Abstract
Predictors of responsiveness to opioid analgesic medications are not well understood. This study tested whether individual differences in endogenous opioid (EO) function are associated with analgesic responsiveness to morphine. In randomized, counterbalanced order over three sessions, 45 chronic low back pain participants (CLBP) and 31 healthy controls received an opioid antagonist (8mg naloxone), morphine (0.08 mg/kg), or placebo. Participants then engaged in two laboratory evoked pain tasks (ischemic, thermal). Outcomes included pain threshold, pain tolerance, and pain ratings. Indexes of EO function and morphine analgesic responsiveness were derived for each measure as the difference in pain responses between the placebo condition, and naloxone or morphine conditions respectively. For all 7 pain measures across the two laboratory pain tasks, greater EO function was associated with significantly lower morphine analgesic responsiveness (p ≤ .001 − p=.02). Morphine reduced pain responses of low EO individuals to levels similar to high EO individuals under placebo. Higher placebo condition evoked pain sensitivity was associated with significantly greater morphine analgesic responsiveness for 5 of 7 pain measures (p<.001 − p=.02). These latter associations were significantly mediated by EO function for 4 of these 5 pain outcomes (p’s<.05). In the laboratory evoked pain context, opioid analgesic medications may supplement inadequate EO analgesia, with little incremental benefit in those with pre-existing high EO function. Implications for personalized medicine are discussed.
Keywords: Endogenous Opioids, Pain, Chronic Pain, Opioid Analgesic, Mediation, Personalized Medicine
The concept of personalized medicine, optimizing medications for individual patients based upon genetic, biomarker, and other patient-related factors, has received increasing attention25,32. Its potential application to the field of pain medicine is tantalizing, although supporting data are as yet inadequate6. Factors that determine, and could be clinical predictors of, responses to commonly used opioid analgesic medications are not fully understood. One surprising gap in this literature is the absence of human studies addressing the impact of individual differences in endogenous opioid (EO) system function on responses to opioid analgesic medications. Such interactions might be expected given that both EOs and opioid medications act on the same opioid receptors41. Animal studies suggest that EO function does influence opioid analgesic responses, although direction of these effects appears inconsistent. While several animal studies indicate possible cross-tolerance between EOs and opioid analgesic medications22,36,48, others suggest synergistic effects, with direct application of EOs (e.g., beta-endorphin) or manipulations that trigger release of EOs (e.g., forced swim, tail suspension, exercise) enhancing responsiveness to opioid analgesics2,5,12,13,17,31,46,47,49,50. Human PET imaging findings are indirectly supportive as well, indicating that a placebo manipulation with EO enhancing effects20 increases opioid analgesic responses4. Improved knowledge regarding the impact of EO system function on responses to opioid analgesic medications in humans might facilitate progress towards the goal of personalized pain medicine.
One individual difference factor previously reported to predict opioid analgesic responses in humans is sensitivity to laboratory evoked pain stimuli18,21. In a clinical trial examining the effects of daily morphine administration in postherpetic neuralgia patients over a 6 week period, greater morphine-related reductions in clinical pain over the trial were observed in patients showing lower baseline (pre-drug) sensitivity to acute heat pain18. The only prior controlled laboratory study examining the influence of pre-drug acute pain sensitivity on responses to a single opioid analgesic dose (in healthy individuals) found similar results, reporting that lower pre-drug laboratory heat pain sensitivity predicted greater reductions in cold pressor pain responses following oxycodone administration21. While intriguing, neither of the existing studies of this issue directly controlled for placebo effects in predictive analyses. Assuming that links between pre-drug pain sensitivity and subsequent opioid analgesic responsiveness are a real effect, the literature above suggests the hypothesis that these predictive associations might be mediated by underlying EO function differences that are known to contribute to acute pain sensitivity1,26,41.
This study therefore had three primary aims: 1) determine whether individual differences in EO function are related to analgesic responses to morphine, 2) determine whether laboratory evoked pain sensitivity under placebo is related to both EO function and analgesic responses to morphine, and 3) test whether individual differences in EO function mediate any observed associations between evoked pain sensitivity and morphine analgesic responses.
Method
Design
A double-blind, placebo-controlled crossover design with administration of an opioid antagonist (naloxone) and an opioid analgesic (morphine) was employed to evaluate whether endogenous opioid function (indexed by opioid blockade effects) is related to efficacy of opioid analgesic medications (morphine effects). Order of drug administration was randomized and counterbalanced. This design rather than one involving pre-post drug evaluation of evoked pain responses within a single session was chosen to permit direct control for placebo effects. The study was a multisite study, with identical data collection procedures and equipment employed in a closely coordinated fashion at two sites (Vanderbilt University Medical Center and Rush University Medical Center).
Participants
Participants included 45 individuals with chronic low back pain (CLBP) and 31 healthy controls (Healthy). All participants were recruited either through on-line advertisements on the Vanderbilt e-mail recruitment system, the Rush Pain Clinic, advertisements in local print media, or posted flyers. General criteria for participation included age between 18–55; no self-reported history of cardiovascular disease, hypertension, liver or kidney disorders, posttraumatic stress disorder, bipolar disorder, psychotic disorder, diabetes, seizure disorder, or alcohol or drug dependence; no use of anti-hypertensive medications; and no daily use of opioid analgesics (with absence of recent use confirmed via urine opiate screen before each laboratory study session). As in our past opioid blockade studies7–9, additional inclusion criteria for the CLBP group were chronic daily low back pain of at least 3 months’ duration with an average past month severity of at least 3 on a 0–10 verbal numeric pain intensity scale. Individuals with chronic pain related to malignancy, autoimmune disorders, or fibromyalgia were excluded. Potential participants who were pregnant (determined by urine pregnancy screens) were excluded to avoid unknown effects of naloxone on the fetus. No participants in the Healthy group were taking antidepressants or as-needed opioid analgesics. In the CLBP group, 5 participants reported occasional use of opioid analgesics (but none in the preceding 3 days or more), and 2 reported use of antidepressant medications. Regarding specific opioid analgesics prescribed in the CLBP group, 4 were taking hydrocodone/acetaminophen (10/325mg) as needed, and 1 was taking oxycodone (15mg) as needed.
Study Drugs
Blockade of opioid receptors was achieved by administration of naloxone, an opioid antagonist with a brief half-life (1.1 hours)35. As in past work7–9, an 8 mg dose in 20 ml normal saline was infused intravenously over a 10-minute period through an intravenous cannula placed in the non-dominant arm. At this dosage, naloxone provides effective blockade of all three major opioid receptor subtypes34. Peak naloxone activity is achieved within approximately 15 min3.
The opioid analgesic medication examined in this study was morphine sulfate, the prototypic mu opioid receptor agonist. As in similar laboratory evoked pain studies with morphine23, the current study employed a dosage of 0.08 mg/kg (in 20ml normal saline), which was infused in the same manner as naloxone. This dosage (approximately 7mg for an average sized male) was selected because it was judged to be sufficient to produce analgesia, but low enough to avoid ceiling effects that might obscure key individual differences in morphine responding. Peak morphine activity is achieved within approximately 15 min3.
Laboratory Evoked Pain Tasks
After receiving the assigned study drug in each session, participants engaged in two laboratory evoked pain tasks, both of which had previously been shown to be sensitive to the effects of morphine23. First, participants underwent an ischemic pain task based on procedures described by Maurset et al.37, similar to our past opioid blockade studies7–9. Participants first engaged in two minutes of dominant forearm muscle exercise using a hand dynamometer at 50% of his or her maximal grip strength as determined prior to beginning the laboratory procedures. Then they were asked to raise their dominant forearm over their head for 15 sec. A manual BP cuff was then inflated on the participant’s dominant biceps to 200 mmHg SBP, the arm was lowered, and the cuff remained inflated until tolerance was reached, up to a maximum of 8 min. Participants were instructed to indicate when they first experienced pain after the cuff was inflated, with this ischemic pain threshold defined as the time elapsed from task onset to when the sensation was first described as “painful.” Ischemic pain tolerance was defined as the time elapsed between onset of the pain task and participants’ expressed desire to terminate the task (set at a maximum of 8 min). Nine participants failed to indicate that the ischemic task had become “painful” prior to tolerance being reached during at least one session; in such cases, ischemic pain threshold was recorded as missing for analyses.
The second laboratory pain task was a heat pain task using a Medoc TSAII NeuroSensory Analyzer (Medoc US., Minneapolis, MN). This equipment was used to assess heat pain threshold and tolerance using an ascending method of limits protocol as in several previous studies10,14,23. Four trials each were conducted for heat pain threshold and tolerance, with each trial conducted sequentially at one of four different non-overlapping sites on the non-dominant ventral forearm. An interval of 30 sec between successive stimuli was employed. For pain threshold trials, the probe started at an adaptation temperature of 32°C, with the temperature increasing at a ramp rate of 0.5°C/sec until the participant indicated that the stimulus had begun to feel “painful” by depressing a button on a computer mouse. For each tolerance trial, the probe started at an adaptation temperature of 40°C, with the temperature increasing at a ramp rate of 0.5°C/sec until the participant indicated maximum tolerance had been reached. Means of the four thermal pain threshold and tolerance trials were separately derived for use in analyses. Maximum possible tolerance temperature was 51°C due to an automatic hardware cutoff in the TSAII device to ensure participant safety. Prior to beginning the first laboratory session, all participants underwent standardized training to familiarize them with the thermal stimulus device and the concepts of pain threshold and tolerance.
Laboratory Evoked Pain Outcomes
In addition to the pain threshold and pain tolerance outcomes for both pain tasks described above, participants were asked to describe the level of evoked pain experienced during each laboratory pain task using a 100mm visual analog scale (VAS) of overall pain intensity (anchored with “No Pain” and “Worst Possible Pain”)40. For the longer duration ischemic pain task only, participants were also asked to rate their current pain using a 0–100 verbal numeric rating scale (NRS; anchored with 0 = “no pain” and 100 = “worst possible pain”) at 30-sec intervals throughout the task, with the mean value representing overall NRS intra-task pain intensity.
Procedure
All procedures were conducted at the Vanderbilt General Clinical Research Center or a dedicated research room at the Rush University Pain Center. All procedures were approved by the Institutional Review Boards at the respective institutions. After providing informed consent, participants completed a packet of questionnaires, including information regarding demographics and chronic pain. Individuals then participated in three identical experimental sessions (placebo, naloxone, morphine) that were scheduled one week apart, at the same time of day to control for variance due to circadian rhythms.
Participants remained seated upright in a comfortable chair throughout all laboratory procedures. During each session, participants initially completed a 10-min seated rest period, after which an indwelling venous cannula was inserted into the dominant arm by a trained research nurse under physician supervision. After a 30-min resting adaptation period, participants received (via the cannula) either saline placebo, naloxone, or morphine, with order of drug administration across the three sessions randomly determined and counterbalanced. The investigational pharmacy at each institution prepared and provided the study drugs in blinded fashion to the study nurses.
After a 15-min rest period to allow peak drug activity to be achieved, participants engaged in the ischemic task using procedures described above, after which the VAS Intensity measure was immediately completed to describe responses to this evoked pain stimulus. Then, participants engaged in the thermal pain task to assess heat pain threshold and tolerance, with the VAS Intensity measure again immediately completed to describe the pain experienced during the heat pain tolerance trials. All participants remained in the lab under observation for 2 hours after peak drug activity had been achieved to allow drug effects to remit, after which they were released to a responsible adult. The most significant adverse effect noted with any of the study drugs was nausea and/or vomiting with morphine in some participants. The morphine session protocol was stopped before completing all tasks due to transient intense nausea/vomiting in three participants, but with these exceptions, there were no instances of vomiting reported at the assessment 15 min following completion of the morphine infusion.
Statistical Analysis
All analyses were conducted using IBM SPSS for Windows Version 20 (SPSS Inc., Chicago, IL). Prior to conducting analyses, an index of EO function was derived for each evoked pain measure, reflecting the difference between placebo and naloxone condition pain values, calculated so that higher scores reflected greater EO function (i.e., greater pain sensitivity under opioid blockade compared to placebo). Similarly, morphine analgesic response scores were derived for each measure, reflecting the difference between morphine and placebo condition values, calculated such that higher scores indicated greater morphine analgesia. In total, there were 4 evoked pain outcomes for the ischemic task (pain threshold, pain tolerance, VAS Intensity, and intra-task NRS intensity ratings) and three pain outcomes for the thermal pain task (pain threshold, pain tolerance, and VAS Intensity). Placebo condition, EO function, and morphine analgesic response measures were analyzed for each of these measures.
Analyses of participant characteristics across participant types used independent samples t-tests (for continuous measures) and Chi Square tests (for categorical measures). Preliminary analyses of within-subject differences in laboratory evoked pain responses between drug conditions were conducted using paired sample t-tests. Given the number of potentially interrelated measures being examined, the first step in the primary analyses was to conduct multivariate general linear model analysis of variance (MANOVA) procedures to examine hypothesized associations between each set of EO function, morphine analgesic response, and placebo condition pain response variables. For example, the multivariate set of EO function measures was examined as it related to the multivariate set of morphine analgesic response measures separately for each laboratory pain task. Similar MANOVAs were conducted to examine multivariate associations between placebo pain responses and morphine analgesic response measures, and for multivariate associations between placebo pain responses and EO function measures. To evaluate the direction and magnitude of the univariate associations contributing to the multivariate links between each continuous EO function measure and corresponding continuous placebo condition and morphine analgesic response measures, follow-up analyses used Pearson correlation coefficients. These correlation coefficients are directly interpretable as an effect size measure45.
Solely for purposes of graphically presenting the overall association between EO function and morphine analgesic responses, a summary Z score variable was derived separately for each, reflecting the mean of the combined standardized values across all evoked pain measures and both pain tasks. All analyses used the maximum number of available cases and a two-tailed probability value of p<.05 as the criterion for significance.
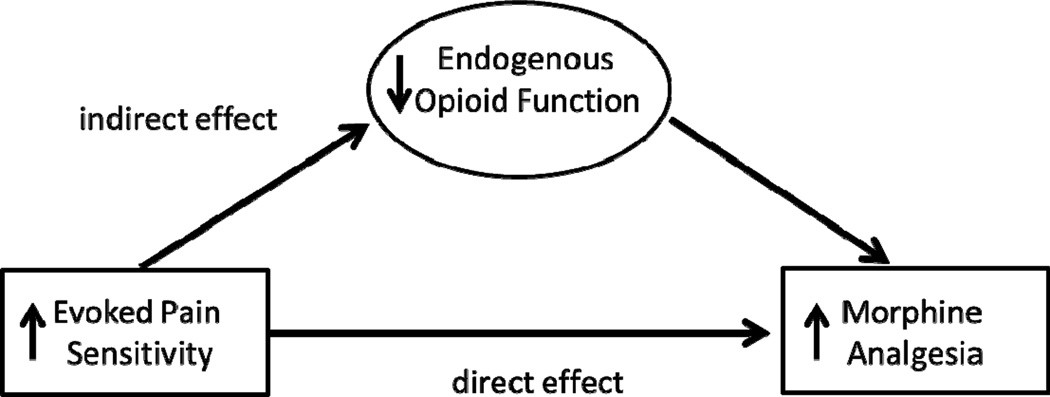
Results of the primary analyses were consistent with the conceptual mediation model that was hypothesized. Therefore, a statistical mediation model was tested in which associations between placebo condition evoked pain sensitivity and morphine analgesic responses were mediated by differences in EO function (see Figure 1). As described by Preacher and Hayes43, two criteria were necessary to demonstrate mediation: 1) there was an effect to be mediated (i.e., a significant association between placebo evoked pain responses and morphine analgesic responses), and 2) the indirect effect of placebo pain sensitivity on morphine responses via EO function was significant. For those evoked pain outcomes for which there was an effect to be mediated, the approach of Preacher and Hayes43 was used to test the significance of the indirect effect. Custom SPSS dialogue (the Indirect Procedure; http://www.afhayes.com/spss-sas-andmplus-macros-and-code.html#sobel) was used to conduct the mediation analyses. As portrayed in Figure 1, these analyses determined the significance for both the direct effect of placebo condition evoked pain sensitivity on morphine analgesic responses and its indirect effect on morphine responses via EO function (indexed by opioid blockade effects). In theory, both direct and indirect effects might be significant in the case of partial mediation. Significance of the indirect effects was determined two ways. First, it was tested with the Sobel test, which although often used in mediation analyses, assumes a normal distribution and may be underpowered in samples that are not large43. Significance of indirect effects was therefore also tested using more powerful bootstrap estimates that make no assumptions about the distribution of the variables43. This bootstrap methodology was used to test each mediation model in a series of 1000 random subsamples repeatedly drawn from the full sample, generating 95% confidence intervals (bias corrected) around the indirect effect test statistic. If the 95% confidence intervals for the indirect effect generated by the model do not include zero, this indicates that the hypothesized indirect (mediated) effect is significant at the p<.05 level. Based on previously published empirical power estimates for the bias corrected bootstrap methodology employed in the current study24 and the moderate or larger effect sizes observed for associations between evoked pain responsiveness and EO function and between EO function and morphine analgesic responsiveness, a sample size of 71 participants was required to achieve a power of .80 to reject the null hypothesis regarding mediation.
Figure 1.
Proposed model in which the link between evoked pain sensitivity and morphine analgesic responsiveness is mediated by individual differences in endogenous opioid function.
Results
Participant Characteristics
Of the total sample, 68.4% participated at the Vanderbilt site, with no differences in the proportion of CLBP participants versus controls or in the overall past month intensity of CLBP across the two sites (p’s>.10). Characteristics of both study subgroups are summarized in Table 1. The two subgroups did not differ significantly in gender, race, or ethnicity, and were of statistically comparable age (p’s > .10).
Table 1.
Participant characteristics by participant type.
| Measure | Healthy Controls (n=31) |
CLBP (n=45) |
|---|---|---|
| Gender (% female) | 58.1 | 66.7 |
| Race: | ||
| Caucasian | 48.4 | 62.2 |
| African-American | 45.2 | 33.3 |
| Ethnicity: | ||
| Non-Hispanic | 93.5 | 95.6 |
| Age (years) | 33.7±9.61 | 37.9±11.03 |
| VAS Chronic Pain Intensity (0–100) | 54.5±24.27 | |
| Pain Duration (median, in months) | 90.2 |
Note: Summary statistics are presented as percentages or means ( SD). All statistical comparisons across participant types were nonsignificant. CLBP = Chronic Low Back Pain. VAS Chronic Pain Intensity was a retrospective measure of overall past month chronic pain intensity.
Potential effects of participant type and gender on results of primary analyses were considered. None of the EO function or morphine analgesic response measures differed between genders or participant types (all p’s>.10). To further evaluate the extent to which participant type differences might confound results, MANOVAs paralleling the primary analyses described below with morphine analgesic response outcomes as the dependent variable were run including main and interactive effects of participant type. In no case did the main effect or two-way interactions (with EO function or placebo pain measures) reach statistical significance. Given the lack of influence of participant type on the primary dependent variable (morphine analgesic responses), the two participant type subgroups were combined in analyses to maximize statistical power. The MANOVAs reported below did not include participant type in the final models.
Pain Responses Across Drug Conditions
Raw pain response values across drug conditions are displayed in Table 2. Paired sample t-tests revealed significant overall analgesic effects for morphine relative to placebo evoked pain responses. Ischemic task threshold [t(63)=−2.66, p=.01] and tolerance [t(71)=−2.21, p=.03] were both significantly higher after morphine than after placebo, and ischemic NRS ratings [t(71)=3.18, p=.002] and VAS Intensity [t(71)=2.10, p=.04] were both significantly lower after morphine. Similarly, for the thermal pain task, heat pain threshold [t(71)=−2.79, p=.007] and tolerance [t(71)=−2.09, p=.04] were significantly higher after morphine than after placebo, with a nonsignificant trend towards lower VAS Intensity ratings after morphine [t(71)=1.83, p=.07]. All other comparisons across drug conditions failed to reach statistical significance (p’s>.10).
Table 2.
Mean (±S.D.) evoked pain responses by drug condition.
| Drug Condition | |||
|---|---|---|---|
| Evoked Pain Measure | Placebo | Naloxone | Morphine |
| ISC Threshold (sec) | 51.3±86.78 * | 50.3±72.29 | 84.3±117.52 |
| ISC Tolerance (sec) | 314.1±175.03 * | 300.0±169.65 | 348.3±151.96 |
| ISC NRS (0–100) | 53.1±28.36 * | 52.0±27.28 | 46.9±28.43 |
| ISC VAS Intensity (0–100) | 52.0±26.00 * | 54.0±26.27 | 45.4±29.20 |
| Thermal Threshold (°C) | 43.8±3.11 * | 43.6±3.36 | 44.6±3.03 |
| Thermal Tolerance (°C) | 47.8±1.69 * | 47.6±1.79 | 48.1±1.76 |
| Thermal VAS Intensity (0–100) | 57.5±25.98 | 58.4±26.68 | 52.6±27.73 |
Note:
p<05 for comparisons between Placebo and Morphine conditions. All comparisons between Placebo and Naloxone conditions were nonsignificant (p’s>.10).
ISC = Ischemic Task; NRS = Numeric Rating Scale; VAS = Visual Analog Scale.
Is Endogenous Opioid Function Related to Morphine Responses?
Planned Analyses
A MANOVA of ischemic task outcomes examining effects of EO function (independent variables) on morphine analgesic responses (dependent variables) revealed significant multivariate effects for both ischemic pain threshold [F(4,53) = 6.84, p<.001; Wilks’ Lambda = 0.659] and tolerance [F(4,53) = 6.27, p<.001; Wilks’ Lambda = 0.679]. Significant multivariate effects were also noted for ischemic intra-task NRS intensity ratings [F(4,53) = 5.24, p=.001; Wilks’ Lambda = 0.717], as well as VAS intensity ratings [F(4,53) = 8.23, p<.001; Wilks’ Lambda = 0.617]. A comparable MANOVA for thermal task outcomes revealed similarly significant effects for thermal pain threshold [F(3,64) = 8.83,p<.001; Wilks’ Lambda = 0.707] and thermal pain tolerance [F(3,64) = 14.45,p<.001; Wilks’ Lambda = 0.596], as well as thermal task VAS intensity ratings [F(3,64) = 2.91, p=.041; Wilks’ Lambda = 0.880]. In sum, these results indicate significant associations between all EO function measures for each laboratory evoked pain task and the multivariate set of morphine analgesic response measures for the respective task.
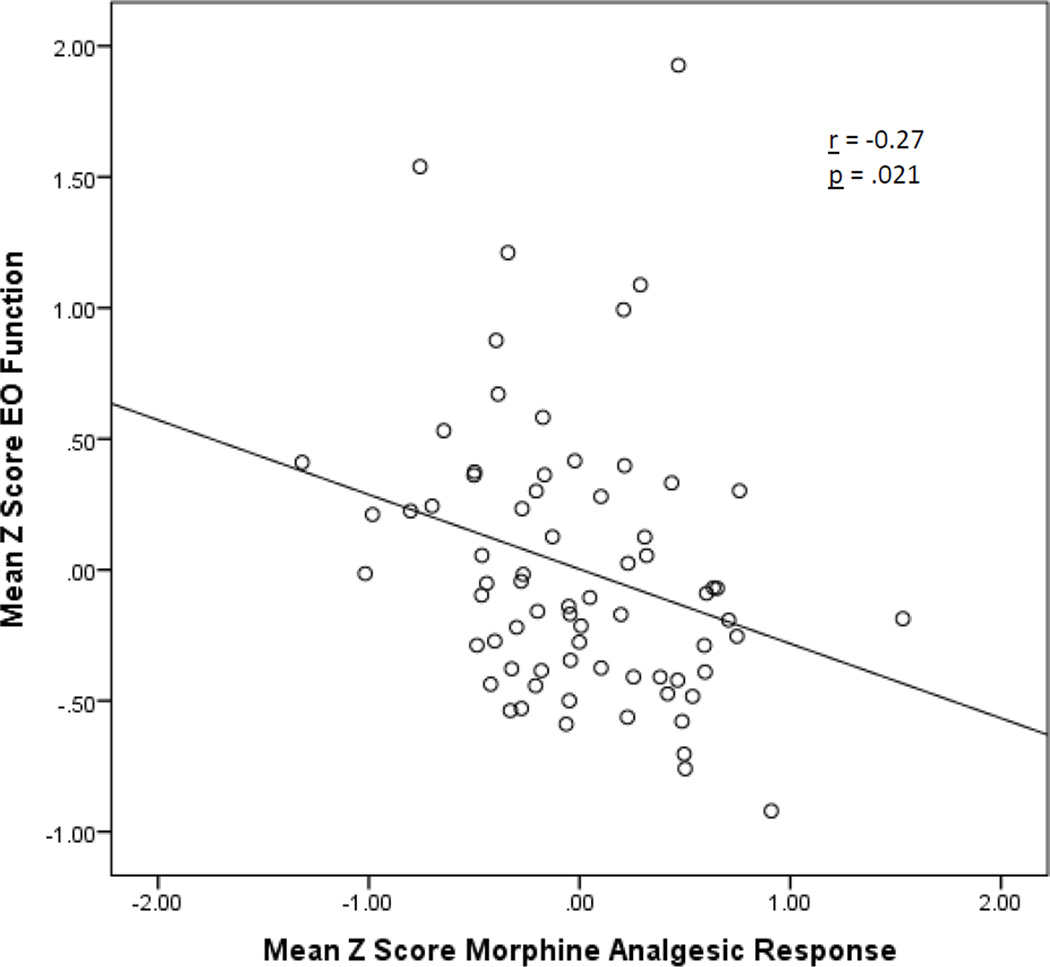
To highlight the direction and magnitude of the effects above, bivariate correlations between EO function and the corresponding morphine analgesic response measure for each pain outcome are summarized in Table 3. For all 7 pain measures examined across the two pain tasks, significant inverse correlations were observed between EO function and analgesic responses to morphine (p’s = .001 − .02). Based on categorizations described by Cohen15, effect sizes for 6 pain measures were medium, and for one measure (ischemic pain tolerance) was large. A scatterplot displaying summary Z scores for EO function and morphine analgesic responses across all measures and both pain tasks is presented in Figure 2, highlighting the association between lower EO function and greater morphine analgesia.
Table 3.
Correlations between EO activity and morphine analgesic responsiveness for each evoked pain measure.
| Evoked Pain Measure | Correlation (r) |
|---|---|
| ISC Threshold | −0.42*** |
| ISC Tolerance | −0.54*** |
| ISC NRS | −0.36** |
| ISC VAS Intensity | −0.41*** |
| Thermal Threshold | −0.39*** |
| Thermal Tolerance | −0.47*** |
| Thermal VAS Intensity | −0.36** |
p<01
p<001
Note: The index of endogenous opioid (EO) activity was derived as the difference between placebo and naloxone conditions, coded so that higher values indicate greater endogenous opioid activity. The index of morphine analgesic responsiveness was derived as the difference between placebo and morphine conditions, coded so that higher values indicate greater morphine analgesia.
Figure 2.
Scatterplot of association between endogenous opioid function (opioid blockade effects) and morphine analgesic responses for summary Z-score variables across all pain measures and both evoked pain tasks.
Post-Hoc Analyses: Source and Clinical Relevance of Effects
Follow-up analyses were conducted to explore the source of significant associations between EO function and differential morphine responsiveness. For each laboratory evoked pain measure, a median split was used to dichotomize participants with regards to EO activity. Then, placebo condition and morphine condition evoked pain responses were compared across these dichotomized groups. We hypothesized that the greater morphine responsiveness among individuals with low EO activity might be due to morphine “making up the difference” in opioid receptor activation relative to those with pre-existing high EO activity. We were therefore particularly interested in comparisons between the placebo condition pain ratings for the high EO group and morphine condition ratings for the low EO group. Results are summarized in Table 4. For all measures but ischemic task pain tolerance, placebo condition pain responses in the high EO group were found to be statistically comparable to morphine condition pain responses in the low EO group (indicated by the label “nonsignificant” in the rightmost column of Table 4). This pattern suggests that morphine in effect supplemented inadequate natural opioid activity in low EO participants, making these individuals look more similar to high EO participants who had received no analgesic medication.
Table 4.
Placebo and morphine condition pain responses by EO function category.
| EO Function | High EO Placebo vs. | |||
|---|---|---|---|---|
| Evoked Pain Measure | Category | Placebo Pain | Morphine Pain | Low EO Morphine |
| ISC Threshold | High | 59.7±98.99 | 74.8±108.93 | Nonsignificant |
| Low | 25.7±32.34 | 90.2±127.37 | ||
| ISC Tolerance | High | 394.7±135.53 | 381.3±146.57 | |
| Low | 124.8±84.61 | 268.7±137.71 | ||
| ISC NRS | High | 45.4±28.64 | 44.5±30.34 | Nonsignificant |
| Low | 60.8±25.88 | 49.8±26.47 | ||
| ISC VAS Intensity | High | 47.4±27.48 | 47.2±32.05 | Nonsignificant |
| Low | 56.8±24.21 | 44.3±26.37 | ||
| Thermal Threshold | High | 44.7±3.19 | 45.2±3.09 | Nonsignificant |
| Low | 42.9±2.80 | 43.9±2.87 | ||
| Thermal Tolerance | High | 48.1±1.36 | 48.0±1.39 | Nonsignificant |
| Low | 47.5±1.95 | 48.1±2.09 | ||
| Thermal VAS Intensity | High | 50.8±23.58 | 54.1±23.02 | Nonsignificant |
| Low | 64.5±26.91 | 52.1±31.62 |
Note: High vs. Low EO function categories were based on median splits. Table 5 indicates that for all measures, associations between placebo pain responses and EO function status were significant.
To display the impact that EO activity has on morphine responsiveness in a more clinically meaningful way, we compared the magnitude of morphine effects on mean ischemic intra-task NRS ratings (0–100) between participants at or below the 10th percentile and at or above the 90th percentiles on EO function for this measure. Results indicate that while the low EO participants displayed a mean decrease in evoked pain intensity ratings of 25.2 NRS scale points with morphine administration, participants with the highest EO activity actually exhibited a slight increase in evoked pain reports with morphine (5.5 NRS scale points). Thus, there was a difference of greater than 30 scale points on a 0–100 scale for morphine responses between those with the lowest versus highest EO activity.
Post-Hoc Analyses: Potential Confounding Effects of Placebo Pain Intensity
While magnitude of EO function scores and morphine analgesic effects should in principle be independent of the common placebo values used as a reference in the derivation of both, inverse associations between these measures could be an artifact of very high or low placebo condition pain sensitivity. For example, if pain sensitivity were already very low under placebo, then morphine would be unable to reduce it further (small morphine analgesic effects) and there would be ample room for opioid blockade to increase pain sensitivity (larger EO function scores), resulting in an inverse correlation. To rule out the possible confounding influence of extremes in placebo condition pain sensitivity, the correlations above were re-run as partial correlations controlling for the common placebo pain measure used as the reference point in deriving each. All 7 correlations that were significant in Table 3 remained significant (p’s = .002 − .049) after removing variance attributable to differences in placebo pain sensitivity. Thus, the inverse association observed between EO function and morphine analgesic responses did not appear to be an artifact of floor or ceiling effects in placebo condition pain sensitivity.
Is Placebo Pain Sensitivity Related to Morphine Responses?
A MANOVA of ischemic task outcomes examining effects of placebo condition pain sensitivity on morphine analgesic responses revealed significant multivariate effects for placebo ischemic pain tolerance [F(4,55) = 9.24,p<.001; Wilks’ Lambda = 0.598], ischemic intra-task NRS intensity ratings [F(4,55) = 5.25,p<.001; Wilks’ Lambda = 0.724], and VAS intensity ratings [F(4,55) = 7.31,p<.001; Wilks’ Lambda = 0.653]. The multivariate effect for placebo ischemic pain threshold was nonsignificant (p=.351). A similar MANOVA for thermal task outcomes revealed significant multivariate effects for thermal pain threshold [F(3,64) = 8.83,p<.001; Wilks’ Lambda = 0.707] and thermal task VAS intensity ratings [F(3,64) = 2.91, p=.041; Wilks’ Lambda = 0.880]. The multivariate effect for placebo thermal pain tolerance was nonsignificant (p=.17). Taken together, these results indicate significant associations across both pain tasks for 5 of 7 placebo condition evoked pain measures and the multivariate set of morphine analgesic responses for the given task.
Table 5 summarizes the bivariate correlations between placebo pain sensitivity and morphine responses for all individual evoked pain outcomes. Of the 7 measures across the two pain tasks, 5 were significant (p’s = .001 − .02) and one approached significance (p=.089). All of these associations were in the same direction, indicating greater morphine responsiveness among those with higher placebo condition evoked pain sensitivity. Magnitude of the significant correlations indicated medium to large (for ischemic tolerance only) effect sizes.
Table 5.
Correlations between placebo condition pain responses and both EO function and morphine analgesic responsiveness for each evoked pain measure.
| Placebo Evoked Pain Measure | EO Function | Morphine Responsiveness |
|---|---|---|
| ISC Threshold | −0.65*** | 0.22† |
| ISC Tolerance | −0.53*** | 0.55*** |
| ISC NRS | −0.42*** | 0.29* |
| ISC VAS Intensity | −0.37*** | 0.36** |
| Thermal Threshold | −0.29** | 0.35** |
| Thermal Tolerance | −0.25* | 0.18 |
| Thermal VAS Intensity | −0.44**** | 0.38*** |
p<10
p<05
p<.01
p<001
Note: Placebo condition threshold and tolerance values have been reverse-scored for these analyses so that direction of effects is similar to pain ratings.
Is Placebo Condition Evoked Pain Sensitivity Related to Endogenous Opioid Function?
Placebo pain responses appeared to have been influenced by individual differences in EO function. A MANOVA of ischemic task outcomes revealed significant multivariate associations between EO function and placebo pain sensitivity for the following EO measures: ischemic pain threshold [F(4,57) = 14.22,p<.001; Wilks’ Lambda = 0.501], ischemic pain tolerance [F(4,57) = 13.41,p<.001; Wilks’ Lambda = 0.515], intra-task NRS intensity ratings [F(4,57) = 8.09,p<.001; Wilks’ Lambda = 0.638], and VAS intensity ratings [F(4,57) = 9.26,p<.001; Wilks’ Lambda = 0.606]. A comparable MANOVA of thermal pain task responses indicated significant multivariate associations between EO function and placebo pain responses for thermal pain threshold [F(3,68) = 2.94, p=.039; Wilks’ Lambda = 0.885] and thermal task VAS intensity ratings [F(3,68) = 6.87,p<.001; Wilks’ Lambda = 0.768]. The multivariate effect for thermal pain tolerance approached significance [F(3,68) = 2.53, p=.064; Wilks’ Lambda = 0.900]
Bivariate correlations between placebo condition evoked pain sensitivity and corresponding EO function measures for all individual pain outcomes are detailed in Table 5. For all 7 pain measures examined, correlations were inverse and significant (p’s = .001 − .021). Each of these associations indicated that lower EO function was associated with greater evoked pain sensitivity under placebo, consistent with a role for EO function in modulating pain sensitivity.
Mediation Models
A mediation model was hypothesized in which the association between placebo condition laboratory evoked pain sensitivity and morphine analgesic responses was mediated by individual differences in EO function. Although not strictly required for testing mediation, the pattern of correlations described above was consistent with the proposed mediation model. That is, higher placebo condition pain sensitivity was associated with both greater morphine analgesia and lower EO function, and lower EO function was itself associated with greater morphine responsiveness. Five of the 7 pain measures across the two evoked pain tasks displayed an effect that could be mediated (placebo pain sensitivity was associated with degree of morphine analgesia), thereby meeting the first requirement for demonstrating mediation. Table 6 displays the results of mediation tests in the proposed model for the 5 relevant measures. Indirect effects in bootstrap tests were significant for 4 of the 5 measures, supporting the hypothesized mediation model for these measures. For the remaining measure (thermal VAS intensity), the normal theory Sobel mediation test statistic approached significance (p=.08). The pattern of findings indicates that high laboratory evoked pain sensitivity under placebo was related to high levels of analgesic effects from morphine in part via the influences of low EO function. Direct effects were also significant for 4 of these 5 measures, indicating that a portion of the influence of placebo pain sensitivity on morphine responses is independent of EO function.
Table 6.
Summary of significance of direct and indirect (mediated) effects for model in which the association between placebo condition evoked pain sensitivity and morphine analgesic responsiveness is mediated by EO function.
| Indirect Effect via EO Function |
||||
|---|---|---|---|---|
| Bootstrap 95% Confidence Intervals |
||||
| Evoked Pain Measure | Direct Effect | Sobel Test | Lower | Upper |
| ISC Tolerance | p=002 | p=.007 | −0.27 | −0.04* |
| ISC NRS | n.s. | p=.041 | 0.01 | 0.17* |
| ISC VAS Intensity | p=037 | p=.032 | 0.02 | 0.30* |
| Thermal Threshold | p=.027 | p=051 | −0.14 | −0.02* |
| Thermal VAS Intensity | p=.021 | p=.072 | −0.004 | 0.24 |
p<.05
Note: Mediation models were only tested for individual evoked pain measures demonstrating an effect to be mediated (i.e., a significant association between placebo pain responses and morphine analgesic responses).
Discussion
The influence of individual differences in EO system function on responses to opioid analgesic medications has not been explored in humans. This mechanistic information may be important to facilitate development of personalized medicine protocols for use of opioid analgesics6. The primary aim of this study therefore was to examine for the first time whether naturally occurring differences in EO function are associated with differential responsiveness to a prototypic opioid analgesic, morphine. Results revealed significant inverse associations between EO function and morphine analgesic responses on all evoked pain measures examined across two different laboratory pain stimuli. Up to 29% of the variance in morphine analgesic responses could be accounted for by individual differences in EO function, although clearly a substantial portion of differential morphine responding was related to non-opioid determinants as well. Opiate-free individuals with higher natural levels of EO activity obtained significantly less analgesic benefit for a given weight-adjusted dose of morphine. These findings were not an artifact of floor or ceiling effects in placebo condition pain sensitivity. Post-hoc subgroup analyses suggested these associations were due to morphine in effect supplementing inadequate EO function, thereby making evoked pain responses of individuals with naturally low EO function appear more similar to individuals with naturally high EO function. Individuals with high natural EO function appear to derive little incremental benefit from opioid analgesic medications, at least in the laboratory evoked pain context.
If replicated, the present findings may have clinical implications. To the extent that biomarkers obtainable in the clinic setting that reflect differing EO function can be identified, such biomarkers might be used to assist in making mechanism-based decisions on whether to initiate opioid therapy and to help guide opioid dosing and titration6. A key issue to address in future work in this regard is whether EO function predicts responses to chronic opioid dosing, as is often the case in chronic pain management. At present, it is unknown whether the current results can be generalized to the chronic pain context.
A second potential clinical implication relates to the question of whether EO function can be manipulated for clinical benefit. Although speculative, results of this study suggest that nonpharmacological interventions that appear to have EO enhancing effects (e.g., acupuncture, aerobic exercise, relaxation training)27,28,38,39 might in some contexts provide a degree of analgesia similar to that achieved with opioid analgesic medications, or reduce the amount of opioid analgesic medications required to achieve adequate analgesia (i.e., less opioid deficit to make up). If confirmed, clinical use of strategic combinations of nonpharmacological approaches and low dose opioid therapy might be indicated, potentially reducing side effects and abuse risk often associated with high-dose opioid prescribing16,30,42.
A second aim of this study was to provide a placebo-controlled replication of findings from two past studies suggesting that natural variations in evoked pain sensitivity predict opioid analgesic responses18,21. Both studies found that lower pre-drug evoked pain responsiveness predicted greater analgesic responses to opioid medications18,21. It does not appear that either prior study directly controlled for placebo effects in these predictive analyses, with both examining changes in pain responses from pre-drug baseline to post-drug. The current study examined laboratory evoked pain sensitivity under placebo as it relates to analgesic responses to morphine. Results were in the opposite direction to the two prior studies: greater placebo-condition pain sensitivity was associated with significantly greater analgesic efficacy of morphine. The consistency of the current findings across multiple evoked pain outcomes and both laboratory pain tasks argue against these being spurious associations. Causes of discrepancies between the current work and the only two past controlled studies of this topic cannot be definitively determined. However, methodological differences may have contributed. Unlike the current study, Edwards et al.18 studied a chronic neuropathic pain population, and examined long-term effects on clinical pain outcomes of daily morphine given for approximately 6 weeks at a maximum tolerable dose differing across individuals. Eisenberg et al.21 employed a single dose of oral oxycodone rather than intravenous morphine as in the current study, examined its effects on evoked pain responses evaluated over a period of several hours, and focused on responses to the cold pressor task as the analgesic outcome measure rather than heat or ischemic pain as in the present work. In addition, this latter study was restricted to analyzing a small subset of 23 study participants who persisted in the cold pressor task for at least 15 seconds, potentially biasing the results in favor of less pain sensitive individuals. Whether differing opioid medication types, acute versus chronic dosing, or differing outcome measures account for the contrasting findings across studies cannot be determined. Whatever their source, replication of the current findings is desirable.
A final aim of this study was to test the hypothesis that individual differences in EO function mediate associations between placebo condition evoked pain sensitivity and morphine analgesic responses. Mediation appeared possible given the clear role of EO activity in modulating laboratory evoked pain responses in this study, a finding consistent with numerous other studies1,11,26,33,51. Bootstrap testing of mediation models indicated that for 4 of the 5 evoked pain outcomes demonstrating an effect to be mediated (i.e., placebo evoked pain sensitivity predicted morphine analgesic responses), the indirect (mediated) effect via EO function was significant. These findings suggest that people with high evoked pain sensitivity may derive high levels of analgesic relief from morphine partly because of low EO function. For 3 of the 4 measures for which indirect effects via EO were significant, the direct effect of placebo pain on morphine responses was also significant, supporting partial mediation by EO function. Thus, people with high laboratory evoked pain sensitivity also show high levels of morphine responsiveness in part through nonopioid mechanisms. The current results supporting partial EO mediation would be consistent with a role for deficient EO function (and therefore greater opioid analgesic responsiveness and possible greater associated rewarding effects) as a contributor to recent reports that chronic pain patients with higher evoked pain sensitivity19 and higher clinical pain intensity after opioid analgesic detoxification29 are at increased risk of opioid misuse and more likely to resume using opioid analgesics, respectively. Results of the present work for the first time reveal a mechanism underlying a putative predictor of responses to opioid analgesic medications. It is likely that improved understanding of mechanisms underlying clinical predictors of opioid analgesic responses will be useful in optimizing such predictors and identifying new ones. This process may, in turn, help facilitate progress towards the goal of personalized pain medicine. y
Several study limitations are noted. Given the absence of an “active” placebo manipulation (e.g., sedating antihistamine), it is likely that blinding particularly for the morphine condition was not maintained in some participants. Whether and how this might have impacted on the results is unknown. Another limitation is that although individuals with chronic back pain were included in the study sample, the present work was restricted to laboratory evoked pain outcomes and did not directly address whether natural variations in EO function would influence the efficacy of opioid analgesics for relief of clinical pain. Nonetheless, it was notable that participants with chronic back pain did not differ from pain-free individuals in their acute analgesic responses to morphine. It also bears mentioning that the chronic pain subsample in this study was comprised largely of individuals with moderate pain who remained relatively functional; this sample may have been qualitatively different than typical pain clinic samples. Chronic pain participants in this study were also distinct from the typical chronic pain clinic population in that none were taking daily opioid analgesic medications. This inclusion criterion was necessary for the safety of the participants, as administration of naloxone to individuals taking daily opioid analgesics would trigger acute withdrawal symptoms. Whether and how differing EO function might influence responses to chronic daily opioid analgesics (and possibly vice versa) remains to be determined. Observed associations in the latter context might be different in character given the dynamic adaptation of opioid receptors to ongoing high levels of opioid agonists (downregulation)52. Nonetheless, the work by Edwards et al.18 described above indirectly supports the possibility that baseline EO differences might predict longer-term responses to chronic opioid therapy. A final potential study limitation is that only a single opioid analgesic agent was tested. It is unknown whether EO function might impact differently on responses to opioid analgesic medications other than morphine, a possibility not inconceivable given that different opioid analgesic agents are known to activate distinct signaling pathways44.
In summary, the current study found that individuals with higher naturally-occurring levels of EO activity exhibit less analgesia in response to morphine administration. Morphine appears to supplement inadequate EO analgesia in those with low natural opioid levels, but provides a much smaller incremental analgesic benefit in individuals with high EO activity in the laboratory evoked pain context. Low EO activity partially mediated observed associations between higher evoked pain sensitivity under placebo and greater morphine analgesic responses. Replication and extension of these results to other analgesic agents, clinical pain outcomes, and chronic opioid dosing is needed.
Acknowledgments
This research was supported by NIH Grant R01-DA031726 and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Contents of this work are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. The authors would like to express their appreciation to Dr. Yu Lin for his contributions to this project, and to the research nurses of the Vanderbilt General Clinical Research Center and the department of Anesthesiology at Rush University for their assistance in data collection. The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson WS, Sheth RN, Bencherif B, Frost JJ, Campbell JN. Naloxone increases pain induced by topical capsaicin in healthy human volunteers. Pain. 2002;99:207–216. doi: 10.1016/s0304-3959(02)00103-3. [DOI] [PubMed] [Google Scholar]
- 2.Benedek G, Szikszay M. Sensitization or tolerance to morphine effects after repeated stresses. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9:369–380. doi: 10.1016/0278-5846(85)90189-7. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz BA, Ngai SH, Hempstead J, Spector S. Disposition of naloxone: use of a new radioimmunoassay. J Pharmacol Exp Ther. 1975;195:499–504. [PubMed] [Google Scholar]
- 4.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 5.Bodnar RJ, Kelly DD, Steiner SS, Glusman M. Stress-produced analgesia and morphine-produced analgesia: lack of cross-tolerance. Pharmacol Biochem Behav. 1978;8:661–666. doi: 10.1016/0091-3057(78)90263-0. [DOI] [PubMed] [Google Scholar]
- 6.Bruehl S, Apkarian AV, Ballantyne JC, Berger A, Borsook D, Chen WG, Farrar JT, Haythornthwaite JA, Horn SD, Iadarola MJ, Inturrisi CE, Lao L, Mackey S, Mao J, Sawczuk A, Uhl GR, Witter J, Woolf CJ, Zubieta JK, Lin Y. Personalized medicine and opioid analgesic prescribing for chronic pain: opportunities and challenges. J Pain. 2013;14:103–113. doi: 10.1016/j.jpain.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruehl S, Burns JW, Chung OY, Chont M. Interacting effects of trait anger and acute anger arousal on pain: the role of endogenous opioids. Psychosom Med. 2011;73:612–619. doi: 10.1097/PSY.0b013e318227cb88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruehl S, Burns JW, Chung OY, Ward P, Johnson B. Anger and pain sensitivity in chronic low back pain patients and pain-free controls: The role of endogenous opioids. Pain. 2002;99:223–233. doi: 10.1016/s0304-3959(02)00104-5. [DOI] [PubMed] [Google Scholar]
- 9.Bruehl S, Chung OY. Parental history of chronic pain may be associated with impairments in endogenous opioid analgesic systems. Pain. 2006;124:287–294. doi: 10.1016/j.pain.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Bruehl S, Dengler-Crish CM, Smith CA, Walker LS. Hypoalgesia related to elevated resting blood pressure is absent in adolescents and young adults with a history of functional abdominal pain. Pain. 2010;149:57–63. doi: 10.1016/j.pain.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchsbaum MD, Davis GC, Bunney WE. Naloxone alters pain perception and somatosensory evoked potentials in normal subjects. Nature. 1977;270:620–622. doi: 10.1038/270620a0. [DOI] [PubMed] [Google Scholar]
- 12.Calcagnetti DJ, Holtzman SG. Potentiation of morphine analgesia in rats given a single exposure to restraint stress immobilization. Pharmacol Biochem Behav. 1992;41:449–453. doi: 10.1016/0091-3057(92)90125-y. [DOI] [PubMed] [Google Scholar]
- 13.Chapman DB, Hu J, Way EL. Methionine-enkephalin antagonism and endorphin potentiation of narcotic-induced analgesia. Eur J Pharmacol. 1980;65:369–377. doi: 10.1016/0014-2999(80)90341-6. [DOI] [PubMed] [Google Scholar]
- 14.Chung OY, Bruehl S, Diedrich L, Diedrich A, Chont M, Robertson D. Baroreflex sensitivity associated hypoalgesia in healthy states is altered by chronic pain. Pain. 2008;138:87–97. doi: 10.1016/j.pain.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Second Edition. Hillsdale, NJ: Lawrence Erlbaum; 1988. pp. 75–108. [Google Scholar]
- 16.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dymshitz J, Amir S. Opposite effects of restraint on morphine analgesia and naloxone-induced jumping. Pharmacol Biochem Behav. 1988;30:905–910. doi: 10.1016/0091-3057(88)90118-9. [DOI] [PubMed] [Google Scholar]
- 18.Edwards RR, Haythornthwaite JA, Tella P, Max MB, Raja S. Basal heat pain thresholds predict opioid analgesia in patients with postherpetic neuralgia. Anesthesiology. 2006;104:1243–1248. doi: 10.1097/00000542-200606000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Edwards RR, Wasan AD, Michna E, Greenbaum S, Ross E, Jamison RN. Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. J Pain. 2011;12:953–963. doi: 10.1016/j.jpain.2011.02.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Eisenberg E, Midbari A, Haddad M, Pud D. Predicting the analgesic effect to oxycodone by 'static' and 'dynamic' quantitative sensory testing in healthy subjects. Pain. 2010;151:104–109. doi: 10.1016/j.pain.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Fazli-Tabaei S, Yahyavi SH, Alagheband P, Samie HR, Safari S, Rastegar F, Zarrindast MR. Cross-tolerance between antinociception induced by swim-stress and morphine in formalin test. Behav Pharmacol. 2005;16:613–619. doi: 10.1097/00008877-200512000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, Staud R. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18:233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ginsburg GS, Willard HF. Genomic and personalized medicine: foundations and applications. Transl Res. 2009;154:277–287. doi: 10.1016/j.trsl.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Gracely RH, Dubner R, Wolskee PJ, Deeter WR. Placebo and naloxone can alter post-surgical pain by separate mechanisms. Nature. 1983;306:264–265. doi: 10.1038/306264a0. [DOI] [PubMed] [Google Scholar]
- 27.Harris RE, Zubieta JK, Scott DJ, Napadow V, Gracely RH, Clauw DJ. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs) Neuroimage. 2009;47:1077–1085. doi: 10.1016/j.neuroimage.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koltyn KF. Analgesia following exercise: a review. Sports Med. 2000;29:85–98. doi: 10.2165/00007256-200029020-00002. [DOI] [PubMed] [Google Scholar]
- 29.Krumova EK, Bennemann P, Kindler D, Schwarzer A, Zenz M, Maier C. Low pain intensity after opioid withdrawal as a first step of a comprehensive pain rehabilitation program predicts long-term nonuse of opioids in chronic noncancer pain. Clin J Pain. 2013 Jan 24; doi: 10.1097/AJP.0b013e31827c7cf6. [Epub ahead of print]; PubMed PMID: 23567163. [DOI] [PubMed] [Google Scholar]
- 30.Kuehn BM. Efforts aim to curb opioid deaths, injuries. JAMA. 2009;301:1213–1215. doi: 10.1001/jama.2009.367. [DOI] [PubMed] [Google Scholar]
- 31.Lee NM, Leybin L, Chang JK, Loh HH. Opiate and peptide interaction: effect of enkephalins on morphine analgesia. Eur J Pharmacol. 1980;68:181–185. doi: 10.1016/0014-2999(80)90319-2. [DOI] [PubMed] [Google Scholar]
- 32.Lesko LJ. Personalized medicine: elusive dream or imminent reality? Clin Pharmacol Ther. 2007;81:807–816. doi: 10.1038/sj.clpt.6100204. [DOI] [PubMed] [Google Scholar]
- 33.Levine JD, Gordon NC, Jones RT, Fields HL. The narcotic antagonist naloxone enhances clinical pain. Nature. 1978;272:826–827. doi: 10.1038/272826a0. [DOI] [PubMed] [Google Scholar]
- 34.Lewis J, Mansour A, Khachaturian H, Watson SJ, Akil H. Opioids and pain regulation. Pain Headache. 1987;9:129–159. [PubMed] [Google Scholar]
- 35.Martin WR. Drugs five years later: naloxone. Ann Intern Med. 1976;85:765–768. doi: 10.7326/0003-4819-85-6-765. [DOI] [PubMed] [Google Scholar]
- 36.Mathes WF, Kanarek RB. Wheel running attenuates the antinociceptive properties of morphine and its metabolite, morphine-6-glucuronide, in rats. Physiol Behav. 2001;74:245–251. doi: 10.1016/s0031-9384(01)00577-7. [DOI] [PubMed] [Google Scholar]
- 37.Maurset A, Skoglung LA, Hustveit O, Klepstad P, Oye I. A new version of the ischemic tourniquet pain test. Meth Find Exp Clin Pharmacol. 1992;13:643–647. [PubMed] [Google Scholar]
- 38.McCubbin JA, Cheung R, Montgomery TB, Bulbulian R, Wilson JF. Aerobic fitness and opioidergic inhibition of cardiovascular stress reactivity. Psychophysiology. 1992;29:687–697. doi: 10.1111/j.1469-8986.1992.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 39.McCubbin JA, Wilson JF, Bruehl S, Ibarra P, Carlson CR, Norton JA, Colclough GW. Relaxation training and opioid inhibition of blood pressure response to stress. J Consult Clin Psychol. 1996;64:593–601. doi: 10.1037//0022-006x.64.3.593. [DOI] [PubMed] [Google Scholar]
- 40.Melzack R. The short form of the McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 41.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 42.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 43.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–31. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 44.Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60:58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. New York: Sage; 1994. pp. 232–243. [Google Scholar]
- 46.Smith FL, Brase DA, Dombrowski DS, Dewey WL. Endogenous opioids released by suspending mice by the tail selectively enhance spinal mu opioid analgesia. J Pharmacol Exp Ther. 1994;270:1177–1185. [PubMed] [Google Scholar]
- 47.Smith MA, McClean JM, Bryant PA. Sensitivity to the effects of a kappa opioid in rats with free access to exercise wheels: differential effects across behavioral measures. Pharmacol Biochem Behav. 2004;77:49–57. doi: 10.1016/j.pbb.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 48.Tseng LF, Loh HH, Li CH. beta-Endorphin: cross tolerance to and cross physical dependence on morphine. Proc Natl Acad Sci U S A. 1976;73:4187–4189. doi: 10.1073/pnas.73.11.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaught JL, Takemori AE. A further characterization of the differential effects of leucine enkephalin, methionine enkephalin and their analogs on morphine-induced analgesia. J Pharmacol Exp Ther. 1979;211:280–283. [PubMed] [Google Scholar]
- 50.Vaught JL, Takemori AE. Differential effects of leucine and methionine enkephalin on morphine-induced analgesia, acute tolerance and dependence. J Pharmacol Exp Ther. 1979;208:86–90. [PubMed] [Google Scholar]
- 51.Willer JC, Dehen H, Cambier J. Stress-induced analgesia in humans: endogenous opioids and naloxone-reversible pain reflexes. Science. 1981;212:689–691. doi: 10.1126/science.6261330. [DOI] [PubMed] [Google Scholar]
- 52.Zhu ZP, Badisa RB, Palm DE, Goodman CB. Regulation of rat MOR-1 gene expression after chronic intracerebroventricular administration of morphine. Mol Med Report. 2012;5:513–516. doi: 10.3892/mmr.2011.677. [DOI] [PMC free article] [PubMed] [Google Scholar]