Abstract
Background
At present, there are no specific nationwide epidemiological studies representing the whole Italian population. This study is aimed at describing the epidemiological and economic burden that HCV will generate in the next few years in Italy. Furthermore, the impact that future anti-HCV treatments may have on the burden of disease was considered. This analysis was developed for the period 2012–2030 from the perspective of the Italian National Health Service (NHS).
Methods
A published system dynamic model was adapted for Italy in order to quantify the HCV-infected population in terms of disease progression and the associated costs from 1950 to 2030. The model structure was based on transition probabilities reflecting the natural history of the disease. In order to estimate the efficacy of current anti-HCV treatment strategies for genotypes 1 and 4, the sustained virological response (SVR) rate in registration clinical trials for both boceprevir and telaprevir was estimated. It was assumed that the efficacy for patients treated with peginterferon + ribavirin was equal to the placebo arm of a randomized clinical trial (RCT) relating to boceprevir and telaprevir. For genotypes 2/3 patients it was assumed that treatment efficacy with dual therapy was equal to a SVR rate from the literature. According to the aim of this study, only direct health care costs (hospital admissions, drugs, treatment, and care of patients) incurred by the Italian NHS have been included in the model. Costs have been extrapolated using the published scientific literature available in Italy and actualized with the 2012 ISTAT (Istituto Nazionale di Statistica) Price Index system for monetary revaluation. Three different scenarios were assumed in order to evaluate the impact of future anti-HCV treatments on the burden of disease.
Results
Overall, in Italy, 1.2 million infected subjects were estimated in 2012. Of these, about 211,000 patients were diagnosed, while only about 11,800 subjects were actually being treated with anti-HCV drugs. A reduction of health care costs is associated with a prevalence decrease. Indeed, once the spending peak is reached during this decade (about €527 million), the model predicts a cost reduction in the following 18 years. In 2030, based on the more effective treatments currently available, the direct health care cost associated with the management of HCV patients may reach €346 million (−34.3% compared to 2012). The first scenario (new treatment in 2015 with SVR =90% and same number of treated patients) was associated with a significant reduction in HCV-induced clinical consequences (prevalence =−3%) and a decrease in direct health care expenses, corresponding to €11.1 million. The second scenario (increase in treated patients to 12,790) produced an incremental cost reduction of €7.3 million, reaching a net decrease equal to €18.4 million. In the third scenario (treated patients =16,770), a higher net direct health care cost decrease versus the base-case (€44.0 million) was estimated.
Conclusion
Our model showed that the introduction of new treatments that are more effective could result in a quasi-eradication of HCV, with a very strong reduction in prevalence.
Keywords: chronic hepatitis, cost of illness, forecast, new HCV treatment
Background
In 2010, the World Health Organization (WHO) recognized that hepatitis C virus (HCV) is a major global public health problem.1 It is estimated that about 3% of the world’s population is HCV positive.2 The prevalence of the disease varies around the world.
According to the study conducted by the European Centre for Disease Prevention and Control (ECDC), Italy has the highest number of HCV positive subjects in Europe and the highest death rate from hepatocellular carcinoma (HCC) and cirrhosis.3
In fact, chronic HCV infection is a primary cause of cirrhosis, HCC, and liver transplantation.4 HCV is currently the major etiologic agent in patients needing medical assistance due to chronic hepatic diseases5,6 and, as in the rest of the world, is the most common cause of liver transplantation.7 However, despite being the only therapeutic treatment for terminal liver disease, transplantation does not treat HCV infection, and recurrence may take place after transplantation.8
At present, there are no specific nationwide epidemiological studies representing the whole Italian population. However, HCV prevalence has been evaluated in some local or regional studies.9 HCV ribonucleic acid prevalence is normally around 3% (with an average value of about 6%–10% in a 1940–1949 birth cohort); it is usually lower than 2% (mean: 1.6%) in people born in 1950–1959 and tends to decrease in younger people.10 In addition to age correlation, the North–South geographical gradient generates a remarkable epidemiological variability. In fact, prevalence is higher in southern regions (7.3%) in comparison to central (6.1%) and northern ones (1.6%).11
It is clear that in future, society and the health service will have to face the complications of HCV-induced pathologies, involving a growing demand in liver transplantations and HCC treatments.12 The costs of viral hepatitis are high and tend to increase according to disease severity. In addition to the direct costs incurred for disease management, there are indirect costs linked to the loss of productivity due to disability and premature death in patients suffering from the chronic HCV infections.13
This study is aimed at describing the epidemiological and economic burden that HCV will generate in the next few years in Italy. Furthermore, the impact that future anti-HCV treatments may have on the burden of disease was considered. The analysis has been developed for the period 2012–2030 from the perspective of the Italian National Health Service (NHS).
Methods
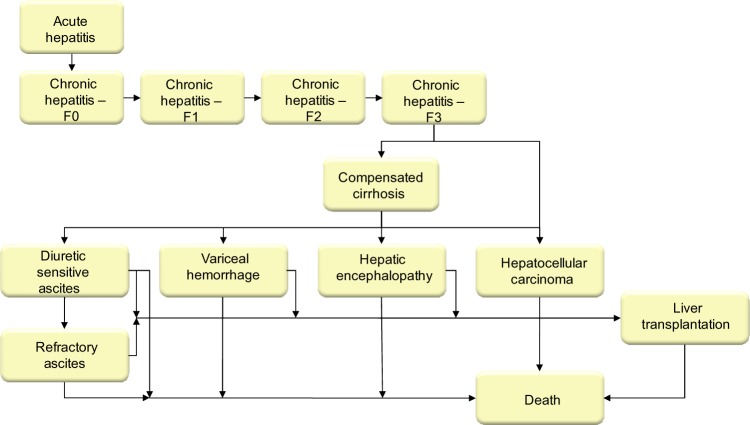
A published system dynamic model14 was adapted for Italy in order to quantify the HCV-infected population in terms of disease progression and the associated costs from 1950–2030. The model structure was based on transition probabilities reflecting the natural history of the disease (Figure 1). The time horizon was settled in 19 years (2012–2030) and a lag transition of 1 year was considered. Transition probabilities have been associated with cost variables of each pathological state and related treatment able to predict - in addition to the epidemiological burden - the economic cost incurred by NHS. Further details on the methods used are reported in the appendix of a previous study.14
Figure 1.
Forecasting model structure. Data taken from Razavi et al.14
Epidemiological data
The model predicted the future projection of epidemiological effects, starting from historical data. Data reported by the United Nations in terms of a historical series in the residential Italian population from 1950 to 2012 were used.15 In addition to this, the death rates of the Italian population16 were taken into account. Therefore, a projection of effects between 2008 and 2030 was calculated on the basis of historical demographic data in which the age of the population was grouped into 5-year clusters.
The model assumed an incidence rate equal to 0.016%. Furthermore, the predictive model included a redistribution of prevalence data by age and sex. With reference to this parameter, an American model was adjusted to the Italian context using national literature data.17,18 Hence, the model was calibrated by comparing the output trend with the age-specific prevalence of HCV infection (Figure 2).
Figure 2.
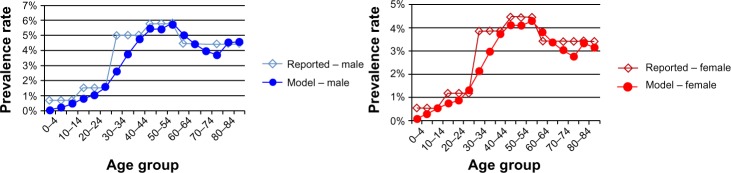
Prevalence by sex and age in Italy, 2012.
Note: Data comparison of literature and model estimate taken from Ansaldi et al17 and Cozzolongo et al.18
The number of diagnosed and treated patients in Italy is considerably lower than in the US. Based on published data, it was assumed that in Italy, only 8% of chronic HCV patients are currently treated in clinical centers,19 and among these diagnosed patients, only 20% are treated with dual or triple therapy.20 Finally, patients were divided by genotypes according to national epidemiological data21,22 where a prevalence of 56%, 30%, 10%, and 5% in terms of genotypes 1, 2, 3, and 4, respectively, was estimated.
Treatment efficacy
In order to estimate the efficacy of current anti-HCV treatment strategies in genotype 1 patients, the sustained virological response (SVR) rate was estimated in registration trials for both boceprevir (70.0% and 64.4% in treatment-naïve and treatment-experienced patients, respectively) and telaprevir (70.0% in treatment-naïve and 67.4% in treatment- experienced patients).23–26 It was assumed that the efficacy of peginterferon + ribavirin in genotype 1 patients was equal to the placebo arm of a randomized clinical trial (RCT) relating to boceprevir (SVR rate of 36.6% and 24.2% in treatment- naïve and treatment-experienced patients, respectively).23,24 It was assumed that in genotypes 2/3, treatment efficacy with dual therapy was equal to a SVR rate of 65% in treatment-naïve patients27 and 59% in treatment-experienced ones.28 Finally, it was assumed that the SVR rate for genotype 4 was comparable to that for genotype 1 in this model.
In the base-case, the model assumed that only 10% of genotype 1 patients eligible for treatment in Italy were treated with boceprevir and telaprevir. The remaining diagnosed patients were assumed to be treated with peginterferon + ribavirin or kept under observation.
Cost data
According to the aim of this study, only direct health care costs (hospital admissions, drugs, treatment, and care of patients) incurred by the Italian NHS have been included in the model. Table 1 reports direct cost data associated with each health condition. Costs have been extrapolated using the published scientific literature available in Italy and actualized with the 2012 ISTAT (Istituto Nazionale di Statistica) Price Index system for monetary revaluation.29 Furthermore, transplantation costs have been estimated using the surgery cost calculated through the Diagnosis Related Group (DRG) reimbursement tariffs30 to which the treatment cost following the first year of transplantation has been added.10,31,32
Table 1.
Direct health care costs associated with health conditions considered in the analysis
| Direct annual costs | Initial value | Actualized cost | Reference |
|---|---|---|---|
| Chronic hepatitis | €246 | €288 | 10,31,32 |
| Compensated cirrhosis | €347 | €407 | 10,31,32 |
| Decompensated cirrhosis | €5,466 | €6,409 | 10,31,32 |
| Carcinoma | €6,075 | €7,124 | 10,31,32 |
| Transplantation (surgery) | €80,199 | €80,199 | 30 |
| Transplantation (1st year treatment) | €4,729 | €4,729 | 10,31,32 |
| Drug costs | Weekly cost | Treatment (number of weeks) | Reference |
|
| |||
| Peginterferone-2a + ribavirin | €283 | 48 | 20 |
| Peginterferone-2b + ribavirin | €261 | 48 | 20 |
| Boceprevir | €643 | 35 | 33,34 |
| Telaprevir | €1,880 | 12 | 33,35 |
In addition to the above reported costs, in chronic HCV hepatitis and compensated cirrhosis patients, costs associated with dual and triple therapy have been estimated. Table 1 reports input data to estimate treatment costs with dual and triple therapy. The weekly cost of dual therapy has been estimated on the basis of the spending data published in the literature.31 For triple therapy, ex-factory prices of boceprevir and telaprevir published in the Official Gazette of the Italian Republic33 were used. This price was multiplied by the estimated average number of treatment weeks as given by the European Medicine Agency (EMA) guidelines.34,35
Scenario analysis
The model included three future scenarios and a base-case. The base-case scenario assumed that all eligible patients were treated with peginterferon + ribavirin, and that 10% of the genotype 1 patients were treated with the most recent protease inhibitors (50% with telaprevir and 50% with boceprevir). In this base-case scenario, it was assumed that no new treatments will be available in Italy, and therefore, the treatment of patients suffering from chronic HCV is not substantially changed. The base-case scenario has been compared with three alternative scenarios, each one including different assumptions, as indicated in Table 2. The assumptions of each scenario have been based on the expert opinion of a clinical board composed by the authors of this work. The scenarios aim to evaluate the epidemiological and economic impact that the new anti-HCV treatments might have from the Italian NHS perspective. In all scenarios, it has been assumed that the cost of the new treatment is comparable to protease inhibitor therapy (conservative assumption).
Table 2.
Model parameters by analysis scenario
| Parameter | Scenario 1 | Scenario 2 | Scenario 3 |
|---|---|---|---|
| Initiation of new treatment | 2015 | 2015 | 2015 |
| Patients eligible for treatment (genotypes) | G1 | G1–G4 | G1–G4 |
| Fibrosis status (Metavir score) | ≥F2 | ≥F2 | ≥F2 |
| Rate of eligible patients being treated | 60% | 80% | 90% |
| Rate of patients treated with the new treatment | 10% (2015), 15% (2016), 25% (2018) | 10% (2015), 15% (2016), 25% (2018) | 15% (2015), 25% (2016), 35% (2018) |
| SVR attributed to the new treatment | 90% (naïve) | 90% (naïve) | 90% (naïve) |
| 80% (experienced) | 80% (experienced) | 80% (experienced) | |
| Compliance to new treatment | 80% | 80% | 90% |
| The number of treated patients is | Constant over time (n=11,860) | Increased (from 11,860–12,790) | Increased (from 11,860–16,770) |
Abbreviations: F, fibrosis grade; G, genotype; n, number of subjects; SVR, sustained virological response.
Sensitivity analysis
Sensitivity analysis is recommended any time there is uncertainty.36 In order to verify the uncertainty of the model results, a probabilistic sensitivity analysis (PSA)37 was performed. PSA provides a useful technique to quantify the level of confidence of a decision maker in drawing the conclusions of an economic evaluation.37 At each model parameter, a probability distribution (Table 3) was assigned to perform 5,000 Monte Carlo simulations in order to test the robustness of the results and define a 95% confidence interval (CI) for the data.
Table 3.
Probabilistic sensitivity analysis parameter (range and distribution)
| Range
|
Distribution | Reference | ||
|---|---|---|---|---|
| Min | Max | |||
| Epidemiological and efficacy parameter | ||||
| Prevalence rate: male | 2.0% | 3.5% | BETA | 17,18 |
| Prevalence rate: female | 2.0% | 3.5% | BETA | 17,18 |
| Incidence rate | 0.010% | 0.020% | BETA | |
| SVR base-case G1/G4 | 24.0% | 37.0% | BETA | 23–26 |
| SVR base-case G2/G3 | 59.0% | 65.0% | BETA | 23–26 |
| Annual cost | ||||
| Chronic hepatitis | €231 | €346 | GAMMA | 10,31,32 |
| Compensated cirrhosis | €325 | €489 | GAMMA | 10,31,32 |
| Decompensated cirrhosis | €5,125 | €7,691 | GAMMA | 10,31,32 |
| Carcinoma | €5,699 | €8,548 | GAMMA | 10,31,32 |
| Transplantation (surgery) | €72,175 | €88,221 | GAMMA | 30 |
| Transplantation (1st year treatment) | €3,784 | €5,675 | GAMMA | 10,31,32 |
Abbreviations: G, genotype; SVR, sustained virological response; Min, minimum; Max, maximum.
Results
Overall, in Italy, 1.2 million infected subjects were estimated in 2012. Of these, about 11,800 subjects are actually being treated with anti-HCV drugs.
The virus peak of chronic HCV prevalence was reached during the 1990s, with the number of infected subjects exceeding 1.8 million (Figure 3A). Thereafter, it is estimated that the total prevalence will decrease until 2030 due to the effect of new treatments currently being used in Italy. In fact, observing the prevalence decrease by HCV sequelae, it is possible to note that cirrhotic patients show a larger drop in prevalence, while more serious pathologies, like HCC and liver transplantation, initially increase and then slightly decrease (Figure 3B).
Figure 3.
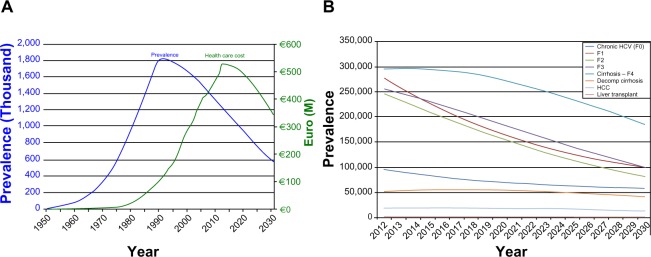
Prevalence estimate and yearly health care costs: Italy base-case.
Notes: (A) Total prevalence and health care costs: Italy 1950–2030; (B) HCV sequelae and total prevalence: Italy 2012–2030.
Abbreviations: HCC, hepatocellular carcinoma; HCV, hepatitis C virus; F, fibrosis grade; Decomp, decompensated.
A reduction in health care costs is of course associated with a decrease in prevalence. Indeed, once the spending peak is reached during this decade (about €527 million), the model predicts a spending reduction in the following 18 years (Figure 3A). In 2030, due to the more effective treatments currently available, the direct health care spending associated with the management of patients suffering from HCV may reach €346 million (−34.3% compared to 2012).
The first scenario was associated with a significant reduction in HCV-induced clinical consequences (prevalence of −3%) and a decrease in direct health care expenses corresponding to €11.1 million (95% CI: −€7.1 to −€15.0) (Table 4 and Figure 4). The second scenario produced an incremental cost reduction of €7.3 million, reaching a net decrease equal to €18.3 million (95% CI: −€16.2 to −€20.5). In the third scenario, a higher net direct health care cost decrease versus the base-case (€44.0 million, 95% CI: −€42.2 to −€45.8) was estimated.
Table 4.
Results by analysis scenario and base-case comparison
| Scenario 1 | 2012 | 2030 scenario 1 | 2030 scenario 1 vs 2012 | 2030 scenario 1 vs 2030 base-case | ||||
|---|---|---|---|---|---|---|---|---|
| Prevalence (n) | 1,242,682 | 566,521 | −676,161 | −15,688 | ||||
| 95% CI (min, max) | 917,150 | 1,568,213 | 402,842 | 730,200 | −514,309 | −838,013 | −9,336 | −22,039 |
| Health care cost (€million) | €527.0 | €334.9 | −€192.0 | −€11.1 | ||||
| 95% CI (min, max) | €388 | €666 | €232 | €438 | −€156 | −€228 | −€7.1 | −€15.0 |
| Scenario 2 | 2012 | 2030 scenario 2 | 2030 scenario 2 vs 2012 | 2030 scenario 2 vs 2030 base-case | ||||
|
| ||||||||
| Prevalence (n) | 1,242,682 | 556,323 | −686,358 | −25,885 | ||||
| 95% CI (min, max) | 917,150 | 1,568,213 | 389,842 | 722,805 | −527,308 | −845,408 | −22,336 | −29,434 |
| Health care costs (€million) | €527.0 | €327.6 | −€199.3 | −€18.3 | ||||
| 95% CI (min, max) | €388 | €666 | €223 | €432 | −€165 | −€234 | −€16.2 | −€20.5 |
| Scenario 3 | 2012 | 2030 scenario 3 | 2030 scenario 3 vs 2012 | 2030 scenario 3 vs 2030 base-case | ||||
|
| ||||||||
| Prevalence (n) | 1,242,682 | 520,909 | −721,772 | −61,299 | ||||
| 95% CI (min, max) | 917,150 | 1,568,213 | 348,154 | 693,665 | −568,996 | −874,548 | −64,024 | −58,574 |
| Health care costs (€million) | €527.0 | €302.0 | −€224.9 | −€44.0 | ||||
| 95% CI (min, max) | €388 | €666 | €194 | €410 | −€194 | −€256 | −€42.2 | −€45.8 |
Abbreviations: CI, confidence interval; n, number of subjects; min, minimum; max, maximum; vs, versus.
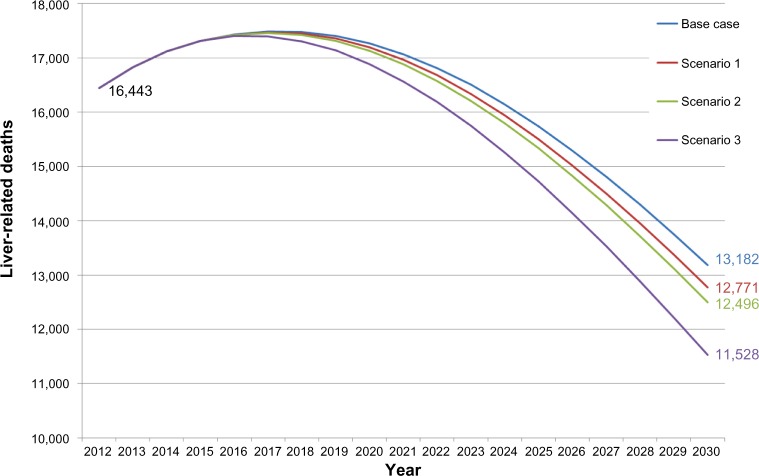
Figure 4.
Liver-related deaths of base-case and scenario: Italy 2012–2030.
Moreover, the second scenario almost doubled the reduction of HCV prevalence (−5%), as well as the number of prevented deaths (+7.5% versus the first scenario). However, the third scenario estimated −11% in terms of prevalence during the considered follow-up period and a higher number of prevented deaths (+33.9% versus the first scenario and +24.5% versus the second scenario). Although conservative, all scenarios can be deemed reliable and realistic from the Italian NHS perspective.
Discussion
Chronic HCV infection is a pathological condition with high economic and social impacts worldwide, particularly in Italy, in which the prevalence is around 2.0%–3.5%.17,18 The Italian situation is peculiar and ECDC epidemiological analysis shows that this is the highest prevalence rate in Europe.3 Unfortunately, these are only estimates and not collected data.
Our study was aimed at dealing with the lack of reliable information on the Italian burden of HCV-induced diseases. Available estimates on HCV pathologies and the expected epidemiological trend are very limited, if existing at all. Furthermore, the objective was to provide decision makers with fundamental information for reflection and discussion, in order to allow them to plan the implementation of rational and economically sustainable actions aimed at the control and eradication of the infection.
Following its historical evolution, viral HCV infection peaked in the 1990s and decreased in the new millennium. From 2012, the model predicted a steady decrease in prevalence that can be attributed to new available treatments. A further thrust to the eradication of this disease could be given by the use of new and more effective treatments that, on one hand, would allow us to increase the typology of patients to be treated, and on the other, increase the number of those actually treated. This would allow us to reach two primary objectives for the NHS: to reduce the impact of disease transmission and obtain economic benefits associated with the prevention of dramatically expensive disease complications such as decompensated cirrhosis, HCC, and liver transplantation.
Despite being conservative, the analyzed scenarios have allowed us to evaluate the epidemiological and economic impacts that the new anti-HCV treatments may have from the Italian NHS perspective. The opportunity to treat patients with a higher eligibility rate and therapy compliance, especially at an early stage, is not only ethically indisputable, but also economically efficient (scenario 3 allows better epidemiological and economic performance).
As of today, there are neither national studies that allow us to evaluate the economic burden of HCV-induced pathologies, nor studies able to predict their evolution. However, a recent work of Razavi et al14 in 2013, from which this study derives, has allowed us to predict the infection evolution of HCV-induced pathologies in the US. In accordance with the American study, in our country, HCV prevalence is bound to decrease, even if with very different age dynamics. The extent of this decrease will be determined by the scenario that future anti-HCV treatments will follow and by the choices of decision makers.
The model has limitations. First of all, epidemiological parameters need to be taken into account. If the shortage of national data makes reliability of results difficult, a model and a systematized method of information, like the one presented in this study, allows us to obtain estimates that are as reliable as possible. Secondly, there is a difficulty in considering further variables that may potentially influence the Italian demographic and social dynamics. Indeed, the model does not take into account any changes in the social behavior of the population, and nor does it consider the possible influence of migratory dynamics or the possible variation of risk factors influencing viral transmission (ie, injection drug use, accidental needlestick injuries, minor nosocomial surgical procedures, acupuncture, tattooing, or piercing). Finally, no monitoring or side effects were considered for anti-HCV drugs, which may cause underestimation of the savings attributable to a better safety of new drugs.
In conclusion, the analysis showed that the introduction of new treatments that are more effective could result in a quasi-eradication of HCV, with a very strong reduction in prevalence. In addition, a large number of lives could be saved from HCV-related deaths accompanied by a large reduction in the number of liver transplantations.
In addition, the model suggests that the potential of new treatments produces significant consequences not only in greater efficiency, but also in terms of security and shorter treatment durations.
All this, according to the logic of economic evaluations, represents a valuable tool for future health policies relating to HCV, and the benefits to health and economics that the new treatments will cause can be ensured in the near future.
We must not forget the great impact in terms of reducing indirect costs (both in terms of reduction of lost productivity, and above all, in terms of the impact on social security and social expenditure, which in Italy is financed by means of public expenditure).
This study does not have the pretension of being or creating a perfect model of epidemiological projection. The objective of this study was to supply data and carefully consider a dialectical confrontation among different professionals involved in the management of patients with HCV-induced chronic infection, and to suggest a valuable tool for future health policy strategy.
Acknowledgments
Thanks to Homie Razavi and Erin Gower for their technical support. This study was supported with unrestricted funding from Gilead, Italy.
Footnotes
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data; drafted the article and revised it critically for important intellectual content; and gave final approval of the version to be published.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization . Sixty-third World Health Assembly. Viral hepatitis: WHA 63.18; Geneva, Switzerland: May 21, 2010. [Google Scholar]
- 2.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control Technical Report Hepatitis B and C in the EU neighborhood: prevalence, burden of disease and screening policies. Sep, 2010. [Accessed April 14, 2014]. Available at: http://www.ecdc.europa.eu/en/publications/Publications/TER_100914_Hep_B_C%20_EU_neighbourhood.pdf.
- 4.Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362(9401):2095–2100. doi: 10.1016/s0140-6736(03)15109-4. [DOI] [PubMed] [Google Scholar]
- 5.Gane EJ, Portmann BC, Naoumov NV, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334(13):815–820. doi: 10.1056/NEJM199603283341302. [DOI] [PubMed] [Google Scholar]
- 6.Sagnelli E, Stroffolini T, Mele A, et al. The importance of HCV on the burden of chronic liver disease in Italy: a multicenter prevalence study of 9,997 cases. J Med Virol. 2005;75(4):522–527. doi: 10.1002/jmv.20313. [DOI] [PubMed] [Google Scholar]
- 7.Fagiuali S, Mirante VG, Pompili M, et al. Monotematica AISF 2000-OLT Study Group Liver transplantation: the Italian experience. Dig Liver Dis. 2002;34(9):640–648. doi: 10.1016/s1590-8658(02)80207-9. [DOI] [PubMed] [Google Scholar]
- 8.Gane E. The natural history and outcome of liver transplantation in hepatitis C virus-infected recipients. Liver Transpl. 2003;9(11):S28–S34. doi: 10.1053/jlts.2003.50248. [DOI] [PubMed] [Google Scholar]
- 9.Mariano A, Scalia Tomba G, Tosti ME, Spada E, Mele A. Estimating the incidence, prevalence and clinical burden of hepatitis C over time in Italy. Scand J Infect Dis. 2009;41(9):689–699. doi: 10.1080/00365540903095358. [DOI] [PubMed] [Google Scholar]
- 10.White Book of AISF Proposal of a National Plan for the Monitoring of Hepatic Diseases: Definition of Environments and Possible Interventions. 2011. [Accessed May 13, 2014]. Available from: http://www.webaisf.org/media/13891/libro-bianco-aisf-2011.pdf. Italian.
- 11.Ansaldi F, Bruzzone B, Salmaso S, et al. Different seroprevalence and molecular epidemiology patterns of hepatitis C virus infection in Italy. J Med Virol. 2005;76(3):327–332. doi: 10.1002/jmv.20376. [DOI] [PubMed] [Google Scholar]
- 12.Mele A, Tosti ME, Spada E, Mariano A, Bianco E, SEIEVA Collaborative Group . Epidemiology of acute viral hepatitis: twenty years of surveillance through SEIEVA in Italy and a review of the literature. Rome: ISTISAN; 2006. [Accessed May 19, 2014]. Available from: http://www.iss.it/binary/publ/cont/06-12.1149070762.pdf. [Google Scholar]
- 13.Carosi G, Caporaso N, Gardini I, et al. Epatiti Summit 2010 – A Hidden Emergency: A Comparison of Opinions and Strategies. [Accessed May 13, 2014]. 2010. Available from: http://www.sosfegato.it/camo/onlus/es/Executive_Summary.pdf. Italian.
- 14.Razavi H, Elkhoury AC, Elbasha E, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57(6):2164–2170. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United Nations [webpage on the Internet] Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat, World Population Prospects: The 2010 Revision and World Urbanization Prospects: The 2010 Revision. New York, NY: United Nations; 2010. [Accessed November 1, 2013]. Available from: http://esa.un.org/unpd/wpp/unpp/panel_indicators.htm. [Google Scholar]
- 16.UC Berkeley, Max Planck Institute for Demographic Research [webpage on the Internet] The Human Mortality Database. Rostock, Germany: Max Planck Institute for Demographic Research; [Accessed November 1, 2013]. Available from: http://www.mortality.org. [Google Scholar]
- 17.Ansaldi F, Bruzzone B, Salmaso S, et al. Different seroprevalence and molecular epidemiology patterns of hepatitis C virus infection in Italy. J Med Virol. 2005;76(3):327–332. doi: 10.1002/jmv.20376. [DOI] [PubMed] [Google Scholar]
- 18.Cozzolongo R, Osella AR, Elba S, et al. Epidemiology of HCV infection in the general population: a survey in a southern Italian town. Am J Gastroenterol. 2009;104(11):2740–2746. doi: 10.1038/ajg.2009.428. [DOI] [PubMed] [Google Scholar]
- 19.Colombo M. How to measure the efficacy of antiviral treatments. The evaluation through “substitute” healing; Second national workshop on economics and drugs in hepatology. WEF-E 2012; Rome, Italy. February 2, 2012; 2012. pp. 12–13. Italian. [Google Scholar]
- 20.Mariano A, Caserta C, Pendino GM, et al. Antiviral treatment for hepatitis C virus infection: effectiveness at general population level in a highly endemic area. Dig Liver Dis. 2009;41(7):509–515. doi: 10.1016/j.dld.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Matera G, Lamberti A, Quirino A, et al. Changes in the prevalence of hepatitis C virus (HCV) genotype 4 in Calabria, Southern Italy. Diagn Microbiol Infect Dis. 2002;42(3):169–173. doi: 10.1016/s0732-8893(01)00350-9. [DOI] [PubMed] [Google Scholar]
- 22.Marascio N, Matera G, Quirino A, et al. Eleven-year distribution pattern of hepatitis C virus in southern Italy. J Pathog. 2012;2012:631095. doi: 10.1155/2012/631095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwo PY, Lawitz EJ, McCone J, et al. SPRINT-1 investigators Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376(9742):705–716. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 24.Bacon BR, Gordon SC, Lawitz E, et al. HCV RESPOND-2 Investigators Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McHutchison JG, Everson GT, Gordon SC, et al. PROVE1 Study Team Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360(18):1827–1838. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- 26.Zeuzem S, Andreone P, Pol S, et al. REALIZE Study Team Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364(25):2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson IM, Brown RS, Jr, Freilich B, et al. WIN-R Study Group Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology. 2007;46(4):971–981. doi: 10.1002/hep.21932. [DOI] [PubMed] [Google Scholar]
- 28.Poynard T, Colombo M, Bruix J, et al. Epic Study Group Peginterferon alfa-2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology. 2009;136(5):1618–1628.e2. doi: 10.1053/j.gastro.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 29.ISTAT (Italian National Institute of Statistics). Consumer Price Index for Currency Revaluation. [Accessed October 1, 2013]. Available at: http://rivaluta.istat.it/ Italian.
- 30.DRG 480 Liver transplantation and/or intestinal transplantation -Reimbursement rate - Lazio Region. Italian.
- 31.Cammà C, Petta S, Enea M, et al. WEF Study Group Cost-effectiveness of boceprevir or telaprevir for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2012;56(3):850–860. doi: 10.1002/hep.25734. [DOI] [PubMed] [Google Scholar]
- 32.Ravasio R. Cost efficacy of peginterferon α-2a+ ribavirin versus peginterferon α-2b+ ribavirin in chronic C hepatitis treatment of HIV co-infected patients. PharmacoEconomics – Italian Research Articles. 2008;10(1):37–47. Italian. [Google Scholar]
- 33.Ex-factory Cost AIFA – G.U. n. 287 del 10.12.2012. [Accessed November 1, 2013]. Available from: http://www.farmacovigilanza.asl3.liguria.it/pdf/determinazione_1_incivo.pdf.
- 34.European Medicines Agency . EMA/431387/2011. London, UK: European Medicines Agency; 2012. [Accessed November 1, 2013]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002332/WC500109790.pdf. [Google Scholar]
- 35.European Medicines Agency . EMA/644234/2011. London, UK: European Medicines Agency; 2013. [Accessed November 1, 2013]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002313/WC500115507.pdf. [Google Scholar]
- 36.Briggs A. Economics notes: handling uncertainty in economic evaluation. BMJ. 1999;319(7202):120. doi: 10.1136/bmj.319.7202.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor M. What is sensitivity analysis? Newmarket, UK: Hayward Medical Communications; 2009. [Accessed April 14, 2014]. Available from: http://www.medicine.ox.ac.uk/bandolier/painres/download/whatis/What_is_sens_analy.pdf. [Google Scholar]