Abstract
Conclusions
This study showed a transient increase of ocular vestibular evoked myogenic potential (oVEMP) amplitudes in the affected ear after successful liberatory maneuvers and no changes in cervical VEMP (cVEMP) amplitudes. These findings support the hypothesis that successful liberatory maneuvers can lead to a repositioning of otoconia to the utricle.
Objectives
To evaluate whether oVEMP amplitudes increase after successful liberatory maneuvers in patients with posterior semicircular canal benign paroxysmal positioning vertigo (pc-BPPV), while cVEMP amplitudes do not change. These findings may indicate a successful repositioning of dislodged otoconia to the utricular macula, but not to the saccular macula.
Methods
Thirty patients with unilateral pc-BPPV were prospectively examined with bone-conducted oVEMP and air-conducted cVEMP at four time points: before, after, 1 week after, and 1 month after the liberatory maneuvers (Sémont maneuvers).
Results
At the 1-week follow-up, 20 of 30 patients were asymptomatic (responders); BPPV could still be induced in the other 10 (non-responders). In responders the mean n10 amplitude on the affected side increased from 12 ± 6.5 μV at baseline (before the treatment) to 15.9 ± 7.1 μV at 1 week after treatment; this increase was significantly (p = 0.001) higher in responders than in non-responders. cVEMP did not differ significantly.
Keywords: Vestibular evoked myogenic potentials, nystagmus, Sémont maneuvers
Introduction
Benign paroxysmal positioning vertigo (BPPV) is the most common cause of vertigo [1]. In the majority of cases it is caused by canalolithiasis, i.e. dislodged otoconia that freely move within a semicircular canal – most often in the posterior semicircular canal (pc-BPPV) – for anatomic reasons [2]. More than 90% of patients can be successfully treated with either the Epley repositioning maneuvers or the Sémont liberatory maneuvers [3,4]. Although there is so far no direct evidence concerning the underlying mechanism, these maneuvers may lead to a repositioning of the dislodged otoconia to the utricle and – due to the small diameter of the utriculosaccular duct – not to the saccule [5]. This theory is also supported by the so-called type 2 BPPV [6], which is presumably caused by the chronic canalolithiasis of posterior canal short arm leading to the chronic deflection of the utricular side of the cupula. The presence of the otoconia at this particular place can be tested in the head-hanging position. Furthermore, in accordance with the previous work referring to the anatomic configuration of the labyrinth [7], in the head hanging position during the Dix-Hallpike maneuver, the posterior canal cupula hangs vertically downward, so that the crystals fall down from the cupula into the utricle.
The utricular function can be evaluated by ocular vestibular evoked myogenic potentials (oVEMP) and the saccular function by cervical VEMPs (cVEMPs) [8]. oVEMP reflect the excitatory utricular response. The utricular signals are conveyed through the superior vestibular nerve to the superior vestibular region in the brainstem, crossing to the oculomotor nucleus and to the extraocular muscles. cVEMPs measure saccular function representing inhibitory responses conducted through the inferior vestibular nerve to the accessory nucleus in the brainstem and via accessory nerves to the ipsilateral sternocleidomastoid muscle [9]. Recently two reports were published on oVEMP abnormalities in patients with BPPV: one study showed reduced oVEMP amplitude in 66.7% and reduced cVEMP in 25.0% on the affected side, with abnormal oVEMP in 50% and abnormal cVEMP in 16.7% on the non-affected side [10,11]. In another study a higher rate of abnormal oVEMP was found in the recurrent BPPV group than in the non-recurrent group [11].
Are there changes in oVEMP and cVEMP amplitudes after successful treatment that might indicate a repositioning of otoconia to the otolith organs, in particular to the utricle? Due to the high density and specific gravity of otoconia (consisting of calcium carbonate/calcite, density ρp = 2.7 g/cm3) compared with the endolymph, they are responsible for the effective transmission of linear acceleration on the otolith organs. In terms of the application of VEMPs to evaluate a repositioning of otoconia to the otolith organs, a simple biomechanical explanation would be that if more otoconia are attached or reattached to the otolith maculae, a higher VEMP amplitude results because the mass of otoconia to be stimulated by the mechanical vibrator or sound increases after repositioning. In the current study, the following four hypotheses were evaluated. First, otoconia are mainly repositioned to the utricle. Second, repositioning to the utricle leads to an increase of oVEMP amplitude. Third, the increase of oVEMP amplitude does not occur immediately after repositioning because the otoconia first have to be reattached to the mucociliar matrix. Fourth, the otoconia are not repositioned to the saccule, i.e. the cVEMP amplitude does not change.
Material and methods
Subjects
The prospective study included 30 patients with unilateral pc-BPPV from the German Center for Vertigo and Balance Disorders who met the following inclusion criteria: a history of short-lasting (<1 min) rotational vertigo provoked by head position changes relative to gravity; a mixed torsional/up-beating nystagmus beating toward the lower ear and elicited by positional testing in the lateral Dix-Hallpike position for <60 s on one side (observed with Frenzel’s glasses); and no evidence of central positioning/positional nystagmus. Patients with bilateral, anterior or horizontal canal BPPV were excluded to standardize the study and to build up the homogeneous population.
All patients underwent a standardized neurological, neuro-ophthalmological, and neurological examination, including video-oculography with caloric irrigation as well as cVEMP and oVEMP testing (see below). After diagnosis of pc-BPPV and examination of the patients with cVEMP and oVEMP, they were treated with the Sémont maneuvers three times in the morning, three times at noon and three times at night for at least 3 days. Subjects were instructed to perform the self-treatment until the symptoms (vertigo when performing maneuvers) disappeared.
In the follow-up visits (see below), the Dix-Hallpike maneuver was used to assess the response to treatment by the presence of canalolithiasis. After 1 week, successful treatment was defined as the absence of positioning vertigo and positioning nystagmus. Patients with a positive positioning maneuver at the 1-week or the 1-month follow-up were considered non-responders and were instructed to continue the self-treatment until no vertigo could be induced.
The study was performed in accordance with the Helsinki II Declaration and was approved by the Ethics Committee of the Ludwig-Maximilians University Medical Faculty (no. 379-12). All participants gave their informed consent before their inclusion in the study.
Methods
oVEMP and cVEMP were measured separately at four time points: before, immediately after, 1 week after, and 1 month after the initial repositioning. The oVEMP n10 amplitude was evaluated on the basis of evidence that this part of the response was most clearly vestibular. As regards the cVEMP, the p13 component of the PP potential (=peak-to-peak; sum of the p13 and n23 potentials) was analyzed as an analog to the n10 component. However, preceding studies had demonstrated a good inter-rater reliability for oVEMP testing, while a fair-to-good inter-rater reliability was demonstrated for cVEMP [12].
Recording of oVEMP
Subjects were examined in a semi-recumbent position with their upper bodies elevated at a 30 angle from the horizontal. Maximum up-gaze was maintained during oVEMP stimulation and recording at all four time points, while subjects gazed supermedially at a small fixed target fastened at the mini-shaker margin. The visual angle was approximately 30°. This angle has been found to evoke the largest responses compared with other eye positions.
‘Mini taps’ were already broadly applied to assess the otolithic function. They were delivered with a Bruel and Kjaer Mini-Shaker Type 4810 (2 ms clicks of positive polarity, with a repetition rate of two per second) at the Fz cranial site (in the midline at the hairline, 30% of the distance between the inion and nasion). Fz taps have been shown to provide an acceleration wave that spreads through the cranium to the mastoid on either side, predominantly causing an outward linear stimulation of the utricles bilaterally. After cleaning and de-fatting with abrasive paste, oVEMP were recorded with an electrode alignment consisting of a recording electrode placed over the contralateral inferior oblique muscle, approximately 3 mm below the eye and centered beneath the pupil, a reference electrode on the chin, and a ground electrode placed under the chin. The responses to 50–100 stimuli were averaged. The first negative and positive peaks of the oVEMP response that occurred between 10 and 20 ms after stimulus onset were designated n1 (n10) and p1 (p15), respectively. The responses recorded from the different electrodes were amplified by a Bruel and Kjaer Type 2718 power amplifier (the voltage gain was 30 dB), and the unrectified signals were averaged (filter cut-offs: 20 Hz to 500 Hz).
Recording of cVEMP
Subjects assumed a semi-recumbent position with their upper bodies elevated at a 30° angle from horizontal and with the head lifted so as to cause active neck flexion during cVEMP stimulation and recording for tonic background muscle activity. Air-conducted 500 Hz, 125 dB SPL tone bursts were delivered monaurally via intro-aurical speakers with foam ear-tips. cVEMP were recorded from an electrode montage consisting of a recording electrode placed at the midpoint of the belly of the ipsilateral sternocleido-mastoid muscle, a reference electrode placed on the manubrium, and a ground electrode placed on the forehead. Electomyogram (EMG) activity was recorded (Nicolet Biomedical Inc., Madison, WI, USA), amplified, and bandpass filtered, and the responses to 50–100 stimuli were averaged. The first positive and negative peaks that occurred between 13 and 23 ms after stimulus onset were designated p13 and n23, respectively.
Statistical analysis
On the basis of the hypotheses outlined in the introduction, an increase of oVEMP amplitudes after 1 week compared with the time point before treatment is expected in responders compared with non-responders. This was analyzed separately for the affected and non-affected sides using t tests for independent samples after box plots had been inspected and outliers (defined as an increase in amplitude of >10 μV) had been removed. The t test for dependent samples was used to compare the size of oVEMP amplitudes separately between time points in responders and non-responders. To describe the time course of VEMP amplitudes in BPPV patients during treatment, measurements were taken immediately after, 1 week after, and 1 month after repositioning. No marked increases or differences were expected (see supplemental material) in cVEMP between responders and non-responders, since cVEMP reflect saccular function. Analysis was performed using SPSS, version 20.0.0 (IBM, New York, NY, USA), R version 2.15 (R Foundation for Statistical Computing, Vienna, Austria), and StatWeave (http://www.stat.uiowa.edu/~rlenth/StatWeave/) (for details see supplementary material).
Results
Thirty patients with typical unilateral pc-BPPV were included (19 women, mean age ± SD of 68,8 ± 12,9 years; median 72.5 years, range 33–87 years). The symptoms at the time of diagnosis had lasted less than 1 month in 3 patients, more than 1 month but less than 6 months in 5 patients, and more than 6 months in 22 patients. In 66.7% of the patients the right side was affected. Twelve patients could not be tested at all four time points (loss to follow-up: ten patients were tested three times, two patients twice). After 1 week, 66,6% of the patients treated with liberatory maneuvers were free of symptoms. After 1 month, the therapy response rate rose to 73.9%.
oVEMP
In responders the mean n10 amplitude on the affected side increased from 12 ± 6.5 μV at baseline (immediately before the treatment) to 15.9 ± 7.1 μV 1 week after treatment, yielding a mean increase of 3.9 μV. This increase was significantly (p = 0.02) greater in responders than in non-responders (Figure 1). In non-responders, the mean n10 amplitudes were 11.9 ± 6.05 μV before treatment and 9.1 ± 4.3 μV 1 week after treatment. The analysis of the PP amplitudes (n10-p15) gave similar results. The absolute mean difference of n10 amplitudes (Δn10) between the affected and non-affected ears in responders was 0.94 μV before the initial repositioning and 2.28 after the initial repositioning. In responders, the mean difference was 1.31 μV after 1 week and 0.3 μV after 1 month. In non-responders, the mean difference was 0.42 μV after 1 week and 4 μV after 1 month. The mean values and standard deviations of the oVEMP n10 and PP amplitudes for all testing sessions in affected and non-affected ears are shown in Tables I and II.
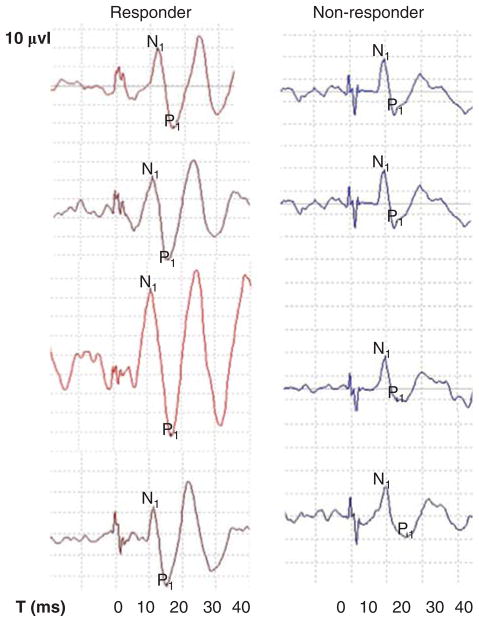
Figure 1.
Sample recordings of affected ears over the course of time in responder and non-responder.
Table I.
Mean values and standard deviations of the oVEMP n10 amplitudes for all testing sessions in affected and non-affected ears.
| N10 oVEMP | Ear | Before | After | After one week | P Value | After one month | P Value |
|---|---|---|---|---|---|---|---|
| Responders | Affected | 12.0 ± 6.5 | 14.9 ± 9.5 | 15.9 ± 7.1 | 0.001 | 16.6 ± 9.1 | 0.08 |
| Non-affected | 11.1 ± 4.8 | 12.7 ± 9.1 | 14.6 ± 7.5 | 0.006 | 17.0 ± 7.2 | 0.384 | |
| Non-responders | Affected | 11.9 ± 6.1 | 8.0 ± 5.1 | 9.1 ± 4.3 | 0.057 | 11.0 ± 3.6 | 0.66 |
| Non-affected | 9.3 ± 4.3 | 9.9 ± 3.9 | 9.5 ± 4.7 | 0.857 | 15.1 ± 7.8 | 0.035 |
Table II.
Mean values and standard deviations of the oVEMP PP amplitudes for all testing sessions in affected and non-affected ears.
| PP oVEMP | Ear | Before | After | One week after | P Value | One month after | P Value |
|---|---|---|---|---|---|---|---|
| Responders | Affected | 27.6 ± 13.8 | 32.0 ± 16.7 | 33.7 ± 15.1 | 0.004 | 35.1 ± 17.8 | 0.086 |
| Non-affected | 24.7 ± 10.7 | 27.8 ± 19.0 | 31.3 ± 16.0 | 0.018 | 37.5 ± 14.6 | 0.013 | |
| Non-responders | Affected | 20.3 ± 7.7 | 16.4 ± 10.7 | 20.6 ± 8.9 | 0.883 | 22.2 ± 8.3 | 0.096 |
| Non-affected | 21.7 ± 10.7 | 20.7 ± 9.5 | 29.6 ± 7.1 | 0.788 | 29.6 ± 16.0 | 0.222 |
oVEMP sample recordings of affected ears over the course of time in responders and non-responders are shown in Figure 1. The mean and range of the oVEMP n10 amplitudes in affected ears for all testing sessions in responders are presented in Figure 2. The mean increase from baseline (TP0) to 1 week after therapy (TP2) in responders and non-responders is presented in Figure 3.
Figure 2.
Characteristics of the oVEMP n10 amplitudes in responders and non-responders. The bottom and top of the box represent the 25th and 75th percentiles, and the band near the middle of the box represents the 50th percentile (the median). The ends of the whiskers represent the minimum and maximum of all the data.
Figure 3.
Increase of amplitudes from baseline (TP0) to 1 week after therapy onset (TP2) compared between responders and non-responders.
Patients who had a good response to the therapy exhibited a significant increase of n10 and PP oVEMP amplitudes on the affected side 1 week after the initial liberatory maneuver. This increasing trend was observed on the non-affected side, too. It appeared that patients with persistent symptoms showed markedly lower amplitudes that varied less over time. An increase in n10 amplitudes from baseline to the 1-week follow-up was observed less frequently in these patients (4/11) than in responders (11/15). This trend also held for the 1-month follow-up and is consistent with the modeling results presented in the supplementary material.
cVEMP
No significant differences were detected in the p13 cVEMP amplitudes between responders and non-responders at different time points. The mean values and standard deviations of the cVEMP p13 and PP amplitudes for all testing sessions in the affected and the non-affected ears are shown in Tables III and IV.
Table III.
Mean values and standard deviations of the cVEMP p13 amplitudes for all testing sessions in the affected and the non-affected ears.
| P13 cVEMP | Ear | Before | After | One week after | One month after |
|---|---|---|---|---|---|
| Responders | Affected | 14.5 ± 14.6 | 29.9 ± 23.2 | 25.6 ± 19.0 | 21.5 ± 16.3 |
| Non-affected | 23.5 ± 20.8 | 32.2 ± 21.8 | 24.6 ± 23.9 | 26.7 ± 24.9 | |
| Nonresponders | Affected | 17.6 ± 23.2 | 19.3 ± 19.5 | 16.3 ± 20.0 | 33.0 ± 21.0 |
| Non-affected | 17.9 ± 13.7 | 22.3 ± 15.8 | 22.3 ± 10.5 | 20.5 ± 19.2 |
Table IV.
Mean values and standard deviations of the cVEMP PP amplitudes for all testing sessions in the affected and the non-affected ears.
| PP cVEMP | Ear | Before | After | One week after | One month after |
|---|---|---|---|---|---|
| Responders | Affected | 39.2 ± 35.2 | 68.2 ± 50.9 | 56.7 ± 45.3 | 55.0 ± 42.0 |
| Non-affected | 60.0 ± 43.3 | 75.5 ± 56.2 | 54.1 ± 54.2 | 67.0 ± 47.4 | |
| Non-responders | Affected | 43.3 ± 44.6 | 55.7 ± 29.4 | 76.3 ± 41.3 | 89.3 ± 48.7 |
| Non-affected | 42.5 ± 33.7 | 53.7 ± 40.7 | 54.2 ± 21.6 | 44.5 ± 37.3 |
Discussion
This prospective study on 30 patients with pc-BPPV found a significant increase in oVEMP amplitudes 1 week after liberatory maneuvers in responders. No significant changes were found for non-responders, the non-affected ear, and the other time points investigated (immediately and 1 month after treatment onset). Since oVEMP measure utricular function, the increase in oVEMP amplitude may indicate a repositioning of otoconia to the utricle, as originally assumed by Epley when he called his treatment maneuvers ‘repositioning maneuvers’ [14]. The relocation of the otoconia on the utricle after the repositioning is in line with the probable pathomechanism of type 2 BPPV characterized by brief vertigo spells due to the head position changes, with no nystagmus during either Dix-Hallpike positioning and by short episodes of vertigo during and immediately after sitting up. These have been shown by posturographic recordings during and immediately after sitting up, which showed an anterior–posterior sway or trunk oscillations, corroborating the hypothesis of a repositioning of otoconia back onto the cupula in the upright position. This is further supported by the occurrence of so-called positional down-beating nystagmus of the peripheral origin, which may also be caused by the atypical canalolithiasis of the posterior canal [15].
Remarkably, the increase was observed not immediately but after 1 week. One possible explanation is that the reattachment of the otoconia to the mucociliar matrix takes time and occurs within the first week. This may be due to the histological structure of the utricular cupula: the otoconial mass forming the utricular cupula is made of thousands of otoconia (each c. 10 μm in length) and it is overlaid by a gelatinous matrix called the otoconial membrane. This membrane has two layers: the upper one is a dense, isotropic network of filaments to which the otoconia are attached; the lower one is more rigid and thus responsible for the linear force transduction. The successful performance of repositioning relocates at least part of these clots and results in a resolution of the symptoms. In contrast, there were no changes in cVEMP amplitudes in the two groups and at all time points. This indicates that there was no change in saccular function, i.e. these findings provide no evidence of a repositioning of the otoconia to the saccule. Such a repositioning is not expected because of the small dimensions of the utriculosaccular duct (the mean diameter of 0.16 × 0.41 mm at the internal aperture of the vestibular aqueducts and 0.09 × 0.20 mm at the isthmus) that connects the utricle and the saccule [16]. Further, from a methodological point of view, the recording of the cVEMP also served as a control condition to determine nonspecific effects of the liberatory maneuvers on the peripheral or central vestibular systems.
Our explanation of a repositioning of otoconia is supported by other studies. First, after successful repositioning maneuvers, 20–40% of patients with BPPV complain of transient postural imbalance and in posturographic studies increased body sway was found [17]. Second, a deviation of subjective visual vertical (SVV), which normalized within weeks to months, was described after liberatory maneuvers [18]. This finding is in agreement with our finding that 1 month after therapy the oVEMP amplitudes of responders were not statistically significant any more in comparison to those before therapy. This can be explained by central vestibular compensation, which occurs within many days to a few weeks [19]. Another possible explanation for the decrease in amplitude after 4 weeks is that the otoconia became dislodged again [20], but none of the patients examined had a recurrence of BPPV. How can the increased amplitudes on the non-affected side be explained? This might also be caused by the central compensation process acting to improve response asymmetry and thus equalize the imbalance in the otolithic function of the affected and non-affected sides.
Taken together, these findings support the hypothesis that a repositioning of otoconia to the utricle may occur during the liberatory/repositioning maneuvers in BPPV. The otoconia have to be reattached to the mucociliar matrix of the utricle before changes in the oVEMP amplitude are apparent; as this process can take some time, an increase in oVEMP amplitudes was not found immediately after the positioning maneuver. Finally, the cVEMP recordings indicate that a repositioning to the saccule is unlikely for anatomic reasons.
Acknowledgments
We thank Ms J. Benson and Ms K. Ogston for copy editing the manuscript and Mr S. Krafczyk for technical support. This study was supported by a grant from the BMBF to the IFB (grant code 01 EO 0901).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, Lempert T, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. 2007;78:710–15. doi: 10.1136/jnnp.2006.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandt T, Steddin S. Current view of the mechanism of benign paroxysmal positioning vertigo: cupulolithiasis or canalolithiasis? J Vestib Res. 1993;3:373–82. [PubMed] [Google Scholar]
- 3.Honrubia V. Self-treatment of benign paroxysmal positional vertigo: Semont maneuver vs Epley procedure. Neurology. 2005;64:583–4. doi: 10.1212/wnl.64.3.583. [DOI] [PubMed] [Google Scholar]
- 4.Fife TD, Iverson DJ, Lempert T, Furman JM, Baloh RW, Tusa RJ, et al. Practice parameter: therapies for benign paroxysmal positional vertigo (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2008;70:2067–74. doi: 10.1212/01.wnl.0000313378.77444.ac. [DOI] [PubMed] [Google Scholar]
- 5.Baloh RW, Honrubia V, Jacobson K. Benign positional vertigo: clinical and oculographic features in 240 cases. Neurology. 1987;37:371–8. doi: 10.1212/wnl.37.3.371. [DOI] [PubMed] [Google Scholar]
- 6.Büki B, Simon L, Garab S, Lundberg YW, Jünger H, Straumann D. Sitting-up vertigo and trunk retropulsion in patients with benign positional vertigo but without positional nystagmus. J Neurol Neurosurg Psychiatry. 2011;82:98–104. doi: 10.1136/jnnp.2009.199208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckingham RA. Anatomical and theoretical observations on otolith repositioning for benign paroxysmal positional vertigo. Laryngoscope. 1999;109:717–22. doi: 10.1097/00005537-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Rosengren SM, Welgampola MS, Colebatch JG. Vestibular evoked myogenic potentials: past, present and future. Clin Neurophysiol. 2010;121:636–51. doi: 10.1016/j.clinph.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Rosengren SM, Govender S, Colebatch JG. Ocular and cervical vestibular evoked myogenic potentials produced by air- and bone-conducted stimuli: comparative properties and effects of age. Clin Neurophysiol. 2011;122:2282–9. doi: 10.1016/j.clinph.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Nakahara H, Yoshimura E, Tsuda Y, Murofushi T. Damaged utricular function clarified by oVEMP in patients with benign paroxysmal positional vertigo. Acta Otolaryngol. 2013;133:144–9. doi: 10.3109/00016489.2012.720030. [DOI] [PubMed] [Google Scholar]
- 11.Lee JD, Park MK, Lee BD, Lee TK, Sung K-B, Park JY. Abnormality of cervical vestibular-evoked myogenic potentials and ocular vestibular-evoked myogenic potentials in patients with recurrent benign paroxysmal postitional vertigo. Acta Otolaryngol. 2013;133:150–3. doi: 10.3109/00016489.2012.723823. [DOI] [PubMed] [Google Scholar]
- 12.House MG, Honrubia V. Theoretical models for the mechanisms of benign paroxysmal positional vertigo. Audiol Neurootol. 2003;8:91–9. doi: 10.1159/000068998. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010;31:793–802. doi: 10.1097/MAO.0b013e3181e3d60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epley JM. Canalith repositioning maneuver. Otolaryngol Head Neck Surg. 1994;111:688–90. doi: 10.1177/019459989411100530. [DOI] [PubMed] [Google Scholar]
- 15.Cambi J, Astore S, Mandalà M, Trabalzini F, Nuti D. Natural course of positional down-beating nystagmus of peripheral origin. J Neurol. 2013;260:1489–96. doi: 10.1007/s00415-012-6815-9. [DOI] [PubMed] [Google Scholar]
- 16.Lo WW, Daniels DL, Chakeres DW, Linthicum FH, Jr, Ulmer JL, Mark LP, et al. The endolymphatic duct and sac. AJNR Am J Neuroradiol. 1997;18:881–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Blatt PJ, Georgakakis GA, Herdman SJ, Clendaniel RA, Tusa RJ. The effect of the canalith repositioning maneuver on resolving postural instability in patients with benign paroxysmal positional vertigo. Am J Otol. 2000;21:356–63. doi: 10.1016/s0196-0709(00)80045-9. [DOI] [PubMed] [Google Scholar]
- 18.Faralli M, Manzari L, Panichi R, Botti F, Ricci G, Longari F, et al. Subjective visual vertical before and after treatment of a BPPV episode. Ann N Y Acad Sci. 2011;38:307–11. doi: 10.1016/j.anl.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Takeda T, Kitahara M. Compensation of otolith function. Acta Otolaryngol Suppl. 1989;468:283–8. doi: 10.3109/00016488909139062. [DOI] [PubMed] [Google Scholar]
- 20.Brandt T, Huppert D, Hecht J, Karch C, Strupp M. Benign paroxysmal positioning vertigo: a long-term follow-up (6–17 years) of 125 patients. Acta Otolaryngol. 2006;126:160–3. doi: 10.1080/00016480500280140. [DOI] [PubMed] [Google Scholar]