Abstract
1) Aims: To evaluate sympathetic system activity in BPS/IC patients and to investigate if chronic adrenergic stimulation in intact rats induces BPS/IC-like bladder modifications.
2) Methods: Clinical study – In BPS/IC patients and aged and body mass index matched volunteers Tilt test was undertaken and catecholamines were measured in plasma and 24h urine samples. Experimental study – Phenylephrine was injected subcutaneously (14d) to female Wistar rats. Pain behavior, spinal Fos expression, urinary spotting, number of fecal pellets expelled, frequency of reflex bladder contractions and urothelial height were analyzed. Urothelium permeability was investigated by trypan blue staining. Immunoreactivity against caspase 3 and bax were studied in the urothelium and against alpha-1-adrenoreceptor and TRPV1 in suburothelial nerves. Mast cell number was determined in the sub-urothelium. In rats with lipopolysaccharide-induced cystitis, urinary catecholamines and Vesicular Monoamine Transporter 2 expression in bladder nerves were analyzed.
3) Results: The TILT test showed an increase of sympathetic activity. Noradrenaline levels in blood at resting conditions and in 24-hour urine samples were higher in BPS/IC patients. Phenylephrine administration increased visceral pain, spinal Fos expression, bladder reflex activity, urinary spotting and the number of expelled fecal pellets. The mucosa showed urothelial thinning and increased immunoreactivity for caspase 3 and bax. Trypan blue staining was only observed in phenylephrine treated animals. Suburothelial nerves co-expressed alpha1 and TRPV1. Mastocytosis was present in the suburothelium. Cystitis increased sympathetic nerve density and urinary noradrenaline levels.
4) Conclusions: Excessive adrenergic stimulation of the bladder may contribute to the pathophysiological mechanisms of BPS/IC.
Keywords: Bladder Pain Syndrome/Interstitial Cystitis, Sympathetic system, Primary afferents, Urothelium
Introduction
Bladder pain syndrome/interstitial cystitis (BPS/IC) is a chronic painful condition of the bladder that affects up to 7 % of the female population of the western world1. Commonly, bladder pain arises at low volumes of bladder filling and may be referred to close areas such as lower abdomen, lower back, vagina and pelvis, but may also be referred to distant body areas, including the thighs, neck and head2.
The pathophysiologic mechanism of BPS/IC is unclear. However, several findings have been observed in the bladder of some of these patients, such as thinner urothelium, with abnormal cell-to-cell adhesion and impaired barrier function 3. Urothelial cells release an excess of ATP, acetylcholine, nitric oxide, among other neurotransmitters 3. The suburothelium is also abnormal containing more sensory nerves and inflammatory cells, including mast cells4. However it remains unclear if the urothelial and suburothelial defects correspond to a local problem or are bladder manifestations of a systemic disease. Several facts may support the latter hypothesis. BPS/IC is associated with other somatic and visceral chronic painful conditions including irritable bowel syndrome (IBS), fibromyalgia, Sjogren syndrome and anxiety disorders3. BPS/IC patients often exhibit an exaggerated startle response also seen in patients with IBS or anxiety disorders5.
The involvement of the sympathetic system in BPS/IC has been little explored. However it is known that BPS/IC patients excrete high levels of urinary catecholamines6 and have denser sympathetic innervation4. Moreover, there is an increasing body of evidence suggesting that sympathetic system is implicated in chronic painful conditions. Recent studies have demonstrated that irritable bowel syndrome and fibromyalgia course with a state of sympathetic hyperactivity 7. In complex regional pain syndrome, the pain response to adrenergic stimulation is substantially enhanced and pain can be improved by sympathectomy 8. A link between chronic pain and the sympathetic system may also be concluded from several animal experiments. Blockade of sympathetic outflow may reduce neuropathic pain following partial sciatic nerve injury 9. Sympathetic nerve fibres sprout around dorsal root ganglia cells and tangle with cutaneous peptidergic sensory fibres in animal models of neuropathic pain10, 11 or articular inflammation 12. Therefore, in this work we aimed to evaluate sympathetic system activity in BPS/IC patients and to verify if a chronic systemic adrenergic stimulation, intended to mimic a state of sympathetic overactivity, induces bladder modifications that mimic those observed in BPS/IC patients.
Materials and Methods
Noradrenaline levels and autonomic nervous system activity in BPS/IC patients
In 18 BPS/IC patients with active disease (from a cohort previously described elsewhere13), blood and 24 hours urine samples were collected and noradrenaline levels were determined by HPLC (Kit 195-6087, Gilson). Sympathetic activity was investigated with the TILT test in 10 of the 18 patients, who accepted the risks inherent to the test which include episodes of syncope, dizziness or headache, hypotension, nausea and changes in heart rate. The increment in sympathetic activity induced by the change in position increases the heart rate which decreases the interval between P waves and decreases the normal variability of P wave intervals. This physiological event is measured by the variation of the standard deviation of the P wave interval (ΔSDPP). Two other parameters were also recorded, that investigated the activity of the parasympathetic system. The root-mean-square difference among successive normal R-R intervals measures the variability of the heart period width based on R waves (root mean square successive differences, or rMSSD). The baroreflex sensitivity, a homeostatic mechanism for maintaining blood pressure was assessed as the average of all baroreflex sequences registered as the test progresses (BRS, milliseconds/mmHg). Ten age and body mass index matched healthy volunteers with no lower urinary tract symptoms recruited among the staff members of the Institution were used as controls for noradrenaline measurements and TILT test.
Rat model of chronic adrenergic stimulation
Since clinical results showed sympathetic hyperactivity and excess of noradrenaline in 24h urine samples, the effect of chronic adrenergic stimulation on bladder function was investigated in female Wistar rats (250-275g, Charles River), naïve or depleted of nociceptive fibres by systemic capsaicin (75 mg /kg, 24h before the experiments).
Treatment consisted on the daily subcutaneous injection for 14 days of phenylephrine at doses of 0.5 (PHE0.5) or 2.5 mg/kg (PHE2.5). In preliminary experiments these doses did not cause animal death. In contrast, doses of 5 and 10 mg/kg induced an important mortality rate and were therefore disregarded (data not shown).
Each experiment described below used 6 animals per group and where performed during light cycle.
Visceral pain behavioural tests, bladder voiding pattern and colonic activity
Visceral pain behavioural tests were performed in animals after a period of 5 days of habituation to the handler and to the experimental environment. Visceral pain was assessed by performing 3 different tests. In test 1, adapted from Boucher et al., 200014, animal breathing rate, eyes aperture and body posture using were analysed. An arbitrary 0-10 scale was used for each parameter. For the breathing rate, every decrease of 10 cycle/minute compared with day 1 record was scored 1. For the eye aperture test, the following scale was used: 0 for complete opening, 2 for intermediate opening, 5 for half-closed, 7 for intermediate opening and 10 for complete closing. For body posture, if animals presented rounded-back with body aligned or motionlessness the score was 10. Otherwise the score was 0. In test 2, adapted from Olivar & Laird, 199915, the following parameters were scored: normal 0, piloerection 1, strong piloerection 2, laboured breathing 3, licking the abdomen 4 and stretching and contractions of the abdomen 5. In test 3, adapted from Laird et al., 200116, referred pain was assessed using Von Frey analysis in the abdomen, using a series of eight Von Frey monofilaments (rated at 4, 6, 8, 10, 15, 26, 60 g). A positive response was recorded when the animal reacted to the filament (abdomen retraction or jump) in three out of the five filament applications. Tests were carried out at baseline and at day 8 and 14 after the onset of PHE treatment.
To characterize voiding pattern the animals after 14 days of PHE2.5 were placed in cages for 1 hour with filter paper covering the bottom. The number of urinary spots was counted. Due to the association between BPS/IC and IBS, the same animals were afterwards placed in another cage with normal bedding to evaluate colonic activity. After 6 hours, the number of faecal pellets expelled per hour was determined.
Cystometries
Bladder reflex activity was analysed through cystometry under anaesthesia (urethane 1.2 g/kg weight, subcutaneously) 14 days after PHE treatment. After bladder exposure saline was infused through the bladder dome (6 ml/h) and traces obtained for 2 hours after a period of 30 minutes for stabilization. Animal temperature was maintained at 37°C.
Bladder histology
The urinary bladders of saline and PHE2.5 animals were instilled at day 14 with 0.5 ml of 0.5% Trypan blue, under sodium pentobarbital anaesthesia (200 mg/kg, intraperitoneal). Animals were immediately decapitated, the bladders were harvested, fixed in 10% buffered formalin, for 24 hours, embedded in paraffin and sectioned at 5 μm. Bladder sections were stained for Hematoxylin-eosin to analyse urothelium morphology and trypan blue staining. Toluidine Blue 0.1% was used to stain mast cell infiltration. Images were acquired and urothelium thickness and bladder mucosa areas were determined using ImageJ 1.43u software.
Immunohistochemistry
At day 14, immediately after cystometry, animals treated with saline, PHE0.5, PHE2.5 and CAP+PHE2.5 were perfusion-fixed with 4% paraformaldehyde. L6 spinal cord segments were sectioned at 40 μm and immunoreacted against Fos protein. The urinary bladders were further fixed by immersion in 10% buffered formalin, sectioned at 5 μm and immunoreacted against Bax (1:1000), Caspase 3 (1:500) and cytokeratin 20 (1:200), alpha1 adrenoreceptor (1:500) and TRPV1 (1:500).
Effects of cystitis on sympathetic fibres density and on urinary levels of noradrenaline
As sympathetic nerve density was shown to increase during chronic inflammation12, sympathetic innervation was investigated in rat bladders inflamed with intravesical instillation of 2 mg/kg lipopolysaccharide (LPS) for one hour. Saline instillation was used as control. Urine from each animal was collected 24h later and pooled for each group to measure urinary noradrenaline by HPLC (Kit 195-6087, Gilson). LPS- and saline-instilled animals were perfused fixed with paraformaldehyde, the bladders sectioned at 50 μm and immunoreacted against vesicle monoamine transporters 2 1:1000 (VMAT2). VMAT2 is a membrane protein that transports monoamines, such as noradrenaline, from the neuroplasm into synaptic vesicles, in sympathetic nerve endings.
Statistics
Comparisons between the paired data were performed using paired Student’s t tests. For multiple comparisons, Kruskal-Wallis test followed by Dunn’s test was used. P values inferior to 0.05 were considered statistical significant.
Results
Noradrenalin levels and TILT test
In 24h urine samples, noradrenaline was significantly higher in BPS/IC patients than in controls (102.1 ± 43.7 μg vs 47.8 ± 17.8 μg, P<0.05, table 1). After resting in supine position, plasmatic noradrenaline in BPS/IC patients was higher than in controls (397.0 ± 36.4 pg/ml versus 204.3 ± 46.4 pg/ml; respectively, P<0.001, table 1). At upright position, plasmatic noradrenaline was similar between BPS/IC patients and controls (596.3 ± 30.4 pg /ml vs 578.3 ± 69.0 pg /ml; P>0.05, table 1).
| Control | BPS/IC | |
|---|---|---|
| ΔSDPP | 57.2 ± 23.0 | 24.2 ± 18.2* |
| rMSSD | 6.3 ± 2.8 | 5.6 ± 8.4 |
| BRS | 7.1 ± 3.8 | 7.7 ± 8.2 |
| 24h urinary NA(μ/day) | 47.8 ± 17.8 | 102.1 ± 43.7* |
|
plasmatic NA
(supine) (μ/mL) |
204.3 ± 46.6 | 397.0 ± 36.4*** |
|
plasmatic NA
(upright) (μ/mL) |
578.3 ± 69.0 | 596.3 ± 30.4 |
The mean ΔSDPP of BPS/IC patients was significantly lower than in controls (24.2 ± 18.6 vs. 57.2 ± 23.0, P<0.05, table 1). The rMSSD and the BRS were similar in BPS/IC patients and in healthy subjects (5.6 ± 8.4 vs 6.3 ± 2.8 units and 7.7± 8.2 vs 7.1 ± 3.8 units; P>0.05, table 1).
Rat visceral pain behaviour
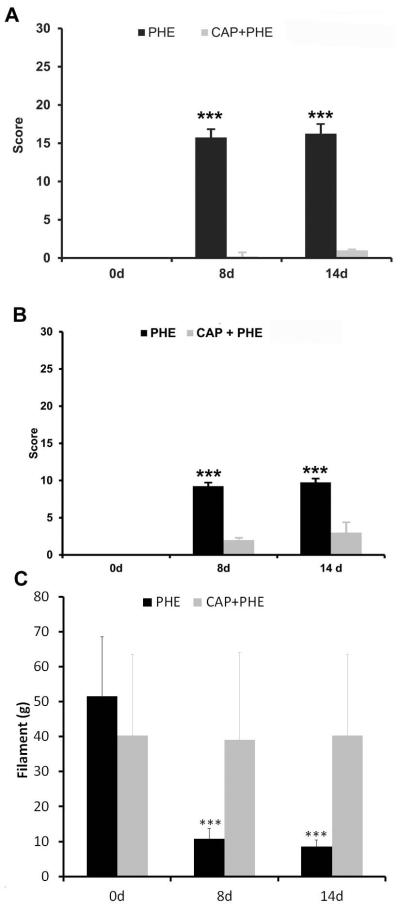
The pain scores in control animals were 0 (Fig.1A,B). After 8 days of PHE2.5, test 1 increased to 16±1and and test 2 to 9±0 (P<0.001 for both tests; Fig.1A,B). At day 14, scores were 16±1and 10±0, for test 1 and 2, respectively (P<0.001 for both tests; Fig.1A,B). Capsaicin pre-treatment blocked the pain behaviour induced by PHE2.5 in the 2 tests (1±0 for test 1 and 3±1 for test 2; P>0.05, compared to control; Fig. 1A,B).
Fig 1.
(A) Graph bar showing test 1 mean score. After chronic adrenergic stimulation with 2.5 mg of PHE/kg body weight, animals presented a decrease in breathing rate, in eye aperture and rounded-back posture characteristic of pain-related behaviour. Pre-treatment with capsaicin abolished visceral pain behavior (*** P < 0.001 comparing day 8 or day 14 with day 0, Kruskal-Wallis test followed by Dunn’s test). (B) Graph bar showing test 2 mean score. After chronic adrenergic stimulation with 2.5 mg of PHE/kg body weight, animals presented strong piloerection, heavy breathing and abdomen stretched characteristic of visceral pain crisis. Pre-treatment with capsaicin abolished visceral pain crisis (P>0.05, comparing day 8 and day 14 in both tests, P>0.05 comparing day 0 with day 8 and day 14 of animals pre-treated with capsaicin, *** P < 0.001 comparing day 8 or day 14 with day 0 of PHE treated animals, in both test, Kruskal-Wallis test followed by Dunn’s test). (C) Graph bar showing lower abdominal mechanical pain threshold using Von Frey filaments. After chronic adrenergic stimulation with 2.5 mg of PHE/kg body weight, animals presented a decrease in the mechanical pain threshold (*** P < 0.001 comparing day 0 with day 8 and day 14, Kruskal-Wallis test followed by Dunn’s test). Pre-treatment with capsaicin abolished abdominal mechanical pain threshold.
PHE2.5 decreased the mechanical pain threshold from 54±13g to 10±3g at day 8, and to 92g at day 14 (P<0.001; Fig. 1C). Capsaicin pre-treatment normalised mechanical pain threshold at days 8 and 14 to 39±25 and 40±23, respectively (P>0.05, compared to basal values; Fig. 1C).
Cystometry and spinal Fos expression
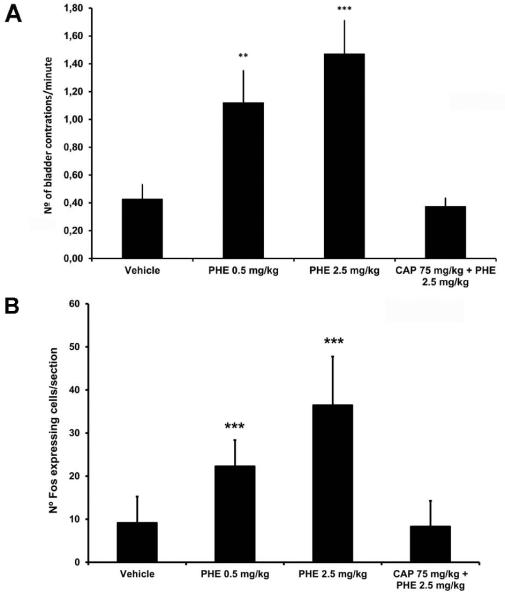
During cystometry control animals had 0.43 ± 0.11 contractions/minute (Fig. 2A). After PHE0.5 frequency was 1.12 ± 0.23 contractions/minute and after PHE2.5 frequency was 1.47 ± 0.24 contractions/minute (P<0.01 and P<0.001, respectively; Fig. 2A). Capsaicin blocked the effect of PHE2.5 (0.38 ± 0.06 contractions/minute; P>0.05, compared to control; Fig. 2A). Following cystometry, control animals presented a bilateral Fos expression of 9 ± 6 cells/section at the superficial lamina of L6 spinal cord segment. PHE0.5 and PHE2.5 increased the number of Fos expressing cells/section to 22 ± 6 and to 36 ± 11, respectively (P<0.001 for both doses; Fig. 2B). Capsaicin prevented the PHE2.5-induce increase of Fos (8 ± 6 per section; P>0.05, compared to control; Fig. 2B).
Fig 2.
(A) Bar graph showing a dose dependent increase in the mean number of bladder reflex contractions induced by saline (vehicle), 0.5 mg PHE/Kg of body weight (PHE0.5), 2.5 mg PHE/Kg of body weight (PHE2.5), 2.5 mg PHE/Kg of body weight following capsaicin pre-treatment (CAP+PHE2.5). (B) Bar graph shows that PHE dose-dependently increased the mean number of Fos immunoreactive cells/section at L6 spinal cord segment after 2h of cystometry. Pre-treatment with capsaicin abolished PHE-induced Fos immunoreactivity (P>0.05, comparing vehicle with CAP 75 mg/kg + PHE 2.5 mg/kg, *** P < 0.001 comparing 0.5 mg of PHE /kg body weight or 2.5 mg of PHE /kg body weight with vehicle, Kruskal-Wallis test followed by Dunn’s test).
Voiding pattern and colonic activity
The number of urinary spots/hour of control animals were 2.8 ± 1.1. After PHE2.5, it increased to 8.2 ± 3.6 spots/h (P<0.05).
In control animals expelled faecal pellets was 0.64 ± 0.36 per hour. PHE2.5 increased the number to 1.64 ± 0.29 (P<0.001).
Urothelial and suburothelial morphology
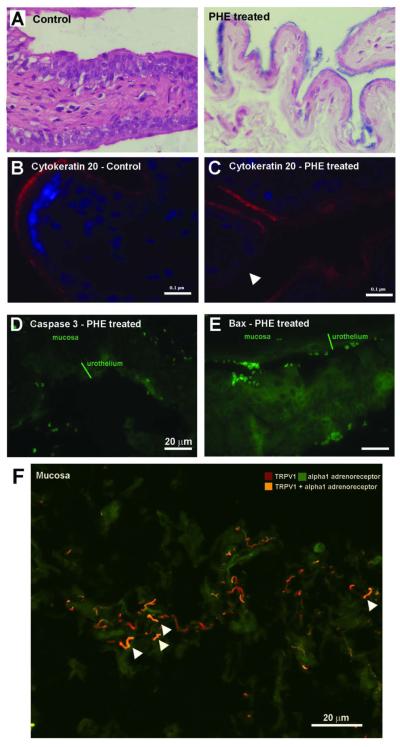
The urothelial thickness of control animals was 40.4 ± 2.4 μm. PHE treatment caused the appearance of large areas of urothelium with a reduced thickness (24.7 ± 2.5 μm for PHE 0.5 rat body weight and 22.4 ± 1.5 μm, for PHE2.5, P<0.001 for both doses). Thin urothelium in PHE2.5 treated rats showed increased trypan blue staining indicating an increase in urothelium permeability (Fig. 3A). Cytokeratin 20 stained the urothelium of control animals (Fig. 3B) but disappeared from large urothelial areas after PHE2.5 (Fig. 3C). A robust caspase and bax staining could be seen in large urothelium areas of PHE2.5 animals (Fig. 3D,E).
Fig. 3.
(A) Micrograph of a typical control bladder and of a typical area of urothelial thinning following administration of 2.5 mg PHE/Kg body weight, subcutaneously, daily for 14 days. (B) Micrograph showing intense cytokeratin 20 immunolabelling in the umbrella cells of the urothelium of control animals. (C) Micrograph showing areas of decrease or absent cytokeratin 20 immunolabelling (white arrows head) following administration of 2.5 mg PHE/Kg body weight, indicating a loss of umbrella cells. (D) Micrograph showing immunolabelling of the active form of caspase 3 in the urothelium after administration of 2.5 mg PHE/Kg body weight. (E) Micrograph showing bax immunolabelling in the urothelium after administration of 2.5 mg PHE/Kg body weight. (F) Micrograph showing co-localization of alpha 1 adrenoreceptor and TRPV1 imunnolabelling in suburothelial nerve fibres of control animals.
In PHE2.5 bladders, sub-urothelium contained 21.3±10.2 mast cells/mm2 while control animals had 8.2±4.0 mast cells/mm2 (P<0.01). In the sub-urothelium of control animals, alpha 1-adrenoreceptor and TRPV1immunoreactivity co-localized in nerve fibres (Fig. 3F).
Effects of cystitis on sympathetic fibres density and on urinary levels of noradrenaline
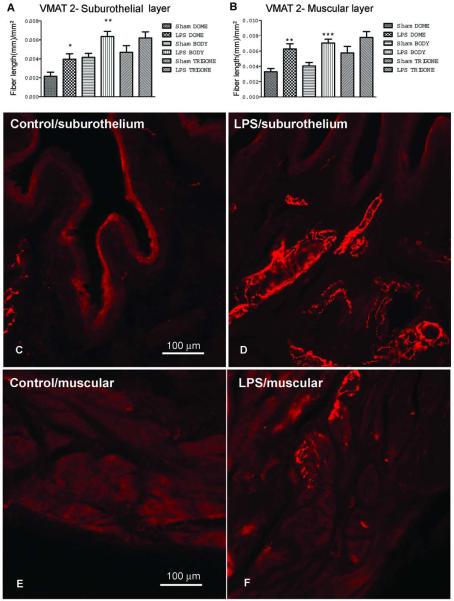
Sympathetic fibres were statistically significant denser in the body and dome of muscular and suburothelial layers of lipopolysaccharide (LPS)-inflamed bladders than in control animals (P<0.05, comparing sham suburothelium dome with LPS suburothelium dome, P<0.01 comparing sham suburothelium body with LPS suburothelium body, and sham muscular dome with LPS muscular dome, P<0.001 comparing sham muscular body with LPS muscular body; Fig. 4). No differences were observed in the trigone (Fig. 4).
Fig. 4.
Cystitis induces sympathetic fiber sprouting. Bar graph showing the mean number of VMAT2-immunopositive fiber length/mm2, in (A) suburothelium of the dome, the body and trigone and in the (B) muscular layers of the dome, body and trigone, of both vehicle and 2 mg/kg of LPS treated animals. (*P<0.05, comparing sham suburothelium dome with LPS suburothelium dome, **P<0.01 comparing sham suburothelium body with LPS suburothelium body, and sham muscular dome with LPS muscular dome, ***P<0.001 comparing sham muscular body with LPS muscular body, Kruskal-Wallis test followed by Dunn’s test). Micrographs representative of sections from the dome immunoreactive for VMAT2 are shown in C-F. (C) Bladder suburothelium of vehicle treated animals. (D) Bladder suburothelium of LPS treated animals. (E) Bladder muscular layer of vehicle treated animals. (F) Bladder suburothelium layer of LPS-treated animals.
LPS treatment induce a massive increase in urinary levels of noradrenaline (2341.2 μg) when compared to control animals (<3 μg).
Discussion
This study found sympathetic nervous system overactivity in BPS/IC patients, as demonstrated by the decreased variability between P wave intervals, assessed by the ΔSDPP during the TILT test. This finding was consistent with the higher urinary noradrenaline levels in patients than the aged-matched healthy individuals, a finding that confirms a previous report 6. The expected subsequent adrenergic overactivity is in line with the higher mean blood pressure and elevated heart rate during bladder hydrodistention reported by others in BPS/IC patients 17. Also this agrees with an increased sympathetic innervation of BPS/IC bladders4.
To establish a causal relationship between an adrenergic overactivity and the bladder changes occurring in BPS/IC, we induced in naïve rats a prolonged stimulation with PHE, a potent non-catecholamine predominantly alpha adrenoreceptor agonist. This drug was chosen due to the fact that urothelial cells express alpha1 adrenoreceptors18. Adrenergic stimulation with PHE reproduced in many aspects the clinical picture of BPS/IC. PHE increased the score of pain behaviour tests used for visceral pain and enhanced spinal Fos expression after innocuous filling (cystometry). PHE also increased urinary spotting and the frequency of bladder reflex contractions. Hence, our data suggests that in rats under adrenergic stimulation, voiding occurs at lower volumes and is accompanied by an intense barrage of nociceptive input, which may be considered equivalent to frequency and pain reported by BPS/IC patients during bladder filling.
PHE treatment also induced histological changes in the bladder that resemble the typical pathologic findings observed in BPS/IC patients. PHE induced extensive urothelial thinning essential due to a loss of cytokeratin 20-positive umbrella cells and increased urothelial cell permeability to trypan blue. The mast cells number in the rat suburothelial layer was increased. In addition, PHE activated pro-apoptotic proteins like Bax and active caspase 3 in the superficial layers of the urothelium, which is similar as a finding recently described in BPS/IC patients19. The stimulation of alpha adrenoreceptors in these cells might explain these apoptotic changes. In fact, overstimulation of the adrenoreceptors induces apoptosis through activation of oxidative stress pathways20.
We observed the co-localization of alpha1 adrenoreceptors and TRPV1 in bladder nerves. TRPV1 is essential for pain and bladder hyperactivity during cystitis and is overexpressed in the suburothelial nerves of BPS/IC patients21. Such co-localization explains why capsaicin, a desensitizing agent of TRPV1, totally abolished the PHE-induced increase of bladder reflex activity and Fos overexpression in the spinal cord. However, more relevant, elucidation of the pathways linking alpha1 adrenoreceptors and TRPV1 in nociceptive fibres may be rewarding to understand the role of the sympathetic system in other forms of chronic pain.
Pan cystitis is a pathological finding that could be found in bladder samples of some BPS/IC patients. The origin of inflammation is yet unclear although urothelial changes have been suggested to increase the permeability of the urothelium to urine content22. Our data, suggests that chronic cystitis may also have an adrenergic contribution. Mast cell migration and degranulation are enhanced by adrenergic stimulation, which might involve the stimulation of alpha adrenoreceptor expressed by these cells23. The sympathetic nervous system was also shown to regulate human immune system function through noradrenaline activation of alpha1 adrenoreceptors expressed by immunocompetent cell such as lymphocytes24.
An increased density of sub-urothelial nerve fibres seems to be a rule in chronic cystitis. In previous studies sensory fibres had been shown to increase in the suburothelium of inflamed bladders25. The present study has also shown an increased density of sympathetic nerves in the suburothelium and muscular layer of the inflamed bladders. This is in line with the recent report of an increased sympathetic innervation in chronic inflamed rat joints 12. Interestingly, sympathetic sprouts were described very close to sensory fibres11. Thus, noradrenaline release from sympathetic nerve endings may have a paracrine influence on sensory fibres which, as seen before, express adrenoreceptors. As the rat urinary noradrenaline levels were markedly increased during cystitis, such paracrine effect might be quite intense.
BPS/IC is often associated with other painful syndromes, such as fibromyalgia and IBS5, a fact that itself indicate a systemic mechanism common for all of them. In IBS increased levels of urinary noradrenaline 26 and an altered noradrenergic activity have also been reported27. In the present work, chronic adrenergic stimulation in rats mimics those associations. PHE treatment increased somatic pain and colon motility, as seen by the mechanical hyperalgesia in the abdomen and increased number of expelled faecal pellets. Therefore, these findings should be considered as a further reinforcement of the relevance of the sympathetic system as a pathophysiological mechanism of BPS/IC and associated pathologies.
Our study has several limitations. The number of patients and healthy volunteers that performed the TILT test was low, as already mention in material and methods. The origin of the sympathetic dysfunction was not revealed. However, a recent study suggested that non-bladder pelvic surgeries might be related with the onset of BPS/IC 28, indicating that surgeries or other unrecognized pelvic insults may trigger the sympathetic dysfunction. Also, this study does not elucidate the adrenoreceptor subtypes that may be implicated in the bladder changes induced by adrenergic stimulation. Noradrenaline might stimulate both alpha and beta adrenoreceptors, while PHE, although acting preferentially on alpha adrenoreceptors, might also induce beta-adrenergic mediated effects29. Concerning beta adrenoreceptors, human bladder express the beta3 subtype30. When stimulated, this adrenoreceptor decreases detrusor contractility and urinary frequency. Mirabegron, which is the first beta3 agonist to be clinically approved for the treatment of bladder overactivity, does not cause bladder pain or any change in bladder morphology31. Concerning alpha adrenoreceptors, only 1A and 1D are highly expressed in the urinary bladder urothelium32. Thus, this study warrants further research to explore the therapeutic role of alpha1A and D adrenoreceptors antagonists in chronic bladder pain.
In conclusion, the present clinical and experimental observations suggest that sympathetic overactivity is implicated in the mechanism leading to BPS/IC, which opens new opportunities in BPS/IC research.
Acknowledgements
The authors thank S. Barros, V. Nunes, J. Freita and A. Wolf Johnston for technical assistance. Ana Charrua is supported by SFRH/BPD/68716/2010. This work was partially supported by InComb FP7 HEALTH project no 223234, and NIH R37 DK54824 and R01 DK57284. Francisco Cruz is a consultant for Allergan, Astellas and Recordati. There are no conflicts of interest.
References
- 1.Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tripp DA, Nickel JC, Wong J, et al. Mapping of pain phenotypes in female patients with bladder pain syndrome/interstitial cystitis and controls. Eur Urol. 2012;62:1188. doi: 10.1016/j.eururo.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 3.Birder LA, Hanna-Mitchell AT, Mayer E, et al. Cystitis, co-morbid disorders and associated epithelial dysfunction. Neurourol Urodyn. 2011;30:668. doi: 10.1002/nau.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omoigui S. The biochemical origin of pain: the origin of all pain is inflammation and the inflammatory response. Part 2 of 3 - inflammatory profile of pain syndromes. Med Hypotheses. 2007;69:1169. doi: 10.1016/j.mehy.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullones Rodriguez MA, Afari N, Buchwald DS, et al. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol. 2013;189:S66. doi: 10.1016/j.juro.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein PC, Torri A, Parsons CL. Elevated urinary norepinephrine in interstitial cystitis. Urology. 1999;53:1140. doi: 10.1016/s0090-4295(98)00663-3. [DOI] [PubMed] [Google Scholar]
- 7.Chalaye P, Goffaux P, Bourgault P, et al. Comparing pain modulation and autonomic responses in fibromyalgia and irritable bowel syndrome patients. Clin J Pain. 2012;28:519. doi: 10.1097/AJP.0b013e31823ae69e. [DOI] [PubMed] [Google Scholar]
- 8.Mailis-Gagnon A, Bennett GJ. Abnormal contralateral pain responses from an intradermal injection of phenylephrine in a subset of patients with complex regional pain syndrome (CRPS) Pain. 2004;111:378. doi: 10.1016/j.pain.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Shir Y, Seltzer Z. Effects of sympathectomy in a model of causalgiform pain produced by partial sciatic nerve injury in rats. Pain. 1991;45:309. doi: 10.1016/0304-3959(91)90056-4. [DOI] [PubMed] [Google Scholar]
- 10.Chung K, Yoon YW, Chung JM. Sprouting sympathetic fibers form synaptic varicosities in the dorsal root ganglion of the rat with neuropathic injury. Brain Res. 1997;751:275. doi: 10.1016/s0006-8993(96)01408-4. [DOI] [PubMed] [Google Scholar]
- 11.Yen LD, Bennett GJ, Ribeiro-da-Silva A. Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J Comp Neurol. 2006;495:679. doi: 10.1002/cne.20899. [DOI] [PubMed] [Google Scholar]
- 12.Longo G, Osikowicz M, Ribeiro-da-Silva A. Sympathetic fiber sprouting in inflamed joints and adjacent skin contributes to pain-related behavior in arthritis. J Neurosci. 2013;33:10066. doi: 10.1523/JNEUROSCI.5784-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto R, Lopes T, Frias B, et al. Trigonal injection of botulinum toxin A in patients with refractory bladder pain syndrome/interstitial cystitis. Eur Urol. 2010;58:360. doi: 10.1016/j.eururo.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 14.Boucher M, Meen M, Codron JP, et al. Cyclophosphamide-induced cystitis in freely-moving conscious rats: behavioral approach to a new model of visceral pain. J Urol. 2000;164:203. [PubMed] [Google Scholar]
- 15.Olivar T, Laird JM. Cyclophosphamide cystitis in mice: behavioural characterisation and correlation with bladder inflammation. Eur J Pain. 1999;3:141. doi: 10.1053/eujp.1998.0105. [DOI] [PubMed] [Google Scholar]
- 16.Laird JM, Martinez-Caro L, Garcia-Nicas E, et al. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 17.Stav K, Lang E, Fanus Z, et al. Autonomic response during bladder hydrodistention in patients with bladder pain syndrome. J Urol. 2012;188:117. doi: 10.1016/j.juro.2012.02.2561. [DOI] [PubMed] [Google Scholar]
- 18.Yanase H, Wang X, Momota Y, et al. The involvement of urothelial alpha1A adrenergic receptor in controlling the micturition reflex. Biomed Res. 2008;29:239. doi: 10.2220/biomedres.29.239. [DOI] [PubMed] [Google Scholar]
- 19.Shie JH, Liu HT, Kuo HC. Increased cell apoptosis of urothelium mediated by inflammation in interstitial cystitis/painful bladder syndrome. Urology. 2012;79:484–e7. doi: 10.1016/j.urology.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 20.Neri M, Cerretani D, Fiaschi AI, et al. Correlation between cardiac oxidative stress and myocardial pathology due to acute and chronic norepinephrine administration in rats. J Cell Mol Med. 2007;11:156. doi: 10.1111/j.1582-4934.2007.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukerji G, Yiangou Y, Agarwal SK, et al. Transient receptor potential vanilloid receptor subtype 1 in painful bladder syndrome and its correlation with pain. J Urol. 2006;176:797. doi: 10.1016/j.juro.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 22.Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007;69:9. doi: 10.1016/j.urology.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 23.Moroni F, Fantozzi R, Masini E, et al. The modulation of histamine release by alpha-adrenoceptors: evidences in murine neoplastic mast cells. Agents Actions. 1977;7:57. doi: 10.1007/BF01964881. [DOI] [PubMed] [Google Scholar]
- 24.Grisanti LA, Perez DM, Porter JE. Modulation of immune cell function by alpha(1)-adrenergic receptor activation. Curr Top Membr. 2011;67:113. doi: 10.1016/B978-0-12-384921-2.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickson A, Avelino A, Cruz F, et al. Peptidergic sensory and parasympathetic fiber sprouting in the mucosa of the rat urinary bladder in a chronic model of cyclophosphamide-induced cystitis. Neuroscience. 2006;141:1633. doi: 10.1016/j.neuroscience.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Heitkemper M, Jarrett M, Cain K, et al. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. Am J Gastroenterol. 1996;91:906. [PubMed] [Google Scholar]
- 27.Berman S, Suyenobu B, Naliboff BD, et al. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. Neuroimage. 2012;63:1854. doi: 10.1016/j.neuroimage.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren JW, Howard FM, Morozov VV. Is there a high incidence of hysterectomy and other nonbladder surgeries before and after onset of interstitial cystitis/bladder pain syndrome? Am J Obstet Gynecol. 2013;208:77–e1. doi: 10.1016/j.ajog.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Chess-Williams RG, Williamson KL, Broadley KJ. Whether phenylephrine exerts inotropic effects through alpha- or beta-adrenoceptors depends upon the relative receptor populations. Fundam Clin Pharmacol. 1990;4:25. doi: 10.1111/j.1472-8206.1990.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 30.Igawa Y, Yamazaki Y, Takeda H, et al. Functional and molecular biological evidence for a possible beta3-adrenoceptor in the human detrusor muscle. Br J Pharmacol. 1999;126:819. doi: 10.1038/sj.bjp.0702358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khullar V, Amarenco G, Angulo JC, et al. Efficacy and tolerability of mirabegron, a beta(3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European-Australian phase 3 trial. Eur Urol. 2013;63:283. doi: 10.1016/j.eururo.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Moro C, Tajouri L, Chess-Williams R. Adrenoceptor function and expression in bladder urothelium and lamina propria. Urology. 2013;81:211–e1. doi: 10.1016/j.urology.2012.09.011. [DOI] [PubMed] [Google Scholar]