Abstract
Background
There is concern about the safety of homebirths, especially in women transferred to hospital during or after labour. The scope of transfer in planned home births has not been assessed in a systematic review. This review aimed to describe the proportions and indications for transfer from home to hospital during or after labour in planned home births.
Methods
The databases Pubmed, Embase, Cinahl, Svemed+, and the Cochrane Library were searched using the MeSH term “home childbirth”. Inclusion criteria were as follows: the study population was women who chose planned home birth at the onset of labour; the studies were from Western countries; the birth attendant was an authorised midwife or medical doctor; the studies were published in 1985 or later, with data not older than from 1980; and data on transfer from home to hospital were described. Of the 3366 titles identified, 83 full text articles were screened, and 15 met the inclusion criteria. Two of the authors independently extracted the data. Because of the heterogeneity and lack of robustness across the studies, there were considerable risks for bias if performing meta-analyses. A descriptive presentation of the findings was chosen.
Results
Fifteen studies were eligible for inclusion, containing data from 215,257 women. The total proportion of transfer from home to hospital varied from 9.9% to 31.9% across the studies. The most common indication for transfer was labour dystocia, occurring in 5.1% to 9.8% of all women planning for home births. Transfer for indication for foetal distress varied from 1.0% to 3.6%, postpartum haemorrhage from 0% to 0.2% and respiratory problems in the infant from 0.3% to 1.4%. The proportion of emergency transfers varied from 0% to 5.4%.
Conclusion
Future studies should report indications for transfer from home to hospital and provide clear definitions of emergency transfers.
Keywords: Planned home birth, Transfer to hospital, Emergency transfer, Systematic review
Background
In Western countries, women planning to give birth at home are transferred to hospital in case of complications, or if conditions indicating a higher risk for adverse outcomes occur. Although a growing body of evidence points to less intervention in labour in low-risk women who planned home births [1-4], there is still a concern about safety. Guidelines on home births state that only low-risk women should be accepted for, or have recommended, home birth [5-8]. “Low-risk women” are defined as women without medical diseases or conditions that may influence outcomes of pregnancy, without serious complications in previous pregnancies, with a single foetus in the cephalic position, and with a spontaneous onset of labour at term [5-8]. Low-risk women are expected to have a low risk for adverse outcomes, such as perinatal death and other serious complications. This does not rule out the possibility that women who are assessed as low-risk upon onset of labour may need interventions or other medical assistance during labour, or immediately after birth.
To the best of our knowledge, the scope of transfer in planned home births has not been assessed in a systematic review. There is little systematic knowledge on the frequency of women and neonates who are transferred from home to hospital in planned home births, and indications for transfers. A systematic review will be useful for women in making informed choices, and for the planning of care for these women.
The aims of the present systematic review were as follows: (1) to describe how often women and neonates are transferred from home to hospital during labour or after birth; (2) to describe the proportion of women transferred for reasons that may indicate higher risks for adverse outcomes, such as “foetal distress”, “postpartum haemorrhage” and “respiratory problems in the infant”; and (3) to describe the proportion and definitions for emergency transfers.
Methods
A systematic review is a research method that aims to identify and compare individual studies on one topic and summarise their findings. The “MOOSE statement”, which is the recommended guidelines for publication of systematic reviews of observational studies in epidemiology [9], was used to prepare this manuscript. We also used the “PRISMA statement”, which recommends preferred reporting items for systematic reviews and meta-analyses [10].
Sources
We conducted electronic searches in Medline, Embase, Cinahl, Swemed, and the Cochrane Library combining the MeSH term “home childbirth” to identify all published studies on home births. The reason for using such broad search terms was that all attempts of narrowing the searches led to few citations found. The searches were conducted between September 15th and October 10th, 2012, with an update on December 11th, 2013. We also searched the reference lists of all relevant studies. Language restrictions were not applied.
Study selection
Criteria for selecting studies were as follows. For the required population, pregnant women attempted home birth, meaning that they were accepted for a planned home birth at the onset of labour. The included studies had to report at least one of the following outcome measures: proportion (n/N) transferred from home to hospital during labour; n/N transferred from home to hospital after birth”; n/N transferred for the indications of foetal distress, postpartum haemorrhage, and respiratory problems in the neonate; n/N transferred for other reasons; “n/N had emergency transfer during labour”; “n/N had emergency transfer after the birth”; and the definition of emergency transfer in the study.
Studies included were from Western countries, published in 1985 or later, with data not older than 1980. Western countries were defined as North America, Australia, New Zealand, and all countries in Europe except for the previous Soviet Union. The review was limited to include studies from Western countries to achieve some homogeneity across study populations and health care systems. Since the late 1970s, women with an increased risk for adverse outcomes have not been recommended, and usually not accepted, for home birth or birth in other midwifery-led settings. Only studies with births assisted by an authorised midwife or medical doctor were included.
One of the reviewers (EB) conducted the electronic searches, and screened titles and abstracts to remove duplicates and studies that were obviously not relevant. Each study retrieved in full text was independently assessed by two reviewers for quality (EB, MK, or HL). Any disagreement was resolved by conference or by a third reviewer (HK or PØ). Studies including women with booked home births (e.g., women had booked a home birth, but could have been transferred to hospital care during pregnancy), and those with unplanned home births or with “freebirths” (e.g., home births were planned without the assistance of a midwife or a physician) were not included.
Methodological quality was assessed by using the Norwegian Knowledge Centre for the Health Services tool for assessing the risk of bias [11]. Studies were evaluated according to whether they had a prospective design, if analyses were stratified for nulli- and multiparity, if the study population represented at least 75% of the total home birth group, and if information on parity, caregivers, and duration of observational time was described. Studies were scored as either “good” if they met all of the quality criteria, “medium” if they did not meet all of the criteria, but had no serious flaws, and “poor” if they met none of the criteria, or if 50% or more of the study population failed to be included or followed up. Studies scored as poor were excluded from the review.
Data extraction and analyses
A data extraction form (Additional file 1) was developed according to our study protocol. The data were extracted from each study and entered into the form independently by two reviewers (EB, MK, or HL). Heterogeneity was assessed by calculating inconsistency (I2), and by visual inspection of data and forest plots [12,13]. Sensitivity analyses were performed to assess the robustness [14]. We assessed whether performing a meta-analyses was appropriate. StatDirect (Version 2.7.9; Cheshire, UK) was used for analyses.
Results
Literature searches and study selection
The electronic searches generated 3366 citations. After screening titles and abstracts, 76 studies were retrieved in full text. Searching the literature lists in these 76 articles generated another seven citations. Therefore, 83 studies were reviewed in full text, 15 were included in the review, and 68 were excluded (Figure 1).
Figure 1.
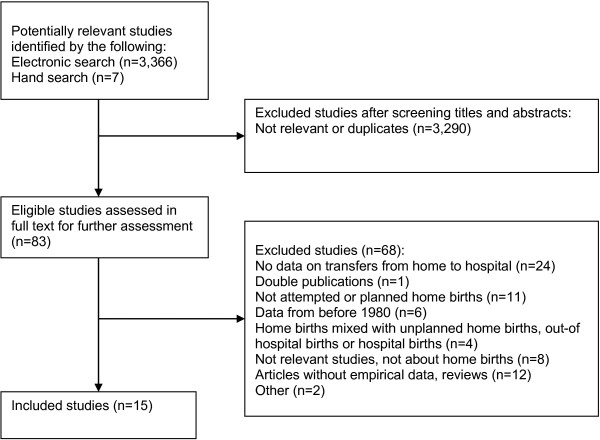
Selection process of eligible studies from all identified studies.
There were only minor disagreements in assessing study quality and whether studies should be included. Disagreements were results of oversights and were solved by consensus.
Reasons for exclusions and a bibliography of excluded studies are shown in Additional file 2.
Description of included studies
Of the 15 included studies, three were from Australia [15-17], three were from Canada [18-20], two were from the USA [21,22], one was jointly from Canada and the USA [4], two were from the UK [1,23], one was from the Netherlands [24], one was from Norway [25], one was from Sweden [26], and one was from Denmark [27]. One study was published in Danish [27], and the others were in English.
The 15 studies included a total of 215,257 women with a planned home birth upon onset of labour. The Dutch study [24] included 168,618 women representing 78% of all women included in the review. The other 14 studies included 46,639 women, and the study populations varied from 70 to 16,848 women. Eight of the studies performed stratified analyses for nulli- and multiparity, and these studies included 8171 nulliparous and 20,581 multiparous women [1,17,18,20,21,23,25,26]. In 10 of the studies, indications for transfers were described [4,15-17,19-23,26].
All of the studies included women who had planned for, and were selected to have homebirth, at the onset of labour. Six of the studies were from settings where home births were an integrated and regulated part of the national or regional health care system [1,15,19,23,24,27], while the other studies described home births assisted by independent midwives. The studies from regulated settings described that only low-risk women were accepted for home birth, and some of the studies provided references to guidelines or other regulations [1,15,19,24]. In the independent settings, the proportion of women with high-risk pregnancies (e.g., post-term delivery, previous caesarean section, or medical conditions that may affect birth outcomes) varied from 4–17% in the four studies, with detailed descriptions of the study populations [4,18,25,26].
One of the studies was assessed as good quality [1], and the others as medium quality. Study characteristics and quality assessments are shown in Table 1.
Table 1.
Description and quality assessments of included studies
| Study | Inclusion criteria | Participants (% P0 1 ) | Caregivers | Study design | Data source | Duration of observation time after birth | Analyses stratified for parity | Study population representative | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Amelink-Verburg et al.[24] |
All women under midwifery care and with an intended home birth in the Netherlands during 01.01.2001-31.12.2003 |
N = 168,618 |
Primary level midwifes |
Prospective |
The Dutch Midwifery Perinatal Database (LVR1) |
2 h after the birth of the placenta |
No |
Data from LVR1 covers 95% of midwifery practices. |
Medium |
| (Parity not described) | |||||||||
| Anderson et al.[22] |
All Nurse-midwifery practices in the USA during 1987-1991 |
N = 11,084 |
Independent midwives |
Retrospective |
Data collection forms from the midwives |
..”early postpartum period” |
No |
66% of midwifery practices participated. |
Medium |
| (Parity not described) | |||||||||
| BECG2[1] |
All NHS trusts providing intrapartum care at home in England (UK) during April 2008-April 2010 |
N = 16,840 |
National Health Service midwives |
Prospective |
Data collection forms from midwives and hospitals |
48 h postpartum |
Yes |
97% of trusts providing home birth services participated. (Home births attended by independent midwives in the region were not included) |
Good |
| (27.2%) | |||||||||
| Blix et al.[25] |
All planned home births in Norway during 01.01.1990-31.12.2007 |
N = 1631 |
Independent midwives |
Retrospective |
Midwives’ patient files |
5 days postpartum |
Yes |
Unclear, probably >70% of all planned home births during the study period |
Medium |
| (22.6% ) | |||||||||
| Davies et al.[23] |
All women in the North Regional Health Authority area (UK) who planned for a home birth and expected to deliver in 1993 |
N = 177 |
National Health Service midwives |
Prospective |
Data collection forms from midwives, women and GP’s |
Not described |
Partly |
Unclear, probably were all planned home births attended by NHS midwives included. |
Medium |
| (9.1%) | |||||||||
| Hansen and et al.[27] |
All home births assisted by midwives employed by the local health authorities in the Municipality of Copenhagen (Denmark) during 1980-1982 |
N = 102 |
Midwives employed at Hvidovre Hospital |
Retrospective |
Hospital patient files |
Not described |
No |
All planned home births assisted by midwives employed by the local health authorities were included. (Home births attended by independent midwives in the region were not included) |
Medium |
| (about 50%) | |||||||||
| Howe [17] |
All home births attended by a registered midwife in the south-west of Western Australia during 01.01.1983-31.12.1986 |
N = 165 |
Independent midwives |
Retrospective |
Midwifery registers |
Not described |
Partly |
All midwives participated |
Medium |
| (31.5%) | |||||||||
| Hutton et al.[18] |
All home births attended by Ontario midwives during 01.04.2003-31.03.2006 (Canada) |
N = 6,692 |
Certified midwives who are required to submit all data to a regional database |
Retrospective |
The Ontario Ministry of Health Database |
Not described |
Partly |
All planned home births were included |
Medium |
| (34.3%) | |||||||||
| Johnson and Daviss [4] |
All home births involving certified professional midwives across the USA and Canada during 01.01.2000-31.12.2000 |
N = 5,418 |
Independent midwives |
Prospective |
Data collection forms from the midwives |
Not described |
No |
73% of the midwives asked, participated. <1% of the women declined participation |
Medium |
| (31.2%) | |||||||||
| Janssen et al.[19] |
All planned home births attended by regulated midwives in British Columbia (Canada) during 01.01.1998-31.12.1999 |
N = 797 |
Regulated midwives |
Prospective |
Data collection forms |
Not described |
No |
>99% of the data collection forms were received |
Medium |
| (about 47%) | |||||||||
| Lindgren et al.[26] |
All planned home births in Sweden during 01.01.1992-31.07.2005 |
N = 1,025 |
Independent midwives |
Retrospective |
Data collection forms to the mothers |
…”shortly after planned home birth” |
Yes |
99% of the women asked, agreed to participate. Unclear if all home births were identified. |
Medium |
| (23.8%) | |||||||||
| McMurtrie et al.[15] |
The first 100 booked home births at the St. George Homebirth Program during Nov 2005-March 2009 in New South Wales (Australia) |
n = 70 attempted home births |
Midwives employed at St George Hospital |
Prospective |
Databases at the birth centre |
Not described |
No |
All planned homebirths were included. (Home births attended by independent midwives in the region were not included) |
Medium |
| (Parity not described) | |||||||||
| Murphy et al.[21] |
All nurse-midwifery practices providing home birth services in the USA during Dec 1994-Dec 1995 |
N = 1,221 |
Independent midwives |
Prospective |
Data collection forms from the midwives, data from hospital files |
Not described |
Partly |
64% of midwifery practices participated. 20% of women transferred to hospital were lost-to-follow-up |
Medium |
| (22.0%) | |||||||||
| Parratt et al.[16] |
All planned home births in Victoria (Australia) during 1995-1998 |
N = 419 |
Independent midwives |
Retrospective |
Midwives’ patient files |
Not described |
No |
50-60 births were not included |
Medium |
| (about 31%) | |||||||||
| Tyson [20] | All planned midwife-attended home births in Toronto (Canada) during Jan 1983-Jul 1988 | N = 1,001 |
Independent midwives | Retrospective | Midwives’ patient files | 4 days postpartum | Yes | All midwives participated | Medium |
| (Parity not described) |
1P0 = nulliparous women. 2Birthplace in England Collaborative Group.
Heterogeneity, robustness, and risks for bias
We detected considerable heterogeneity across the studies through reading the studies, and inspecting tables and forest plots. I2 was above 90% in most of the outcomes (Additional file 3). The reason for this finding is probably because of differences in study populations and clinical practice (e.g., guidelines and traditions for transfer indications).
We performed sensitivity analyses by comparing the pooled prevalence when the largest study was excluded [24], in studies where parity was described [1,4,17-19,21,23,25-27], in studies where parity was not described [15,20,22,24], in studies where home births were booked with independent midwives [3,4,16-18,20-22,25], in studies from settings where home births were an integrated part of the regional or national health care system [1,15,19,23,24,27], and in studies with 10,000 to 100,000 women included [1,22], with 1000 to 10,000 women included [3,4,20,21,25], and with less than 1000 women included [15-17,19,23,27]. In some cases, there were considerable differences between the estimates when comparing a fixed-effect model and a random-effect model, which indicated large differences in results and study sizes. Estimation of the proportion of women transferred to hospital lacked robustness across the sensitivity analyses, while estimation of the proportion of women and neonates transferred for foetal distress, postpartum haemorrhage, respiratory problems, and emergency transfers remained more stable (Additional file 3).
Risks of selection bias are linked to what degree the study populations are representative for all planned home births in the country or region. Some of the studies did not include all home births in the country or region, and it is unclear if the study populations were representative for the total populations [4,16,21,22,25] (Table 1).
Prospective data collection usually provides better study quality than retrospective data collection. Seven of the 15 included studies had a prospective study design [1,4,15,19,21,23,24] (Table 1).
Because of heterogeneity and lack of robustness across the studies, there were considerable risks for bias if performing meta-analyses of the prevalence of transfers (Additional file 3). Therefore, we decided to descriptively present the findings.
All transfers
The total proportion of women transferred to hospital during labour or after birth, varied from 9.9% to 31.9% across the studies (Table 2).
Table 2.
Outcome events and prevalence of transfers from home to hospital in planned home births
| Outcome events, (n/N) | Prevalence | (95% CI) | |
|---|---|---|---|
|
All transfers
1,2
|
|
|
|
| Amelink-Verburg et al.[24] |
53809/168618 |
31.9 |
(31.7-32.1) |
| Anderson et al.[22] |
1093/11081 |
9.9 |
(9.3-10.4) |
| BECG3[1] |
3530/16840 |
21.0 |
(20.3-21.6) |
| Blix et al.[25] |
197/1631 |
12.1 |
(10.5-13.8) |
| Davies et al.[23] |
39/177 |
22.0 |
(16.2-28.9) |
| Hansen et al.[27] |
29/102 |
28.4 |
(19.9-38.2) |
| Howe [17] |
34/165 |
20.6 |
(14.7-27.6) |
| Hutton et al.[18] |
954/6692 |
14.3 |
(13.4-15.1) |
| Janssen et al.[19] |
165/797 |
20.7 |
(17.9-23.7) |
| Johnson and Daviss [4] |
655/5418 |
12.1 |
(11.2-13.0) |
| Lindgren et al.[26] |
128/1025 |
12.5 |
(10.5-14.7) |
| McMurtrie et al.[15] |
10/70 |
14.3 |
(7.1-24.7) |
| Murphy et al.[21] |
126/1221 |
10.3 |
(8.7-12.2) |
| Parratt et al.[16] |
64/419 |
15.3 |
(12.0-19.1) |
| Tyson [20] |
165/1001 |
16.5 |
(14.2-18.9) |
|
Transfers during labour
2
|
|
||
| Amelink-Verburg et al.[24] |
40636/168618 |
24.1 |
(23.9-24.3) |
| Anderson et al.[22] |
905/11081 |
8.2 |
(7.7-8.7) |
| BECG3[1] |
2387/16840 |
14.2 |
(13.7-14.7) |
| Blix et al.[25] |
156/1631 |
9.6 |
(8.2.11.1) |
| Davies et al.[23] |
35/177 |
19.8 |
(14.2-26.4) |
| Howe [17] |
23/165 |
13.9 |
(9.0-20.2) |
| Hutton et al.[18] |
835/6692 |
12.5 |
(11.7-13.3) |
| Janssen et al.[19] |
142/797 |
17.8 |
(15.2-20.7 |
| Johnson and Daviss [4] |
546/5418 |
10.1 |
(9.3-10.9) |
| Lindgren et al.[26] |
109/1025 |
10.6 |
(8.8-12.7) |
| McMurtrie et al.[15] |
7/70 |
10.0 |
(4.1-19.5) |
| Murphy et al.[21] |
102/1221 |
8.4 |
(6.9-10.0) |
| Parratt et al.[16] |
51/419 |
12.2 |
(9.2-15.7) |
| Tyson [20] |
141/1001 |
14.1 |
(12.0-16.4) |
|
Transfers after birth
2
|
|
||
| Amelink-Verburg et al.[24] |
3204/168618 |
1.9 |
(1.8-2.0) |
| Anderson et al.[22] |
188/11081 |
1.7 |
(1.5-2.0) |
| BECG3[1] |
1046/16040 |
6.2 |
(5.9-6.6) |
| Blix et al.[25] |
41/1631 |
2.5 |
(1.8-3.4) |
| Davies et al.[23] |
4/177 |
2.3 |
(0.6-5.7) |
| Howe [17] |
12/165 |
7.3 |
(3.8-12.4) |
| Hutton et al.[18] |
119/6692 |
1.8 |
(1.5-2.1) |
| Janssen et al.[19] |
23/797 |
2.9 |
(1.8-4.3) |
| Johnson and Daviss [4] |
37/5418 |
0.7 |
(0.5-0.9) |
| Lindgren et al.[26] |
19/1025 |
1.9 |
(1.1-2.9) |
| McMurtrie et al.[15] |
3/70 |
4.3 |
(0.9-12.0) |
| Murphy et al.[21] |
24/1221 |
2.0 |
(1.3-2.9) |
| Parratt et al.[16] |
13/419 |
3.1 |
(1.7-5.2) |
| Tyson [20] |
24/1001 |
2.4 |
(1.5-3.5) |
|
Emergency transfers |
|
|
|
| Amelink-Verburg et al.[24] |
5735/168618 |
3.4 |
(3.3-3.5) |
| Anderson et al.[22] |
202/11081 |
1.8 |
(1.6-2.1) |
| Blix et al.[25] |
16/1631 |
1.0 |
(0.6-1.6) |
| Davies et al.[23] |
0/177 |
0.0 |
(0.0-2.1) |
| Hansen et al.[27] |
1/102 |
1.0 |
(0.0-5.3) |
| Hutton et al.[18] |
361/6692 |
5.4 |
(4.9-6.0) |
| Janssen et al.[19] |
27/797 |
3.4 |
(2.2-4.9) |
| Johnson and Daviss [4] |
185/5418 |
3.4 |
(2.9-3.9) |
|
Transfers for slow progress in labour
2
| |||
| Anderson et al.[22] |
612/11081 |
5.5 |
(5.1-6.0) |
| Blix et al.[25] |
108/1631 |
6.6 |
(5.5-7.9) |
| Anderson et al.[22] |
13/177 |
7.3 |
(4.0-12.2) |
| Howe [17] |
13/165 |
7.9 |
(4.3-13.1) |
| Janssen et al.[19] |
56/797 |
7.0 |
(5.4-9.0) |
| Johnson and Daviss [4] |
326/5418 |
6.0 |
(5.4-6.7) |
| Lindgren et al.[26] |
66/1025 |
6.4 |
(5.0-8.1) |
| McMurtrie et al.[15] |
6/70 |
8.6 |
(3.2-17.7) |
| Murphy et al.[21] |
63/1221 |
5.2 |
(4.0-6.7) |
| Parratt et al.[16] |
26/419 |
6.2 |
(4.1-9.0) |
| Tyson [20] |
98/1001 |
9.8 |
(8.0-11.8) |
|
Transfers for fetal distress
2
|
|
||
| Anderson et al.[22] |
170/11081 |
1.5 |
(1.3-1.8) |
| Davies et al.[23] |
2/177 |
1.1 |
(0.1-4.0) |
| Howe [17] |
2/165 |
1.2 |
(0.1-4.3) |
| Janssen et al.[19] |
29/797 |
3.6 |
(2.5-5.2) |
| Johnson and Daviss [4] |
119/5418 |
2.2 |
(1.8-2.6) |
| Lindgren et al.[26] |
11/1025 |
1.1 |
(0.5-1.9) |
| McMurtrie et al.[15] |
1/70 |
1.4 |
(0.0-7.7) |
| Murphy et al.[21] |
13/1221 |
1.1 |
(0.6-1.8) |
| Tyson [20] |
24/1001 |
2.4 |
(1.5-3.5) |
|
Transfers for PPH
2
|
|
||
| Anderson et al.[22] |
44/11081 |
0.4 |
(0.3-0.5) |
| Davies et al.[23] |
0/177 |
0.0 |
(0.0-0.2) |
| Howe [17] |
1/165 |
0.6 |
(0.0-3.3) |
| Janssen et al.[19] |
4/797 |
0.5 |
(0.1-1.3) |
| Johnson and Daviss [4] |
34/5418 |
0.6 |
(0.4-0.9) |
| Lindgren et al.[26] |
9/1025 |
0.9 |
(0.4-1.7) |
| McMurtrie et al.[15] |
1/70 |
1.4 |
(0.0-7.7) |
| Murphy et al.[21] |
3/1221 |
0.2 |
(0.0-0.7) |
| Parratt et al.[16] |
6/419 |
1.4 |
(0.5-3.1) |
| Tyson [20] |
7/1001 |
0.7 |
(0.3-1.4) |
|
Transfers for respiratory problems
2
| |||
| Anderson et al.[22] |
62/11081 |
0.6 |
(0.4-0.7) |
| Howe [17] |
1/165 |
0.6 |
(0.0-3.3) |
| Janssen et al.[19] |
7/797 |
0.9 |
(0.4-1.8) |
| Johnson and Daviss [4] |
33/5418 |
0.6 |
(0.4-0.9) |
| McMurtrie et al.[15] |
1/70 |
1.4 |
(0.0-7.8) |
| Murphy et al.[21] |
7/1221 |
0.6 |
(0.2-1.2) |
| Parratt et al.[16] |
2/419 |
0.5 |
(0.1-1.7) |
| Tyson [20] |
3/1001 |
0.3 |
(0.1-0.8) |
|
All transfers in settings where home births are an integrated and regulated part of the national or regional health care system
1,2
| |||
| Amelink-Verburg et al.[24] |
53809/168618 |
31.9 |
(31.7-32.1) |
| BECG3[1] |
3530/16840 |
21.0 |
(20.3-21.6) |
| Davies et al.[23] |
39/177 |
22.0 |
(16.2-28.9) |
| Hansen et al.[27] |
29/102 |
28.4 |
(19.9-38.2) |
| Janssen et al.[19] |
165/797 |
20.7 |
(17.9-23.7) |
| McMurtrie et al.[15] |
10/70 |
14.3 |
(7.1-24.7) |
|
All transfers in settings where the births were booked with independent midwives
1,2
| |||
| Anderson et al.[22] |
1093/11081 |
9.9 |
(9.3-10.4) |
| Blix et al.[25] |
197/1631 |
12.1 |
(10.5-13.8) |
| Howe [17] |
34/165 |
20.6 |
(14.7-27.6) |
| Hutton et al.[18] |
954/6692 |
14.3 |
(13.4-15.1) |
| Johnson and Daviss [4] |
655/5418 |
12.1 |
(11.2-13.0) |
| Lindgren et al.[26] |
128/1025 |
12.5 |
(10.5-14.7) |
| Murphy et al.[21] |
126/1221 |
10.3 |
(8.7-12.2) |
| Parratt et al.[16] |
64/419 |
15.3 |
(12.0-19.1) |
| Tyson [20] |
165/1001 |
16.5 |
(14.2-18.9) |
|
Nulliparas, all transfers
1
|
|
||
| BECG3[1] |
2057/4568 |
45.4 |
(44.0-46.9) |
| Blix et al.[25] |
117/369 |
31.7 |
(27.0-36.7) |
| Howe [17] |
14/52 |
26.9 |
(15.6-41.0) |
| Hutton et al.[18] |
704/2293 |
30.7 |
(28.8-32.6) |
| Lindgren et al.[26] |
57/244 |
23.4 |
(18.2-29.2) |
| Tyson [20] |
116/360 |
32.2 |
(27.4-37.3) |
|
Nulliparas, transfers during labour | |||
| BECG3[1] |
1605/4568 |
35.1 |
(33.8-36.5) |
| Blix et al.[25] |
100/369 |
27.1 |
(22.6-31.9) |
| Davies et al.[23] |
9/16 |
56.3 |
(29.9-80.2) |
| Hutton et al.[18] |
638/2293 |
27.8 |
(26.0-29.7) |
| Lindgren et al.[26] |
53/244 |
21.7 |
(16.7-27.4) |
| Murphy et al.[21] |
73/269 |
27.1 |
(21.9-32.9) |
| Tyson [20] |
102/360 |
28.3 |
(23,7-33.3) |
|
Nulliparas, transfers after birth | |||
| BECG3[1] |
407/4568 |
8.9 |
(8.1-9.8) |
| Blix et al.[25] |
17/369 |
4.6 |
(2.7-7.2) |
| Lindgren et al.[26] |
4/244 |
1.6 |
(0.4-4.1) |
| Tyson [20] |
14/360 |
3.9 |
(2.1-6.4) |
|
Multiparas, all transfers
1
|
|
||
| BECG3[1] |
1472/12272 |
12.0 |
(11.4-12.6) |
| Blix et al.[25] |
80/1262 |
6.3 |
(5.1-7.8) |
| Howe [17] |
12/113 |
10.6 |
(5.6-17.8) |
| Hutton et al.[18] |
250/4339 |
5.8 |
(5.1-6.5) |
| Lindgren et al.[26] |
71/781 |
9.1 |
(7.2-11.3) |
| Tyson [20] |
49/641 |
7.6 |
(5.7-10.0) |
|
Multiparas, transfers during labour | |||
| BECG3[1] |
782/12272 |
6.4 |
(5.9-6.8) |
| Blix et al.[25] |
56/1262 |
4.4 |
(3.4-5.7) |
| Davies et al.[23] |
26/161 |
16.1 |
(10.8-22.8) |
| Hutton et al.[18] |
197/4339 |
4.5 |
(3.9-5.2) |
| Lindgren et al.[26] |
56/781 |
7.2 |
(5.5-9.2) |
| Murphy et al.[21] |
54/952 |
5.7 |
(4.3-7.3) |
| Tyson [20] |
39/641 |
|
|
|
Multiparas, transfers after birth | |||
| BECG3[1] |
639/12272 |
5.2 |
(4.8-5.6) |
| Blix et al.[25] |
24/1262 |
1.9 |
(1.2-2.8) |
| Lindgren et al.[26] |
15/781 |
1.9 |
(1.1-3.1) |
| Tyson [20] | 10/641 | 1.6 | (0.8-2.9) |
1“All transfers” refers to total transfers during labour and after birth.
2In both nulli- and multiparous women.
3BECG = Birthplace in England Collaborative Group.
In nulliparous women, the proportion of all transfers varied from 23.4% to 45.4%, and in multiparous women it ranged from 5.8% to 12.0%. There was a higher rate of transfer in studies from settings where home births were an integrated and regulated part of the national or regional health care system [1,15,19,23,24,27] than in settings with independent midwives [4,16-18,20-22,25,28] (Table 2).
Transfer during labour
Most transfers to hospital occurred during labour and before the birth of the neonate. Across the 15 included studies, 8.2% to 24.1% were transferred. Seven studies that performed analyses stratified for nulli-and multiparity reported that 22.5% to 56.3% of all nulliparous women were transferred. In multiparous women, these proportions ranged from 4.4% to 16.1%.
Slow progress in labour was the most frequent indication for transfer in nulli- and multiparous women, occurring in 5.2% to 9.8% of all planned home births. Transfers because of foetal distress ranged from 1.0% to 3.6% (Table 2).
Transfer after birth
Between 1.7% and 7.3% of women and neonates were transferred to hospital after birth. Four studies provided analyses stratified for parity; between 1.6% and 8.9% of nulliparous women and between 1.6% and 5.5% of the multiparous women were transferred after birth. Nine of the 15 included studies described the time span for transfers after birth, and this time varied from 2 hours to 5 days.
Between 0% and 0.2% of the women were transferred because of postpartum haemorrhage, and between 0.3% and 1.4% of neonates were transferred because of respiratory problems (Table 2).
Emergency transfers
Eight of the included studies reported the proportion of emergency transfers, and it varied from 0% to 5.4% (Table 2).
The definitions of an emergency transfer varied across the studies. Some studies gave an overall definition, while others listed the indications defined as emergencies (Table 3).
Table 3.
Definitions of “emergency transfer” across the studies
| Study | Study definitions of emergency transfers |
|---|---|
| Amelink-Verburg et al.[24] |
“…a referral for a complication that cannot be treated at the primary care level and that requires immediate diagnostics or treatment at the secondary care level” (Mother: Fetal distress, placental problems, abnormal presentation together with ruptured membranes, postpartum haemorrhage > 1000 ml, intrapartum fetal death. Neonate: early postnatal Apgar score >7 at 5 minutes, respiratory problems including meconium aspiration, congenital malformations with need of immediate care). |
| Anderson et al.[22] |
Failure to progress, fetal distress, meconium in liquor, nonvertex presentations, postpartum haemorrhage, neonatal asphyxia, serious anomalies. |
| Blix et al.[25] |
That the condition of the mother, fetus or infant demanded medical assistance as soon as possible. |
| Davies et al.[23] |
Need for obstetric intervention within one hour after transfer. |
| Janssen et al.[19] |
Fetal distress, meconium in liquor, breech presentation, active herpes, midwife not available, obstructed labour, retained placenta, repair episiotomy, postpartum haemorrhage, asphyxia, neonatal respiratory distress, distended abdomen in infant. |
| Johnson and Daviss [4] |
Based on primary reason for transport. |
| Hansen et al.[27] |
Poor fetal heart rate. |
| Hutton et al.[18] | Transported from home to hospital by ambulance during labour or immediately after delivery. |
Discussion
In the present review, we found that the proportion of transfer from home to hospital during and after planned home births varied from 9.9% to 31.9% across the study populations. In nulliparous, this proportion varied from 23.4% to 45.4%, and in multiparous, it ranged from 5.8% to 12.0%.
The proportion of transfer from home to hospital was higher in studies from settings where home births were an integrated part of the health care system compared with home births assisted by independent midwives. The study populations from regulated settings probably had slightly more nulliparous women included than in studies where independent midwives assisted births (Table 1). However, this was difficult to assess because not all of the studies reported parity. The proportion of nulliparous women in a study population affects transfer rates because nulliparous women are transferred more often than multiparous women. Another reason for the difference in transfer rates could be that in regulated settings, there are more strict guidelines for transfers and less room for individual assessments than in settings with independent midwives. In addition, in settings where home births are not part of the system, women might receive less information regarding home births. Those who choose home birth are probably a selected and motivated group, and less likely to be transferred. Assessment of what transfer rates should be to provide the best outcomes is difficult. Rates of transfer are not necessarily indicators of quality of care or a potential for adverse outcomes. High rates of transfer may be due to weather or traffic conditions, with the need for anticipatory planning. However, a low transfer rate may lead to cases of death and serious morbidity that could have been avoided. A high transfer rate may lead to unnecessary interventions and less patient satisfaction.
Whether there are different outcomes of home births in settings where home births are an integrated part of the health care system compared with home births assisted by independent midwives is unknown. A study from the UK compared outcomes of 1462 women assisted by independent midwives and 8676 women assisted by National Health Service midwives in all settings (obstetric units, midwifery-led units, and home births) [29]. Only 0.4% of the women assisted by National Health Service midwives gave birth at home, while 66.0% of the women assisted by independent midwives did so. These analyses did not adjust for place of birth, and the study design did not allow for conclusions in home births per se. This previous study found that although many outcomes were significantly better in women assisted by independent midwives compared with those assisted by National Health Service midwives, the perinatal mortality rate was higher among high-risk cases. When excluding high-risk cases from the analyses, there was no significant difference in the perinatal mortality rate between the two cohorts. The reasons for accepting high-risk cases in home birth settings should be further explored. This raises the issue of whether independent midwives are more willing to accept such women, or whether the women themselves are exerting pressure on midwives to accept them for home birth.
Our review showed that there was less variability across the included studies, and also less heterogeneity, when analysing transfers for specific indications, such as slow progress in labour, foetal distress, postpartum haemorrhage, and neonatal respiratory problems. One Canadian study reported a higher proportion of transfers because of foetal distress [19,20]. We could not find any methodological reasons why this study had a higher prevalence than the other studies.
Emergency transfers were differently defined across the studies. In one study, slow progress was one of the definitions for an emergency transfer [22]. However, this is usually not regarded as an emergency situation. In the study with the highest proportion of emergency transfers, the definition of an emergency transfer was if the mother or neonate was transported to hospital by ambulance [18]. To compare results across studies, having a standard definition of emergency transfers in planned home births would be useful. We considered that the definition of emergency transfer from the study in the Netherlands [24] was the best and most detailed (Table 3).
Women and neonates who experience emergency transfers during labour and immediately after the birth are probably a vulnerable group with higher risks for adverse outcomes. The studies in our review reported outcomes according to the principle of intention-to-treat, and provided no detailed description on outcomes in women and neonates after an emergency transfer. Mori et al. found that women who had planned for a home birth in England and Wales between 1994 and 2003, but were transferred to hospital, had the highest risk for intrapartum-related perinatal mortality. The authors emphasised that the results should be interpreted with caution because of inconsistencies in the recorded data [30]. A critical appraisal found weaknesses in the study design and that estimates of risk were inaccurate [31]. Evers et al. found an increased risk for perinatal death in women referred from midwifery care to obstetric care during labour in Utrecht in the Netherlands [32]. The results and conclusions of the study by Evers et al. [32] have also been discussed and questioned [33,34]. De Jonge et al. found that low-risk women with planned home births had a lower rate of severe maternal outcomes than those with planned hospital births [35]. Severe adverse outcomes were defined as postpartum haemorrhage >1000 ml, manual removal of the placenta and severe acute maternal morbidity (admission to an intensive care unit, eclampsia, blood transfusion of four or more packed cells, and other serious events). Among planned home births, severe acute maternal morbidity was 1.5/1000, postpartum haemorrhage occurred in 29.2/1000, and manual removal of the placenta occurred in 16.8/1000.
Performing audits to evaluate adverse outcomes during or after transfer to hospital would probably be useful. Audits may lead to improvements in health services (eg., better information between the home birth midwife and hospital, preventing delay in decisions, and transport plans).
Our study has a limitation. Four of the 15 included studies did not describe any indications for the transfers [1,18,24,27]. These four studies represented 89% of women included in the 15 studies.
Conclusions
Future studies should report indications for transfer in planned home births, and also describe proportions and indications for emergency transfers. Analyses should be stratified for parity. Future studies also need to examine the difference in transfer rates in different settings.
Abbreviations
n/N: Proportion; P0: Nulliparous; BECG: Birthplace in England collaborative group.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EB and HEL initiated and designed the study. EB performed the literature searches and all of the analyses. EB, MK, and HEL extracted data and assessed the literature. All of the authors participated in interpretation of results and participated in the writing process. EB revised the manuscript together with HEL, PØ, and MK. HK died in December 2013. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Data extraction form.
Studies excluded after assessment in full text.
Sensitivity analyses.
Contributor Information
Ellen Blix, Email: ellen.blix@unn.no.
Merethe Kumle, Email: merethe.kumle@unn.no.
Pål Øian, Email: pal.oian@unn.no.
Helena E Lindgren, Email: helena.lindgren@gu.se.
Acknowledgements
The authors did not receive any particular funding for conducting the present study.
References
- Birthplace in England Collaborative Group. Perinatal and maternal outcomes by planned place of birth for healthy women with low risk pregnancies: the birthplace in England national prospective cohort study. BMJ. 2011;343:d7400. doi: 10.1136/bmj.d7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PA, Saxell L, Page LA, Klein MC, Liston RM, Lee SK. Outcomes of planned home birth with registered midwife versus planned hospital birth with midwife or physician. CMAJ. 2009;181:377–383. doi: 10.1503/cmaj.081869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren HE, Rådestad IJ, Christensson K, Hildingsson IM. Outcome of planned home births compared to hospital births in Sweden between 1992 and 2004: a population-based register study. Acta Obstet Gynecol Scand. 2008;87:1–9. doi: 10.1080/00016340802199903. [DOI] [PubMed] [Google Scholar]
- Johnson KC, Daviss BA. Outcomes of planned home births with certified professional midwives: large prospective study in North America. BMJ. 2005;330:1416. doi: 10.1136/bmj.330.7505.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE. Intrapartum care: care of healthy women and their babies during childbirth. London: National Institute for Health and Clinical Excellence; 2007. [PubMed] [Google Scholar]
- Helsedirektoratet. Nasjonal retningslinje for hjemmefødsel. Oslo: Helsedirektoratet; 2012. [Google Scholar]
- Sundhedsstyrelsen. Anbefalinger for svangreomsorgen. København: Sundhedsstyrelsen; 2009. [Google Scholar]
- College voor Zorgverzekeringen. Commissie Verloskunde van het CVZ. Verloskundig Vademecum 2003. Diemen, The Netherlands: College voor Zorgverzekeringen; 2003. [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting: meta-analysis af observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasjonalt kunnskapssenter for helsetjenesten. Slik oppsummerer vi forskning. Oslo: Nasjonalt kunnskapssenter for helsetjenesten; 2009. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnier JJ, Moher D, Boon H, Beyene J, Bombardier C. Investigating clinical heterogeneity in systematic reviews: a methodologic review of guidance in the literature. BMC Med Res Methodol. 2012;12:111. doi: 10.1186/1471-2288-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD, Altman GD. Systematic reviews in health care. London: BMJ Books; 2001. [Google Scholar]
- McMurtrie J, Catling-Paull C, Teate A, Caplice S, Chapman M, Homer C. The St. George homebirth program: an evaluation of the first 100 booked women. Aust N Z J Obstet Gynaecol. 2009;49:631–636. doi: 10.1111/j.1479-828X.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Parratt J, Johnston J. Planned homebirths in Victoria, 1995–1998. Aust J Midwifery. 2002;15:16–25. doi: 10.1016/S1445-4386(02)90000-5. [DOI] [PubMed] [Google Scholar]
- Howe KA. Home births in South-West Australia. Med J Aust. 1988;149:296–7. doi: 10.5694/j.1326-5377.1988.tb120628.x. 300, 302. [DOI] [PubMed] [Google Scholar]
- Hutton EK, Reitsma AH, Kaufman K. Outcomes associated with planned home and planned hospital births in low-risk women attended by midwives in Ontario, Canada, 2003–2006: a retrospective cohort study. Birth. 2009;36:180–189. doi: 10.1111/j.1523-536X.2009.00322.x. [DOI] [PubMed] [Google Scholar]
- Janssen PA, Lee SK, Ryan ER, Saxell L. An evaluation of process and protocols for planned home birth attended by regulated midwives in British Columbia. J Midwifery Womens Health. 2003;48:138–145. doi: 10.1016/S1526-9523(02)00418-X. [DOI] [PubMed] [Google Scholar]
- Tyson H. Outcomes of 1001 midwife-attended home births in Toronto, 1983–1988. Birth. 1991;18:14–19. doi: 10.1111/j.1523-536X.1991.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Murphy PA, Fullerton J. Outcomes of intended home births in nurse-midwifery practice: a prospective descriptive study. Obstet Gynecol. 1998;92:461–470. doi: 10.1016/S0029-7844(98)00182-3. [DOI] [PubMed] [Google Scholar]
- Anderson RE, Murphy PA. Outcomes of 11,788 planned home births attended by certified nurse-midwives: a retrospective descriptive study. J Nurse Midwifery. 1995;40:483–492. doi: 10.1016/0091-2182(95)00051-8. [DOI] [PubMed] [Google Scholar]
- Davies J, Hey E, Reid W, Young G. Prospective regional study of planned home births: home birth study steering group. BMJ. 1996;313:1302–1306. doi: 10.1136/bmj.313.7068.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amelink-Verburg MP, Verloove-Vanhorick SP, Hakkenberg RM, Veldhuijzen IM, Bennebroek GJ, Buitendijk SE. Evaluation of 280,000 cases in Dutch midwifery practices: a descriptive study. BJOG. 2008;115:570–578. doi: 10.1111/j.1471-0528.2007.01580.x. [DOI] [PubMed] [Google Scholar]
- Blix E, Huitfeldt AS, Oian P, Straume B, Kumle M. Outcomes of planned home births and planned hospital births in low-risk women in Norway between 1990 and 2007: a retrospective cohort study. Sex Reprod Healthc. 2012;3:147–153. doi: 10.1016/j.srhc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Lindgren HE, Hildingsson IM, Christensson K, Radestad IJ. Transfers in planned home births related to midwife availability and continuity: a nationwide population-based study. Birth. 2008;35:9–15. doi: 10.1111/j.1523-536X.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- Hansen JH, Christoffersen C. Hjemmefødsler i Københavns kommune 1980–1982: I. Obstetriske data. Ugeskr Laeger. 1985;147:2783–2785. [PubMed] [Google Scholar]
- Lindgren HE, Rådestad IJ, Hildingsson IM. Transfer in planned home births in Sweden–effects on the experience of birth: a nationwide population-based study. Sex Reprod Healthc. 2011;2:101–105. doi: 10.1016/j.srhc.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Symon A, Winter C, Inkster M, Donnan PT. Outcomes for births booked under an independent midwife and births in NHS maternity units: matched comparison study. BMJ. 2009;338:b2060. doi: 10.1136/bmj.b2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori R, Dougherty M, Whittle M. An estimation of intrapartum-related perinatal mortality rates for booked home births in England and Wales between 1994 and 2003. BJOG. 2008;115:554–559. doi: 10.1111/j.1471-0528.2008.01669.x. [DOI] [PubMed] [Google Scholar]
- Gyte G, Dodwell M, Newburn M, Sandall J, Macfarlane A, Bewley S. Estimating intrapartum-related perinatal mortality rates for booked home births: when the ‘best’ available data are not good enough. BJOG. 2009;116:933–942. doi: 10.1111/j.1471-0528.2009.02147.x. [DOI] [PubMed] [Google Scholar]
- Evers AC, Brouwers HA, Hukkelhoven CW, Nikkels PG, Boon J, Egmond-Linden A, Hillegersberg J, Snuif YS, Sterken-Hooisma S, Bruinse HW, Kwee A. Perinatal mortality and severe morbidity in low and high risk term pregnancies in the Netherlands: prospective cohort study. BMJ. 2010;341:c5639. doi: 10.1136/bmj.c5639. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke JP. Dutch perinatal mortality: study did a good job. BMJ. 2010;341:c7042. doi: 10.1136/bmj.c7042. [DOI] [PubMed] [Google Scholar]
- Meijer E. Study did a good job, however. BMJ. 2011. Available at: http://www.bmj.com/rapid-response/2011/11/03/study-did-good-job-however.
- de Jonge A, Mesman JA, Mannien J, Zwart JJ, van DJ, van RJ. Severe adverse maternal outcomes among low risk women with planned home versus hospital births in the Netherlands: nationwide cohort study. BMJ. 2013;346:f3263. doi: 10.1136/bmj.f3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data extraction form.
Studies excluded after assessment in full text.
Sensitivity analyses.


