholar]
- 27. Irwin DM, Kocher TD, Wilson AC (1991) Evolution of the Cytochrome b gene of mammals. J Mol Evol 32: 128–144 Available: http://www.researchgate.net/publication/21255081_Evolution_of_the_cytochrome_b_gene_of_mammals/file/9fcfd50a3ada225691.pdf. Accessed 20 June 2013 [DOI] [PubMed] [Google Scholar]
- 28. Bickham JW, Wood CC, Patton JC (1995) Biogeographic Implications of Cytochrome b Sequences and Allozymes in Sockeye (Oncorhynchus nerka). J Hered 86: 140–144 Available: http://jhered.oxfordjournals.org/content/86/2/140.abstract [DOI] [PubMed] [Google Scholar]
- 29. Bickham JW, Patton JC, Schlitter D a, Rautenbach IL, Honeycutt RL (2004) Molecular phylogenetics, karyotypic diversity, and partition of the genus Myotis (Chiroptera: Vespertilionidae). Mol Phylogenet Evol 33: 333–338 Available: http://www.ncbi.nlm.nih.gov/pubmed/15336668. Accessed 20 June 2013 [DOI] [PubMed] [Google Scholar]
- 30. Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41: 95–98. [Google Scholar]
- 31. Teeling EC, Springer MS, Madsen O, Bates P, O'brien SJ, et al. (2005) A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307: 580–584 Available: http://www.ncbi.nlm.nih.gov/pubmed/15681385. Accessed 6 June 2013 [DOI] [PubMed] [Google Scholar]
- 32. Almeida F, Giannini N, DeSalle R, Simmons NB (2011) Evolutionary relationships of the old world fruit bats (Chiroptera, Pteropodidae): Another star phylogeny? BMC Evol Biol 11: 281 Available: http://www.biomedcentral.com/1471-2148/11/281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Souza Luna LK, Heiser V, Regamey N, Panning M, Drexler JF, et al. (2007) Generic detection of coronaviruses and differentiation at the prototype strain level by reverse transcription-PCR and nonfluorescent low-density microarray. J Clin Microbiol 45: 1049–1052 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1829107&tool=pmcentrez&rendertype=abstract. Accessed 19 June 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crochu S, Cook S, Attoui H, Charrel RN, De Chesse R, et al. (2004) Sequences of flavivirus-related RNA viruses persist in DNA form integrated in the genome of Aedes spp. mosquitoes. J Gen Virol 85: 1971–1980 Available: http://www.ncbi.nlm.nih.gov/pubmed/15218182. Accessed 21 May 2013 [DOI] [PubMed] [Google Scholar]
- 35. Moureau G, Temmam S, Gonzalez J-P, Charrel RN, Grard G, et al. (2007) A real-time RT-PCR method for the universal detection and identification of flaviviruses. Vector-Borne 7: 467–477 Available: http://online.liebertpub.com/doi/abs/10.1089/vbz.2007.0206. Accessed 18 June 2013 [DOI] [PubMed] [Google Scholar]
- 36. Towner JS, Amman BR, Sealy TK, Carroll S a R, Comer J a, et al. (2009) Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog 5: e1000536 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2713404&tool=pmcentrez&rendertype=abstract. Accessed 7 June 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jánoska M, Vidovszky M, Molnár V, Liptovszky M, Harrach B, et al. (2011) Novel adenoviruses and herpesviruses detected in bats. Vet J 189: 118–121 Available: http://www.ncbi.nlm.nih.gov/pubmed/20813566. Accessed 30 May 2013 [DOI] [PubMed] [Google Scholar]
- 38. Fortin M, Keitt TH, Maurer BA, Taper ML, Kaufman DM, et al. (2005) Species' geographic ranges and distributional limits: pattern analysis and statistical issues. Oikos 1: 7–17. [Google Scholar]
- 39. Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290 Available: http://bioinformatics.oxfordjournals.org/cgi/doi/10.1093/bioinformatics/btg412. Accessed 20 June 2013 [DOI] [PubMed] [Google Scholar]
- 40. Garland T, Harvey P, Ives A (1992) Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol 41: 18–32 Available: http://sysbio.oxfordjournals.org/content/41/1/18.short. Accessed 20 June 2013 [Google Scholar]
- 41. Kembel S, Cowan P, Helmus M, Cornwell W, Morlon H, et al. (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26: 1463–1464. [DOI] [PubMed] [Google Scholar]
- 42. Streicker DG, Turmelle AS, Vonhof MJ, Kuzmin I V, McCracken GF, et al. (2010) Host phylogeny constrains cross-species emergence and establishment of rabies virus in bats. Science 329: 676–679 Available: http://www.ncbi.nlm.nih.gov/pubmed/20689015. Accessed 28 May 2013 [DOI] [PubMed] [Google Scholar]
- 43. Hochberg M, Ives A (1999) Can natural enemies enforce geographical range limits? Ecography (Cop) 22: 268–276 Available: http://onlinelibrary.wiley.com/doi/10.1111/j.0030-1299.2005.13146.x/full. Accessed 19 June 2013 [Google Scholar]
- 44. Kemp G, Le Gonidec G, Karabatsos N, Rickenbach A, Cropp C (1988) IFE: a new African orbivirus isolated from Eidolon helvum bats captured in Nigeria, Cameroon and the Central African Republic. Bull Soc Pathol Exot Fil 81: 40–48. [PubMed] [Google Scholar]
- 45. Hayman DTS, Suu-Ire R, Breed AC, McEachern J a, Wang L, et al. (2008) Evidence of henipavirus infection in West African fruit bats. PLoS One 3: e2739 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2453319&tool=pmcentrez&rendertype=abstract. Accessed 30 May 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hayman DTS, Fooks AR, Horton D, Suu-Ire R, Breed AC, et al. (2008) Antibodies against Lagos bat virus in megachiroptera from West Africa. Emerg Infect Dis 14: 926–928 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2600291&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kuzmin I V, Niezgoda M, Franka R, Agwanda B, Markotter W, et al. (2008) Lagos bat virus in Kenya. J Clin Microbiol 46: 1451–1461 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2292963&tool=pmcentrez&rendertype=abstract. Accessed 3 June 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tong S, Conrardy C, Ruone S, Kuzmin I V, Guo X, et al. (2009) Detection of novel SARS-like and other coronaviruses in bats from Kenya. Emerg Infect Dis 15: 482–485 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2681120&tool=pmcentrez&rendertype=abstract. Accessed 4 June 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Razafindratsimandresy R, Jeanmaire EM, Counor D, Vasconcelos PF, Sall AA, et al. (2009) Partial molecular characterization of alphaherpesviruses isolated from tropical bats. J Gen Virol 90: 44–47 Available: http://www.ncbi.nlm.nih.gov/pubmed/19088271. Accessed 30 May 2013 [DOI] [PubMed] [Google Scholar]
- 50. Hayman DTS, Emmerich P, Yu M, Wang L-F, Suu-Ire R, et al. (2010) Long-term survival of an urban fruit bat seropositive for Ebola and Lagos bat viruses. PLoS One 5: e11978 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2915915&tool=pmcentrez&rendertype=abstract. Accessed 1 April 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wright E, Hayman DTS, Vaughan A, Temperton NJ, Wood JLN, et al. (2010) Virus neutralising activity of African fruit bat (Eidolon helvum) sera against emerging lyssaviruses. Virology 408: 183–189 Available: http://www.ncbi.nlm.nih.gov/pubmed/20951400. Accessed 24 May 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dzikwi A a, Kuzmin II, Umoh JU, Kwaga JKP, Ahmad A a, et al. (2010) Evidence of Lagos bat virus circulation among Nigerian fruit bats. J Wildl Dis 46: 267–271 Available: http://www.ncbi.nlm.nih.gov/pubmed/20090042 [DOI] [PubMed] [Google Scholar]
- 53. Drexler JF, Corman VM, Gloza-Rausch F, Seebens A, Annan A, et al. (2009) Henipavirus RNA in African bats. PLoS One 4: e6367 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2712088&tool=pmcentrez&rendertype=abstract. Accessed 2 May 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Drexler JF, Corman VM, Müller MA, Maganga GD, Vallo P, et al. (2012) Bats host major mammalian paramyxoviruses. Nat Commun 3: 796 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3343228&tool=pmcentrez&rendertype=abstract. Accessed 27 February 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Canuti M, Eis-Huebinger AM, Deijs M, de Vries M, Drexler JF, et al. (2011) Two novel parvoviruses in frugivorous New and Old World bats. PLoS One 6: e29140 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3246463&tool=pmcentrez&rendertype=abstract. Accessed 25 March 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Esona MD, Mijatovic-Rustempasic S, Conrardy C, Tong S, Kuzmin I V, et al. (2010) Reassortant group A rotavirus from straw-colored fruit bat (Eidolon helvum). Emerg Infect Dis 16: 1844–1852 Available:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3294550&tool=pmcentrez&rendertype=abstract. Accessed 20 June 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Markotter W, Kuzmin I, Rupprecht CE, Nel LH (2008) Phylogeny of Lagos bat virus: Challenges for lyssavirus taxonomy. Virus Res 135: 10–21 Available: http://linkinghub.elsevier.com/retrieve/pii/S0168170208000579. Accessed 4 May 2012 [DOI] [PubMed] [Google Scholar]
- 58. Konstantinov O, Diallo S (2006) The mammals of Guinea as reservoirs and carriers of arboviruses]. Med Parazitol (Mosk) 1: 34–39 Available: http://www.ncbi.nlm.nih.gov/pubmed/16562748. Accessed 20 June 2013 [PubMed] [Google Scholar]
- 59. Kalunda M, Mukwaya LG, Mukuye A, Lule M, Sekyalo E, et al. (1986) Kasokero virus: a new human pathogen from bats (Rousettus aegyptiacus) in Uganda. Am J Trop Med Hyg 35: 387–392 Available: http://europepmc.org/abstract/MED/3082234 [DOI] [PubMed] [Google Scholar]
- 60. Swanepoel R, Smit SB, Rollin PE, Formenty P, Leman Pa, et al. (2007) Studies of reservoir hosts for Marburg virus. Emerg Infect Dis 13: 1847–1851 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2876776&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Müller MA, Paweska JT, Leman PA, Drosten C, Grywna K, et al. (2007) Coronavirus antibodies in African bat Species. Emerg Infect 13: 1367–1370 Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2857293/. Accessed 22 October 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kuzmin IV, Niezgoda M, Franka R, Agwanda B, Markotter W, et al. (2010) Marburg virus in Fruit bat, Kenya. Emerg Infect Dis 16: 352–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rector A, Mostmans S, Van Doorslaer K, McKnight Ca, Maes RK, et al. (2006) Genetic characterization of the first chiropteran papillomavirus, isolated from a basosquamous carcinoma in an Egyptian fruit bat: the Rousettus aegyptiacus papillomavirus type 1. Vet Microbiol 117: 267–275 Available: http://www.ncbi.nlm.nih.gov/pubmed/16854536. Accessed 11 July 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pfefferle S, Oppong S, Drexler JF, Gloza-Rausch F, Ipsen A, et al. (2009) Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229E in bats, Ghana. Emerg Infect Dis 15: 1377–1384 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2819850&tool=pmcentrez&rendertype=abstract. Accessed 6 June 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Quan P, Firth C, Street C, Henriquez J, Petrosov A, et al. (2010) Identification of a severe acute respiratory syndrome coronavirus-like virus in a leaf-nosed bat in Nigeria. MBio 1: 1–9 Available: http://mbio.asm.org/content/1/4/e00208-10.short. Accessed 24 June 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hayman D, Fooks a R, Rowcliffe JM, McCrea R, Restif O, et al. (2012) Endemic Lagos bat virus infection in Eidolon helvum. Epidemiol Infect 1–9 Available: http://www.ncbi.nlm.nih.gov/pubmed/22370126. Accessed 8 August 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kemp GE (1975) Viruses other than arenaviruses from West African wild mammals. Factors affecting transmission to man and domestic animals. Bull World Health Organ 52: 615–620 Available:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2366648&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed] [Google Scholar]
- 68. Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T (2006) Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev 19: 531–545 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1539106&tool=pmcentrez&rendertype=abstract. Accessed 3 February 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.IUCN (2012) IUCN Red List of Threatened Species. Version 20122.