Abstract
Objective
To describe the performance of healthy older adults on common clinical vestibular tests.
Patients
Fifty community-dwelling older adults aged 70 and older, with mean age of 77.2 ± 6.1 years and range of 70 to 95 years.
Intervention(s)
Clinical vestibular tests, including spontaneous and head-shaking nystagmus, head impulse test (HIT), bucket test of subjective visual vertical, modified Romberg test (MRT), and Dizziness Handicap Inventory (DHI).
Main Outcome Measure(s)
Prevalence of abnormal vestibular tests and DHI score.
Results
We observed a 36% and 44% prevalence of abnormal right and left horizontal HIT, respectively. The bucket test was abnormal in 18% of participants; head-shaking nystagmus was present in 2%, and no participant had spontaneous nystagmus. Approximately 68% of participants had abnormal MRT. Abnormal horizontal HIT and MRT were significantly more prevalent among individuals age 80 years and older compared with those age 70 to 79 years (p < 0.05). Mean DHI score was 5.6 ± 11.2, consistent with no self-reported dizziness handicap.
Conclusion
This study documents the expected performance of normative older adults on vestibular tests commonly administered in the neurotology clinic. We observed a high prevalence of abnormalities on clinical vestibular testing in healthy older adults, although self-perceived dizziness handicap was low. Further studies using newly available clinical testing methods (e.g., video HIT) may identify finer gradations of vestibular function in older individuals and the levels of vestibular loss associated with functional impairment.
Keywords: Aging, Bucket test, Dizziness, Falls, Head-impulse test, Romberg, Vestibular dysfunction
A decline in vestibular function with age has been described in numerous physiologic and histologic studies (1-5). A recent epidemiologic study of the US population reported that vestibular dysfunction—defined based on abnormal modified Romberg testing—appears to be highly common among older adults, with a prevalence of 69% among 70- to 79-year-olds and 85% among 80-year-olds and older (6). Moreover, abnormal modified Romberg testing was found to significantly increase the risk of falls (6), which is a highly morbid outcome in older individuals (7).
The modified Romberg test is a global measure of the ability to use vestibular information (6,8,9); therefore, we sought to evaluate the prevalence of vestibular dysfunction based on more specific vestibular tests frequently used in the neurotologic clinic in a sample of community-dwelling older adults. Specifically, we considered the prevalence of spontaneous and head-shaking nystagmus, which are indicative of acute and latent asymmetric vestibular losses, respectively (10). We also evaluated the prevalence of abnormalities in the head impulse test (HIT), which assesses the function of each semicircular canal (11). Furthermore, we examined the prevalence of subjective visual vertical abnormalities using the “bucket” test, which is thought to reflect utricular function (12). We also evaluated performance on the Dizziness Handicap Inventory as a measure of self-perceived functional impairment associated with any positive clinical vestibular signs. Little is known about the normative performance of healthy older individuals on vestibular clinical tests. We anticipate that these findings will guide clinicians regarding expected levels of vestibular function in older individuals and how to interpret clinical findings in older patients.
METHODS
Subjects
Subjects were recruited from a registry of older individuals interested in participating in clinical studies. Eligible subjects were age 70 and older and able to provide informed consent. Exclusion criteria included blindness, poor neck range of motion or cervical spine instability (for the HIT), and diabetes mellitus, given previous data showing increased vestibular dysfunction in diabetics (13). This study was approved by the hospital’s institutional review board.
The following clinical tests were administered to assess vestibular function:
Spontaneous and Head-Shaking Nystagmus
Participant’s eyes were observed behind Frenzel lenses for 1 minute to assess for spontaneous nystagmus. The participant’s head was then moved in the yaw plane at 2 Hz for 20 to 30 seconds with closed eyes. The head was abruptly stopped, and the participant was asked to open their eyes. The examiner observed for nystagmus behind Frenzel lenses for 1 minute.
Head Impulse Test
The subject’s head was moved a small amplitude (approximately 20–30 degrees) with high acceleration (approximately 3,000–4,000 degrees per square second) in the yaw plane (with ~30 degrees forward head pitch) for the right and left horizontal canals and in the planes of the superior and posterior semicircular canals on each side. The subject was asked to keep their gaze on the examiner’s nose, and the examiner observed their eyes for a corrective saccade at the end of each head movement. The test was marked as positive (i.e., abnormal) if a corrective saccade was present and was interpreted as hypofunction of the canal in the direction to which the head movement was performed. Typically 1 to 2 impulses were delivered in each of the 6 canal planes and were given in a randomized manner to be unpredictable (14).
Subjective Visual Vertical or “Bucket” Test
A bucket was fashioned per published specifications with a straight line on the inside and the true vertical position indicated on the outside (12). The subject sat upright with their head inside the bucket, covering their peripheral vision to obscure any visual cues. The bucket was rotated to the right or left randomly in 5 trials then rotated back, and the subject was asked to indicate when the line inside the bucket was vertical. The degrees of deviation from true vertical were recorded. A mean of the absolute values of the degrees recorded in each trial was calculated. The direction was given by the negative or positive sign of the sum of the values of all trials. Values from −3 to +3 degrees were considered normal (12,15).
Modified Romberg Test Condition 4
We also performed the modified Romberg test (MRT), to be consistent with the epidemiologic study discussed previously (6). Subjects were asked to stand on a 4-inch foam-padded cushion with feet together, arms crossed on the chest, and eyes closed, eliminating proprioceptive and visual stimuli. The test was scored on a pass/fail basis and was marked as a failure if the participant could not maintain this position for 30 seconds in at least one of 2 trials.
Participants were also administered the Jacobson Dizziness Handicap Inventory (DHI) (16), which is a 25-point validated tool that categorizes the self-perceived handicap associated with dizziness in four groups: no handicap, mild handicap, moderate handicap, and severe handicap (16).
Statistical Analysis
Descriptive statistics were used to describe the prevalence of abnormalities on vestibular tests. Comparisons were made between participants age 70 to 79 versus 80 years and older. Pearson’s chi-square test was used to compare proportions; the Fisher’s exact test was used for cell counts less than 5. The DHI scores were not normally distributed based on the Shapiro-Wilk test; therefore, the nonparametric Mann-Whitney U test was used to compare mean DHI scores. The p value was set at 0.05 to define significance. SPSS Statistics release version 17.0 (Chicago, IL, USA) was used for all analyses.
RESULTS
Fifty subjects with a mean age of 77.2 ± 6.1 years (range, 70–95 yr) participated in the study. Male subjects comprised 48%; 88% of participants were white, and 12% were black.
The MRT was the most prevalent abnormal test (68%; Table 1). A positive left and right horizontal HIT was present in 44% and 36% of subjects, respectively. Thirty percent of participants had bilateral positive horizontal HIT, and 50% of participants had either an abnormal right or left horizontal HIT. The prevalence of vertical canal (superior and posterior) HIT abnormalities was much lower. We observed that 83% of those with a positive right horizontal HIT also had a positive left horizontal HIT (OR, 17.857; p < 0.001). Additionally, we found that all of participants with a positive bilateral horizontal HIT also failed the MRT (p < 0.05). Less prevalent signs were an abnormal bucket test (18%) and head-shaking nystagmus (2%). No participant in this cohort had spontaneous nystagmus (Table 1). Table 2 compares the prevalence of vestibular test between 70- and 79-year-olds and 80-year-olds and older. Abnormal testing on the MRT and the HIT (right horizontal and right posterior HIT) were significantly more prevalent in those aged 80 and older compared with those aged 70 to 79 years (p < 0.05).
TABLE 1. Prevalence of abnormal clinical tests.
| Test | Prevalence n (%) |
|---|---|
| Spontaneous nystagmus | 0 (0) |
| Head shaking nystagmus | 1 (2) |
| Right horizontal HIT | 18 (36) |
| Left horizontal HIT | 22 (44) |
| Right superior HIT | 3 (6) |
| Left superior HIT | 2 (4) |
| Right posterior HIT | 2 (4) |
| Left posterior HIT | 6 (12) |
| Bilateral horizontal HIT | 15 (30) |
| Any horizontal HIT | 25 (50) |
| Condition 4 of the modified Romberg test | 34 (68) |
| Bucket test | 9 (18) |
Condition 4 of the modified Romberg test: abnormal if not able to stand on a foam-padded cushion with eyes closed at least 30 seconds in 1 of 2 trials. Spontaneous nystagmus: abnormal if spontaneous nystagmus was present. Head-shaking nystagmus: abnormal if nystagmus was present after shaking head for 40 seconds. Bucket test: abnormal if the average of 5 trials was more than ±3 degrees. HIT; Head Impulse test: Abnormal if corrective saccade occurs after passive head impulse in the plane of the canal.
TABLE 2. Prevalence of abnormal clinical vestibular tests by age group.
| Test | Abnormal test in 70 to 79 age group, n (%) | Abnormal test in ≥80 age group, n (%) | p |
|---|---|---|---|
| Spontaneous nystagmus | 0 (0) | 0 (0) | NA |
| Head-shaking nystagmus | 1 (3) | 0 (0) | 1.000 |
| Right horizontal HIT | 11 (28) | 7 (64) | 0.041 |
| Left horizontal HIT | 15 (39) | 7 (64) | 0.178 |
| Right superior HIT | 3 (8) | 0 (0) | 1.000 |
| Left superior HIT | 2 (5) | 0 (0) | 1.000 |
| Right posterior HIT | 0 (0) | 2 (18) | 0.045 |
| Left posterior HIT | 3 (8) | 3 (27) | 0.111 |
| Bilateral horizontal HIT | 9 (23) | 6 (55) | 0.052 |
| Any horizontal HIT | 17 (44) | 22 (57) | 0.088 |
| Condition 4 of the modified Romberg test | 23 (59) | 11 (100) | 0.010 |
| Bucket test | 7 (18) | 2 (18) | 1.000 |
Significant p values (p < 0.05) are marked in bold. Condition 4 of the modified Romberg test: abnormal if not able to stand on a foam-padded cushion with eyes closed at least 30 seconds in 1 of 2 trials. Spontaneous nystagmus: abnormal if spontaneous nystagmus was present. Head-shaking nystagmus: abnormal if nystagmus was present after shaking head for 40 seconds. Bucket test: abnormal if the average of 5 trials was more than ±3 degrees. HIT; Head Impulse test: abnormal if corrective saccade occurs after passive head impulse in the plane of the canal.
The mean DHI score among the 50 participants was 5.6 ± 11.2, which lies within the “no dizziness handicap” category. Figure 1 shows the distribution of participants by DHI category. Only participants who failed the MRT and who had abnormal left superior HIT had significantly poorer DHI scores compared with those without abnormalities on those tests (p < 0.05).
FIG. 1.
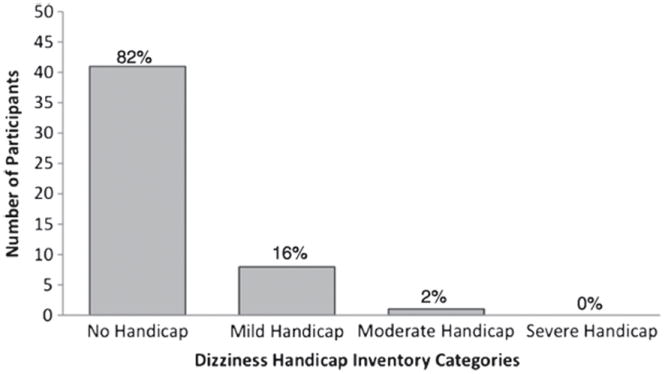
Frequency of self-perceived dizziness handicap by category (none, mild, moderate, and severe handicap) in a cohort of 50 community-dwelling older adults aged 70 and older.
DISCUSSION
These data support the epidemiologic findings discussed previously that loss of vestibular function appears to be prevalent among older individuals (6,17). Using a more specific vestibular clinical test, the HIT, we observed a 30% to 40% prevalence of horizontal semicircular canal impairment in this cohort. This prevalence increased to 64% among individuals age 80 years and older. Our findings on the MRT in this cohort are comparable to the population-based study described previously: we observed a failure rate of 59% compared with 69% among 70- to 79-year-olds and a failure rate of 100% compared with 85% among 80-year-olds and older. Interestingly, we observed that all patients with bilateral positive horizontal HIT failed the MRT, suggesting the ability to maintain upright stance with eyes closed on a foam pad requires adequate functioning of the horizontal semicircular canals bilaterally.
We found that loss of semicircular canal function was more prevalent than loss of otolith function—specifically utricular function (measured with the bucket test). Analyses of age-related changes in the temporal bone have also reported a predominance of semicircular canal relative to otolith abnormalities (4), and specifically, a preservation of utricular structures has been observed (18,19). As might be expected, the age-related vestibular abnormalities we observed appear to be bilateral, as evidenced by the lack of spontaneous nystagmus or head-shaking nystagmus and the propensity for bilateral horizontal HIT abnormalities (2,10).
It should be noted that, despite the high prevalence of abnormalities on vestibular clinical testing in this community-dwelling sample, the perceived handicap as measured by the DHI was minimal. It is possible that the DHI is not sufficiently sensitive to identify levels of vestibular impairment not associated with specific vestibular pathologies (e.g., Méniére’s disease and benign paroxysmal positional vertigo). Alternatively, healthy older individuals may have compensated for their vestibular deficiency and may not experience an associated functional impairment.
Limitations of this study include the small sample size. Moreover, vestibular clinical tests with binary outcomes (normal versus abnormal) were used as measures of vestibular function. Nevertheless, this study provides insight into the expected performance of normative older adults on vestibular tests commonly administered in the neurotology clinic. Further studies using newly available clinical testing methods (e.g., video HIT) may identify finer gradations of vestibular function in older individuals and the levels of vestibular loss associated with functional impairment.
Acknowledgments
This work was supported by the American Neurotology Society Silverstein Award (Y. A.) and the Older Americans Independence Center Pilot Core Grant (Y. A.).
Footnotes
The authors disclose no conflicts of interest.
References
- 1.Baloh RW, Enrietto J, Jacobson KM, Lin A. Age-related changes in vestibular function: a longitudinal study. Ann N Y Acad Sci. 2001;942:210–9. doi: 10.1111/j.1749-6632.2001.tb03747.x. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal Y, Zuniga MG, Davalos-Bichara M, et al. Decline in semicircular canal and otolith function with age. Otol Neurotol. 2012;33:832–9. doi: 10.1097/MAO.0b013e3182545061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basta D, Singbartl F, Todt I, Clarke A, Ernst A. Vestibular rehabilitation by auditory feedback in otolith disorders. Gait Posture. 2008;28:397–404. doi: 10.1016/j.gaitpost.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Rosenhall U. Degenerative patterns in the aging human vestibular neuro-epithelia. Acta Otolaryngol. 1973;76:208–20. doi: 10.3109/00016487309121501. [DOI] [PubMed] [Google Scholar]
- 5.Mhoon EE, Bernstein LP, Towle VL. Saccular influence on the otolith-spinal reflex and posture during sudden falls of the cat. Am J Otol. 1997;18:86–92. [PubMed] [Google Scholar]
- 6.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med. 2009;169:938–44. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Falls among older adults: an overview. 2012 Dec 2; [Google Scholar]
- 8.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction of balance. Suggestion from the field. Phys Ther. 1986;66:1548–50. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 9.Cohen H, Blatchly CA, Gombash LL. A study of the clinical test of sensory interaction and balance. Phys Ther. 1993;73:346–51. doi: 10.1093/ptj/73.6.346. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi S, Fetter M, Koenig E, Dichgans J. The clinical significance of head-shaking nystagmus in the dizzy patient. Acta Otolaryngol. 1990;109:8–14. doi: 10.3109/00016489009107409. [DOI] [PubMed] [Google Scholar]
- 11.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–9. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 12.Zwergal A, Rettinger N, Frenzel C, Dieterich M, Brandt T, Strupp M. A bucket of static vestibular function. Neurology. 2009;72:1689–92. doi: 10.1212/WNL.0b013e3181a55ecf. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Diabetes, vestibular dysfunction, and falls: analyses from the National Health and Nutrition Examination Survey. Otol Neurotol. 2010;31:1445–50. doi: 10.1097/MAO.0b013e3181f2f035. [DOI] [PubMed] [Google Scholar]
- 14.Schubert MC, Tusa RJ, Grine LE, Herdman SJ. Optimizing the sensitivity of the head thrust test for identifying vestibular hypofunction. Phys Ther. 2004;84:151–8. [PubMed] [Google Scholar]
- 15.Dieterich M, Brandt T. Ocular torsion and tilt of subjective visual vertical are sensitive brainstem signs. Ann Neurol. 1993;33:292–9. doi: 10.1002/ana.410330311. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424–7. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal Y, Carey JP, Hoffman HJ, Sklare DA, Schubert MC. The modified Romberg Balance Test: normative data in U.S. adults. Otol Neurotol. 2011;32:1309–11. doi: 10.1097/MAO.0b013e31822e5bee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igarashi M, Saito R, Mizukoshi K, Alford BR. Otoconia in young and elderly persons: a temporal bone study. Acta Otolaryngol Suppl. 1993;504:26–9. doi: 10.3109/00016489309128117. [DOI] [PubMed] [Google Scholar]
- 19.Johnsson LG, Hawkins JE., Jr Sensory and neural degeneration with aging, as seen in microdissections of the human inner ear. Ann Otol Rhinol Laryngol. 1972;81:179–93. doi: 10.1177/000348947208100203. [DOI] [PubMed] [Google Scholar]


