Abstract
Objectives
High maternal weight before and during pregnancy contributes to child obesity. To assess the additional role of weight change after delivery, we examined associations between pre- and post-pregnancy weight changes and preschooler overweight.
Methods
Sample: 4359 children from the Children and Young Adults of the 1979 National Longitudinal Survey of Youth (NLSY) born to 2816 NLSY mothers between 1979 and 2006 and followed to age 4–5 years old. Exposures: gestational weight gain (GWG) and post-delivery maternal weight change (PDWC). Outcome: child overweight (body mass index (BMI) ≥85th percentile).
Results
Adjusted models suggested that both increased GWG (OR: 1.08 per 5 kg GWG, 95% CI: 1.01, 1.16) and excessive GWG (OR: 1.29 versus adequate GWG, 95% CI: 1.06, 1.56) were associated with preschooler overweight. Maternal weight change after delivery was also independently associated with child overweight (OR: 1.12 per 5 kg PDWC, 95% CI: 1.04, 1.21). Associations were stronger among children with overweight or obese mothers.
Conclusions
Increased maternal weight gain both during and after pregnancy predicted overweight in preschool children. Our results suggest that healthy post-pregnancy weight may join normal pre-pregnancy BMI and adequate GWG as a potentially modifiable risk factor for child overweight.
Keywords: Body mass index, Child, Mothers, Overweight, Obesity, Pregnancy, Weight gain, Weight loss, Postpartum period, Longitudinal studies
Introduction
More than one in four U.S. children age 2–5 are overweight or obese (Ogden et al., 2012), and the prevalence of overweight and obesity among 4 year-olds in European Union countries ranges from 12 to 32% (Cattaneo et al., 2010). Indeed, child obesity is considered a global epidemic (Wang and Lobstein, 2006). Child obesity is associated with poor lifelong health; understanding early life determinants as part of the environmental, social, and genetic multifactorial pathways to child overweight (Butland et al., 2007) is essential for primary prevention (Biro and Wien, 2010; IOM et al., 2011).
Several mechanisms have been proposed to explain how pregnancy-related maternal weight changes may contribute to child obesity (Oken and Gillman, 2003; Tarry-Adkins and Ozanne, 2011). Prepregnancy obesity as well as prenatal maternal diet, excessive or inadequate gestational weight gain, or gestational diabetes may contribute to offspring adiposity through developmental programming via the thrifty obesity or fetal over nutrition pathways (Dabelea and Crume, 2011; Drake and Reynolds, 2010; Poston et al., 2011). After birth, maternal obesity, diet and diabetes may also program neural development of the neonatal hypothalamus, influencing long-term appetite (Armitage et al., 2008). Both maternal and paternal BMI are associated with child’s BMI (Durmuş et al., 2013), suggesting that genetics as well as shared environments, and post-birth behaviors, including breastfeeding and physical activity, are linked to both maternal and infant weights (Chu et al., 2012).
Postpartum weight retention and interpregnancy weight gain are growing concerns: for example, 50% of low income U.S. women retain 10 lb and 25% retain more than 20 lb after pregnancy (IOM, 2009). Weight retention after pregnancy is a risk factor for both permanent maternal obesity (Gore et al., 2003; Linee et al., 2004; Rooney and Schauberger, 2002) and adverse outcomes in subsequent pregnancies (Ehrlich et al., 2011; Jain et al., 2013; Villamor and Cnattingius, 2006). While there is growing evidence that high prepregnancy body mass index (BMI) and excessive gestational weight gain (GWG) are each associated with child obesity (independent of pregnancy-related characteristics such as smoking or length of gestation, post-pregnancy characteristics such as breastfeeding, and child characteristics such as birthweight) (de Hoog et al., 2011; Fraser et al., 2010; Hinkle et al., 2012a; Margerison Zilko et al., 2010; Nehring et al., 2013; Oken et al., 2007; Olson et al., 2009), the contribution of maternal weight changes after delivery to child obesity has not been explored.
We investigated how excessive GWG and post-delivery maternal weight change are associated with preschooler (age 4–5 years old) overweight, and how these associations vary by maternal prepregnancy weight in a nationally representative cohort. We hypothesized that overweight preschool children would have higher odds of having a mother with less weight loss between delivery and three years postpartum.
Methods
Sample
This secondary data analysis utilized the 1979 National Longitudinal Survey of Youth (NLSY79), a nationally representative cohort study of U.S. youth aged 14–22 in 1979, and the Children and Young Adults of the 1979 National Longitudinal Survey of Youth (NLSY79-CYA), a cohort of all children born to NLSY79 mothers starting in 1986. Detailed information on sampling design, data collection, and response rates is reported elsewhere (CHRR, 2008, 2010). The Office for the Protection of Human Subjects at the University of California, Berkeley did not require formal review because these data are deidentified and publicly available.
The analytic data set included all singleton births born to each NLSY79 mother between 1979 and 2010 with at least one follow-up survey when the child was 4–5 years old (n = 7613) and a reported maternal weight at 3–36 months postpartum, and excluded births with a gestational age of <22 or >44 weeks (n = 19) or births with implausible birthweight–gestational age combinations (n = 25) (Alexander et al., 1996). The sample was further restricted to cases with complete data for all variables of interest described below (Table 1), yielding a final analytic sample of 4359 children born to 2816 mothers, which represented about 58% of NLSY participants. Similar retention rates are common among secondary analyses of cohort studies (Mei et al., 2004; Wake et al., 2007).
Table 1.
Weighted descriptive statistics for complete case analysis sample by child overweight status at 4–5 years old, Children and Young Adults of the 1979 National Longitudinal Survey of Youth Cohort, United States, 1979–2010. Mean (SD) reported unless indicated otherwise.
| Characteristic | BMI <85th percentile | BMI ≥85th percentile | Analytic sample (N = 4359) | Excluded (n varies) |
|---|---|---|---|---|
| Full analytic sample, % | 77.1% | 22.9% | 100% | 0% |
| Maternal demographics | ||||
| Race–ethnicity, % | (n = 3151) | |||
| Black | 12.4% | 16.3% | 13.3% | 16.0% |
| Hispanic | 6.4% | 6.8% | 6.5% | 8.5% |
| Non-Black non-Hispanic | 81.2% | 76.9% | 80.2% | 75.6% |
| Education at child’s birth, % | (n = 2918) | |||
| Less than high school | 12.3% | 15.3% | 13.0% | 15.4% |
| High school to some college | 68.8% | 66.7% | 68.3% | 59.2% |
| College graduate and higher | 19.0% | 18.0% | 18.8% | 25.4% |
| Employment at child’s birth, % | (n = 2988) | |||
| Employed | 27.9% | 30.0% | 28.4% | 41.8% |
| Unemployed | 32.3% | 28.3% | 31.4% | 24.4% |
| Out of labor force | 39.8% | 41.7% | 40.3% | 33.8% |
| Marital status at child’s birth, % | (n = 2929) | |||
| Married | 79.5% | 74.9% | 78.4% | 74.7% |
| Single | 20.5% | 25.1% | 21.6% | 25.3% |
| Equivalized household income at child’s birth (in thousands of 2000 U.S. dollars) | 9.9 (1.1) | 9.8 (1.1) | 9.8 (1.1) | (n = 2302) 10.0 (1.2) |
| Pregnancy characteristics | ||||
| Smoking during pregnancy, % | (n = 2441) | |||
| Never smoked | 73.4% | 69.7% | 72.5% | 73.5% |
| Smoked | 26.6% | 30.3% | 27.5% | 26.5% |
| Prepregnancy BMI (kg/m2) | 22.9 (4.4) | 24.4 (5.4) | 23.3 (4.7) | (n = 2237) 23.7 (5.3) |
| Prepregnancy BMI category | (n = 2237) | |||
| Underweight (<18.5) | 8.5% | 5.1% | 7.7% | 8.2% |
| Normal weight (18.5–24.9) | 68.4% | 60.7% | 66.6% | 63.5% |
| Overweight (25–29.9) | 15.5% | 21.2% | 16.8% | 16.9% |
| Obese (>30) | 7.6% | 13.0% | 8.9% | 11.4% |
| Maternal age at child’s birth (y) | 26.3 (4.6) | 26.9 (5.1) | 26.4 (4.7) | (n = 2237) 27.4 (5.6) |
| Maternal height (m) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | (n = 2237) 1.6 (0.1) |
| Length of gestation (wks) | 38.7 (1.9) | 38.7 (1.9) | 38.7 (1.9) | (n = 2237) 38.6 (2.0) |
| Gestational weight gain (kg) | 14.3 (6.0) | 14.7 (6.4) | 14.4 (6.0) | (n = 2300) 14.5 (6.4) |
| Adequacy of GWG (2009 IOM), % | (n = 2300) | |||
| Inadequate | 21.8% | 18.2% | 20.8% | 28.2% |
| Adequate | 34.6% | 30.4% | 33.7% | 27.8% |
| Excessive | 43.6% | 51.4% | 45.5% | 44.0% |
| Post-delivery weight change (kg) | −12.3 (6.5) | −12.0 (7.5) | −12.3 (6.7) | −11.1 (7.5) |
| Postpartum weight difference (kg) | 1.91 (5.3) | 2.7 (6.0) | 2.1 (5.4) | 1.7 (6.4) |
| Survey characteristics | (n = 2238) | |||
| Maternal birth year | 1961.2 (2.2) | 1961.5 (2.3) | 1961.2 (2.3) | 1961.8 (2.3) |
| Child’s birth order | 1.9 (1.0) | 2.0 (1.1) | 1.9 (1.0) | 2.2 (1.2) |
| Child’s age at postpartum weight (mo.) | 13.3 (7.6) | 13.8 (7.9) | 13.4 (7.6) | 13.5 (7.4) |
| Year child was 4–5 years old | 1992.4 (4.6) | 1993.3 (5.3) | 1992.6 (4.8) | 1995.5 (6.5) |
| Child demographics | ||||
| Gender | (n = 3151) | |||
| Male | 51.9% | 49.7% | 51.4% | 50.8% |
| Female | 48.1% | 50.3% | 48.6% | 49.2% |
| Birthweight | (n = 2342) | |||
| Low (500–2499 g) | 6.0% | 4.7% | 5.7% | 6.5% |
| Normal (2500–3999 g) | 82.0% | 79.8% | 81.5% | 83.1% |
| High (>4000 g) | 12.0% | 15.5% | 12.8% | 10.5% |
Percentages may not add up to 100% due to rounding.
Measures
Maternal pregnancy-related weight
In the first survey after each pregnancy, NLSY79 mothers recalled their prepregnancy weight and weight before delivery. Height was reported in 1985. Maternal BMI was calculated as weight (in kilograms) divided by height (in meters) squared (kg/m2). We calculated observed GWG as the difference (kilograms) between prepregnancy weight and delivery weight (Fig. 1) and then categorized GWG as inadequate, adequate, or excessive based on adequacy for gestational age and BMI using the Institute of Medicine (IOM) recommendations (IOM, 2009) as described in detail elsewhere (Bodnar et al., 2010; Deardorff et al., 2013).
Fig. 1.
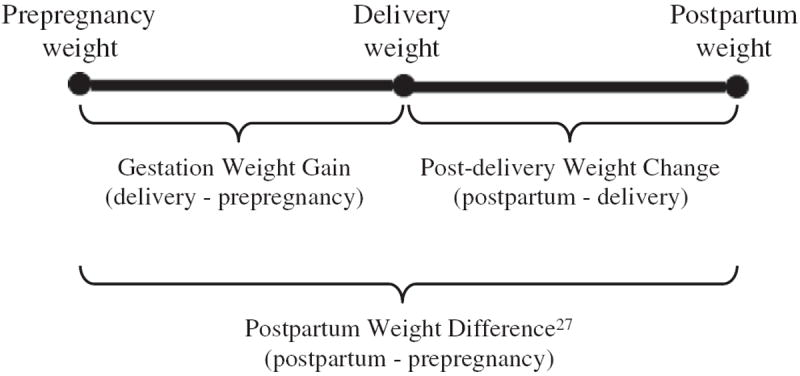
Timing and definitions of different pregnancy-related maternal weight change measures.
Postpartum weight
Mothers reported their current weight, which was regression-calibrated to account for potential self-reporting bias (Strauss and Thomas, 1996; Thomas and Frankenberg, 2002). We created a post-delivery weight change variable (PDWC), defined as the difference (kilograms) between the current weight within 3–36 months after delivery (mean 13.4 months, SD 7.6 months) and delivery weight (Fig. 1). If a mother became pregnant again before completing her first postpartum survey for the index child (13%), we used the prepregnancy weight for the next child as the postpartum weight for the previous child.
The PDWC variable was created to decompose the prepregnancy through postpartum weight into GWG and PDWC (Fig. 1). Traditionally, the difference between postpartum weight and prepregnancy weight has been defined as postpartum weight retention (IOM, 2009). However, because new weight gain postpartum can be misclassified as retained GWG, others suggest using “postpartum weight difference” (PPWD) (Lipsky et al., 2012) to describe weights obtained after 1 year. For purposes of comparison to previous studies (i.e., Kac et al., 2004; Nehring et al., 2011; Olson et al., 2003; Rode et al., 2012; Walker et al., 2004), we also calculated PPWD (kilograms) as the difference between the first postpartum weight and prepregnancy weight (Fig. 1).
Child overweight
The outcome of interest was child overweight (BMI ≥85th percentile which includes both overweight: BMI ≥85th and <95th percentiles, and obese: BMI ≥95th percentile) (Ogden and Flegal, 2010) at age 4–5 years old. Children’s weights and heights were either measured at the in-home interview (by a trained interviewer using a tape measurement for height and a portable scale for weight) or reported by the mother; 74% of weights and 82% of heights were measured. Others have found no bias resulting from mother report of child weight and height (Weden et al., 2012), but we nevertheless regression-calibrated the mother-reported weights and heights to reduce potential bias (Strauss and Thomas, 1996; Thomas and Frankenberg, 2002). We calculated sex-specific BMI-for-age percentiles using the SAS Program for the Centers for Disease Control and Prevention (CDC) Growth Charts (CDC, 2007). This age range (4 to 5 years old) was chosen to capture the time before the potential critical period in the development of obesity known as the adiposity rebound (Dietz, 1994) at 5–7 years of age, when body fatness reaches a nadir before increasing into adolescence and adulthood (Boonpleng et al., 2012; Dietz, 1994; Whitaker et al., 1998).
Potential confounders were selected based on previous studies of both pregnancy-related weight exposures of interest and child overweight (Fraser et al., 2010; Hinkle et al., 2012a; Lawlor et al., 2011; Oken et al., 2007; Olson et al., 2009). Demographic confounders (defined in Table 1) were measured in the child’s birth year and included race/ethnicity, maternal educational attainment, maternal employment status, maternal marital status, and equivalized household income (continuous variable in year 2000 U.S. dollars that accounts for household size; Rehkopf et al., 2010). Pregnancy-related confounders were: smoking during pregnancy, prepregnancy BMI (continuous), maternal age at child’s birth, maternal prepregnancy height (meters), and length of gestation (weeks). Maternal birth year, child’s birth order, and year in which the child was 4–5 years old were also included in the model as survey characteristics to account for any potential birth cohort effects. The models for PDWC and PPWD additionally adjusted for GWG (kilograms), child’s birthweight (grams), and child’s age (months) when maternal postpartum weight was obtained. All continuous covariates were median-centered.
Data analysis
Separate models were used to examine how maternal weight changes pre- and post-delivery (expressed as per 5 kg weight change) were associated with child overweight, adjusting for potentially confounding demographic and pregnancy variables. Given the wide time range of reported postpartum weights, we also conducted subgroup analyses to compare results for mothers reporting within the first 12 months of delivery versus more than 1 year post-delivery. To determine if prepregnancy BMI category (BMI <25, BMI ≥25) modified the association between maternal pre- or post-pregnancy weight, we also ran models with interaction terms, using an a priori cutoff of 0.10.
All models used generalized estimating equations (GEE) in Stata 12.1 (Stata Corporation, 2011) to: a) account for the clustering of births by mothers, since about 90% of children had at least one sibling, and b) make a population average estimate, which is more clinically relevant than random effects (Hubbard et al., 2010). These GEE models allow us to report odds ratios that are adjusted for co-variates in the model. We used custom sampling weights to make the sample nationally representative to account for oversampling and loss to follow-up, and robust standard errors were calculated.
Results
Almost one-fourth of the 4359 children in the analytic sample were overweight at age 4–5 (Table 1). A similar proportion of mothers were overweight or obese before pregnancy. Mean maternal GWG was 14.4 kg (SD 6.0 kg) and almost half of all the mothers in our analytic sample had excessive GWG. On average, at 13.4 months postpartum, mothers had lost 12.3 kg (SD 6.7 kg) from their delivery weight (PDWC) and gained 2.1 kg (SD 5.4 kg) over their prepregnancy weight (PPWD). Comparing the analytic sample to those excluded, a larger proportion of included mothers were non-Black non-Hispanic, out of the labor force or unemployed, and married; a larger proportion of mothers excluded due to incomplete data had less than a high school education, were obese before pregnancy, and had inadequate GWG. The prevalence of child overweight was the same between included and excluded children, but included children had a lower birth order, earlier birth date, and were more likely to have a high birthweight (Table 1).
Table 1 also compares characteristics by child overweight status. Compared to mothers of normal weight children, a larger proportion of mothers of overweight children were Black, had excessive GWG, were overweight prepregnancy, did not graduate from high school, were single, and smoked during pregnancy. Additionally, a larger proportion of overweight children had a high birthweight.
After adjusting for demographic, pregnancy, and survey characteristics, both maternal overweight (OR: 1.48, 95% CI: 1.18, 1.97) and obesity (OR: 1.78, 95% CI: 1.34, 2.36) before pregnancy were significantly associated with preschooler overweight when compared to normal weight mothers. Table 2 displays the odds ratios for overweight in preschoolers by GWG and PDWC after adjustment for prepregnancy BMI and covariates. A 5-kilogram increase in GWG was associated with a statistically significant eight percent increase in the odds of preschooler overweight. Children whose mothers had excessive GWG had significantly increased odds of overweight when compared to children exposed to adequate GWG. In contrast, children exposed to inadequate GWG did not have significantly different odds of overweight when compared to children exposed to adequate GWG. A 5-kilogram increase in PDWC was associated with a statistically significant 12% increase in the odds of preschooler overweight. In subgroup analyses stratified by when women reported their post-delivery weight, the adjusted odds ratios for PDWC and child overweight were closer to the null for postpartum weights obtained more than 12 months after delivery (OR: 1.05, 95% CI:.95, 1.22) than for postpartum weights obtained within 12 months of delivery (OR: 1.22, 95% CI: 1.05, 1.34). We also tested the association between PPWD and child overweight and found similar results to PDWC (OR: 1.10, 95% CI: 1.05–1.22).
Table 2.
Associations of maternal gestational weight gain (GWG), adequacy of GWG, and maternal post-delivery weight change with preschooler overweight, Children and Young Adults of the 1979 National Longitudinal Survey of Youth Cohort, United States, 1979–2010.
| Odds ratio (95% CI)a | |
|---|---|
| Gestational weight gainb (per 5 kg) | 1.08 (1.01, 1.16) |
| Adequacy of GWGb (2009 IOM) | |
| Inadequate | 0.90 (0.70, 1.16) |
| Adequate | 1.00 (Reference) |
| Excessive | 1.29 (1.06, 1.56) |
| Post-delivery weight changec (per 5 kg) | 1.12 (1.04, 1.21) |
CI—confidence interval.
Observations were weighted using custom sample weights and robust standard errors were calculated.
Odds ratios are adjusted for demographic characteristics (race–ethnicity, maternal education at child’s birth, maternal employment status at child’s birth, equivalized household income at child’s birth, marital status at child’s birth), pregnancy characteristics (smoking during pregnancy, prepregnancy BMI, maternal age at child’s birth, maternal prepregnancy height, length of gestation), and survey characteristics (maternal birth year, child’s birth order, and year child was 4–5 years old).
Model adjusted for all variables in GWG models plus gestational weight gain, child’s birthweight, and child’s age at maternal postpartum weight.
Finally, when we tested for interaction by prepregnancy BMI, the adjusted association between each maternal weight measure with child overweight appeared limited to the overweight/obese group of mothers (Table 3). In separate models, GWG examined as a continuous variable and as excessive GWG compared to adequate GWG was associated with increased odds of child overweight. Gaining inadequately also increased the odds of having an overweight preschool child, though the 95% CI for inadequate GWG was wide. After adjusting for GWG, each 5-kilogram of PDWC was associated with a 17% increased odds of child overweight.
Table 3.
Associations of maternal gestational weight gain (GWG), adequacy of GWG, and maternal post-delivery weight change with preschooler overweight by prepregnancy BMI, Children and Young Adults of the 1979 National Longitudinal Survey of Youth Cohort, United States, 1979–2010.
| Prepregnancy BMIa
|
||
|---|---|---|
| Underweight or normal, OR (95% CI) | Overweight or obese, OR (95% CI) | |
| Gestational weight gainb (per 5 kg) | Wald test p = 0.11 | |
| 1.02 (0.95, 1.10) | 1.15 (1.07, 1.25) | |
| Adequacy of GWGb (2009 IOM) | Wald test p = 0.002 | |
| Inadequate vs. adequate | 0.82 (0.62, 1.07) | 1.62 (0.99, 2.66) |
| Excessive vs. adequate | 1.13 (0.91, 1.40) | 1.57 (1.23, 2.01) |
| Post-delivery weight changec (per 5 kg) | Wald test p = 0.07 | |
| 1.05 (0.94, 1.16) | 1.17 (1.07, 1.29) | |
OR—odds ratio.
CI—confidence interval.
Observations were weighted using custom sample weights and robust standard errors were calculated.
Odds ratios are adjusted for demographic characteristics (race–ethnicity, maternal education at child’s birth, maternal employment status at child’s birth, equivalized household income at child’s birth, marital status at child’s birth), pregnancy characteristics (smoking during pregnancy, prepregnancy BMI, maternal age at child’s birth, maternal prepregnancy height, length of gestation), and survey characteristics (maternal birth year, child’s birth order, and year child was 4–5 years old).
Model adjusted for all variables in GWG models plus gestational weight gain, child’s birthweight, and child’s age at maternal postpartum weight.
Discussion
In this national sample of U.S. women, decreased maternal weight loss after delivery was associated with increased odds of preschooler overweight, even after adjusting for GWG. This finding suggests that healthy post-pregnancy weight may join normal prepregnancy BMI and adequate GWG as potentially modifiable risk factor for child overweight and obesity. Our data also reinforce that prepregnancy BMI (Li et al., 2005; Olson et al., 2010; Weden et al., 2012; Whitaker, 2004) and GWG (IOM, 2009; Nehring et al., 2013) are each independently associated with child overweight. The association between both GWG and PDWC with child overweight appeared driven by overweight and obese mothers.
The 2009 IOM report suggests that to fully implement their recommended GWG guidelines, all postpartum women might require counseling with the goal of achieving their prepregnancy weight during the first year after birth to ensure a healthier prepregnancy BMI at a subsequent pregnancy (IOM, 2009). Our results suggest that another benefit of this approach could be a lower risk of child overweight in the recently delivered child. Further studies are required to confirm our findings.
Maternal food intake influences infant and young child feeding practices (Thompson, 2013). It is possible that women who manage their own weight after delivery provide a healthier food and physical activity environment that translates into less child overweight, though we identified no published data to demonstrate this. Reduced breastfeeding may enhance intergenerational transfer of child overweight (Horta et al., 2007; Ip et al., 2007; Thompson, 2013), and longer term, more intensive breastfeeding has been associated with greater maternal weight loss (Baker et al., 2008; Martin et al., 2012) though data are inconsistent (Neville et al., 2013; Thompson, 2013). Although we could not directly examine these pathways in this study, these behaviors are potentially modifiable, with a possible outcome that improves overall family health. Finally, genes from overweight mothers may predispose a child to adiposity (Manco and Dallapiccola, 2012; Warrington et al., 2013) and may also predispose a mother to a higher weight after delivery; if so, both might benefit from intervention.
Associations of GWG, GWG adequacy, and PDWC with child overweight were stronger in mothers with BMI ≥25 before pregnancy, suggesting that this group could be particularly targeted for intervention. Though some previous studies of child overweight found no evidence of a BMI–GWG interaction (e.g. Fraser et al., 2010; Oken et al., 2007) others found evidence of effect modification by prepregnancy BMI (e.g. Hinkle et al., 2012a; Lawlor et al., 2011; Olson et al., 2009), though results have been inconsistent by BMI group. A recent meta-analysis of child overweight concluded that most studies had adjusted for maternal prepregnancy BMI, making it impossible to examine the potential role of effect modification (Nehring et al., 2013). Though inadequate GWG has been either unrelated or associated with reduced risk of child obesity (Nehring et al., 2013), we note that in our sample there was a positive association between inadequate GWG and child overweight in overweight/obese mothers and a negative association between inadequate GWG and child overweight underweight/normal weight mothers. As our study was not powered to detect differences at this level of stratification, further studies of sufficient sample size are needed to explore effect modification by prepregnancy BMI for GWG and maternal postpartum weight.
We acknowledge our study’s limitations. First, self-reported weights are likely to introduce misclassification of BMI and without measured weights for cross validation it is impossible to predict the direction or magnitude of possible errors in our findings. Although previous validation studies of self-reported pregnancy-related weights, including prepregnancy weight (Lederman and Paxton, 1998), GWG (Hinkle et al., 2012b), and delivery weight (Schieve et al., 1999), suggest that self-reported and anthropometrically-measured weights are highly correlated, we regression-calibrated all weights and heights that had available standards based on NHANES (mothers’ heights; mothers’ weights at time of survey; and mother-reported weights and heights for children) to address possible error. Second, while postpartum weight was collected on average 13.4 months post-delivery, the time frame ranged widely. We adjusted for the time when postpartum weight was collected, but we could not specify whether the weight changes were due to maternal weight loss, retention, or regain. Subgroup analyses showed that the PDWC–child overweight association was stronger for post-delivery weights collected within the first year after delivery compared to after more than one year; longitudinal studies with frequent measurements are needed to explore this sinding. Third, we did not control for maternal or child body composition, diet, physical activity, shared genes, breastfeeding, or the shared environment, all of which could be potential confounding factors. Finally, we have complete information for only 58% of respondents; as noted earlier, this is relatively common among secondary data analyses of cohorts, but nevertheless may limit our internal validity.
Despite these limitations, our study’s unique strengths include examining the independent associations of maternal weight before, during, and after pregnancy with child overweight in a diverse, national, longitudinal sample of women and their children, which adds prospective data to previous cross-sectional findings (Keane et al., 2012; Whitaker et al., 1997).
Fig. 2 illustrates several potential opportunities for health care professionals to prevent child overweight. This includes providers emphasizing healthy GWG during prenatal visits, with the goal of optimizing the fetal environment and reducing pregnancy complications related to increased maternal adiposity (IOM, 2009, 2013; Phelan et al., 2011), and supporting women to maintain healthy weight between pregnancies. Routine well child visits (Hagan et al., 2008), especially in the first year of life, could provide a potential opportunity for practitioners to promote healthy behaviors to both mother and child. However, one small, non-randomized trial that explored merging management of maternal postpartum health with infant care in a pediatric practice found that the intervention produced changes in infant health, but not maternal health-related behaviors (Taveras et al., 2011). Another study reported that maternal attempts to lose weight postpartum through exercise were associated with increased child obesity at 3 years (Sonneville et al., 2011). These findings suggest that while the postpartum period may be an important time to intervene to improve both maternal and child weight, new research is needed to identify effective multidisciplinary approaches (Dixon et al., 2012).
Fig. 2.
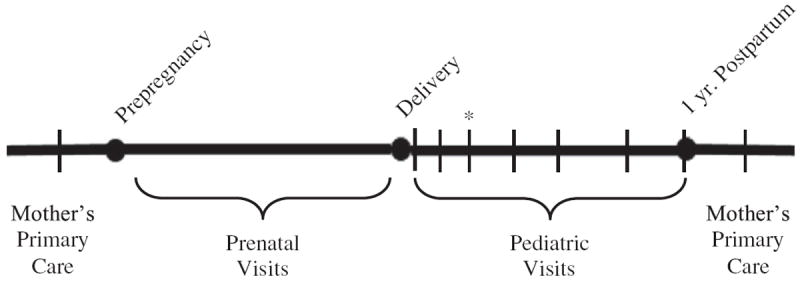
Recommended health care visits before, during, and after pregnancy.
Note: * is time point for both a pediatric visit and a postpartum obstetric/gynecology visit
Conclusion
To our knowledge, this is the first study to investigate the association between post-delivery maternal weight change and child overweight. Increasing maternal weight gain both during and after pregnancy independently predicted overweight in preschool children whose mothers were overweight or obese prepregnancy. If future research replicates these findings, new models of care in the context of the family, eating environment, and sociocultural environment will be needed to break the link between maternal weight before, during, and after pregnancy and overweight in children (IOM, 2013).
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Poston L, Taylor PD. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res. 2008;36:73–84. doi: 10.1159/000115355. [DOI] [PubMed] [Google Scholar]
- Baker JL, Gamborg M, Heitmann BL, Lissner L, Sorensen TI, Rasmussen KM. Breastfeeding reduces postpartum weight retention. Am J Clin Nutr. 2008;88(6):1543–1551. doi: 10.3945/ajcn.2008.26379. http://dx.doi.org/10.3945/ajcn.2008.26379. [DOI] [PubMed] [Google Scholar]
- Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91(5):1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Siega-Riz AM, Simhan HN, et al. Severe obesity, gestational weight gain, and adverse birth outcomes. Am J Clin Nutr. 2010;91:1642–1648. doi: 10.3945/ajcn.2009.29008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonpleng W, Park CG, Gallo AM. Timing of adiposity rebound: a step toward preventing obesity. Pediatr Nurs. 2012;38(1):37–42. [PubMed] [Google Scholar]
- Butland B, Jebb S, Kpelman P, et al. Tackling Obesities: Future Choices—Project Report. 2. UK Government Foresight Programme, Government Office for Science; 2007. [Google Scholar]
- Cattaneo A, Monasta L, Stamatakis E, et al. Overweight and obesity in infants and pre-school children in the European Union: a review of existing data. Obes Rev. 2010;11(5):389–398. doi: 10.1111/j.1467-789X.2009.00639.x. http://dx.doi.org/10.1111/j.1467-789X.2009.00639.x. [DOI] [PubMed] [Google Scholar]
- Center for Human Resource Research (CHRR) NLSY79 user’s guide. Ohio State University; Columbus: 2008. [Google Scholar]
- Center for Human Resource Research (CHRR) NLSY79 Child and Young Adult data user’s guide. Ohio State University; Columbus: 2010. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) A SAS program for the CDC growth charts. Division of Nutrition, Physical Activity and Obesity, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 2007. Available at: http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. [Google Scholar]
- Chu L, Retnakaran R, Zinman B, Hanley AJG, Hamilton JK. Impact of maternal physical activity and infant feeding practices on infant weight gain and adiposity. Int J Endocrinol. 2012;2012:293821. doi: 10.1155/2012/293821. http://dx.doi.org/10.1155/2012/293821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabelea D, Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes. 2011;60(7):1849–1855. doi: 10.2337/db11-0400. http://dx.doi.org/10.2337/db11-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoog ML, van Eijsden M, Stronks K, Gemke RJ, Vrijkotte TG. Overweight at age two years in a multi-ethnic cohort (ABCD study): the role of prenatal factors, birth outcomes and postnatal factors. BMC Public Health. 2011;1:11–611. doi: 10.1186/1471-2458-11-611. http://dx.doi.org/10.1186/1471-2458-11-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff J, Millett RB, Rehkopf D, Luecke E, Lahiff M, Abrams B. Maternal pre-pregnancy BMI, gestational weight gain, and age at menarche in daughters. Matern Child Health J. 2013;17(8):1391–1398. doi: 10.1007/s10995-012-1139-z. http://dx.doi.org/10.1007/s10995-012-1139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59:955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- Dixon B, Peña MM, Taveras EM. Lifecourse Approach to Racial/Ethnic Disparities in Childhood Obesity. Adv Nutr Int Rev J. 2012;3(1):73–82. doi: 10.3945/an.111.000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140(3):387–398. doi: 10.1530/REP-10-0077. http://dx.doi.org/10.1530/REP-10-0077. [DOI] [PubMed] [Google Scholar]
- Durmuş B, Arends LR, Ay L, et al. Parental anthropometrics, early growth and the risk of overweight in pre-school children: the Generation R Study. Pediatr Obes. 2013;8(5):339–350. doi: 10.1111/j.2047-6310.2012.00114.x. http://dx.doi.org/10.1111/j.2047-6310.2012.00114.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich SF, Hedderson MM, Feng J, Davenport ER, Gunderson EP, Ferrara A. Change in Body Mass Index Between Pregnancies and the Risk of Gestational Diabetes in a Second Pregnancy. Obstet Gynecol. 2011;117:1323–1330. doi: 10.1097/AOG.0b013e31821aa358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A, Tilling K, Macdonald-Wallis C, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–2564. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Ann Behav Med. 2003;26(2):149–159. doi: 10.1207/S15324796ABM2602_07. [DOI] [PubMed] [Google Scholar]
- Hagan JF, Shaw JS, Duncan P, editors. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. Third edition. American Academy of Pediatrics; Elk Grove Village, IL: 2008. [Google Scholar]
- Hinkle SN, Sharma AJ, Swan DW, Schieve LA, Ramakrishnan U, Stein AD. Excess gestational weight gain is associated with child adiposity among mothers with normal and overweight prepregnancy weight status. J Nutr. 2012a;142:1851–1858. doi: 10.3945/jn.112.161158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle SN, Sharma AJ, Schieve LA, Ramakrishnan U, Swan DW, Stein AD. Reliability of gestational weight gain reported postpartum: a comparison to the birth certificate. Matern Child Health J. 2012b;17:756–765. doi: 10.1007/s10995-012-1057-0. [DOI] [PubMed] [Google Scholar]
- Horta BL, Bahl R, Martines JC, Victora CG. World Health Organization. Evidence on the long-term effects of breastfeeding: systematic reviews and meta-analysis. WHO Press; Geneva: 2007. [Google Scholar]
- Hubbard AE, Ahern J, Fleischer NL, et al. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology. 2010;21(4):467–474. doi: 10.1097/EDE.0b013e3181caeb90. http://dx.doi.org/10.1097/EDE.0b013e3181caeb90. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Weight Gain during Pregnancy: Reexamining the Guidelines. The National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Leveraging Action to Support Dissemination of Pregnancy Weight Gain Guidelines: Workshop Summary. The National Academies Press; Washington, DC: 2013. [PubMed] [Google Scholar]
- Birch LL, Parker L, Burns A, editors. Institute of Medicine (IOM) Early Childhood Obesity Prevention Policies. National Academy of Sciences. The National Academies Press; Washington, DC: 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 2007:1–186. [PMC free article] [PubMed] [Google Scholar]
- Jain AP, Gavard JA, Rice JJ, Catanzaro RB, Artal RA, Hopkins SA. The impact of interpregnancy weight change on birthweight in obese women. Am J Obstet Gynecol. 2013;208(205):205.e1–7. doi: 10.1016/j.ajog.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Kac G, Benicio MH, Velasquez-Melendez G, Valente JG. Nine months postpartum weight retention predictors for Brazilian women. Public Health Nutr. 2004;7(5):621–628. doi: 10.1079/PHN2003579. [DOI] [PubMed] [Google Scholar]
- Keane E, Layte R, Harrington J, Kearney PM, Perry IJ. Measured parental weight status and familial socio-economic status correlates with childhood overweight and obesity at age 9. PLoS One. 2012;7(8):e43503. doi: 10.1371/journal.pone.0043503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Lichtenstein P, Fraser A, Långström N. Does maternal weight gain in pregnancy have long-term effects on offspring adiposity? A sibling study in a prospective cohort of 146,894 men from 136,050 families. Am J Clin Nutr. 2011;94(1):142–148. doi: 10.3945/ajcn.110.009324. http://dx.doi.org/10.3945/ajcn.110.009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman SA, Paxton A. Maternal reporting of prepregnancy weight and birth outcome: consistency and completeness compared with the clinical record. Matern Child Health J. 1998;2:123–126. doi: 10.1023/a:1022996924094. [DOI] [PubMed] [Google Scholar]
- Li C, Kaur H, Choi WS, Huang TTK, Lee RE, Ahluwalia JS. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obes Res. 2005;13(2):362–371. doi: 10.1038/oby.2005.48. [DOI] [PubMed] [Google Scholar]
- Linee Y, Dye L, Barkeling B, Rossner S. Long-term weight development in women: a 15-year follow-up of the effects of pregnancy. Obes Res. 2004;12(7):1166–1178. doi: 10.1038/oby.2004.146. [DOI] [PubMed] [Google Scholar]
- Lipsky LM, Strawderman MS, Olson CM. Maternal weight change between 1 and 2 years postpartum: the important of 1 year weight retention. Obesity. 2012;20:1496–1502. doi: 10.1038/oby.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manco M, Dallapiccola B. Genetics of pediatric obesity. Pediatrics. 2012;130(1):123–133. doi: 10.1542/peds.2011-2717. [DOI] [PubMed] [Google Scholar]
- Margerison Zilko CE, Rehkopf DH, Abrams B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. Am J Obstet Gynecol. 2010;202:574.e1–8. doi: 10.1016/j.ajog.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Martin JE, Hure AJ, Macdonald-Wicks L, Smith R, Collins CE. Predictors of post-partum weight retention in a prospective longitudinal study. Matern Child Nutr. 2012 doi: 10.1111/j.1740-8709.2012.00437.x. http://dx.doi.org/10.1111/j.1740-8709.2012.00437.x (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- Mei Z, Grummer-Strawn LM, Thompson D, Dietz WH. Shifts in percentiles of growth during early childhood: analysis of longitudinal data from the California Child Health and Development Study. Pediatrics. 2004;113:e617. doi: 10.1542/peds.113.6.e617. [DOI] [PubMed] [Google Scholar]
- Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr. 2011;94:1225–1231. doi: 10.3945/ajcn.111.015289. [DOI] [PubMed] [Google Scholar]
- Nehring I, Lehmann S, von Kries R. Gestational weight gain in accordance to the IOM/NRC criteria and the risk for childhood overweight: a meta-analysis. Pediatr Obes. 2013;8(3):218–224. doi: 10.1111/j.2047-6310.2012.00110.x. [DOI] [PubMed] [Google Scholar]
- Neville CE, McKinley MC, Holmes VA, Spence D, Woodside JV. The relationship between breastfeeding and postpartum weight change—a systematic review and critical evaluation. Int J Obes. 2013:1–14. doi: 10.1038/ijo.2013.132. http://dx.doi.org/10.1038/ijo.2013.132. [DOI] [PubMed]
- Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Vol. 25. National Health Statistics Reports; Hyattsville, MD: 2010. pp. 1–5. [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents. 1990–2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322e1–8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CM, Strawderman MS, Hinton PS, Pearson TA. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. Int J Obes Relat Metab Disord. 2003;27:117–127. doi: 10.1038/sj.ijo.0802156. [DOI] [PubMed] [Google Scholar]
- Olson CM, Strawderman MS, Dennison BA. Maternal weight gain during pregnancy and child weight at age 3 years. Matern Child Health J. 2009;13:839–846. doi: 10.1007/s10995-008-0413-6. [DOI] [PubMed] [Google Scholar]
- Olson CM, Demment MM, Carling SJ, Strawderman MS. Associations between mothers’ and their children’s weights at 4 years of age. Child Obes. 2010;6(4):201–207. doi: 10.1089/chi.2010.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the fit for delivery study. Am J Clin Nutr. 2011;93(4):772–779. doi: 10.3945/ajcn.110.005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston L, Harthoorn LF, Van Der Beek EM Contributors to the ILSI Europe Workshop. Obesity in pregnancy: implications for the mother and lifelong health of the child. A consensus statement Pediatr Res. 2011;69(2):175–180. doi: 10.1203/PDR.0b013e3182055ede. http://dx.doi.org/10.1203/PDR.0b013e3182055ede. [DOI] [PubMed] [Google Scholar]
- Rehkopf DH, Krieger N, Coull B, Berkman LF. Biologic risk markers for coronary heart disease. Epidemiology. 2010;21(1):38–46. doi: 10.1097/EDE.0b013e3181c30b89. [DOI] [PubMed] [Google Scholar]
- Rode L, Kjaergaard H, Ottesen B, Damm P, Hegaard HK. Association between gestational weight gain according to body mass index and postpartum weight in a large cohort of Danish women. Matern Child Health J. 2012;16(2):406–413. doi: 10.1007/s10995-011-0775-z. [DOI] [PubMed] [Google Scholar]
- Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Am J Obstet Gynecol. 2002;100(2):245–252. doi: 10.1016/s0029-7844(02)02125-7. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Perry GS, Cogswell ME, et al. Validity of self-reported pregnancy delivery weight: an analysis of the 1988 National Maternal and Infant Health Survey. NMIHS Collaborative Working Group. Am J Epidemiol. 1999;150:947–956. doi: 10.1093/oxfordjournals.aje.a010103. [DOI] [PubMed] [Google Scholar]
- Sonneville KR, Rifas-Shiman SL, Oken E, et al. Longitudinal association of maternal attempt to lose weight during the postpartum period and child obesity at age 3 years. Obesity. 2011:1–7. doi: 10.1038/oby.2011.25. http://dx.doi.org/10.1038/oby.2011.25. [DOI] [PMC free article] [PubMed]
- Stata Corporation. Stata Statistical Software: Release 12. StataCorp LP; College Station, TX: 2011. [Google Scholar]
- Strauss J, Thomas D. Measurement and mismeasurement of social indicators. Am Econ Rev. 1996;86(2):30–34. [Google Scholar]
- Tarry-Adkins JL, Ozanne SE. Mechanisms of early life programming: current knowledge and future directions. Am J Clin Nutr. 2011;94(Supplement):1765S–1771S. doi: 10.3945/ajcn.110.000620. [DOI] [PubMed] [Google Scholar]
- Taveras EM, Blackburn K, Gillman MW, et al. First steps for mommy and me: a pilot intervention to improve nutrition and physical activity behaviors of postpartum mothers and their infants. Matern Child Health J. 2011;15:1217–1227. doi: 10.1007/s10995-010-0696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Frankenberg E. The measurement and interpretation of health in social surveys. In: Lopez AD, Mathers CD, Murray CJL, Salomon JA, editors. Summary Measures of Population Health: Concepts, Ethics, Measurement, and Applications. World Health Organization; 2002. pp. 387–420. [Google Scholar]
- Thompson AL. Intergenerational impact of maternal obesity and postnatal feeding practices on pediatric obesity. Nutr Rev. 2013;71(Suppl. 1):555–561. doi: 10.1111/nure.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368:1164–1170. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- Wake M, Nicholson JM, Hardy P, Smith K. Preschooler obesity and parenting styles of mothers and fathers: Australian National Population Study. Pediatrics. 2007;120(6):e1520–e1527. doi: 10.1542/peds.2006-3707. [DOI] [PubMed] [Google Scholar]
- Walker LO, Timmerman GM, Sterling BS, Kim M, Dickson P. Do low-income women attain their pre-pregnant weight by the 6th week of postpartum? Ethn Dis. 2004;14:119–126. [PubMed] [Google Scholar]
- Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1(1):11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- Warrington NM, Wu YY, Pennell CE, et al. Modeling BMI trajectories in children for genetic association studies. PLoS One. 2013;8(1):e53897. doi: 10.1371/journal.pone.0053897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weden MM, Brownell P, Rendall MS. Prenatal, perinatal, early life, and sociodemographic factors underlying racial differences in the likelihood of high body mass index in early childhood. Am J Public Health. 2012;102(11):2057–2067. doi: 10.2105/AJPH.2012.300686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114(1):e29. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Whitaker RC, Pepe MS, Wright JA, Seidel KD, Dietz WH. Early adiposity rebound and the risk of adult obesity. Pediatrics. 1998;101(3):e5. doi: 10.1542/peds.101.3.e5. [DOI] [PubMed] [Google Scholar]


