Abstract
The diagnosis of bilateral vestibulopathy (BV) is typically established based on bilateral semicircular canal dysfunction. The degree to which both otolith organs—the saccule and utricle—are also impaired in BV is not wellestablished, particularly with respect to the etiology and severity of BV. The aim of this study was to evaluate semicircular canal, saccular and utricular function in patients with BV due to aminoglycoside ototoxicity and bilateral Menière’s disease, and with different severities of BV. Caloric and head impulse testing were used as measures of canal function. Cervical vestibular-evoked myogenic potentials (cVEMP) and ocular VEMPs (oVEMP) were used as measures of saccular and utricular function, respectively. We enrolled 34 patients with BV and 55 controls in a prospective case–control study. Patients with BV were less likely to have saccular (61 %) or utricular (64 %) dysfunction relative to canal dysfunction (100 %). Utricular function differed significantly between patients by etiologic group: the poorest function was found in patients with BV due to aminoglycoside toxicity, and the best function in Menière’s disease patients. Canal and saccular function did not vary according to etiology. Further, utricular but not saccular function was significantly correlated with canal function. Saccular and utricular function had the strongest association with Dizziness Handicap Inventory scores relative to canal function. These data suggest that when a patient with BV is identified in a clinical context, oVEMP testing is the most sensitive test in distinguishing between aminoglycoside toxicity and bilateral Menière’s disease. Both cVEMP and oVEMP testing may be considered to evaluate the functional impact on the patient.
Keywords: Bilateral vestibulopathy, Vemp, Otolith, Semicircular canal, Meniere’s disease
Introduction
Bilateral vestibulopathy (BV) is characterized by impairment or loss of function of the peripheral vestibular system bilaterally. The disorder typically manifests with chronic disequilibrium, gait instability and oscillopsia [1, 2]. BV appears to be the final common manifestation of many different pathophysiologic processes, including aminoglycoside antibiotic (e.g. gentamicin) toxicity, exposure to other ototoxins (e.g. diuretics, chemotherapy), bilateral Menière’s disease, infection (e.g. meningitis), aging (presbystasis) and neuro-degeneration. It is usually diagnosed based on reduced or absent caloric responses bilaterally, impaired rotational testing bilaterally, and/or abnormal head impulse testing (HIT) bilaterally [3-5]. Pathologic caloric and rotational testing indicate a low-frequency deficit of the horizontal semicircular canals. Abnormal HIT indicates a high-frequency semicircular canal deficit (of the horizontal, anterior and/or posterior canals depending on the plane of the head rotation stimulus). In addition to the semicircular canals the otolith organs, i.e. the saccule and the utricle, can also be affected in BV [6, 7]. The degree to which the semicircular canals and the saccule and utricle may be differentially affected in BV depending on the degree and etiology of BV is however not known.
Therefore, in this study, the semicircular canal, saccular and utricular deficits associated with BV were examined in patients with BV due to aminoglycoside ototoxicity and bilateral Menière’s disease, and with different severities of BV. We used caloric and HIT as measures of the low- and high-frequency gain of the vestibulo-ocular reflex (VOR) and thereby semicircular canal function [8]. The sound-evoked cervical vestibular-evoked myogenic potential (cVEMP) was applied as a measure of saccular function [9], and the vibration-evoked ocular vestibular-evoked myogenic potential (oVEMP) was used as a measure of utricular function [10]. Thereby, the sensitivity of the different parts of the vestibular end-organs to damage by ototoxic drugs and due to Menière’s disease could be further elucidated. From a clinical standpoint the findings can also have implications for vestibular rehabilitation, i.e. use of exercises for impaired semicircular and/or otolith function depending on the type of dysfunction.
Methods
Subjects
Study subjects were recruited from an outpatient neurology clinic in a tertiary care academic medical center (German Dizziness Center in Munich). Eligible subjects had a diagnosis of BV, which was made based on the following criteria: (1) bilaterally diminished [defined as a mean peak slow phase velocity (SPV) of <5°/s on both sides], or absent caloric responses on electronystagmography, and/or (2) bilaterally pathologic clinical HIT, consistent with prior work [2, 8, 11]. The etiology for the BV was ascertained by querying subjects about a history of exposure to ototoxins (e.g. antibiotics, diuretics, chemotherapeutics), history of otologic symptoms (e.g. vertigo, hearing loss, tinnitus and aural fullness) and otologic disorders (e.g. Menière’s disease based on a history of episodic vertigo, fluctuating hearing loss, tinnitus and aural fullness), and history of other neurologic, auto-immune and neoplastic conditions. All subjects underwent complete neurologic, neuro-ophthalmologic, and neuro-otologic examination, including the HIT, and evaluation for spontaneous nystagmus with Frenzel’s lenses, gaze-evoked nystagmus, smooth pursuit, saccades, optokinetic nystagmus, visual fixation suppression of the VOR, rebound nystagmus and head-shaking nystagmus [12]. Subjects were also administered the Dizziness Handicap Inventory (DHI), and overall as well as subscale (physical, functional and emotional) scores were computed [13]. This study was approved by the Ludwig-Maximilians University institutional review board and all participants gave their informed consent prior to their inclusion in the study.
Vestibular physiologic testing
All patients underwent comprehensive vestibular physiologic testing, including caloric testing, and cervical and ocular VEMP testing.
Caloric testing
Bithermal caloric testing (30 and 44 °C) was performed in all patients on the left and right sides to determine peak SPV using Igor Pro Wave Metric Software (version 3.13).
Cervical VEMP testing
Participants were positioned supine with their upper bodies elevated at a 30° angle from horizontal. The neck was actively flexed by the participant during cVEMP stimulation and recording to provide tonic background muscle activity. Air-conducted 500-Hz, 125-dB SPL tone bursts were delivered monaurally via intra-auricular speakers. An air-conducted stimulus was used given electrophysiological evidence in animal studies that saccular afferents are responsive to air-conducted sounds [14, 15], and given that air conduction sound-evoked cVEMPs are thought to represent the best-characterized measure of saccular responses [16]. Cervical VEMPs were recorded from an electrode montage consisting of a non-inverting electrode placed at the midpoint of the ipsilateral sternocleidomastoid muscle belly, an inverting electrode placed on the manubrium sterni, and a ground electrode placed on the forehead. We found previously that prestimulus EMG activity did not differ between controls and BV patients [6]. EMG activity was recorded (Nicolet Biomedical Inc, Madison WI, USA), amplified and bandpass filtered, and the responses to 50–100 stimuli were averaged. The first positive and negative peaks that occurred between 13 and 23 ms after stimulus onset were designated p13 and n23, respectively. The peak-to-peak amplitude, calculated as the sum of the p13 and the n23 amplitudes, was evaluated given prior data demonstrating a high inter-rater reliability of this measure [17].
Ocular VEMP testing
Participants were positioned supine with their upper bodies elevated at a 30° angle from horizontal. Maximum upgaze was maintained during oVEMP stimulation and recording. “Mini taps,” as described by Iwasaki et al. [18], were delivered with a Bruel and Kjaer Mini-Shaker Type 4810 (1-ms clicks of positive polarity, with a repetition rate of five per second) at the Fz cranial site (in the midline at the hairline, 30 % of the distance between the inion and nasion). Fz taps have been shown to provide an acceleration wave that propagates through the cranium to the mastoid on either side, predominantly causing an outward linear acceleration of the utricles bilaterally [19]. A bone conducted vibration stimulus was used based on studies in guinea pigs which showed a low threshold for stimulation of utricular afferents by bone-conducted vibration [20], and a generally accepted understanding that bone vibration-evoked oVEMPs are the best-characterized measure of utricular responses [16]. Ocular VEMPs were recorded from an electrode montage consisting of a non-inverting electrode placed over the contralateral inferior oblique muscle approximately 3 mm below the eye and centered beneath the pupil, an inverting electrode on the chin, and a ground electrode placed under the chin. The responses to 50–100 stimuli were averaged. The first negative and positive peaks of the oVEMP response that occurred between 10 and 20 ms after stimulus onset were designated n1 and p1, respectively. The oVEMP n1 amplitude was evaluated, given that this is the portion of the response that is most clearly vestibular and previous data demonstrating a high inter-rater reliability of this measure [17, 21]. Sample oVEMP traces from a control subject and a patient are presented (Fig. 1).
Fig. 1.
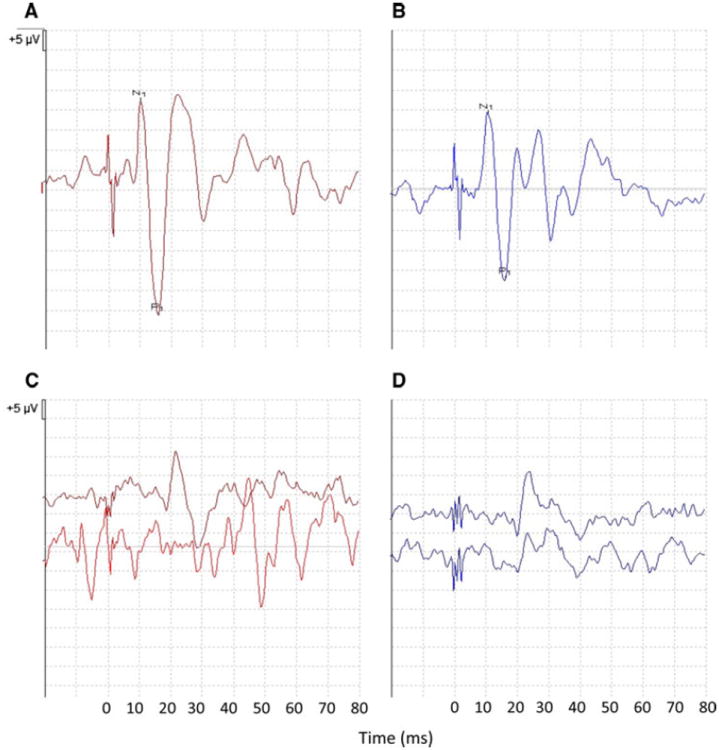
Sample right and left oVEMP traces from a control subject (a, b) and a patient with absent oVEMPs (c, d). N1 and P1 peaks are marked
Statistical analysis
Cervical and ocular VEMP testing results were compared between the study subjects with BV and normative data generated in our laboratory from a control group of 55 subjects [mean (SD) age 33.7 (15.1), range 15–72 years; 20 % male]. Left and right cervical and ocular VEMP values were averaged together, given that these were patients with BV. Among the normal controls, age was significantly correlated with lower cVEMP amplitude (r = −0.47, p = 0.0004) in Spearman rank correlation analyses; age was also correlated with lower oVEMP amplitude although this association was not statistically significant (r = −0.18, p = 0.1807). For cervical VEMP testing, we defined abnormal as a peak-to-peak amplitude below the fifth percentile from control data. For ocular VEMP testing, we defined abnormal as an n1 amplitude below the fifth percentile from control data. Given that the VEMP data were not normally distributed, the non-parametric Wilcoxon rank sum test was used for comparisons of amplitudes, and the χ2 test was used to compare proportions. SAS 9.2 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
We enrolled 34 subjects (16 males) with a mean age ± SD of 65.6 ± 14.3 years (range 24–83; Table 1). Subjects were classified into three etiologic groups: (1) aminoglycoside toxicity (N = 9), (2) bilateral Menière’s disease (N = 11), and (3) other [N = 14; cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS, N = 1), meningitis (N = 1), Sjogren’s syndrome (N = 1), temporal bone radiation exposure (N = 1), auto-immune disease (N = 1), cisplatin chemotherapy exposure (N = 1), and idiopathic (N = 8)]. Of these subjects, 29 (85 %) reported unsteadiness or oscillopsia during locomotion [22]. The mean age of subjects across etiologic groups did not differ significantly; subjects with bilateral Menière’s disease were significantly more likely to be female (Table 1). All subjects (100 %) with BV secondary to aminoglycoside toxicity had pathologic caloric and head-impulse testing. Subjects with Menière’s disease demonstrated a typical pattern of bilateral loss of low-frequency (caloric) VOR function with relative preservation of high-frequency responses. The proportion of patients with pathologic caloric responses did not differ significantly by etiologic group; however, the percentage of bilateral HIT abnormalities was significantly lower in patients with Menière’s disease (Table 1).
Table 1.
Descriptive characteristics and case definition in BV study population
| BV etiology | N | Age in years Mean (SD) | Gender Male | Bilateral pathologic HIT | Bilateral pathologic calorica |
|---|---|---|---|---|---|
| Overall | 34 | 65.6 (14.3) | 47 % | 73 % | 81 % |
| Aminoglycoside | 9 | 64.6 (15.0) | 78 % | 100 % | 100 % |
| Menière’s | 11 | 65.9 (13.6) | 18 % | 33 % | 73 % |
| Other | 14 | 65.9 (15.0) | 50 % | 89 % | 75 % |
| p valueb | 0.9714 | 0.0282 | 0.0035 | 0.2716 |
BV bilateral vestibulopathy, SD standard deviation, HIT head impulse test
Bilateral pathologic caloric defined as a mean slow phase velocity (SPV) less than 5°/s
p value computed for the difference across BV etiologic groups. ANOVA used for continuous variables and Chi-square tests used for categorical variables
We evaluated the relative prevalence of semicircular canal versus saccular versus utricular deficits in our BV study population. By definition, 100 % of patients had evidence of canal dysfunction based on abnormal caloric and/or HIT. To compute the prevalence of saccular dysfunction, we defined abnormal as below the fifth percentile peak-to-peak amplitude from control subjects (<28.58 μV). Sixty-one percent (95 % CI 42, 77) of BV patients had evidence of saccular dysfunction (Table 2), comparable to the 70 % prevalence of saccular dysfunction previously observed in a similar cohort [6]. To compute the prevalence of utricular function, we defined abnormal as below the fifth percentile n1 amplitude from control subjects (<5.15 μV). Sixty-four percent (95 % CI 45, 80) of BV patients had evidence of utricular dysfunction (Table 2); this proportion was not significantly different from the percentage with saccular dysfunction.
Table 2.
Cervical VEMP and ocular VEMP test results in control subjects versus BV patients
| Subjects | N | cVEMP Amplitude μV Median (IQR) | oVEMP Amplitude μV Median (IQR) |
|---|---|---|---|
| Controls | 55 | 104.2 (79.4) | 12.1 (8.1) |
| All BV | 34 | 18.1 (42.3) | 4.2 (5.7) |
| % Abnormal (95 %CI)a | 61 (42, 77) | 64 (45, 80) |
VEMP vestibular-evoked myogenic potential, BV bilateral vestibulopathy, SPV slow phase velocity, IQR interquartile range 25th–75th percentile
For cervical VEMP testing, abnormal was defined as a peak-to-peak amplitude below the fifth percentile from control data. For ocular VEMP testing, abnormal was defined as a n1 amplitude below the fifth percentile from control data
We next compared semicircular canal, saccular and utricular function based on etiology of BV. We observed that only ocular VEMP amplitudes differed significantly among patient groups (p = 0.0047), with the lowest median n1 amplitude observed in BV patients with aminoglycoside toxicity (1.6 μV) and the highest median n1 amplitude seen in patients with bilateral Menière’s disease (8.3 μV; Table 3). Cervical VEMP mean peak-to-peak amplitude and caloric mean peak SPV did not differ significantly by etiology of BV (p = 0.4036 and p = 0.1683, respectively; Table 3).
Table 3.
Caloric, cervical VEMP and ocular VEMP test results in BV patients by diagnosis
| BV etiology | N | Caloric SPV
|
cVEMP Amplitude
|
oVEMP Amplitude
|
|||
|---|---|---|---|---|---|---|---|
| °/s Mean (SD) | p value | μV Median (IQR) | p valuea | μV Median (IQR) | p valuea | ||
| Aminoglycoside | 9 | 4.7 (2.5) | 0.1683 | 0.0 (17.8) | 0.4036 | 1.6 (1.4) | 0.0047 |
| Menière’s | 11 | 16.6 (8.2) | 18.4 (42.2) | 8.3 (9.0) | |||
| Other | 14 | 21.2 (28.8) | 34.4 (58.9) | 3.9 (7.0) | |||
VEMP vestibular-evoked myogenic potential, BV bilateral vestibulopathy, SPV slow phase velocity, IQR interquartile range 25th–75th percentile
p value for non-parametric Wilcoxon rank sum test
We evaluated for associations between utricular function and caloric function, and saccular function and caloric function in patients with BV. We observed a significant correlation between ocular VEMP n1 amplitudes and caloric mean peak slow phase velocities (r = 0.51; p = 0.0023; Fig. 2). In contrast, we did not observe a significant correlation between cervical VEMP peak-to-peak amplitudes and caloric mean peak slow phase velocities (r = 0.22; p = 0.22; Fig. 2).
Fig. 2.
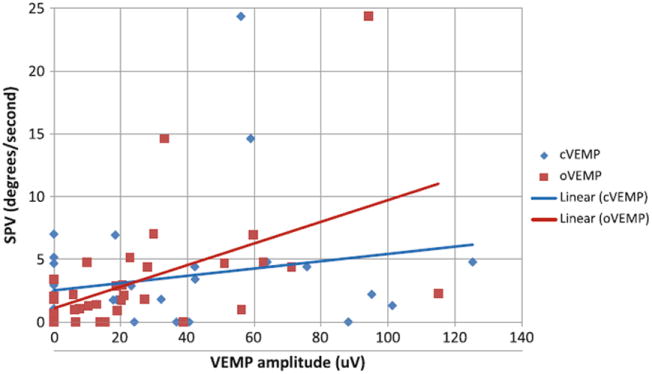
Correlation between cervical and ocular vestibularevoked myogenic potential (VEMP) and caloric function in bilateral vestibulopathy (BV) patients. The correlation between ocular VEMP n1 amplitudes and caloric mean peak slow phase velocities was statistically significant (r = 0.51; p = 0.0023), while there was no significant correlation between cervical VEMP peak-to-peak amplitudes and caloric mean peak slow phase velocities (r = 0.22; p = 0.22)
Finally, we considered overall and subscale (physical, functional and emotional) DHI scores in patients with BV. We evaluated whether DHI scores varied by etiology of BV, and the extent to which semicircular canal versus saccular versus utricular function was associated with DHI scores. DHI-Physical subscale scores were poorest (highest) among BV patients with aminoglycoside toxicity and were lowest in patients with bilateral Menière’s disease (p = 0.021; Table 4). Overall DHI scores, or functional or emotional subscale scores, did not significantly differ among BV patient groups (Table 4).
Table 4.
Dizziness Handicap Inventory (DHI) scores in BV patients by diagnosis
| BV etiology | N | DHI: overall Mean (SD) | DHI: physical Mean (SD) | DHI: functional Mean (SD) | DHI: emotional Mean (SD) |
|---|---|---|---|---|---|
| Aminoglycoside | 9 | 62.5 (12.2) | 19.3 (2.8) | 24.3 (4.5) | 19.0 (6.0) |
| Menière’s | 11 | 42.5 (30.9) | 10.2 (8.9) | 17.9 (11.2) | 14.4 (11.6) |
| Other | 14 | 56.4 (24.1) | 16.2 (6.0) | 21.6 (9.6) | 18.7 (9.7) |
| p valuea | 0.2061 | 0.0210 | 0.3407 | 0.5029 |
BV bilateral vestibulopathy, SD standard deviation
p value for ANOVA computed for the difference in DHI score across BV etiologic groups
We found significant correlations between cervical VEMP peak-to-peak amplitudes and overall DHI score as well as all three DHI subscale scores (Table 5). We also observed a significant correlation between ocular VEMP n1 amplitudes and DHI-physical subscale score (Table 5). There were no significant correlations between caloric SPV and DHI scores.
Table 5.
Correlation between Dizziness Handicap Inventory (DHI) scores and caloric, cervical VEMP and ocular VEMP testing in BV patients
| Vestibular function test | DHI: overall | DHI: physical | DHI: functional | DHI: emotional |
|---|---|---|---|---|
| Caloric SPV (°/s) | r = −0.003 | r = −0.09 | r = −0.09 | r = 0.16 |
| p = 0.9879 | p = 0.6325 | p = 0.6330 | p = 0.4229 | |
| Cervical VEMP (uV)a | r = −0.46 | r = −0.46 | r = −0.47 | r = −0.39 |
| p = 0.0138 | p = 0.0130 | p = 0.0108 | p = 0.0418 | |
| Ocular VEMP (uV)b | r = −0.26 | r = −0.41 | r = −0.24 | r = −0.13 |
| p = 0.1748 | p = 0.0288 | p = 0.2111 | p = 0.4985 |
VEMP vestibular-evoked myogenic potential, BV bilateral vestibulopathy, SPV slow phase velocity, SD standard deviation
Cervical VEMP peak-to-peak amplitudes are used in correlation analyses
Ocular VEMP n1 amplitudes are used in correlation analyses
Discussion
This study is among the first (to our knowledge) to evaluate semicircular canal, saccular and utricular function concurrently in a cohort of patients with BV. We observed in this study that patients with BV experience a global reduction in vestibular function involving both the semicircular canals and the two otolith organs—the saccule and the utricle. This finding is consistent with a prior study that demonstrated reduced cervical VEMP responses (indicative of saccular dysfunction) in BV patients, and another study that reported attenuated responses to eccentric rotation (suggestive of utricular dysfunction) in patients with bilateral semicircular canal dysfunction [23, 24]. We also found that losses of saccular and utricular function were less severe than the loss of semicircular canal function, and that the rate of saccular and utricular dysfunction was similar (61 vs. 64 %). Previous studies have also suggested a relative preservation of saccular function in BV patients [6, 25]. Moreover, a lower prevalence of both utricular and saccular function has been reported in older patients with presbystasis (a form of BV) [26]. Temporal bone studies in older individuals have also found greater reductions in vestibular hair cell counts in the semicircular canal ampullae compared to the otolithic maculae associated with aging [27]; there may be a differential susceptibility across the vestibular end-organ to the toxic and metabolic exposures associated with BV as well. It should be noted that the relative predominance of semicircular canal deficits in our cohort of patients may reflect the fact that the diagnosis of BV is typically established based on bilaterally absent canal function. It has been shown that subtypes of BV characterized by isolated bilateral otolith dysfunction also exist [7]; these patients would not have been captured in our study.
We observed that ocular VEMP testing was the only vestibular test that significantly differed based on etiology of BV, with the highest n1 amplitudes (indicative of greatest utricular function) in patients with Menière’s disease and the lowest n1 amplitudes (indicative of poorest utricular function) in patients with aminoglycoside toxicity. In general, we found that aminoglycoside exposure abolished both canal and otolith function to the greatest extent relative to Menière’s disease and other BV etiologies, although this difference was only statistically significant for utricular function. Aminoglycoside antibiotics, such as gentamicin, have been shown to preferentially target type I vestibular hair cells [28], which are responsive to high-frequency stimulations such as the air-conducted sound and bone vibration associated with cervical and ocular VEMPs respectively [29]. As such, BV secondary to aminoglycoside toxicity may manifest with reduced VEMP responses. Bilateral Menière’s disease appears to be associated with a lesser degree of vestibular loss globally compared to aminoglycoside toxicity. However, given that Menière’s disease typically presents with reduced caloric responses [30] and is characterized by cochleosaccular hydrops [31], only the relative preservation of utricular function in Menière’s disease compared to aminoglycoside toxicity represented a statistically significant difference. These data suggest when a BV patient is identified in a clinical context, ocular VEMP testing may be the most sensitive test in distinguishing the etiology of BV.
We found that utricular function was significantly correlated with horizontal semicircular canal function, whereas saccular function was not significantly associated with canal function. One prior study also noted a parallel between horizontal linear otolith–ocular reflexes (a measure of utricular function) and rotational responses (a measure of horizontal canal function) [32]. The shared susceptibility of these structures may reflect the common embryologic origin and vascular supply of the pars superior of the labyrinth. In the clinical setting, a finding of bilateral semicircular canal dysfunction on routine caloric testing may suggest concomitant utricular dysfunction.
Finally, these data suggest that in patients with BV, dysfunction of the otolith organs had a greater association with functional impairment (captured by the DHI) compared to canal dysfunction. This was particularly true for cervical VEMP amplitudes, which are indicative of saccular function. Thus, in patients who present with BV diagnosed by caloric testing, both cervical and ocular VEMP testing may be considered to characterize otolith function and also to estimate the functional impact on the patient. Moreover, assessing the loss of otolith function in addition to semicircular canal function has important implications for treatment of BV. Current treatment modalities for BV are in their infancy and are not in widespread clinical practice; these include vestibular rehabilitation to promote compensation by central and other peripheral sensory systems (e.g. visual, proprioceptive) [33], sensory substitution devices (e.g. auditory or vibrotactile biofeedback devices) [34], and vestibular prosthetics [35]. Given that otolith dysfunction contributes significantly to the disability associated with BV, effective treatment of BV will likely need to specifically address otolith impairment.
Footnotes
Conflicts of interest The authors declare that they have no conflict of interest.
Ethical standard All human studies must state that they have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Contributor Information
Yuri Agrawal, Email: yagrawa1@jhmi.edu, Department of Neurology and German Dizziness Center, (IFBLMU), University Hospital Munich, Campus Grosshadern, Munich, Germany; Department of Otolaryngology-Head and Neck Surgery, The Johns Hopkins University School of Medicine, 601 N. Caroline Street, Baltimore, MD 21287, USA.
Tatiana Bremova, Department of Neurology and German Dizziness Center, (IFBLMU), University Hospital Munich, Campus Grosshadern, Munich, Germany.
Olympia Kremmyda, Department of Neurology and German Dizziness Center, (IFBLMU), University Hospital Munich, Campus Grosshadern, Munich, Germany.
Michael Strupp, Department of Neurology and German Dizziness Center, (IFBLMU), University Hospital Munich, Campus Grosshadern, Munich, Germany.
References
- 1.Minor LB. Gentamicin-induced bilateral vestibular hypofunction. JAMA. 1998;279(7):541–544. doi: 10.1001/jama.279.7.541. [DOI] [PubMed] [Google Scholar]
- 2.Zingler VC, Cnyrim C, Jahn K. Causative factors and epidemiology of bilateral vestibulopathy in 255 patients. Ann Neurol. 2007;61(6):524–532. doi: 10.1002/ana.21105. [DOI] [PubMed] [Google Scholar]
- 3.Vibert D, Liard P, Hausler R. Bilateral idiopathic loss of peripheral vestibular function with normal hearing. Acta Otolaryngol. 1995;115(5):611–615. doi: 10.3109/00016489509139375. [DOI] [PubMed] [Google Scholar]
- 4.Rinne T, Bronstein AM, Rudge P, Gresty MA, Luxon LM. Bilateral loss of vestibular function: clinical findings in 53 patients. J Neurol. 1998;245(6–7):314–321. doi: 10.1007/s004150050225. [DOI] [PubMed] [Google Scholar]
- 5.Baloh RW, Honrubia V, Yee RD, Hess K. Changes in the human vestibulo-ocular reflex after loss of peripheral sensitivity. Ann Neurol. 1984;16(2):222–228. doi: 10.1002/ana.410160209. [DOI] [PubMed] [Google Scholar]
- 6.Zingler VC, Weintz E, Jahn K, et al. Saccular function less affected than canal function in bilateral vestibulopathy. J Neurol. 2008;255(9):1332–1336. doi: 10.1007/s00415-008-0887-6. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto C, Murofushi T, Chihara Y, Suzuki M, Yamasoba T, Iwasaki S. Novel subtype of idiopathic bilateral vestibulopathy: bilateral absence of vestibular evoked myogenic potentials in the presence of normal caloric responses. J Neurol. 2009;256(9):1488–1492. doi: 10.1007/s00415-009-5147-x. [DOI] [PubMed] [Google Scholar]
- 8.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45(7):737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 9.Welgampola MS, Colebatch JG. Characteristics and clinical applications of vestibular-evoked myogenic potentials. Neurology. 2005;64(10):1682–1688. doi: 10.1212/01.WNL.0000161876.20552.AA. [DOI] [PubMed] [Google Scholar]
- 10.Welgampola MS, Migliaccio AA, Myrie OA, Minor LB, Carey JP. The human sound-evoked vestibulo-ocular reflex and its electromyographic correlate. Clin Neurophysiol. 2009;120(1):158–166. doi: 10.1016/j.clinph.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorns-Haderli M, Straumann D, Palla A. Accuracy of the bedside head impulse test in detecting vestibular hypofunction. J Neurol Neurosurg Psychiatry. 2007;78(10):1113–1118. doi: 10.1136/jnnp.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandt T, Strupp M. General vestibular testing. Clin Neurophysiol. 2005;116(2):406–426. doi: 10.1016/j.clinph.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116(4):424–427. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 14.McCue MP, Guinan JJ., Jr Sound-evoked activity in primary afferent neurons of a mammalian vestibular system. Am J Otol. 1997;18(3):355–360. [PubMed] [Google Scholar]
- 15.Murofushi T, Curthoys IS. Physiological and anatomical study of click-sensitive primary vestibular afferents in the guinea pig. Acta Otolaryngol. 1997;117(1):66–72. doi: 10.3109/00016489709117994. [DOI] [PubMed] [Google Scholar]
- 16.Welgampola MS, Carey JP. Waiting for the evidence: VEMP testing and the ability to differentiate utricular versus saccular function. Otolaryngol Head Neck Surg. 2010;143(2):281–283. doi: 10.1016/j.otohns.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010;31(5):793–802. doi: 10.1097/MAO.0b013e3181e3d60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwasaki S, Smulders YE, Burgess AM, et al. Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin Neurophysiol. 2008;119(9):2135–2147. doi: 10.1016/j.clinph.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 19.Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol. 2010;121(2):132–144. doi: 10.1016/j.clinph.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res. 2006;175(2):256–267. doi: 10.1007/s00221-006-0544-1. [DOI] [PubMed] [Google Scholar]
- 21.Smulders YE, Welgampola MS, Burgess AM, McGarvie LA, Halmagyi GM, Curthoys IS. The n10 component of the ocular vestibular-evoked myogenic potential (oVEMP) is distinct from the R1 component of the blink reflex. Clin Neurophysiol. 2009;120(8):1567–1576. doi: 10.1016/j.clinph.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Oh YM, Koo JW, Kim JS. Bilateral vestibulopathy: clinical characteristics and diagnostic criteria. Otol Neurotol. 2011;32(5):812–817. doi: 10.1097/MAO.0b013e31821a3b7d. [DOI] [PubMed] [Google Scholar]
- 23.Wiest G, Demer JL, Tian J, Crane BT, Baloh RW. Vestibular function in severe bilateral vestibulopathy. J Neurol Neurosurg Psychiatry. 2001;71(1):53–57. doi: 10.1136/jnnp.71.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuzaki M, Murofushi T. Vestibular evoked myogenic potentials in patients with idiopathic bilateral vestibulopathy. Report of three cases. ORL J Otorhinolaryngol Relat Spec. 2001;63(6):349–352. doi: 10.1159/000055772. [DOI] [PubMed] [Google Scholar]
- 25.Brantberg K, Lofqvist L. Preserved vestibular evoked myogenic potentials (VEMP) in some patients with walking-induced oscillopsia due to bilateral vestibulopathy. J Vestib Res. 2007;17(1):33–38. [PubMed] [Google Scholar]
- 26.Agrawal Y, Zuniga MG, Davalos-Bichara M, et al. Decline in semicircular canal and otolith function with age. Otol Neurotol. 2012;33(5):832–839. doi: 10.1097/MAO.0b013e3182545061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merchant SN, Velazquez-Villasenor L, Tsuji K, Glynn RJ, Wall C, 3rd, Rauch SD. Temporal bone studies of the human peripheral vestibular system. Normative vestibular hair cell data. Ann Otol Rhinol Laryngol Suppl. 2000;181:3–13. doi: 10.1177/00034894001090s502. [DOI] [PubMed] [Google Scholar]
- 28.Hirvonen TP, Minor LB, Hullar TE, Carey JP. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J Neurophysiol. 2005;93(2):643–655. doi: 10.1152/jn.00160.2004. [DOI] [PubMed] [Google Scholar]
- 29.Iwasaki S, Chihara Y, Smulders YE, et al. The role of the superior vestibular nerve in generating ocular vestibular-evoked myogenic potentials to bone conducted vibration at Fz. Clin Neurophysiol. 2009;120(3):588–593. doi: 10.1016/j.clinph.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 30.Park HJ, Migliaccio AA, Della Santina CC, Minor LB, Carey JP. Search-coil head-thrust and caloric tests in Meniere’s disease. Acta Otolaryngol. 2005;125(8):852–857. doi: 10.1080/00016480510033667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horner KC. Review: morphological changes associated with endolymphatic hydrops. Scanning Microsc. 1993;7(1):223–238. [PubMed] [Google Scholar]
- 32.Lempert T, Gianna CC, Gresty MA, Bronstein AM. Effect of otolith dysfunction. Impairment of visual acuity during linear head motion in labyrinthine defective subjects. Brain. 1997;120(Pt 6):1005–1013. doi: 10.1093/brain/120.6.1005. [DOI] [PubMed] [Google Scholar]
- 33.Herdman SJ. Role of vestibular adaptation in vestibular rehabilitation. Otolaryngol Head Neck Surg. 1998;119(1):49–54. doi: 10.1016/S0194-5998(98)70195-0. [DOI] [PubMed] [Google Scholar]
- 34.Basta D, Rossi-Izquierdo M, Soto-Varela A, et al. Efficacy of a vibrotactile neurofeedback training in stance and gait conditions for the treatment of balance deficits: a double-blind, placebo-controlled multicenter study. Otol Neurotol. 2011;32(9):1492–1499. doi: 10.1097/MAO.0b013e31823827ec. [DOI] [PubMed] [Google Scholar]
- 35.Della Santina CC, Migliaccio AA, Hayden R, et al. Current and future management of bilateral loss of vestibular sensation—an update on the Johns Hopkins Multichannel Vestibular Prosthesis Project. Cochlear Implants Int. 2010;11(Suppl 2):2–11. doi: 10.1179/146701010X12726366068454. [DOI] [PMC free article] [PubMed] [Google Scholar]


