Abstract
In this post-genomic era, genome-wide functional analysis is indispensable. The recent development of RNA interference techniques has enabled researchers to easily analyze gene function even in non-model organisms. On the other hand, little progress has been made in the identification and functional analyses of cis-regulatory elements in non-model organisms. In order to develop experimental platform for identification and analyses of cis-regulatory elements in a non-model organism, in this case, the ladybird beetle, Harmonia axyridis, we established transgenic transposon-tagged lines using a novel composite vector. This vector enables the generation of two types of insertion products (jumpstarter and mutator). The jumpstarter portion carries a transposase gene, while the mutator segment carries a reporter gene for detecting enhancers. The full-composite element is flanked by functional termini (required for movement); however, the mutator region has an extra terminus making it possible for the mutator to remobilize on its own, thus leaving an immobile jumpstarter element behind. Each insertion type is stable on its own, but once crossed, jumpstarters can remobilize mutators. After crossing a jumpstarter and mutator line, all tested G2 females gave rise to at least one new insertion line in the next generation. This jumping rate is equivalent to the P-element-mediated jumpstarter method in Drosophila. These established transgenic lines will offer us the ideal experimental materials for the effective screening and identification of enhancers in this species. In addition, this jumpstarter method has the potential to be as effective in other non-model insect species and thus applicable to any organism.
Introduction
For the last two decades, our knowledge of gene function in non-model organisms has dramatically increased [1]. Methodological advances in RNA interference (RNAi) enabled researchers to address various biologically interesting phenomena via gene knock down even in non-model organisms [2], [3]. On the other hand, there are very few studies on gene function via over expression or ectopic expression in non-model organisms (but see [4]). Transgenic techniques have often been used for over/ectopic expression analyses [3], [5], [6]. However, limited knowledge of cis-regulatory elements including enhancers in non-model organisms prevents studies from further progress [3], [6], [7]. Enhancer-trapping is an excellent method to detect enhancers in the genome following the expression pattern of a reporter gene [8]. Although the generation of enhancer trap lines by microinjection is time-consuming and labor-intensive, the jumpstarter method allows transposon-tagged lines to be generated by simply crossing two lines, thereby enabling genome-wide assays. In Drosophila, thousands of enhancer-trap lines have been established based on this method [9], [10]. Thus, an essential technique for the genome-wide analysis of gene function is the jumpstarter method, which was initially developed for insertional mutagenesis in Drosophila [11], [12]. This approach allows transposon-tagged lines to be generated en mass by simply crossing a jumpstarter line, containing a transposase gene, with a mutator line, containing a transposable element consisting of two inverted terminal repeat (ITR) sequences (5′ and 3′). The transposon requires both elements–the transposase and two ITRs–for mobilization. However, this valuable method has so far been confined to a few model organisms, for example, in ascidians [13], insects [11], [12], [14], [15], [16], vertebrates [17], [18], [19] and plants [20], because of difficulties associated with the generation of a jumpstarter strain. In particular, an immobilized jumpstarter element has generally thought impossible to generate with a single transposon, with the exception of a special case in which an exogenous DNA fragment without a transposable element was integrated into a mouse genome. Furthermore, even in Drosophila, immobilized jumpstarter elements have been produced accidentally through imprecise excision by the P element itself [11], [12]. To overcome these problems, we applied the transgene stabilization method [21] to a composite piggyBac vector that simultaneously generates two elements that can be used for the jumpstarter technique. This transposon-based transformation vector has an additional 5′ ITR [21]. When 5′ and 3′ ITRs at both ends of the vector are used for transposition, the entire vector is integrated into the genome. By contrast, when the ITR in the middle of the vector is used, only one-half of the vector is integrated; in this case, generating a mutator designed for enhancer trapping. After the integration of the entire vector, the transposase acts between the 5′ and 3′ ITRs, and one-half of the vector is excised leaving the transposase gene with only one 5′ ITR, thereby immobilizing it.
In order to develop an experimental platform for future identification of various enhancers, here we established transgenic transposon-tagged lines in ladybird beetle, Harmonia axyridis. By crossing two established lines (jumpstarter line and mutator line) we will be able to generate various enhancer- trap lines in an efficient manner.
Materials and Methods
Insects
Laboratory stocks of Harmonia axyridis were derived from field collections in Aichi, Japan. They were reared as described by [22].
Construction of piggyBac Vector
A Bgl II-Asc I double digested fragment containing EGFP with the nuclear localization signal (NLS) under the Drosophila heat shock protein 27 (Dmhsp27) minimal promoter was isolated from pSL1180fa[tetO-hsp27mp-NLS-EGFP] as described in [23] and was filled in with a Klenow fragment, cloned into the filled-in site of Asc I of pBac[3xP3-DsRed] [24] and named pBac[3xP3-DsRed, hsp27mp-NLS-EGFP]. A Sac I-Xba I double digested fragment containing 3xP3-ECFP was isolated from pBac[3xP3-ECFPafm] [25] and was filled in with a Klenow fragment, cloned into the filled-in site of EcoR I of pBac[3xP3-DsRed, hsp27mp-NLS-EGFP] and named pBac[3xP3-DsRed, hsp27mp-NLS-EGFP, 3xP3-ECFP]. An Ase I fragment containing the piggyBac transposase under Drosophila heat shock protein 70 (Dmhsp70) promoter from phsp-pBac [26] was filled in with a Klenow fragment, and cloned into the filled-in site of Asc I of pBac[3xP3-DsRed, hsp27mp-NLS-EGFP, 3xP3-ECFP]. This composite vector was named pBac(hsp70-transposase)::(3xP3-ECFP)::hsp27-EGFP::(3xP3-DsRed).
Generation of Transgenic Ladybird Beetles
Transgenic ladybird beetles were generated by microinjection of 500 ng/µl composite vector without a helper plasmid into the posterior pole of embryos during the syncytium stage of development as described by [27]. The G0 adults were crossed with non-injected wild type adults. The G1 newly hatched larvae were examined under a fluorescent stereomicroscope. ECFP, DsRed and EGFP fluorescence was observed using a fluorescent stereomicroscope (MZ FLIII, Leica) equipped with a CFP, a DsRED and a GFP2 filter (Leica), respectively. Detected fluorescent red colors were converted to magenta using DPcontroller software (Olympus Optical Co.) in order to increase visibility for potential color blind readers.
Inverse PCR
Inverse PCR for a mutator strain was performed as described by [27]. Genomic DNA were digested with HaeIII or MspI. PCR was performed using the primers described in [28].
Cloning
Total RNA was extracted from 0 to 2-day-old Harmonia eggs with Trizol (Gibco BRL) according to the manufacture’s instructions. The first-strand cDNA was synthesized with SMART PCR cDNA Amplification Kit (Clontech) using 1 µg of total RNA. Harmonia axyridis ribosomal protein 49 (Ha-rp49) cDNA fragments were amplified using rp49-1 and rp49-2 primer sets. The following degenerate primers were designed corresponding to highly conserved amino acid sequences found in Rp49 among D. melanogaster Rp49 (U92431), mouse Rp49 (M23453), rat Rp49 (X06483) and yeast Rp49 (Y13134).
Degenerate primers for rp49.
rp49-1: 5′-ACIAARMAITTYATIMGICA-3′
rp49-2: 5′-TGIGCIATYTCISCRCARTA-3′
(R, A and G; Y, T and C; S, C and G; M, C and A; I, inosine).
PCRs were performed using 2.5 µl of the 10-fold diluted first-strand cDNA, a pair of primers from the list above, and AmpliTaq Gold (Perkin Elmer).
Sequencing and Sequence Analysis
The PCR product was subcloned into the EcoR V site of the pBluescript KS+ vector (Stratagene). The nucleotide sequences of the PCR products and the flanking regions around the restriction enzyme-digested fragments, which were inserted into the vectors during cloning procedures, were confirmed using the dideoxy chain-termination method by an automatic DNA sequencer (CEQ 2000XL; Beckman Coulter or DNA sequencer 3130 genetic analyser; Applied Biosystems). Sequence analysis was carried out using a DNASIS system (Hitachi Software Engineering).
Sequence Accession Number
DDBJ/EMBL/GenBank accession numbers for Ha-rp49 cDNA is AB552923.
RT-PCR
H. axyridis 0-day-old pupa of a jumpstarter strain or a wild type was used for total RNA extractions. Total RNA was treated with 10 U DNase I for 30 min at 37°C. 500 ng of DNase I treated total RNA was used for first-strand cDNA syntheses as described above. A reaction without reverse transcriptase (RT) was performed with cDNA synthesis and was used as a negative control for the RT-PCR experiment. The PCR cycle numbers are 35 cycles for transposase and Ha-rp49. The following primers were used. Ha-rp49 was used as an internal control. Effect of heat shock on transposase expression has also investigated in differential heat shock temperatures H. axyridis 0-day-old pupae of a jumpstarter strain were exposed to heat shock for 1 h at 25°C, 30°C, 35°C, 40°C and 45°C. Exposed pupae were used for RNA extraction.
Gene-specific primer for transposase.
RT-transposase: 5′-CATCGTTTTCTCGAAGTGTGGGCCG-3′
PLF: 5′-CTTGACCTTGCCACAGAGGACTATTAGAGG-3′
Gene-specific primer for Ha-rp49.
Ha-rp49-#1: 5′-GCGATCGCTATGGAAAACTC-3′
Ha-rp49-#2: 5′-TACGATTTTGCATCAACAGT-3′
PCR for Analyzing Mobilization
PCR were performed using extracted genome DNA as template. Sequence of PLR was according to [28]. Primers HaG1 and HaG3 were designed from original genome integrated site of mutator.
HaG1: 5′-AAGCCGAAGTAACAATTGAAATGTCACTGC-3′
HaG3: 5′-TGATCGTGATGAAGCTTCTCCCTATGCTGC-3′
Results and Discussion
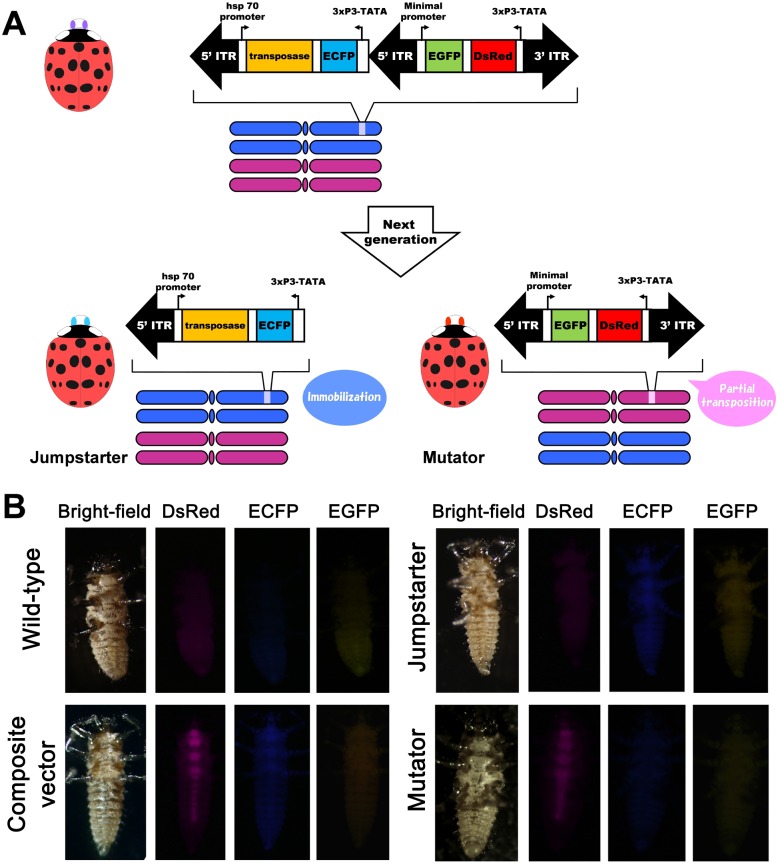
The composite vector is designed to produce an immobilized jumpstarter element and a mutator (Fig. 1A). The left half contains a 5′ ITR and a transposase gene; the right half contains both 5′ and 3′ ITRs. First, the composite vector is integrated into a chromosome. Transposase acts to remobilize the right half of the vector (5′ and 3′ ITRs) and integrate it elsewhere in the genome. This part is used as a mutator. The remainder, the left half with the 5′ ITR and transposase gene, are no longer mobile and remain in the original insertion site. This part is used as a jumpstarter.
Figure 1. Schematic of composite vector and overview of genomic integration.
(A) The left half of the composite vector contains a 5′ ITR, a transposase gene under the control of the Dmhsp70 promoter and the 3xP3-ECFP transformation marker, while the right half contains both 5′ and 3′ ITRs, and is marked by both an EGFP gene under the control of the Dmhsp27 minimal promoter for enhancer trapping and a 3xP3-DsRed transformation marker. The transposase gene can be immobilized by the precise excision of the sequence between the central 5′ ITR and the 3′ ITR; in this case, larvae expressed ECFP alone. (B) Transformation markers expressed in the CNS of first instar larvae of H. axyridis indicate vector integration. From left to right, bright field, DsRed, ECFP and EGFP are presented in wild-type, composite vector (DsRed and ECFP are expressing), Jumpstarter (only ECFP is expressing) and Mutator (only DsRed is expressing). EGFP expression is not detected in all of strains. All panels display a ventral view (anterior uppermost).
In this study, we constructed a piggyBac-based transposon vector, pBac(hsp70-transposase)::(3xP3-ECFP)::hsp27-EGFP::(3xP3-DsRed), containing the piggyBac transposase gene under the control of Drosophila heat shock protein 70 (Dmhsp70) promoter [26] as the jumpstarter element and the Enhanced Green Fluorescent Protein (EGFP) gene under the hsp 27 minimal promoter as the mutator element (Fig. 1B) to detect a genomic enhancer. The Enhanced Cyan Fluorescent Protein (ECFP) gene and Discosoma Red Fluorescent Protein (DsRed) gene under the control of the 3xP3 element, which promotes expression in visual systems and the central nervous system (CNS) [29], were used as transformation markers for the jumpstarter and mutator, respectively. Two types of integration into the genome in G1 were obtained by microinjection of the vector into early embryos (G0): G1 larvae expressing both DsRed and ECFP confirmed integration of the entire composite element using the left end of the 5′ and 3′ ITRs, and larvae expressing DsRed alone indicated integration of the right half of the vector using the middle of the 5′ and 3′ ITRs.
To examine the utility of the vector carrying the composite element, we generated transgenic ladybird beetles. The transformation efficiency of the vector was 2.3% (Table 1) and five G0 individuals produced offspring that expressed both DsRed and ECFP (Fig. 1B). Three individuals also gave rise to progeny expressing DsRed alone (Fig. 1B), which were used as a mutator. Single transgenic G1 individuals that expressed both ECFP and DsRed markers were crossed with wild-type. In the G2 generation, larvae expressing ECFP alone were obtained and used as the potential jumpstarters (Fig. 1B). The precise excision of the right half of the vector was confirmed using inverse polymerase chain reaction (PCR) (data not shown). The jumpstarter element was very stable and has been subsequently maintained for 17 generations over eleven years. Insertions possessing the full composite element could possiblly move as a single unit, or because of the preference for local movement of piggyBac, even if the jumpstarter and mutator are no longer a single unit, they could be too close together to detect remobilization via our selection method.
Table 1. Transformation efficiency of piggyBac vector.
| Injected embryos | Hatched larvae | Eclosed adults | Fertile adults | Fertile adults given transgenic insects | Transformation efficiency (%) |
| 914 | 465 | 299 | 219 | 5 | 2.3 |
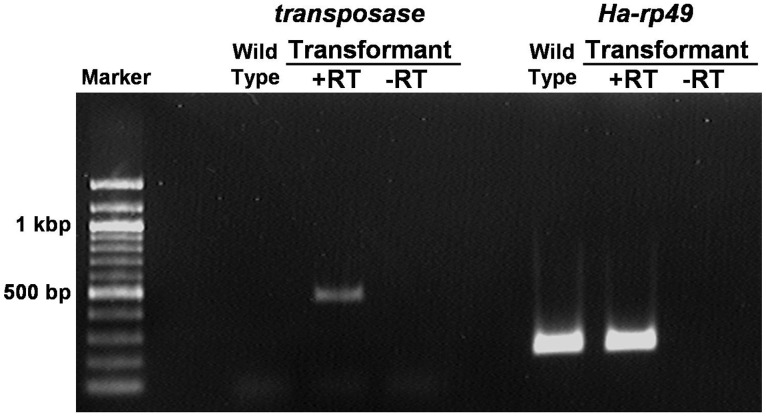
Next, RT-PCR analysis of the jumpstarter line was performed to confirm expression of transposase mRNA. When pupae were reared at a constant temperature of 25°C, transposase mRNA was expressed (Fig. 2). This basal expression of the transposase gene in the absence of heat shock resulted in sufficient activity for transposition in the germline. To determine the heat shock conditions required for the highest expression of the transposase gene by the Dmhsp70 promoter in Harmonia, pupae were exposed to 1-h heat shocks at several temperatures. The highest expression of transposase mRNA was observed after a heat shock at 40°C (Figure S1), suggesting that the Dmhsp70 promoter is heat-inducible in Harmonia.
Figure 2. Reverse transcriptase PCR (RT-PCR) analysis of piggyBac transposase expression.
Total RNA was extracted from transgenic pupae that expressed only the ECFP marker, and from wild-type pupae reared at 25°C. A reaction without reverse transcriptase (-RT) was performed with cDNA synthesis as a negative control. Harmonia axyridis ribosomal protein 49 (Ha-rp49) was used as an internal control.
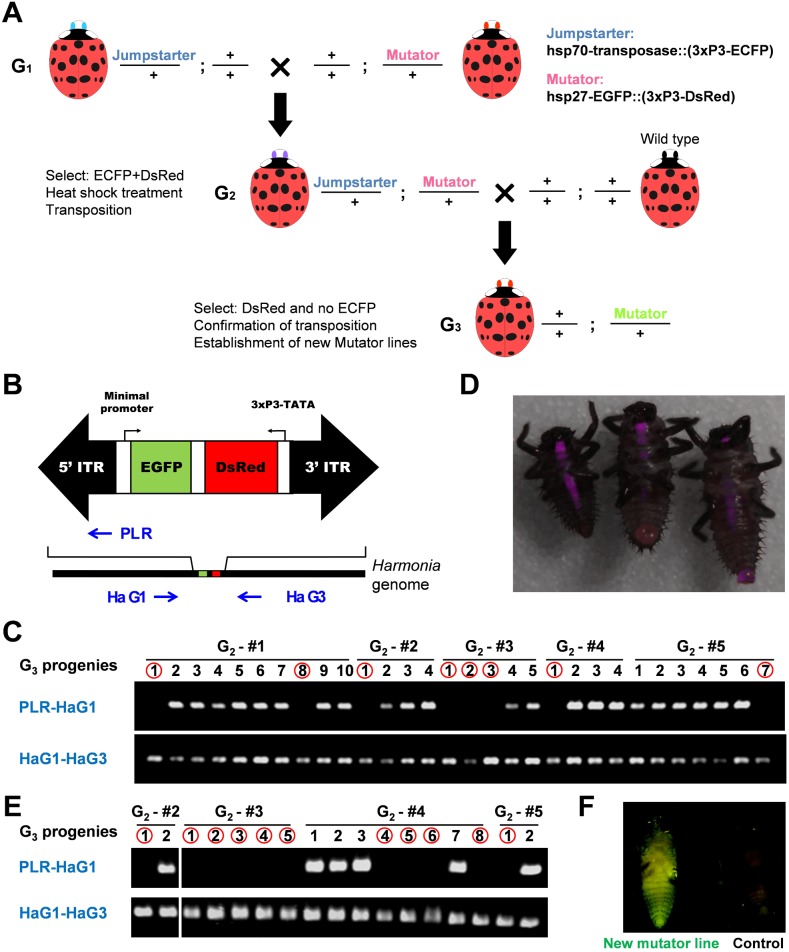
We successfully implemented the jumpstarter method in the ladybird beetles (Fig. 3A). After crossing the jumpstarter line with the mutator line (Fig. 3A, G 1 cross), newly hatched larvae carrying both elements were selected according to the presence of transformation markers.
Figure 3. Jumpstarter method in Harmonia axyridis.
(A) Crossing scheme for jumpstarter method. A mutator line and a jumpstarter line are crossed (G1). Newly hatched larvae expressing both ECFP and DsRed transformation markers in the CNS were selected from the progeny (G2). Larvae carrying both the mutator and jumpstarter elements were heat shocked to induce transposase expression. Newly hatched larvae expressing only the DsRed transformation marker were selected from the progeny (G3), and analyzed by PCR for mobilization of the mutator (i.e. integration elsewhere in the genome). Each unique insertion line was used to establish a new mutator line. (B) Schematic representation of PCR method to analyze mobilization of the original mutator element. Primer set HaG1 and PLR was used to detect the original non-mobilized mutator element. If the original mutator element is remobilized, no PCR product will be detected. Primer set HaG1-HaG3 was used as a positive control to detect the homologous, mutator element-free chromosome. (C) Red circles denote progeny with apparent remobilization events. PCR amplification with the PLR-HaG1 primer set was not detected in progeny marked with a red circle, because the original mutator element was mobilized to other genomic sites. These larvae were established as new mutator lines. Each G2 female gave at least one newly mobilized progeny. (D) Comparison of DsRed fluorescence between original mutator line (middle) and two new mutator lines (left and right). Compare to original mutator line (middle), two new mutator line larvae show higher (left) or lower (right) expression of DsRed. (E) PCR analysis of mutator element mobilization. Newly hatched G3 larvae strongly or weakly expressing DsRed marker compared with original mutator line were selected and subjected to PCR analysis. PCR amplification with the PLR-HaG1 primer set was not detected in progeny marked with a red circle. In this case, detection efficiency of remobilized mutator element is increased compared with random selection (see result in C). (F) An enhancer-trap line of transgenic ladybird beetles. During the generation of mobilized mutator lines using the jumpstarter method, an enhancer expressing GFP throughout the body was detected (left); a wild-type control larva is also shown on the right. Panel displays a ventral view (anterior uppermost).
Since all adult males were sterile, mostly owing to the heat sensitivity of spermatogenesis, five females were individually crossed to wild-type males (Fig. 3A, G 2 cross) and G3 progenies selected for expression of DsRed, but absence of CFP expression. Any such progeny should carry only the mutator element and were randomly selected for PCR transposition analysis (Fig. 3B). Jumping rate was 100%, that is, all five G2 females gave rise to at least one new insertion line in G3 progeny (Fig. 3C). In total, 27% of progeny without a jumpstarter element contained a new insertion of the mutator element; this is on par with the P-element-mediated jumpstarter method used in Drosophila [30].
Further, to increase the efficiency of establishing new mutator lines, we tested the possibility of selecting larvae with new insertions according to the strength of DsRed fluorescence. The position effect acting on genome insertion sites affects expression of the transformation marker, so by selecting larvae with higher or lower DsRed marker expression compared with that of the original mutator (Fig. 3D), we increased the efficiency of selection to 62% (Fig. 3E). In addition to this, we also confirmed remobilization by inverse PCR (Fig. 4). Furthermore, an enhancer-trap line expressing EGFP fluorescence throughout the body was generated (Fig. 3F). Such newly established enhancer-trap line with easily detectable marker also can be used as new mutator line.
Figure 4. Results of inverse PCR for confirming remobilization of mutator.

We performed inverse PCR using extracted DNA from original mutator individual and G4 individuals. Each G4 individual was derived from G2 individuals (#2–5, described in Fig. 3E). Each mutator inserted to different position in each genome.
However, our examination of several larval stages did not detect EGFP expression in other mutator lines. This suggests that the minimal promoter used in this study was not suitable for enhancer detection in Harmonia, as the choice of minimal promoter is crucial in Bombyx [31].
As shown in this study, we have successfully established stable transgenic mutator and jumpstarter lines. The standard methods for establishing jumpstarter and mutator lines requires researchers to perform two independent transformations. The strength of the method described here is the ability to generate both jumpstarter and mutator lines with a single transformation. In addition, we demonstrated the potential for the development of various enhancer-trap lines by crossing both transformed lines. This is the first report of a single transformation vector being used to generate separate helper and donor elements for functional analyses in non-model insects. Especially, ladybird beetles are used as study material in both of basic natural science (e.g. color pattern polymorphism) [32], [33] and applied sciences (e.g. biological control) [34]. Also, this method can be applied to the transgenic analyses of any organism using any two-component system.
Supporting Information
Effect of differential heat shock temperature on piggyBac transposase expression. A reaction without reverse transcriptase (-RT) was performed with cDNA synthesis as a negative control. Harmonia axyridis ribosomal protein 49 (Ha-rp49) was used as an internal control.
(DOC)
Acknowledgments
We thank Y. Sato, M. Kobayashi and M. Ikeda for helpful discussions. We are grateful to E. A. Wimmer for the supply of vectors. We also appreciate Robert A. Zinna for his English grammar corrections on the manuscript.
Funding Statement
This research was supported in part by Formation and Recognition, PRESTO, Japan Science and Technology Agency, Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan, the Japan Society for the Promotion of Science Research for the Future and Grant-in-Aid (BDP) from the Ministry of Agriculture, Forestry and Fisheries. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Müller GB (2007) Evo–devo: extending the evolutionary synthesis. Nat Rev Genet 8: 943–949. [DOI] [PubMed] [Google Scholar]
- 2. Brown SJ, Mahaffey JP, Lorenzen MD, Denell RE, Mahaffey JW (1999) Using RNAi to investigate orthologous homeotic gene function during development of distantly related insects. Evol Dev 1: 11–15. [DOI] [PubMed] [Google Scholar]
- 3. Wimmer EA (2003) Applications of insect transgenesis. Nat Rev Genet 4: 225–232. [DOI] [PubMed] [Google Scholar]
- 4. Lewis DL, DeCamillis MA, Brunetti CR, Halder G, Kassner VA, et al. (1999) Ectopic gene expression and homeotic transformations in arthropods using recombinant Sindbis viruses. Curr Biol 9: 1279–1287. [DOI] [PubMed] [Google Scholar]
- 5. Horn C, Wimmer EA (2000) A versatile vector set for animal transgenesis. Dev Genes Evol 210: 630–637. [DOI] [PubMed] [Google Scholar]
- 6. Masumoto M, Ohde T, Shiomi K, Yaginuma T, Niimi T (2012) A baculovirus immediate-early gene, ie1, promoter drives efficient expression of a transgene in both Drosophila melanogaster and Bombyx mori . PLoS One 7: e49323 http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0049323 Accessed 25 February 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wittkopp PJ, Kalay G (2012) Cis-regulatory elements: molecular mechanisms and evolutionary processes underlying divergence. Nat Rev Genet 13: 59–69. [DOI] [PubMed] [Google Scholar]
- 8. O’Kane CJ, Gehring WJ (1987) Detection in situ of genomic regulatory elements in Drosophila . Proc Natl Acad Sci U S A 84: 9123–9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gerlitz O, Nellen D, Ottiger M, Basler K (2002) A screen for genes expressed in Drosophila imaginal discs. Int J Dev Biol 46: 173–176. [PubMed] [Google Scholar]
- 10. Hayashi S, Ito K, Sado Y, Taniguchi M, Akimoto A, et al. (2002) GETDB, a database compiling expression patterns and molecular locations of a collection of Gal4 enhancer traps. Genesis 34: 58–61. [DOI] [PubMed] [Google Scholar]
- 11. Cooley L, Kelley R, Spradling A (1988) Insertional mutagenesis of the Drosophila genome with single P elements. Science 239: 1121–1128. [DOI] [PubMed] [Google Scholar]
- 12. Robertson HM, Preston CR, Phillis RW, Johnson-Schlitz DM, Benz WK, et al. (1988) A stable genomic source of P element transposase in Drosophila melanogaster . Genetics 118: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sasakura Y, Konno A, Mizuno K, Satoh N, Inaba K (2008) Enhancer detection in the ascidian Ciona intestinalis with transposase-expressing lines of Minos . Dev Dyn 237: 39–50. [DOI] [PubMed] [Google Scholar]
- 14. Horn C, Offen N, Nystedt S, Häcker U, Wimmer EA (2003) piggyBac-based insertional mutagenesis and enhancer detection as a tool for functional insect genomics. Genetics 163: 647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lorenzen MD, Kimzey T, Shippy TD, Brown SJ, Denell RE, et al. (2007) piggyBac-based insertional mutagenesis in Tribolium castaneum using donor/helper hybrids. Insect Mol Biol 16: 265–275. [DOI] [PubMed] [Google Scholar]
- 16. Uchino K, Sezutsu H, Imamura M, Kobayashi I, Tatematsu K, et al. (2008) Construction of a piggyBac-based enhancer trap system for the analysis of gene function in silkworm Bombyx mori . Insect Biochem Mol Biol 38: 1165–1173. [DOI] [PubMed] [Google Scholar]
- 17. Dupuy AJ, Fritz S, Largaespada DA (2001) Transposition and gene disruption in the male germline of the mouse. Genesis 30: 82–88. [DOI] [PubMed] [Google Scholar]
- 18. Horie K, Kuroiwa A, Ikawa M, Okabe M, Kondoh G, et al. (2001) Efficient chromosomal transposition of a Tc1/mariner-like transposon Sleeping Beauty in mice. Proc Natl Acad Sci U S A 98: 9191–9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zagoraiou L, Drabek D, Alexaki S, Guy JA, Klinakis AG, et al. (2001) In vivo transposition of Minos, a Drosophila mobile element, in mammalian tissues. Proc Natl Acad Sci U S A 98: 11474–11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Long D, Martin M, Sundberg E, Swinburne J, Puangsomlee P, et al. (1993) The maize transposable element system Ac/Ds as a mutagen in Arabidopsis: identification of an albino mutation induced by Ds insertion. Proc Natl Acad Sci U S A 90: 10370–10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Handler AM, Zimowska GJ, Horn C (2004) Post-integration stabilization of a transposon vector by terminal sequence deletion in Drosophila melanogaster . Nat Biotechnol 22: 1150–1154. [DOI] [PubMed] [Google Scholar]
- 22. Niimi T, Kuwayama H, Yaginuma T (2005) Larval RNAi applied to the analysis of postembryonic development in the ladybird beetle, Harmonia axyridis . J Insect Biotechnol Sericol 74: 95–102. [Google Scholar]
- 23. Hara K, Kuwayama H, Yaginuma T, Niimi T (2008) Establishment of a tetracycline-Off system using a piggyBac-based vector as a gene functional analysis tool for the temporal targeting of gene expression. J Insect Biotechnol Sericol 77: 159–166. [Google Scholar]
- 24. Horn C, Schmid BGM, Pogoda FS, Wimmer EA (2002) Fluorescent transformation markers for insect transgenesis. Insect Biochem Mol Biol 32: 1221–1235. [DOI] [PubMed] [Google Scholar]
- 25. Horn C, Wimmer EA (2000) A versatile vector set for animal transgenesis. Dev Genes Evol 210: 630–637. [DOI] [PubMed] [Google Scholar]
- 26. Handler AM, Harrell RA (1999) Germline transformation of Drosophila melanogaster with the piggyBac transposon vector. Insect Mol Biol 8: 449–457. [DOI] [PubMed] [Google Scholar]
- 27. Kuwayama H, Yaginuma T, Yamashita O, Niimi T (2006) Germ-line transformation and RNAi of the ladybird beetle, Harmonia axyridis . Insect Mol Biol 15: 507–512. [DOI] [PubMed] [Google Scholar]
- 28. Hediger M, Niessen M, Wimmer EA, Dübendorfer A, Bopp D (2001) Genetic transformation of the housefly Musca domestica with the lepidopteran derived transposon piggyBac . Insect Mol Biol 10: 113–119. [DOI] [PubMed] [Google Scholar]
- 29. Berghammer AJ, Klinger M, Wimmer EA (1999) A universal marker for transgenic insects. Nature 402: 370–371. [DOI] [PubMed] [Google Scholar]
- 30. Berg CA, Spradling AC (1991) Studies on the rate and site-specificity of P element transposition. Genetics 127: 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uchino K, Imamura M, Sezutsu H, Kobayashi I, Kojima K, et al. (2006) Evaluating promoter sequences for trapping an enhancer activity in the silkworm Bombyx mori . J Insect Biotechnol Sericol 75: 89–97. [Google Scholar]
- 32. Osawa N (2000) Population field studies on the aphidophagous ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae): resource tracking and population characteristics. Pop Ecol 42: 115–127. [Google Scholar]
- 33. Komai T (1956) Genetics of ladybeetles. Adv Genet 8: 155–188. [Google Scholar]
- 34. Koch RL (2003) The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts. J insect Sci 3: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of differential heat shock temperature on piggyBac transposase expression. A reaction without reverse transcriptase (-RT) was performed with cDNA synthesis as a negative control. Harmonia axyridis ribosomal protein 49 (Ha-rp49) was used as an internal control.
(DOC)