Abstract
The field of tissue engineering involves design of high-fidelity tissue substitutes for predictive experimental assays in vitro and cell-based regenerative therapies in vivo. Design of striated muscle tissues, such as cardiac and skeletal muscle, has been particularly challenging due to a high metabolic demand and complex cellular organization and electromechanical function of the native tissues. Successful engineering of highly functional striated muscles may thus require creation of biomimetic culture conditions involving medium perfusion, electrical and mechanical stimulation. When optimized, these external cues are expected to synergistically and dynamically activate important intracellular signaling pathways leading to accelerated muscle growth and development. This review will discuss the use of different types of tissue culture bioreactors aimed at providing conditions for enhanced structural and functional maturation of engineered striated muscles.
INTRODUCTION
Since the first successful organ transplantation in 1954, transplant rejection and organ donor shortage rendered the transplant therapies an imperfect solution to organ failure and tissue loss (1, 2). Over the past two decades, tissue engineering (TE) has evolved as a potential strategy for de novo design of replacement tissues and organs. Furthermore, TE techniques have been utilized for the development of in vitro microphysiological systems or ‘organ-on-a-chip’ platforms for pre-clinical drug screening and studying of microtissue physiology and pathology (3–6). Engineering and therapeutic use of striated (cardiac and skeletal) muscle tissues, in particular, while promising, has met with difficulties in replicating aligned cellular organization and adequate functional output characteristic of native muscle. Thus, significant research in the field has been aimed at providing more favorable culture environments to aid survival, growth, differentiation, alignment, and functional maturation of engineered striated muscles in vitro (7–9). In particular, mechanical (stretch, shear) and electrical stimulation as well as medium perfusion have been the key biomimetic signals applied to promote and support functional myogenesis in vitro.
Specifically, mechanical loading has been known to profoundly affect different cells in the body, generally termed mechanocytes, including osteocytes, chondrocytes, cardiomyocytes, and skeletal muscle cells (10, 11). The cellular responses to mechanical stimuli are mediated by mechanotransduction pathways that can either promote tissue development and function or lead to pathological changes (12). For example, mechanical overload of skeletal muscle during exercise can enhance its force generating capacity in vivo (13). Conversely, pressure or volume overload in the heart can lead to physiological or pathological hypertrophy depending on how the stress is applied (12, 14). Similar to mechanical stimulation, electrical stimulation influences the survival, proliferation rate, hypertrophy, and other properties of muscle cells. In vitro, the use of electrical stimulation is aimed at mimicking the action of the motoneurons (in skeletal muscle) or pacemaker cells (in cardiac muscle) found in native physiological environment. Denervated skeletal muscles are known to undergo a decrease of fiber diameter (atrophy) as well as become prone to injury and degeneration (15–17). The application of electrical signals may enable long-term survival and continued differentiation of muscle cells in vitro as well as prepare engineered muscle tissues for electromechanical integration following implantation in vivo.
The need to create a more biomimetic cell culture environment compared to previously used static and unstimulated conditions led to the development of different types of tissue culture bioreactors. Nowadays, the use of bioreactors has become a standard tissue engineering practice to provide a tightly controlled environment for long-term tissue growth as well as to test the acute responses of tissues to external stimuli (18, 19). While the bioreactor types used range widely from simple tissue culture plates to high-end multimodal systems, they are all designed with one or more of the following functions: (1) Maintenance of sterility, (2) Exchange of gases and nutrients, (3) Control of operating conditions (pH, temperature), and (4) Delivery of tissue-specific biophysical and biochemical cues. Bioreactor design is particularly challenging for tissues such as striated muscles that exist in high stress environments, are metabolically active, and perform vital functions in vivo. Between skeletal and cardiac muscle, design requirements for tissue culture bioreactors are comparable owing to the similarities in their cellular density, metabolism, function, and biomechanical growth laws (20). This review will mostly focus on the environmental conditions and biophysical stimuli provided by tissue culture bioreactors that can enable efficient structural and functional maturation of engineered striated muscles in vitro.
USE OF SCAFFOLDING MATERIALS TO PROMOTE ENGINEERED MUSCLE STRUCTURE, DIFFERENTIATION, AND FUNCTION
The ultimate goal of any tissue engineering effort is to generate a completely biomimetic tissue substitute that would structurally and functionally integrate with the host tissue after implantation at both micro- and macroscopic levels. In general, host-donor differences in tissue architecture may hinder graft integration, prevent healing and regeneration, and even compromise safety of the therapy (e.g. by causing cardiac arrhythmias). In the case of striated muscle, for the most efficient and safe repair, engineered tissue grafts should possess structural properties characteristic of native muscle including high cell density, alignment, and proper cell-type distribution (21). Furthermore, striated muscle constructs should be able to support macroscopic tissue contractions and not only generate high active (contractile) stresses but also sustain dynamic changes in passive tension that occur during normal muscle function and exercise.
Biomaterial scaffolds represent a component of tissue engineering design that is critical for the proper structural organization and functional maturation of engineered muscle tissues as well as for their ability to sustain chronic biophysical stimulation in vitro and in vivo. Both synthetic and natural scaffolds have been previously utilized for striated muscle tissue engineering. Up to date, naturally derived hydrogels have proven more capable of producing constructs with high cell density (due to cell-mediated gel compaction), uniform cell distribution, and better cell alignment than synthetic polymeric scaffolds (21–23). Hydrogels can be also micropatterned to reproducibly generate specific tissue structures (21, 24) and can be used to deliver mechanical stimuli to cells when anchored at opposite ends via porous materials, metal wires, or elastomeric posts (25–27). On the other hand, hydrogel-based as well as scaffold-free tissue patches usually fall short of supporting uniform 3D cell alignment over a large tissue area (28–30). Cylindrically-shaped constructs can support both high cell density and alignment (31–33), however, they do not allow local control of the degree or direction of cell alignment. The existing methods to generate uniform 3-D orientation of cells (over multiple cell layers) in cardiac and skeletal muscle tissue patches involve the use of 3-D scaffolds with aligned pores (34, 35), mesoscopic hydrogel molding (25, 36, 37), and stacking of aligned tissue sheets (38). Still, the ability to engineer 3-D biomimetic striated muscle tissues that are soft, free-standing, relatively large, and possess stable aligned architecture and physiological density of muscle cells is yet to be demonstrated.
In addition to being able to support high cell density and alignment, biomaterial scaffolds need to provide a biophysical environment that is supportive of striated muscle differentiation and function. In particular, Discher and others have shown that myogenic cells can sense and react to stiffness of the surrounding matrix and optimally grow and differentiate on 2D substrates with a stiffness similar to that of native muscle (39, 40). Similarly, 3D hydrogels of different stiffness have been shown to distinctly affect gene expression and function of cardiac progenitor cells or neonatal cardiomyocytes (41, 42). More recent studies however suggest that the stem cell fate and thus the process of striated muscle differentiation and maturation may not be directly governed by the substrate stiffness per se, but instead depend on the type, density, and distribution of cell adhesion receptors which are in turn regulated by the hydrogel chemistry and, potentially, the dynamics of muscle cell contractions (43, 44). Furthermore, biophysical and biochemical properties of the surrounding matrix will directly determine how efficiently: 1) individual muscle cell contractions integrate at the macroscopic tissue level, and 2) externally applied mechanical stimuli transmit to individual cells. In general, improved understanding of cell-biomaterial interactions (for both muscle and supporting non-muscle cells) will be required for the design of advanced 3D scaffolds for tissue engineering of functional striated muscles.
USE OF FLOW TO ENHANCE MASS TRANSPORT WITHIN ENGINEERED MUSCLE TISSUES
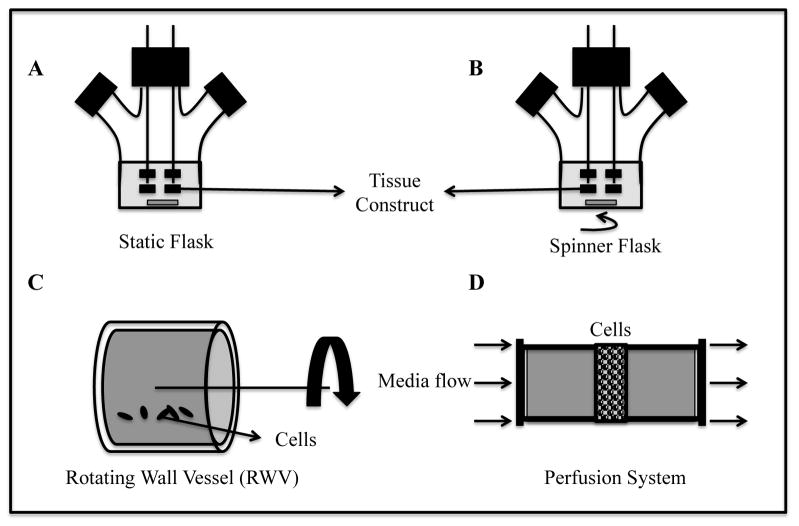
Traditional cell culture systems such as tissue culture plates and flasks maintain cells in static condition whereby gas exchange that occurs via surface aeration and mass transfer is diffusion-limited (45). In static systems, engineered skeletal and cardiac muscle tissues develop a necrotic core due to low mass transfer rates and high metabolic demand of cells (30, 31). In an attempt to mimic physiologic delivery of oxygen, nutrients, and chemical signals by blood flow, researchers have designed tissue culture bioreactors with continuously mixed media that provide significantly higher mass transfer rates compared to static cultures. These bioreactors enabled engineering of thicker tissue constructs, however, physiologically dense (108–109 cells/ml of tissue) engineered striated muscle tissues still remain limited in thickness to 120–150 μm (28, 46). Examples of dynamic tissue culture bioreactors (e.g., spinner flasks, rotating wall vessels (RWVs), perfusion systems) are listed in increasing order of their mass transfer rates in Figure 1. Characteristics of the bioreactors utilized for seeding and cultivation of engineered skeletal and cardiac muscle tissues are summarized in Table 1. In addition, different microfluidic systems can be used to facilitate drug screening studies in striated muscle microtissues by minimizing the fluid volume needed to perfuse such organ-on-a-chip platforms (47, 48).
Fig. 1.
Bioreactors for engineering of striated muscle tissues. Static flask, A; Spinner flask, B. Tissue constructs are threaded onto stainless steel needles; Rotating Wall Vessel, C. Levitating tissue constructs experience “microgravity” condition. Arrow shows direction of vessel rotation; Perfusion bioreactor, D. Arrows shows the direction of media flow through tissue construct.
Table 1.
Bioreactors with media mixing for engineering of striated muscles
| Vessel Type | Type of Flow | Seeding Mechanism | Mass Transfer | Engineered Muscle | Ref. |
|---|---|---|---|---|---|
| Spinner Flask | Stirring/turbulent | Statically or dynamically on microcarrier beads or polymer scaffolds | Convection (recirculation) | Skeletal | (126) |
| Cardiac | (30, 49) | ||||
| Rotating Wall | Laminar | Statically or dynamically on microcarrier beads or polymer scaffolds | Convection (recirculation) | Skeletal | (52, 127) |
| Cardiac | (49, 53, 128) | ||||
| Perfused Chamber | Laminar | Statically or dynamically on polymer scaffolds | Convection (perfusion) | Skeletal | (57, 129) |
| Cardiac | (56, 58, 130) |
Stirred Flasks
Stirred spinner flasks were among the first bioreactors employed to promote circulation of media and supply of oxygen to 3-dimensional cell cultures (19). Although media mixing in these bioreactors yields an increase in overall cell viability compared to static cultures (49), the use of rotating stirrer bars also creates a turbulent shear stress environment which can negatively affect health and viability of shear-sensitive cells such as cardiomyocytes (46, 49).
Rotating Wall Bioreactors
For shear-sensitive cells, such as cardiomyocytes, an ideal culture environment would involve both the presence of high mass transfer rates and low shear stresses. NASA was the first to introduce a tissue culture bioreactor that satisfied these requirements, a rotating wall vessel (RWV). Through control of rotation speed, the RWVs maintain a free-falling (“microgravity”) tissue environment in which cells experience low shear stress but still receive sufficient supply of oxygen and nutrients (50). There are several adaptations of the RWV currently in use, including slow lateral turning vessel (STLV), high aspect ratio vessel (HARV), and the rotating wall perfusion vessel (pRWV) (19). STLVs and HARVs are cylindrical vessels that differ in the base-to-height aspect ratio and size and position of the gas exchange membrane. In STLVs, the gas exchange membrane wraps around an inner cylinder whereas in HARVs, the membrane is flat and positioned at the base of the cylinder (51). In pRWVs, the media perfuses through the rotating cylinders. These bioreactors have been utilized to promote formation of functional skeletal and cardiac muscle tissues. Specifically, Molnar et al. cultured satellite cells on microcarrier beads in HARVs and observed that cells experiencing microgravity showed better cellular organization than those in static cultures (52). Interestingly, application of microgravity improved cell proliferation without the anticipated muscle atrophy. Papadaki and Bursac et al. observed significant improvements in cell viability, hypertrophy, expression of cardiac proteins, and electrical function when engineered cardiac tissues were cultured in HARVs compared to spinner flask bioreactors (46, 53).
Perfusion Bioreactors
Perfusion bioreactors have been developed to allow continuous flow of culture medium and provide high transfer rates of gases and nutrients, stabilization of pH, and effective removal of toxins, all of which are expected to result in better structural organization and functional properties of engineered striated muscles. Specifically, the use of perfusion through porous polymer scaffolds has been shown to improve seeding efficiency leading to increased density and more uniform 3-D distribution of skeletal muscle cells and cardiomyocytes (54–56). In addition to more uniform cell seeding, perfusion of cardiac and skeletal muscle tissues can promote their viability and long-term maintenance in vitro, as shown by Chromiak et al. for avian skeletal muscle organoids (57). In recent studies, Cheng et al. analyzed the individual and combined effects of IGF and bidirectional perfusion on engineered cardiac tissues and showed reduced cell apoptosis, increased connexin-43 expression, stronger contractions, and decreased excitation threshold (58). Similarly, Shansky et al. employed a perfusion bioreactor to study paracrine effects of IGF-1 on avian skeletal muscle constructs and observed overexpression of muscle myosins (59). Brown et al. compared pulsatile and static perfusion of engineered cardiac tissues and showed that pulsatile flow led to improved contractile properties and excitability of cardiomyocytes (60). While these and other studies suggest beneficial roles of in vitro perfusion on the structure and function of engineered striated muscles, for physiologically dense muscle tissues, high shear stresses during interstitial perfusion may negatively affect viability of shear-sensitive muscle cells. Furthermore, removal of perfusion conditions at the time of implantation is likely to result in rapid tissue ischemia and death in vivo.
Recently, Okano’s group reported a novel “vascularization” strategy whereby an underlying vascular bed was used to generate perfused vessel networks within engineered cardiac and skeletal muscle tissue sheets (61, 62). Specifically, co-cultured engineered sheets of neonatal rat cardiomyocytes and endothelial cells were laid on top of either perfused collagen micro-channels or an ex vivo vascular bed. The co-cultured cells showed high viability, formation of vascular structures under perfusion, and increased connectivity between underlying vascular bed and tissue sheets in the presence of bFGF. Theoretically, these vascularized cardiac tissues could be continually ‘grown’ by the repetitive stacking of additional cell sheets. Upon implantation, these tissue patches connected to host vasculature, resulting in increased viability compared to non-vascularized controls (62). While promising, it remains to be studied if this approach can enable in vitro growth of dense striated muscle tissues thicker than 150 μm.
USE OF BIOPHYSICAL STIMULATION TO PROMOTE ENGINEERED MUSCLE MATURATION AND FUNCTION
Engineering of highly functional striated muscle tissues will likely require extensive understanding of the muscle development, structure, physiology, functional adaptation, and injury repair mechanisms. Ideally, advanced bioreactors for muscle tissue engineering are expected to provide active monitoring of tissue viability, metabolism, and function, as well as controlled application of important biophysical cues present in healthy innervated muscle. In particular, controlled electrical stimulation (pacing) to induce active muscle contraction and mechanical stimulation (stretch) to alter passive muscle loading are utilized to mimic the most important aspects of striated muscle work. Overview of bioreactor systems with capabilities for biophysical stimulation utilized for cardiac and skeletal muscle tissue engineering are listed in Table 2.
Table 2.
Bioreactors with biophysical stimulation for engineering of striated muscles
| Cell Source | Electrical Stimulation | Mechanical Stimulation | Perfusion | Ref. |
|---|---|---|---|---|
| Bioreactors for Cardiac Muscle Tissue Engineering | ||||
| ECCMs, NRCMs | − | + | − | (131) |
| NRCMs | − | + | − | (132) (93) |
| NRCMs | + | − | − | (116) |
| NRCMs | + | − | + | (123) |
| NRCMs | − | + | + | (60) |
| NRCMs | + | + | + | (125) |
| Mouse iPSC-CMs | + | + | + | (133) |
| Human Pediatric CMs | − | + | − | (92) |
| Human ESC/iPSC-CMs | − | + | − | (94) (133) |
| Human ESC/iPSC-CMs | + | − | − | (118, 119) |
| Bioreactors for Skeletal Muscle Tissue Engineering | ||||
| C2C12 Line | + | − | − | (104) |
| C2C12 Line | − | + | − | (88) |
| C2C12 Line | + | + | − | (122) |
| Rat MPCs + MDCs | − | + | − | (89) (90) |
| Human Muscle Cells | − | + | − | (134) |
| Human Muscle Precursor Cells | − | + | − | (135) |
CM: Cardiomyocyte; ECCM: Embryonic Chick Cardiomyocyte; NRCM: Neonatal Rat Cardiomyocyte; hESC-CM: Human Embryonic Stem Cell-derived Cardiomyocyte; iPSC-CM: Induced Pluripotent Stem Cell-derived Cardiomyocyte; MPC: Muscle Progenitor Cell; MDC: Muscle Derived Cell.
Mechanotransduction in Native and Engineered Muscle Tissues
Different regimes of mechanical loading have been shown to cause profound alterations in muscle structure and function by the activation of a variety of intracellular signaling cascades. In general, any change in the mechanical stress experienced by striated muscle cells leads to one of the following outcomes: cell elongation (preferential sarcomere addition in series), thickening (preferential sarcomere addition in parallel), elongation and thickening (sarcomere addition in both series and parallel), or apoptosis and death. In skeletal muscle, chronic active overload may lead to myofiber thickening (hypertrophy), while chronic passive stretch leads to myofiber elongation (63). In cardiomyocytes, the mechanosensitive response varies depending on the type of overload, pressure or volume, and can result in either physiological growth (characterized by both cell elongation and thickening), physiological or pathological concentric hypertrophy (cell thickening) and physiological or pathological eccentric hypertrophy (cell elongation) (14).
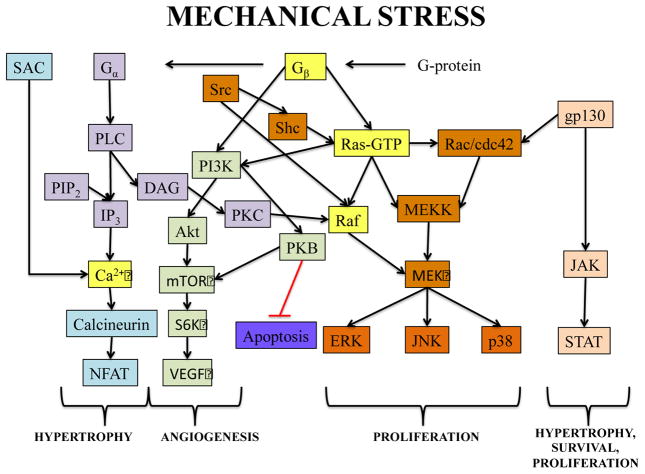
Mechanical loading affects muscle cell growth, differentiation, and morphology via various intracellular molecular pathways, some of which are depicted in Figure 3. In particular, static and cyclic stretch have been shown to upregulate secretion of different autocrine and paracrine factors (64, 65) including insulin-like growth factor I (IGF-I), adenosine mono- and tri-phosphate (AMP, ATP), reactive oxygen species (ROS), and prostaglandins (PG) (65, 66). In striated muscle cells, these cytokines activate a number of downstream effectors among which Phosphoinositide 3-kinase (PI3K), G-protein, Mitogen activated protein kinase (MAPK), calcium, and AMP activated kinase (AMPK) play the most important roles in muscle mechanotransduction (63, 65, 67, 68). Specifically, phosphoinositide 3-kinases play a major role in cell metabolism and directly regulate cell proliferation and differentiation. These enzymes can be activated by autocrine/paracrine action of IGF-I and other growth factors upon stimulation by mechanical stress (69). G-proteins (heterotrimeric guanine nucleotide binding proteins) associate with the cell-surface receptors and increase transcription of muscle-specific genes in cardiac and skeletal muscle cells subjected to mechanical strain to mediate cell growth and hypertrophy (11, 70, 71). Stretch-induced IGF-I and other cytokines act via G-protein coupled receptors (GPCRs) to activate mitogen activated protein kinases (MAPK) pathways known to play integral roles in muscle mechanotransduction (72, 73). MAPKs are divided into c-Jun NH2-terminal kinase (JNK), extracellular regulated kinase (ERK), and p38 families. The JNK family is also known as stress-activated protein kinases. Increased ERK and JNK phosphorylation was observed in response to mechanical stress in skeletal muscle cells, while p38 phosphorylation was either found to be unaffected, or increased for eccentric but not concentric muscle loading (67, 74). Positive growth and differentiation effects of IGF-I on skeletal and cardiac muscle were recapitulated in vitro where addition of IGF-I to culture medium improved structural and contractile properties of engineered muscle tissues (58, 59).
Fig. 3.
Important mechanotransduction signaling pathways in striated muscle cells. Blue, Intracellular calcium-mediated signaling; Purple, Phospholipase C signaling; Orange, MAPK pathway; Green, PI3K pathway; Peach, JAK/STAT pathway; Yellow, Overlap between signaling pathways.
G-protein receptor activated phospholipase C (PLC) pathway is another major contributor to the mechanotransduction signaling in striated muscles that integrates with both the Ras-Raf and Calcium-mediated pathways (75). PLC activation of protein kinase C (PKC) isoforms has been shown to stimulate expression of cytoskeletal protein β-MHC leading to cardiomyocyte hypertrophy (76). Muscle mechanotransduction is also governed by intracellular calcium levels that are mainly regulated by calcium influx through stretch-activated channels (SAC), active force generation, and phospholipase pathways (65, 77, 78). Increased intracellular calcium levels can stimulate activity of PKC and Calmodulin-dependent protein kinase (CaMK) and profoundly affect proliferation and hypertrophy of developing cardiomyocytes (79). Stimulation of the calcium/calmodulin pathway can also activate calcineurin to induce translocation of cytoplasmic nuclear factor of activated T cells (NFATc) into the nucleus and enhance the transcription of a number of muscle-specific genes (e.g. MHC isoforms) leading to cardiac and skeletal muscle hypertrophy (64, 65, 80).
Mechanical Stimulation by Stretch
Due to essential roles that mechanical forces play in muscle growth, differentiation, and function, the ability to apply controlled patterns of mechanical stimulation is critical for promoting functional myogenesis in vitro. A variety of mechanical stimulation regimes have been utilized for muscle tissue engineering including static stretch (i.e., a single step of stretch at the beginning of culture), ramp stretch (i.e., continuous or discrete, step-by-step increase in stretch amplitude), and cyclic stretch (i.e., a periodic train of stretch pulses with a given shape, amplitude, frequency, and duration of intermittent pauses). In theory, these temporal regimes of mechanical stimulation can be applied along one (uniaxial), two (biaxial), or all planar tissue axes (multiaxial) (81) (Fig. 2), with uniaxial stimulation being the simplest and mostly utilized stimulation regime for engineering of striated muscle.
Fig. 2.
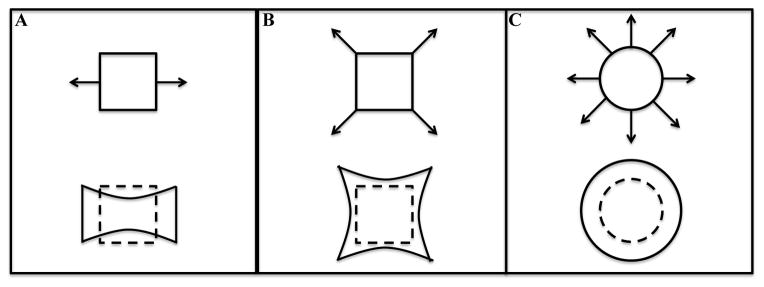
Examples of stretch regimes to be applied to engineered striated muscle: Uniaxial stretch, A; Biaxial stretch, B; Multiaxial stretch, C.
Studies that utilized mechanical stimulation for cardiac and skeletal muscle tissue engineering are summarized in Table 3. Vandenburgh et al. were among the first to utilize bioreactors with mechanical stimulation for culture and testing of cylindrically shaped striated muscle constructs. (82, 83). They and others (84, 85) found that both uniaxial static and cyclic stretch have beneficial effects on engineered muscle survival, alignment, hypertrophy, and differentiation, as well as glucocorticoid-induced skeletal muscular atrophy (86, 87). In a study by Candiani et al., application of a biomimetic stretch regime (with a 2-day steady ramp stretch to 3.3%, followed by cyclic 5-pulse, 0.5 Hz, 3.4% burst stretches for 2 min with 28 min intermittent breaks over a period of 10 days) to C2C12 cells induced an 8-fold increase in the expression of myosin heavy chain (MHC) protein compared to static control (88). More recently, Machingal et al. engineered skeletal muscle constructs using mechanically preconditioned muscle progenitor cells (by 10% stretch, 3 times per minute for the first 5 min of every hour, for 1 week). Two months post-implantation in a volumetric muscle loss injury model in mice, preconditioned constructs had superior biomechanical properties and induced accelerated and prolonged functional recovery following implantation (89, 90).
Table 3.
Use of bioreactors for mechanical stimulation of engineered striated muscle
| Cell Source | Stretch Regime | Strain (%) | Duration | Outcome | Ref. |
|---|---|---|---|---|---|
| ECCMs, NRCMs | 1.5 Hz cyclic | 20 | 6 days | Hypertrophy, improved contractile function | (131) |
| NRCMs | 1.34 Hz cyclic, continuous or 15 min/h | 1, 3.5, 7 | 7 days | Increased tensile strength, CM and EC marker expression | (132) |
| NRCMs | 1 Hz cyclic for 2h, 6h, or 24h per day | 10 | 1 day | No functional differences among groups | (93) |
| Human Pediatric CMs | 1.34 Hz cyclic | 20 | 14 days | Improved CM proliferation, distribution, mechanical strength | (92) |
| Human ESC/iPSC-CMs | 1 Hz cyclic | 5 | 4 days | Increased CM proliferation, hypertrophy | (94) |
| Mouse + Human iPSC-CMs | 1 Hz cyclic | 10 | 7 days | Improved tissue formation, CM hypertrophy, contractile function | (133) |
| Static ramp with 2% every 2nd day | 0–23 | 14 days | |||
| C2C12 Line | 1 Hz for 1 hour every 6 hours | 5 or 10 with 5% static pre-strain | 2–14 days | Increased cell alignment, contractile proteins | (122) |
| C2C12 Line | 5-pulse 0.5 Hz bursts for 2 min, then 28 min rest | 6.7 | 10 days | Increased MHC expression in myofibers | (88) |
| Adult Rat MPCs/MDCs | 0.05 Hz for 5 min every hour | 10 | 7 days | Improved host recovery upon implantation | (89) (90) |
| Human Skeletal Muscle Cells | 5-pulse 0.5 Hz bursts for 2 min, then 28 min rest | 5, day 8–10 10, day 10–12 15, day 12–16 |
8 days | Increased myofiber diameter and density, decreased tissue stiffness | (134) |
| Human Muscle Precursor Cells | 0.05 Hz for 5 min every hour | 10 | 5–21 days | Improved force generation post-implantation | (135) |
Interestingly, application of different mechanical stimulation regimes has been shown to affect the switch between the two skeletal muscle fiber phenotypes, namely, slow oxidative (type I), which is economical in energy consumption and fatigue resistant, and fast anaerobic (type II), which is used when there is a large but short physical demand. Specifically, stretch or mechanical load upregulated type I and downregulated type II muscle fiber genes (e.g. slow MHC), and when the load was removed or reduced, the fibers reverted back to type II along with a reduction in slow MHC expression (11, 91). It is possible that the application of appropriate mechanical stimulation regimes within tissue culture bioreactors could allow engineering of skeletal muscle tissue with the desired fiber type composition, with the goal of better matching the native muscle phenotype present at the injury site.
Similar to studies with engineered skeletal muscle tissues, beneficial effects of mechanical stimulation on tissue structure, differentiation, and function have been also demonstrated for engineered cardiac muscle. Fink et al. investigated the effects of mechanical stimulation on engineered neonatal rat and chick cardiac muscle and found that 6 days of 20%, 1 Hz uniaxial cyclic stretch improved cardiomyocyte alignment, elongation, and hypertrophy yielding a 2–4 fold increase in contractile force amplitude (85). Akhyari et al. subjected human neonatal heart cells seeded in gelatin foam scaffolds to continuous cyclic stretch (20%, 1.3 Hz) for 14 days using the Bio-Stretch apparatus (81) and showed significant improvements in cell spreading, distribution, and proliferation (92). Birla et al. designed a novel bioreactor for stimulation of engineered cardiac tissues but was unable to show any positive effects of a short-term cyclic stretch (10% at 1 Hz up to 24 hours) on cardiac function (93). Recently, Tulloch et al. co-cultured endothelial cells with human pluripotent stem cell-derived cardiomyocytes and applied cyclic stretch (5% at 1 Hz) for 4 days. They found that synergistic action between mechanical loading and endothelial cell presence led to increased cardiomyocyte proliferation (94).
Of note is however that not all regimes of mechanical stimulation have positive effects on engineered striated muscle differentiation, as found by Boonen et al. who showed a decrease in myogenic regulatory factors and delay in sarcomerogenesis upon application of 2% uniaxial ramp stretch followed by 4% uniaxial intermittent dynamic stretch to C2C12 cells (95). Similarly, although not widely used, specific regimes of biaxial stretch have shown increased cell proliferation along with delayed activation and differentiation of satellite cells (96, 97). Taken together, while mechanical loading plays a vital role in myogenic growth and development, the optimal stimulation regimes for structural and functional maturation of engineered muscle remain to be determined and are likely to differ for different muscle types, scaffolds, and culture media formulations.
Electrical Stimulation
Continuous contractile activity of the beating heart muscle (initiated by firing of pacemaker cells) or working skeletal muscle (initiated by release of acetylcholine from motor neurons) is of critical importance for the maintenance of striated muscle cell viability, muscle tone and mass, and, ultimately, human mobility and life. The ability to replicate this activity in vitro requires that appropriate electrical stimulation regimen is applied to cultured cells or engineered tissues in a controlled and biomimetic fashion. Over the last 30 years, large numbers of in vitro and in vivo studies have shown beneficial effects of electrical stimulation on skeletal muscle cell fusion, alignment, hypertrophy, expression of contractile proteins and myogenic markers, electrical excitability, and force generation (98–103). While most of the in vitro studies involved the use of standard monolayer cultures, few tissue culture bioreactors have been designed for long-term stimulation of 3D engineered muscle. For example, Donnelly et al. designed a tissue culture bioreactor with the ability to vary electrical stimulation parameters including frequency, pulse amplitude, and pulse width (104). At higher stimulation frequencies, they found that 2D monolayers of C2C12 cells showed higher rates of protein synthesis, while 3D muscle constructs displayed improved force generation. On the other hand, large amplitude electrical stimuli of more than 6 × threshold value caused significant electrochemical damage in engineered muscle (103).
Specific patterns of muscle activity have been shown to determine the skeletal muscle fiber phenotype in seminal studies by Buller et al., who converted the fast into slow muscle fibers by cross-innervating them with slow fiber neurons (105). In vitro, Naumann et al., showed that different regimes of chronic electrical stimulation can modulate expression of MHC isoforms in primary rat myotubes and that changes from fast to slow fiber phenotype occur during stimulation with high frequency bursts (106). The fast-to-slow fiber type conversion has also been observed during application of chronic low frequency stimulation (CLFS) (107), presumably due to its similarity to neuronal stimulation patterns present in slow muscle fibers (108). The reversal of the fast-to-slow fiber type switch could be achieved by removing stimulation, denervation, administration of tetrodotoxin, stimulation at high frequency, and shortening of the muscle length (109). Still, slow-to-fast fiber type transition is more difficult to achieve due to lower work-to-rest ratio and random firing pattern of the fast muscle fibers (110). Lately, Guo et al. developed a functional human neuromuscular junction system by co-culture of human stem cell derived motoneurons and primary muscle cells (111). Such systems could be adapted to incorporate electrical stimulation, and when miniaturized, used for high-throughput drug and toxicology screening, disease modeling, and studies of neuromuscular synaptogenesis.
Similar to skeletal muscle cells, electrical stimulation of cardiomyocytes has been shown to promote cardiomyocyte ultrastructural organization, hypertrophy, alignment, excitability, and electrical coupling (112, 113). In 2D neonatal rat cardiomyocyte cultures, chronic electrical pacing (1–3 Hz for 2–4 days) stabilized action potential duration and conduction velocity as well as altered expression of ion channel and gap junctional proteins (114, 115). In 3D engineered neonatal rat cardiac tissues, 1 Hz electrical stimulation for 8–9 days yielded increase in cell elongation, volume fraction, expression of gap junctional protein connexin-43, excitability, and maximum tissue capture rate (112, 116). Sauer et al. and Chen et al. have shown that short term (90 s) and chronic (4 day) electrical stimulation can also promote cardiac differentiation of mouse ESC-derived embryoid bodies (EBs) through the generation of intracellular reactive oxygen species (ROS), suggesting the potential utility of electric stimulation for enhanced functional maturation of stem cell-derived cardiomyocytes (113, 117). Recently, Lieu et al. applied cyclic electrical stimulation (2.5V/cm, 1Hz for 14 days) to hESC-derived cardiomyocytes starting at 24–28 days of differentiation and showed compelling evidence for structural and electromechanical maturation of cells including appearance of robust sarcomeres, increased expression of excitation-contraction coupling and calcium cycling genes, increased potassium currents, hyperpolarization of resting potential, and increased calcium transient amplitude (118). Similarly, in recent studies by Nunes et al., cyclic electrical stimulation of variable frequency (1–6 Hz increase for the first 7 days followed by steady 1 Hz for 14 days) promoted structural and functional maturation of human ESC-derived cardiomyocytes within 3D collagen-based tissue constructs (119). While it is almost certain that chronic electrical stimulation can provide significant benefits for differentiation and maturation of engineered striated muscles, the optimal stimulation regimes leading to physiological rather than pathological phenotypic changes remain to be identified (120).
Multimodal Stimulation
The native microenvironment of striated muscle is a complex source of various physical and biochemical signals, which to be fully reproduced in vitro, will require the design of multimodal bioreactors with the ability to simultaneously provide a combination of flow, electrical and mechanical stimuli. Feng et al. were the first to design a bioreactor capable of co-delivering electrical and mechanical stimulation to engineered cardiac muscle. They suggested that application of a 10 V electrical pulse at the beginning of each period of cyclic stretch (3.7%, 1 Hz) enhanced cardiomyocyte size and contractile properties (121). Using a multimodal bioreactor, Liao et al. simultaneously applied intermittent cyclic stretch (5% amplitude, 1 Hz for 1 h with 5 h of rest for total of 7 days) and electrical stimulation (4 V/cm) to tubular skeletal muscle constructs and measured higher fraction of cross-striated myotubes, expression of MHC proteins, and excitability compared to unstimulated cultures (122). In this particular case, combined electromechanical stimulation did not provide additional benefit compared to application of individual (electrical or mechanical) stimulation regimes. Barash et al. (123) and more recently Maidhof et al. (124) constructed bioreactors that delivered combined electrical stimulation (1 Hz) and media perfusion to engineered cardiac tissues and found that the bimodal stimulation improved cardiomyocyte structural and contractile properties. In a recent report, Kensah et al. designed a bioreactor that was capable of electrical stimulation, mechanical stimulation, perfusion, and real-time monitoring of tissue morphology and force production. In these bioreactors, collagen-based neonatal rat cardiac tissue constructs were subjected to mechanical stretch (10%, 1 Hz, for 7 days) and β-adrenergic stimulation. Expression of cardiac hypertrophy markers and contractile force amplitudes in stretched tissues were higher than those cultured in static conditions but lower than those observed under β-adrenergic stimulation alone or with mechanical stretch (125). Overall, multimodal bioreactors hold promise in providing the most biomimetic culture environment for growth and maturation of engineered striated muscles. However, with increased bioreactor complexity, susceptibility to failure increases and so do the difficulties in identifying the optimal regime of stimulation (110). In addition, translational potential of the entire approach may decrease due to potential regulatory and commercialization issues. All of these factors must be considered when designing a viable tissue engineering strategy for use in toxicology screening, drug development, and cell-based therapies.
CONCLUSIONS
Striated muscle tissue engineering has come a long way since the very first attempts to culture and maintain muscle cells in vitro. With better control of pH, temperature, and sterility, improved mass transfer of nutrients and oxygen, application of electrical and mechanical stimulation, and ability to monitor cell differentiation and function in real time, sophisticated tissue engineering bioreactors will pave the way for generation of highly functional biomimetic muscle tissues. When developing a successful bioreactor system, one should have an in-depth knowledge of the native tissue biology and physiology and prioritize the design parameters in accordance with the aims of the study in order to strike a balance between the minimum required complexity and reliability. Understanding potential synergistic or antagonistic effects of different biophysical and biochemical stimuli on engineered muscle structure and function is of particular importance and is yet to be explored in detail. For the optimal tissue growth and maturation, operational parameters for different types of stimuli should be carefully selected and, if not physiologically or developmentally based, may be detrimental to cell health and/or cause compensatory pathological changes. These changes, often transient in nature and eventually followed by tissue deterioration, may be mistakenly interpreted as improved functional outcomes. Development of bioreactors that can support long-term tissue growth and provide continuous tuning of operational parameters based on real-time monitoring of tissue function and maturation could help avoid these issues. Recently developed tissue systems, such as microtissue systems or NMJ systems, show real promise in therapeutic applications, studying the physiopathology of tissue-specific diseases and even enhancing post-implantation integration and survival. We expect that in the years to come new advances in bioreactor technology will be critical for successful engineering of highly functional striated muscle tissues eventually leading to their clinical use for treatment of muscle disease and injury.
Table 4.
Use of bioreactors for electrical stimulation of engineered striated muscle
| Cell Source | Cyclic regime | Duration | Outcome | Ref. |
|---|---|---|---|---|
| NRCMs | 2 ms pulses 5 V/cm, 1 Hz | 8 days | Improved CM ultrastructure, alignment, contraction, excitability | (112) |
| NRCMs | 2 ms pulses 4 V/cm, 1 Hz | 9 days | Increased CM volume fraction, elongation, connexin-43 expression | (116) |
| Human ESC-CMs | 2.5 V/cm, 1 Hz | 14 days | Enhanced CM sarcomerogenesis, Ca handling, electrical properties | (118) |
| Human ESC/iPSC-CMs | 3–4 V/cm, 1–6 Hz for 7 days, then 1 Hz | 7–21 days | Promoted cell organization, conduction velocity, Ca2+ handling | (119) |
| C2C12 Line | 5 V/cm, 1 Hz for 1 hour every 6 hours | 7 days | Enhanced formation of striations, contractile protein expression | (122) |
| C2C12 Line | 12.5, 25, 50 V/cm, 4 pulses at 10 or 100 Hz, then 3.6 s rest | 7 days | Higher pulse amplitude increased force production and excitability, higher pulse frequency increased the rate of protein synthesis | (104) |
Acknowledgments
This work was supported by NIH-NIAMS grants AR055226 and AR065873, NIH-NHLBI grant HL104326, and NIH Common Fund for the Microphysiological Systems Initiative grant UH2TR000505 to N.B. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Harrison JH, Merrill JP, Murray JE. Renal homotransplantation in identical twins. Surgical forum. 1956;6:432–436. [PubMed] [Google Scholar]
- 2.Fuchs JR, Nasseri BA, Vacanti JP. Tissue engineering: a 21st century solution to surgical reconstruction. The Annals of thoracic surgery. 2001;72:577–591. doi: 10.1016/s0003-4975(01)02820-x. [DOI] [PubMed] [Google Scholar]
- 3.Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. Microengineered physiological biomimicry: organs-on-chips. Lab on a chip. 2012;12:2156–2164. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- 4.Sung JH, Esch MB, Prot JM, Long CJ, Smith A, Hickman JJ, Shuler ML. Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab on a chip. 2013;13:1201–1212. doi: 10.1039/c3lc41017j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truskey GAH, Bursac N, Chan HF, Cheng C, Fernandez C, Hong S, Jung Y, Koves T, Kraus W, Leong K, Madden L, Reichert W, Zhao X. Design Considerations for an Integrated Microphysiological Muscle Tissue for Drug and Tissue Toxicity Testing. Stem Cell Research & Therapy. 2013 doi: 10.1186/scrt371. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandenburgh H, Shansky J, Benesch-Lee F, Barbata V, Reid J, Thorrez L, Valentini R, Crawford G. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve. 2008;37:438–447. doi: 10.1002/mus.20931. [DOI] [PubMed] [Google Scholar]
- 7.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 8.Bach AD, Beier JP, Stern-Staeter J, Horch RE. Skeletal muscle tissue engineering. J Cell Mol Med. 2004;8:413–422. doi: 10.1111/j.1582-4934.2004.tb00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 10.Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Ann Rev Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 11.Goldspink G, Scutt A, Loughna PT, Wells DJ, Jaenicke T, Gerlach GF. Gene expression in skeletal muscle in response to stretch and force generation. Am J Physiol. 1992;262:R356–363. doi: 10.1152/ajpregu.1992.262.3.R356. [DOI] [PubMed] [Google Scholar]
- 12.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest. 2013;123:37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93:1318–1326. doi: 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- 14.Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14:38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson SJ, Harris AJ. Formation of myotubes in aneural rat muscles. Dev Biol. 1993;156:509–518. doi: 10.1006/dbio.1993.1097. [DOI] [PubMed] [Google Scholar]
- 16.Fredette BJ, Landmesser LT. A reevaluation of the role of innervation in primary and secondary myogenesis in developing chick muscle. Dev Biol. 1991;143:19–35. doi: 10.1016/0012-1606(91)90051-4. [DOI] [PubMed] [Google Scholar]
- 17.Harris AJ. Embryonic growth and innervation of rat skeletal muscles. I. Neural regulation of muscle fibre numbers. Philos Trans R Soc Lond B Biol Sci. 1981;293:257–277. doi: 10.1098/rstb.1981.0076. [DOI] [PubMed] [Google Scholar]
- 18.Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80–86. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Chen HC, Hu YC. Bioreactors for tissue engineering. Biotechnology letters. 2006;28:1415–1423. doi: 10.1007/s10529-006-9111-x. [DOI] [PubMed] [Google Scholar]
- 20.Taber LA. Biomechanical growth laws for muscle tissue. Journal of theoretical biology. 1998;193:201–213. doi: 10.1006/jtbi.1997.0618. [DOI] [PubMed] [Google Scholar]
- 21.Bian W, Bursac N. Tissue engineering of functional skeletal muscle: challenges and recent advances. IEEE Eng Med Biol Mag. 2008;27:109–113. doi: 10.1109/MEMB.2008.928460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsang VL, Bhatia SN. Three-dimensional tissue fabrication. Advanced drug delivery reviews. 2004;56:1635–1647. doi: 10.1016/j.addr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Costa KD, Lee EJ, Holmes JW. Creating alignment and anisotropy in engineered heart tissue: role of boundary conditions in a model three-dimensional culture system. Tissue Eng. 2003;9:567–577. doi: 10.1089/107632703768247278. [DOI] [PubMed] [Google Scholar]
- 24.Bryant SJ, Cuy JL, Hauch KD, Ratner BD. Photo-patterning of porous hydrogels for tissue engineering. Biomaterials. 2007;28:2978–2986. doi: 10.1016/j.biomaterials.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bian W, Liau B, Badie N, Bursac N. Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues. Nature protocols. 2009;4:1522–1534. doi: 10.1038/nprot.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vandenburgh HH, Karlisch P, Farr L. Maintenance of Highly Contractile Tissue-Cultured Avian Skeletal Myotubes in Collagen Gel. In Vitro Cell Dev Biol. 1988;24:166–174. doi: 10.1007/BF02623542. [DOI] [PubMed] [Google Scholar]
- 27.Boudou T, Legant WR, Mu AB, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS. A Microfabricated Platform to Measure and Manipulate the Mechanics of Engineered Cardiac Microtissues. Tissue Engineering Part A. 2012;18:910–919. doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furuta A, Miyoshi S, Itabashi Y, Shimizu T, Kira S, Hayakawa K, Nishiyama N, Tanimoto K, Hagiwara Y, Satoh T, Fukuda K, Okano T, Ogawa S. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ Res. 2006;98:705–712. doi: 10.1161/01.RES.0000209515.59115.70. [DOI] [PubMed] [Google Scholar]
- 29.Neumann T, Hauschka SD, Sanders JE. Tissue engineering of skeletal muscle using polymer fiber arrays. Tissue Eng. 2003;9:995–1003. doi: 10.1089/107632703322495637. [DOI] [PubMed] [Google Scholar]
- 30.Bursac N, Papadaki M, Cohen RJ, Schoen FJ, Eisenberg SR, Carrier R, Vunjak-Novakovic G, Freed LE. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am J Physiol. 1999;277:H433–444. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- 31.Hinds S, Bian W, Dennis RG, Bursac N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials. 2011;32:3575–3583. doi: 10.1016/j.biomaterials.2011.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 33.Okano T, Satoh S, Oka T, Matsuda T. Tissue engineering of skeletal muscle. Highly dense, highly oriented hybrid muscular tissues biomimicking native tissues. Asaio J. 1997;43:M749–753. [PubMed] [Google Scholar]
- 34.Bursac N, Loo Y, Leong K, Tung L. Novel anisotropic engineered cardiac tissues: studies of electrical propagation. Biochem Biophys Res Commun. 2007;361:847–853. doi: 10.1016/j.bbrc.2007.07.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroehne V, Heschel I, Schugner F, Lasrich D, Bartsch JW, Jockusch H. Use of a novel collagen matrix with oriented pore structure for muscle cell differentiation in cell culture and in grafts. J Cell Mol Med. 2008;12:1582–1838. doi: 10.1111/j.1582-4934.2008.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liau B, Christoforou N, Leong KW, Bursac N. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials. 2011;32:9180–9187. doi: 10.1016/j.biomaterials.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34:5813–5820. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi H, Shimizu T, Nakayama M, Yamato M, Okano T. The use of anisotropic cell sheets to control orientation during the self-organization of 3D muscle tissue. Biomaterials. 2013;34:7372–7380. doi: 10.1016/j.biomaterials.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 39.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes Differentiate Optimally on Substrates With Tissue-Like Stiffness: Pathological Implications for Soft or Stiff Microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shapira-Schweitzer K, Seliktar D. Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial. Acta Biomater. 2007;3:33–41. doi: 10.1016/j.actbio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Guo X, Matsushita S, Guan J. Differentiation of cardiosphere-derived cells into a mature cardiac lineage using biodegradable poly(N-isopropylacrylamide) hydrogels. Biomaterials. 2011;32:3220–3232. doi: 10.1016/j.biomaterials.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 43.Chopra A, Lin V, McCollough A, Atzet S, Prestwich GD, Wechsler AS, Murray ME, Oake SA, Kresh JY, Janmey PA. Reprogramming cardiomyocyte mechanosensing by crosstalk between integrins and hyaluronic acid receptors. J Biomech. 2012;45:824–831. doi: 10.1016/j.jbiomech.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaudhuri O, Mooney DJ. Stem-cell differentiation: Anchoring cell-fate cues. Nature materials. 2012;11:568–569. doi: 10.1038/nmat3366. [DOI] [PubMed] [Google Scholar]
- 45.Freed LE, aV-NG . In Principles of Tissue Engineering. Academic Press; 2000. Tissue Engineering Bioreactors. [Google Scholar]
- 46.Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. Am J Physiol Heart Circ Physiol. 2001;280:H168–178. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 47.Hsu YH, Moya ML, Hughes CC, George SC, Lee AP. A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab on a chip. 2013;13:2990–2998. doi: 10.1039/c3lc50424g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agarwal A, Goss JA, Cho A, McCain ML, Parker KK. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab on a chip. 2013;13:3599–3608. doi: 10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carrier RL, Papadaki M, Rupnick M, Schoen FJ, Bursac N, Langer R, Freed LE, Vunjak-Novakovic G. Cardiac tissue engineering: cell seeding, cultivation parameters, and tissue construct characterization. Biotechnol Bioeng. 1999;64:580–589. doi: 10.1002/(sici)1097-0290(19990905)64:5<580::aid-bit8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 50.Portner R, Nagel-Heyer S, Goepfert C, Adamietz P, Meenen NM. Bioreactor design for tissue engineering. J Biosci Bioeng. 2005;100:235–245. doi: 10.1263/jbb.100.235. [DOI] [PubMed] [Google Scholar]
- 51.Lanza RPLR, Vacanti JP. In Principles of Tissue Engineering. Academic Press; San Diego: 2000. Principles of Tissue Engineering. [Google Scholar]
- 52.Molnar G, Schroedl NA, Gonda SR, Hartzell CR. Skeletal muscle satellite cells cultured in simulated microgravity. In Vitro Cell Dev Biol Anim. 1997;33:386–391. doi: 10.1007/s11626-997-0010-9. [DOI] [PubMed] [Google Scholar]
- 53.Bursac N, Papadaki M, White JA, Eisenberg SR, Vunjak-Novakovic G, Freed LE. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng. 2003;9:1243–1253. doi: 10.1089/10763270360728152. [DOI] [PubMed] [Google Scholar]
- 54.Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002;8:175–188. doi: 10.1089/107632702753724950. [DOI] [PubMed] [Google Scholar]
- 55.Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng. 2003;82:403–414. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 56.Maidhof R, Marsano A, Lee EJ, Vunjak-Novakovic G. Perfusion seeding of channeled elastomeric scaffolds with myocytes and endothelial cells for cardiac tissue engineering. Biotechnol Prog. 2010;26:565–572. doi: 10.1002/btpr.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chromiak JA, Shansky J, Perrone C, Vandenburgh HH. Bioreactor perfusion system for the long-term maintenance of tissue-engineered skeletal muscle organoids. In Vitro Cell Dev Biol Anim. 1998;34:694–703. doi: 10.1007/s11626-998-0065-2. [DOI] [PubMed] [Google Scholar]
- 58.Cheng M, Moretti M, Engelmayr GC, Freed LE. Insulin-like growth factor-I and slow, bi-directional perfusion enhance the formation of tissue-engineered cardiac grafts. Tissue Eng Part A. 2009;15:645–653. doi: 10.1089/ten.tea.2008.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shansky J, Creswick B, Lee P, Wang X, Vandenburgh H. Paracrine release of insulin-like growth factor 1 from a bioengineered tissue stimulates skeletal muscle growth in vitro. Tissue Eng. 2006;12:1833–1841. doi: 10.1089/ten.2006.12.1833. [DOI] [PubMed] [Google Scholar]
- 60.Brown MA, Iyer RK, Radisic M. Pulsatile perfusion bioreactor for cardiac tissue engineering. Biotechnol Prog. 2008;24:907–920. doi: 10.1002/btpr.11. [DOI] [PubMed] [Google Scholar]
- 61.Sakaguchi K, Shimizu T, Horaguchi S, Sekine H, Yamato M, Umezu M, Okano T. In vitro engineering of vascularized tissue surrogates. Scientific reports. 2013;3:1316. doi: 10.1038/srep01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sekine H, Shimizu T, Sakaguchi K, Dobashi I, Wada M, Yamato M, Kobayashi E, Umezu M, Okano T. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hornberger TA, Esser KA. Mechanotransduction and the regulation of protein synthesis in skeletal muscle. The Proceedings of the Nutrition Society. 2004;63:331–335. doi: 10.1079/PNS2004357. [DOI] [PubMed] [Google Scholar]
- 64.Lammerding J, Kamm RD, Lee RT. Mechanotransduction in cardiac myocytes. Ann N Y Acad Sci. 2004;1015:53–70. doi: 10.1196/annals.1302.005. [DOI] [PubMed] [Google Scholar]
- 65.Burkholder TJ. Mechanotransduction in skeletal muscle. Front Biosci. 2007;12:174–191. doi: 10.2741/2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perrone CE, Fenwick-Smith D, Vandenburgh HH. Collagen and stretch modulate autocrine secretion of insulin-like growth factor-1 and insulin-like growth factor binding proteins from differentiated skeletal muscle cells. J Biol Chem. 1995;270:2099–2106. doi: 10.1074/jbc.270.5.2099. [DOI] [PubMed] [Google Scholar]
- 67.Martineau LC, Gardiner PF. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J Appl Physiol. 2001;91:693–702. doi: 10.1152/jappl.2001.91.2.693. [DOI] [PubMed] [Google Scholar]
- 68.Musi N, Yu H, Goodyear LJ. AMP-activated protein kinase regulation and action in skeletal muscle during exercise. Biochem Soc Trans. 2003;31:191–195. doi: 10.1042/bst0310191. [DOI] [PubMed] [Google Scholar]
- 69.Cheema U, Brown R, Mudera V, Yang SY, McGrouther G, Goldspink G. Mechanical signals and IGF-I gene splicing in vitro in relation to development of skeletal muscle. J Cell Physiol. 2005;202:67–75. doi: 10.1002/jcp.20107. [DOI] [PubMed] [Google Scholar]
- 70.Molkentin JD, Dorn GW., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annual review of physiology. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 71.Kudoh S, Akazawa H, Takano H, Zou Y, Toko H, Nagai T, Komuro I. Stretch-modulation of second messengers: effects on cardiomyocyte ion transport. Progress in biophysics and molecular biology. 2003;82:57–66. doi: 10.1016/s0079-6107(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 72.Komuro I, Kudo S, Yamazaki T, Zou Y, Shiojima I, Yazaki Y. Mechanical stretch activates the stress-activated protein kinases in cardiac myocytes. FASEB J. 1996;10:631–636. doi: 10.1096/fasebj.10.5.8621062. [DOI] [PubMed] [Google Scholar]
- 73.Ryder JW, Fahlman R, Wallberg-Henriksson H, Alessi DR, Krook A, Zierath JR. Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle. Involvement Of the mitogen- and stress-activated protein kinase 1. J Biol Chem. 2000;275:1457–1462. doi: 10.1074/jbc.275.2.1457. [DOI] [PubMed] [Google Scholar]
- 74.Wretman C, Lionikas A, Widegren U, Lannergren J, Westerblad H, Henriksson J. Effects of concentric and eccentric contractions on phosphorylation of MAPK(erk1/2) and MAPK(p38) in isolated rat skeletal muscle. The Journal of physiology. 2001;535:155–164. doi: 10.1111/j.1469-7793.2001.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vandenburgh HH, Shansky J, Karlisch P, Solerssi RL. Mechanical stimulation of skeletal muscle generates lipid-related second messengers by phospholipase activation. J Cell Physiol. 1993;155:63–71. doi: 10.1002/jcp.1041550109. [DOI] [PubMed] [Google Scholar]
- 76.Kariya K, Karns LR, Simpson PC. Expression of a constitutively activated mutant of the beta-isozyme of protein kinase C in cardiac myocytes stimulates the promoter of the beta-myosin heavy chain isogene. J Biol Chem. 1991;266:10023–10026. [PubMed] [Google Scholar]
- 77.Tidball JG. Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol. 2005;98:1900–1908. doi: 10.1152/japplphysiol.01178.2004. [DOI] [PubMed] [Google Scholar]
- 78.Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. The Journal of physiology. 2005;562:367–380. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gruver CL, DeMayo F, Goldstein MA, Means AR. Targeted developmental overexpression of calmodulin induces proliferative and hypertrophic growth of cardiomyocytes in transgenic mice. Endocrinology. 1993;133:376–388. doi: 10.1210/endo.133.1.8319584. [DOI] [PubMed] [Google Scholar]
- 80.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 81.Liu M, Montazeri S, Jedlovsky T, Van Wert R, Zhang J, Li RK, Yan J. Bio-stretch, a computerized cell strain apparatus for three-dimensional organotypic cultures. In Vitro Cell Dev Biol Anim. 1999;35:87–93. doi: 10.1007/s11626-999-0006-8. [DOI] [PubMed] [Google Scholar]
- 82.Vandenburgh H. A computerized mechanical cell stimulator for tissue culture: effects on skeletal muscle organogenesis. In Vitro Cellular and Developmental Biology. 1988;24:609–619. doi: 10.1007/BF02623597. [DOI] [PubMed] [Google Scholar]
- 83.Vandenburgh HH, Hatfaludy S, Karlisch P, Shansky J. Mechanically induced alterations in cultured skeletal muscle growth. J Biomech. 1991;24(Suppl 1):91–99. doi: 10.1016/0021-9290(91)90380-6. [DOI] [PubMed] [Google Scholar]
- 84.Cheema U, Yang SY, Mudera V, Goldspink GG, Brown RA. 3-D in vitro model of early skeletal muscle development. Cell Motil Cytoskeleton. 2003;54:226–236. doi: 10.1002/cm.10095. [DOI] [PubMed] [Google Scholar]
- 85.Fink C, Ergun S, Kralisch D, Remmers U, Weil J, Eschenhagen T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J. 2000;14:669–679. doi: 10.1096/fasebj.14.5.669. [DOI] [PubMed] [Google Scholar]
- 86.Chromiak JA, Vandenburgh HH. Glucocorticoid-induced skeletal muscle atrophy in vitro is attenuated by mechanical stimulation. Am J Physiol. 1992;262:C1471–1477. doi: 10.1152/ajpcell.1992.262.6.C1471. [DOI] [PubMed] [Google Scholar]
- 87.Chromiak JA, Vandenburgh HH. Mechanical stimulation of skeletal muscle cells mitigates glucocorticoid-induced decreases in prostaglandin production and prostaglandin synthase activity. J Cell Physiol. 1994;159:407–414. doi: 10.1002/jcp.1041590304. [DOI] [PubMed] [Google Scholar]
- 88.Candiani G, Riboldi SA, Sadr N, Lorenzoni S, Neuenschwander P, Montevecchi FM, Mantero S. Cyclic mechanical stimulation favors myosin heavy chain accumulation in engineered skeletal muscle constructs. Journal of applied biomaterials & biomechanics : JABB. 2010;8:68–75. [PubMed] [Google Scholar]
- 89.Machingal MA, Corona BT, Walters TJ, Kesireddy V, Koval CN, Dannahower A, Zhao W, Yoo JJ, Christ GJ. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng Part A. 2011;17:2291–2303. doi: 10.1089/ten.tea.2010.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Corona BT, MM, ACT, Vadhavkar M, Dannahower AC, Bergman C, Zhao W, Christ GJ. Further Development of a Tissue Engineered Muscle Repair Construct In Vitro for Enhanced Functional Recovery Following Implantation In Vivo in a Murine Model of Volumetric Muscle Loss Injury. Tissue Eng. 2012:18. doi: 10.1089/ten.tea.2011.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loughna PT, Izumo S, Goldspink G, Nadal-Ginard B. Disuse and passive stretch cause rapid alterations in expression of developmental and adult contractile protein genes in skeletal muscle. Development. 1990;109:217–223. doi: 10.1242/dev.109.1.217. [DOI] [PubMed] [Google Scholar]
- 92.Akhyari P, Fedak PW, Weisel RD, Lee TY, Verma S, Mickle DA, Li RK. Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation. 2002;106:I137–142. [PubMed] [Google Scholar]
- 93.Birla RK, Huang YC, Dennis RG. Development of a novel bioreactor for the mechanical loading of tissue-engineered heart muscle. Tissue Eng. 2007;13:2239–2248. doi: 10.1089/ten.2006.0359. [DOI] [PubMed] [Google Scholar]
- 94.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boonen KJ, Langelaan ML, Polak RB, van der Schaft DW, Baaijens FP, Post MJ. Effects of a combined mechanical stimulation protocol: Value for skeletal muscle tissue engineering. J Biomech. 2010;43:1514–1521. doi: 10.1016/j.jbiomech.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 96.Otis JS, Burkholder TJ, Pavlath GK. Stretch-induced myoblast proliferation is dependent on the COX2 pathway. Experimental Cell Research. 2005;310:417–425. doi: 10.1016/j.yexcr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 97.Kumar A, Murphy R, Robinson P, Wei L, Boriek AM. Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF-kappaB transcription factor. Faseb J. 2004;18:1524–1535. doi: 10.1096/fj.04-2414com. [DOI] [PubMed] [Google Scholar]
- 98.Dusterhoft S, Pette D. Effects of electrically induced contractile activity on cultured embryonic chick breast muscle cells. Differentiation. 1990;44:178–184. doi: 10.1111/j.1432-0436.1990.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 99.Connor MK, Irrcher I, Hood DA. Contractile activity-induced transcriptional activation of cytochrome C involves Sp1 and is proportional to mitochondrial ATP synthesis in C2C12 muscle cells. J Biol Chem. 2001;276:15898–15904. doi: 10.1074/jbc.M100272200. [DOI] [PubMed] [Google Scholar]
- 100.Freyssenet D, Connor MK, Takahashi M, Hood DA. Cytochrome c transcriptional activation and mRNA stability during contractile activity in skeletal muscle. Am J Physiol. 1999;277:E26–32. doi: 10.1152/ajpendo.1999.277.1.E26. [DOI] [PubMed] [Google Scholar]
- 101.Brevet A, Pinto E, Peacock J, Stockdale FE. Myosin synthesis increased by electrical stimulation of skeletal muscle cell cultures. Science. 1976;193:1152–1154. doi: 10.1126/science.959833. [DOI] [PubMed] [Google Scholar]
- 102.Serena E, Flaibani M, Carnio S, Boldrin L, Vitiello L, De Coppi P, Elvassore N. Electrophysiologic stimulation improves myogenic potential of muscle precursor cells grown in a 3D collagen scaffold. Neurological research. 2008;30:207–214. doi: 10.1179/174313208X281109. [DOI] [PubMed] [Google Scholar]
- 103.Khodabukus A, Baar K. Defined electrical stimulation emphasizing excitability for the development and testing of engineered skeletal muscle. Tissue Eng Part C Methods. 2012;18:349–357. doi: 10.1089/ten.TEC.2011.0364. [DOI] [PubMed] [Google Scholar]
- 104.Donnelly K, Khodabukus A, Philp A, Deldicque L, Dennis RG, Baar K. A novel bioreactor for stimulating skeletal muscle in vitro. Tissue Eng Part C Methods. 2010;16:711–718. doi: 10.1089/ten.TEC.2009.0125. [DOI] [PubMed] [Google Scholar]
- 105.Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. The Journal of physiology. 1960;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Naumann K, Pette D. Effects of chronic stimulation with different impulse patterns on the expression of myosin isoforms in rat myotube cultures. Differentiation. 1994;55:203–211. doi: 10.1046/j.1432-0436.1994.5530203.x. [DOI] [PubMed] [Google Scholar]
- 107.Huang YC, Dennis RG, Baar K. Cultured slow vs. fast skeletal muscle cells differ in physiology and responsiveness to stimulation. Am J Physiol Cell Physiol. 2006;291:C11–17. doi: 10.1152/ajpcell.00366.2005. [DOI] [PubMed] [Google Scholar]
- 108.Pette D. Historical Perspectives: plasticity of mammalian skeletal muscle. J Appl Physiol. 2001;90:1119–1124. doi: 10.1152/jappl.2001.90.3.1119. [DOI] [PubMed] [Google Scholar]
- 109.Hamalainen N, Pette D. Slow-to-fast transitions in myosin expression of rat soleus muscle by phasic high-frequency stimulation. FEBS Lett. 1996;399:220–222. doi: 10.1016/s0014-5793(96)01325-7. [DOI] [PubMed] [Google Scholar]
- 110.Dennis RG, Smith B, Philp A, Donnelly K, Baar K. Bioreactors for guiding muscle tissue growth and development. Adv Biochem Eng Biotechnol. 2009;112:39–79. doi: 10.1007/978-3-540-69357-4_3. [DOI] [PubMed] [Google Scholar]
- 111.Guo X, Gonzalez M, Stancescu M, Vandenburgh HH, Hickman JJ. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials. 2011;32:9602–9611. doi: 10.1016/j.biomaterials.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sauer H, Rahimi G, Hescheler J, Wartenberg M. Effects of electrical fields on cardiomyocyte differentiation of embryonic stem cells. J Cell Biochem. 1999;75:710–723. doi: 10.1002/(sici)1097-4644(19991215)75:4<710::aid-jcb16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 114.Sathaye A, Bursac N, Sheehy S, Tung L. Electrical pacing counteracts intrinsic shortening of action potential duration of neonatal rat ventricular cells in culture. J Mol Cell Cardiol. 2006;41:633–641. doi: 10.1016/j.yjmcc.2006.06.076. [DOI] [PubMed] [Google Scholar]
- 115.Kujala K, Ahola A, Pekkanen-Mattila M, Ikonen L, Kerkela E, Hyttinen J, Aalto-Setala K. Electrical Field Stimulation with a Novel Platform: Effect on Cardiomyocyte Gene Expression but not on Orientation. International journal of biomedical science : IJBS. 2012;8:109–120. [PMC free article] [PubMed] [Google Scholar]
- 116.Lasher RA, Pahnke AQ, Johnson JM, Sachse FB, Hitchcock RW. Electrical stimulation directs engineered cardiac tissue to an age-matched native phenotype. Journal of tissue engineering. 2012;3:1–15. doi: 10.1177/2041731412455354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen MQ, Xie X, Wilson KD, Sun N, Wu JC, Giovangrandi L, Kovacs GT. Current-Controlled Electrical Point-Source Stimulation of Embryonic Stem Cells. Cell Mol Bioeng. 2009;2:625–635. doi: 10.1007/s12195-009-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lieu DK, Fu JD, Chiamvimonvat N, Tung KC, McNerney GP, Huser T, Keller G, Kong CW, Li RA. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Arrhythm Electrophysiol. 2013;6:191–201. doi: 10.1161/CIRCEP.111.973420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Masse S, Gagliardi M, Hsieh A, Thavandiran N, Laflamme MA, Nanthakumar K, Gross GJ, Backx PH, Keller G, Radisic M. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10:781–787. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H1223–1230. doi: 10.1152/ajpheart.00079.2007. [DOI] [PubMed] [Google Scholar]
- 121.Feng Z, Matsumoto T, Nomura Y, Nakamura T. An electro-tensile bioreactor for 3-D culturing of cardiomyocytes. A bioreactor system that simulates the myocardium’s electrical and mechanical response in vivo. IEEE Eng Med Biol Mag. 2005;24:73–79. doi: 10.1109/memb.2005.1463399. [DOI] [PubMed] [Google Scholar]
- 122.Liao IC, Liu JB, Bursac N, Leong KW. Effect of Electromechanical Stimulation on the Maturation of Myotubes on Aligned Electrospun Fibers. Cell Mol Bioeng. 2008;1:133–145. doi: 10.1007/s12195-008-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barash Y, Dvir T, Tandeitnik P, Ruvinov E, Guterman H, Cohen S. Electric field stimulation integrated into perfusion bioreactor for cardiac tissue engineering. Tissue Eng Part C Methods. 2010;16:1417–1426. doi: 10.1089/ten.TEC.2010.0068. [DOI] [PubMed] [Google Scholar]
- 124.Maidhof R, Tandon N, Lee EJ, Luo J, Duan Y, Yeager K, Konofagou E, Vunjak-Novakovic G. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. Journal of tissue engineering and regenerative medicine. 2012;6:e12–23. doi: 10.1002/term.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kensah G, Gruh I, Viering J, Schumann H, Dahlmann J, Meyer H, Skvorc D, Bar A, Akhyari P, Heisterkamp A, Haverich A, Martin U. A novel miniaturized multimodal bioreactor for continuous in situ assessment of bioartificial cardiac tissue during stimulation and maturation. Tissue Eng Part C Methods. 2011;17:463–473. doi: 10.1089/ten.tec.2010.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bardouille C, Lehmann J, Heimann P, Jockusch H. Growth and differentiation of permanent and secondary mouse myogenic cell lines on microcarriers. Applied microbiology and biotechnology. 2001;55:556–562. doi: 10.1007/s002530100595. [DOI] [PubMed] [Google Scholar]
- 127.Slentz DH, Truskey GA, Kraus WE. Effects of chronic exposure to simulated microgravity on skeletal muscle cell proliferation and differentiation. In Vitro Cell Dev Biol Anim. 2001;37:148–156. doi: 10.1290/1071-2690(2001)037<0148:EOCETS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 128.Akins RE, Schroedl NA, Gonda SR, Hartzell CR. Neonatal rat heart cells cultured in simulated microgravity. In Vitro Cell Dev Biol. 1997:337–343. doi: 10.1007/s11626-997-0003-8. [DOI] [PubMed] [Google Scholar]
- 129.Flaibani M, Luni C, Sbalchiero E, Elvassore N. Flow cytometric cell cycle analysis of muscle precursor cells cultured within 3D scaffolds in a perfusion bioreactor. Biotechnol Prog. 2009;25:286–295. doi: 10.1002/btpr.40. [DOI] [PubMed] [Google Scholar]
- 130.Radisic M, Marsano A, Maidhof R, Wang Y, Vunjak-Novakovic G. Cardiac tissue engineering using perfusion bioreactor systems. Nature protocols. 2008;3:719–738. doi: 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fink C, Ergun S, Kralisch D, Remmers U, Weil J, Eschenhagen T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000;14:669–679. doi: 10.1096/fasebj.14.5.669. [DOI] [PubMed] [Google Scholar]
- 132.Boublik J, Park H, Radisic M, Tognana E, Chen F, Pei M, Vunjak-Novakovic G, Freed LE. Mechanical properties and remodeling of hybrid cardiac constructs made from heart cells, fibrin, and biodegradable, elastomeric knitted fabric. Tissue Eng. 2005;11:1122–1132. doi: 10.1089/ten.2005.11.1122. [DOI] [PubMed] [Google Scholar]
- 133.Kensah G, Roa Lara A, Dahlmann J, Zweigerdt R, Schwanke K, Hegermann J, Skvorc D, Gawol A, Azizian A, Wagner S, Maier LS, Krause A, Drager G, Ochs M, Haverich A, Gruh I, Martin U. Murine and human pluripotent stem cell-derived cardiac bodies form contractile myocardial tissue in vitro. Eur Heart J. 2013;34:1134–1146. doi: 10.1093/eurheartj/ehs349. [DOI] [PubMed] [Google Scholar]
- 134.Powell CA, Smiley BL, Mills J, Vandenburgh HH. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol. 2002;283:C1557–1565. doi: 10.1152/ajpcell.00595.2001. [DOI] [PubMed] [Google Scholar]
- 135.Moon DG, Christ G, Stitzel JD, Atala A, Yoo JJ. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng. 2008:14. doi: 10.1089/tea.2007.0104. [DOI] [PubMed] [Google Scholar]