Abstract
Cell motility is partially dependent on interactions between the integrins and the extracellular matrix. Our previous studies have identified synthetic D-amino acid cell adhesion peptides using a combinatorial screening approach. In this study, we demonstrate that HYD1 (kikmviswkg) completely blocks random haptotactic migration and inhibits invasion of prostate carcinoma cells on laminin-5. This effect is adhesion independent and reversible. The inhibition of migration by HYD1 involves a dramatic remodeling of the actin cytoskeleton resulting in increased stress fiber formation and actin colocalization with cortactin at the cell membrane. HYD1 interacts with α6β1 (not α6β4) and α3β1 integrins and surprisingly elevates laminin-5-dependent intracellular signals including focal adhesion kinase, mitogen-activated protein kinase kinase and extracellular signal-regulated kinase. HYD1 does not contain a previously characterized binding sequence for integrins. A scrambled derivative of HYD1, called HYDS (wiksmkivkg), does not interact with the α6 or β3 integrin subunits and is not biologically active. Taken together, these results indicate that HYD1 is a biologically active integrin-targeting peptide that reversibly inhibits tumor cell migration on laminin-5 and uncouples phosphotyrosine signaling from cytoskeletal-dependent migration.
Introduction
Cell migration is a complex process integral to normal biological events such as wound healing and inflammatory responses as well as the pathological circumstances of tumor invasion and metastasis. The motile nature of all cell types depends upon the actions of many different molecular components (1). Central to this process are the signaling and cytoskeletal responses elicited by the interactions of integrins with the extracellular matrix (ECM). The adhesive complexes formed from integrin ligation and activation regulate intracellular signaling events that dictate the cytoskeletal reorganization necessary for cell movement (2). Several signaling pathways have been shown to be important for cell movement and specific pathways may have crucial roles depending on the extracellular environment (2–5). In addition, tumors associated with an invasive and migratory phenotype may favor a certain integrin repertoire (6–8), displaying a pivotal role of specific integrin/ECM interactions that favor tumor metastasis.
It is well established that the ECM can induce integrin-dependent cell spreading and migration by activation of specific signaling programs that regulate focal adhesion and cytoskeletal dynamics (1,2). Interestingly, specific integrin/ECM pairs have been shown to differentially modulate the activities of these programs (5,9) suggesting that integrin and ECM composition will dictate the signaling response and phenotype. Laminin-5-dependent cell spreading and migratory activities, for example, have been linked to the activities of focal adhesion kinase (FAK), phosphoinositide 3-OH kinase (PI3-K), p21-activated kinase (PAK) and the mitogen-activated protein kinase (MAPK) pathway (5,10,11). Inhibition of these pathways using small molecules can ultimately block a migratory phenotype. However, given the multitude of components in these pathways and their redundancy in function, targeted dysregulation of integin/ligand activity may prove to be a more potent method to inhibit motility.
Integrins are evolutionarily conserved heterodimeric cell surface molecules. To date there are 18 distinct α and 8 distinct β subunits that pair in a restrictive manner to give about 24 different integrins that have individual ligand binding specificities (12). The primary ligands for integrins are proteins of the ECM that consist of Type I and IV collagens, fibronectin, laminins, heparin sulfate proteoglycan and other non-collagenous glycoproteins (13). The integrins α6β1, α6β4 and α3β1 are laminin receptors, (14,15) and these integrin pairs are associated with the progression of many epithelial tumors (16–18). In particular, the α6 subunit is continually expressed during prostate cancer progression and found in micrometastases (8,16,19). Previous studies have shown that biologically active peptides developed from defined regions within laminin chains can have profound effects on biological events including cell migration and metastasis (20–27). These findings prompted us to develop α6-binding cell adhesion peptides.
Our previous work (28,29) has identified human tumor cell adhesion peptides by using a ‘one-bead one-compound’ combinatorial screening method (30). Peptides were selected that were capable of binding prostate carcinoma cells expressing the α6 integrin. We characterized two D-amino acid peptides, HYD1 (kikmviswkg) and RZ3 (kmviywkag), as cell adhesion peptides based on their ability to both support tumor cell adhesion themselves and inhibit tumor cell adhesion to immobilized ECM proteins (29). These peptides do not show homology to any known binding sequences for integrins. In the present study, we examine the effect of these peptides on laminin-5-dependent haptotaxis. HYD1 causes dramatic cytoskeletal reorganization in prostate tumor cells adhered to 1748 laminin-5, resulting in a loss of cell migration. HYD1 interacts with both α6 and α3 integrin complexes and induces signaling through FAK, mitogen-activated protein kinase kinase (MEK) and extracellular signal-regulated kinase (ERK). These data show that HYD1 is a novel synthetic peptide that disconnects pro-migration phosphorylation signals from cytoskeletal-dependent migration.
Materials and methods
Cell lines and culture conditions
All cell lines were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. The human prostate carcinoma cell lines, PC3N and DU-145H, were grown in Iscove’s Modified Dulbecco’s Medium (Gibco BRL, Gaithersburg, MD) plus 10% fetal bovine serum (Gibco BRL). All medium was supplemented with penicillin/streptomycin, 100 U/ml (Gibco BRL). Serum-free medium was supplemented with 0.1% bovine serum albumin (BSA; Sigma, St Louis, MO). PC3N cells are a variant of the human PC3 prostate carcinoma cell line (31). DU-145H cells are DU-145 cells, a prostate carcinoma cell line originating from a brain metastasis, selected for high expression of α6 integrin (7). DU-145 and PC3 cell lines are available from ATCC (Rockville, MD). HaCaT cells (32) were obtained from Dr Norbert E. Fusenig (German Cancer Research Center, University of Heidelberg, Heidelberg, Germany).
Antibodies and reagents
GoH3, a rat anti-α6 functional-blocking antibody was purchased from Serotech (Raleigh, NC). The anti-α3 functional-blocking antibody, P1B5, was purchased from Chemicon (Temecula, CA). AIIB2, a rat anti-β1 functional-blocking antibody, and TS2/16, a mouse anti-β1 antibody, were purified from its hybridoma (Developmental Studies Hybridoma Bank, University of Iowa). Integrin antibody used for western blotting, Anti-α6, AA6A rabbit polyclonal, was generated and purified by Bethyl Laboratories (Montgomery, TX). Anti-α3, AB1920, was purchased from Chemicon (Temecula, CA). Antibodies to FAK, Tyr-397 and 4.47, in addition to phospho-MEK (serine 298) and cortactin (clone 4F11) were purchased from Upstate (Lake Placid, NY). MEK1 antibody was obtained from BD Transduction Laboratories (San Diego, CA). Map kinase antibodies, phospho-p44/42 (Thr202/Tyr204) and total p44/42, were purchased from Cell Signaling Technology (Beverly, MA). Inhibitors for Src (PP2) and ROCK (Y-27632) were purchased from Calbiochem (San Diego, CA). Anti-mouse and anti-rabbit secondary antibodies conjugated to horseradish peroxidase for immunoblotting were obtained from Chemicon (Temecula, CA). Anti-mouse secondary antibody conjugated to Alexa Fluor 568 and phalloidin conjugated to Alexa Fluor 488, used in immunofluorescence, were obtained through Molecular Probes (Eugene, OR).
Synthetic peptides and laminin-5
RZ3 (kmviywkag), HYD1 (kikmviswkg) and HYDS (wiksmkivkg) contain all D-amino acids as previously described (29). AG73 (RKRLQVQLSIRT) is a previously characterized L-amino acid peptide from the G-domain of the laminin α1 chain (22). All peptides were synthesized and purified by high-performance liquid chromatography (HPLC) to >90% by Global Peptide Service (Fort Collins, CO). Laminin-5 was obtained from conditioned media of HaCaT cells. Briefly, HaCaT cells were grown in DMEM/F12 serum-free medium for 1 week in T175 cm2 tissue culture flasks. The media were collected and clarified by centrifugation at 40 for 15 min. The resulting supernatant was filtered through a 0.2 μm acetate filter in the presence of protease inhibitors (50 μM PMSF, 50 μM N-ethylmaleimide and 5 mM EDTA). The resulting material is concentrated and frozen at −80°C and the resulting supernatant is used. This procedure is essential as it takes advantage of the known cold insoluble properties of a potential contaminant, fibronectin. For laminin-5 coating of tissue culture plastic, coverslips and time-lapse video plates, conditioned media were added to the surface for 2 h at room temperature and washed one time with phosphate-buffered saline (PBS) (2.7 mM KCl, 1.5 mM KH2PO4, 138 mM NaCl and 8.1 mM Na2HPO4, pH 7.4) before use. For signaling experiments, plates were blocked with 1% BSA for 30 min at room temperature.
Affinity precipitation with peptides
DU-145H cells (1.2 × 108 cells) were harvested with 2 mM EDTA in PBS. Cells were washed in AP buffer containing 25 mM HEPES (pH 8.0), 100 mM KCl, 1 mM MgCl, 1 mM CaCl, 20% glycerol, 1 mM PMSF and 1 μg/ml leupeptin and aprotinin. Cells were lysed using a dounce homogenizer and then centrifuged for 5 min at 15 000 g at 4°C in an Eppendorf microcentrifuge. Supernatants were further centrifuged for 15 min at 96 000 g at 4°C in a Sorvall RC M150GX ultracentrifuge. Membrane fraction pellets were suspended in AP buffer containing 0.2% NP-40 and analyzed for protein concentration using the BCA protein assay kit (Pierce, Rockford, IL). A total of 500 μg of biotinylated peptide was preloaded on to 30 μl of UltraLink Immobilized NeutrAvidin Plus beads (Pierce) in a buffer containing 0.5 mM KCl, 0.3 mM KH2PO4, 27.6 mM NaCl and 1.6 mM Na2HPO4, pH 7.4, for 1 h at room temperature. The resulting beads were washed twice with AP buffer and incubated with 30 μg of the membrane fraction adjusted to a total volume of 500 μl for 18 h at 4°C. NeutrAvidin beads were washed three times with AP buffer containing 0.2% NP-40. All samples were suspended in 2× laemmli buffer and analyzed by SDS–PAGE electrophoresis.
Cell lysis, western-blot, and immunoprecipitation
For cell signaling experiments involving FAK, MEK and ERK, cells were incubated in serum-free media overnight and harvested with 5 mM EDTA in PBS for 10 min. Cells were washed in serum-free media and added to laminin-5-coated plates or BSA-coated plates for 1 h at 37°C. Cells were then treated with peptide in serum-free media for stated times at 37°C. Two minutes before lysis, 0.5 mM sodium orthovanadate was added into the media. Cells were lysed for 5 min in a modified RIPA lysis buffer containing 40 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, 6 mM EDTA, 100 mM NaF, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 1 mM PMSF and 1 μg/ml leupeptin and aprotinin. Lysates were processed for SDS–PAGE after adjusting for equal loading by using the BCA protein assay kit (Rockford IL). Proteins resolved in the gel were electrotransferred to Millipore Immobilon-P polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford MA). Blots were developed using chemiluminescence (ECL Western Blotting Detection System, Amersham, Arlington Heights, IL) and band densities were analyzed by densitometry using NIH Image software (Scion). For stripping, membranes were incubated in stripping buffer [62.5 mM Tris (pH 6.75), 2% SDS and 100 mM beta-mercaptoethanol] at 50°C for 30 min followed by washing five times in TBS. Immunoprecipitation of cortactin per reaction contained, 200 μg total protein lysate, 35 μl of protein G sepharose and 3 μg of antibody. The final volume was adjusted to 400 μl of lysis buffer described above. The mixture was rotated at 4°C for 18 h and the complexes were washed three times with cold lysis buffer. All samples were suspended in 2× laemmli buffer and analyzed by SDS–PAGE electrophoresis.
Cell motility assays
Both time-lapse video microscopy and modified Boyden chamber transwell assays were performed. Time-lapse video microscopy was used to analyze random haptotaxis of PC3N cells on laminin-5. The effect of the peptides or pharmacological inhibitors was observed with differential interference contrast optics on an inverted Olympus IMT2 microscope (Olympus America, Melville NY). The microscope was equipped with a BiopTechs Delta T live Cell system (BiopTechs, Butler PA) with a humidified CO2/Air atmosphere (5:95) and was maintained at 37°C. Images were obtained with a grayscale CCD camera (ORCA-100, Hamamatsu, Japan) and analyzed using Compix imaging software (SimplePCI 4.0, Compix Imaging, Cranberry Township PA). A total of 70 000 cells were plated for 1 h in serum-free conditions on laminin-5-coated Delta T dishes (0.15 mm, BiopTechs, Butler PA). Coating was done as described above. Soluble peptides or chemical inhibitors in serum-free media at the stated concentrations were added to the adhered cells and video was started at ~30 min post peptide or inhibitor treatment. For peptide reversibility experiments, cells were adhered and treated as above but after 30 min of treatment with peptide the cells were washed one time with PBS and serum-free media or soluble laminin-5 was added back. Videos were 4 h in length with a frame capture every 5 min. The speed of each cell was calculated manually with the Compix software by measuring the distance traveled by the cell nuclei over the 4 h time period. The average rate of migration (15 cells/experiment) in microns was calculated. Each experiment was performed at least three times.
Analysis of invasion was performed using a modified Boyden chamber assay. Falcon polyethylene terephthalate (PET) track-etched membrane 8-μm pore inserts (Becton Dickinson Labware, Franklin Lakes, NJ) were coated on the underside with laminin-5 (HaCaT conditioned media) for 2 h at room temperature and were washed one time with HEPES buffer (20 mM HEPES, 130 mM NaCl, 5 mM KCl, 0.8 mM MgCl2, 1 mM CaCl2, pH 7.4). Membranes were blocked with 1% BSA in PBS for 30 min at room temperature and were washed one time with HEPES buffer. In the upper chamber, in serum-free media 50 000 cells were seeded and allowed to attach for 1 h followed by the indicated treatments with peptide or chemical inhibitor. Peptides and inhibitors were in serum-free media and in both upper and lower chambers. For experiments with integrin-blocking antibodies, cells were pretreated in suspension with the indicated antibody(s) for 15 min before attachment. Cells were allowed to invade for 18 h at 37°C and cells remaining in the upper well were removed with a cotton swab while cells on the lower surface were fixed with methanol and stained with crystal violet. The number of cells on the lower surface of the insert was quantified by solubilizing the dye in 0.1 M sodium citrate and reading the absorbance at 562 nm. Triplicate determinations were done with each treatment and each experiment was performed at least three times.
Immunofluorescence
Coverslips were coated with laminin-5 as stated above and washed with PBS before plating the cells. Cells were plated subconfluently and allowed to adhere for 1 h before treatments. Peptide was added in serum-free media for 30 min before fixation. Coverslips were dipped in PBS and fixed in PBS containing 3.2% formaldehyde (Ted Pella, Irvine, CA) for 5 min. The coverslips were rinsed in water before incubation with 50 mM NH4Cl in PBS for 5 min. Following a wash in PBS for 5 min cells were treated with 0.2% Triton X-100 in PBS for 5 min. Fixation and washing were performed at room temperature. Cells were washed again in PBS and blocked with 1% BSA for 30 min at room temperature before incubating with the primary antibody (cortactin 1:100) and phallodin (1:40) for 1 h at room temperature. Secondary antibody was diluted at 1:200 for 30 min at room temperature. The coverslips were mounted with Anti-Fade (Molecular Probes) and observed at ×40 with an inverted Zeiss Axiophot (Carl Zeiss, Gottingen, Germany) equipped with an Axiocam camera. Captured images were analyzed with Photoshop 7.0 software (Adobe Systems, San Jose, CA).
In vivo actin distribution assay
The distribution of globular actin and filamentous actin was analyzed using an F-actin/G-actin in vivo assay kit (Cytoskeleton, Denver, CO). Briefly, after peptide treatment, cells were lysed in a cell lysis and F-actin stabilization buffer and homogenized using 27 gauge needles. In order to isolate cellular filamentous actin, cell lysates were centrifuged at 100 000 g for 60 min at 37°C and the supernatants (G-actin) were immediately separated from the pellets (F-actin). The pellets were resuspended in the same volume of dH2O as the supernatants and were incubated on ice for 60 min. Cytochalasin D (2 μM) was added to the resuspended pellets to promote dissociation of filamentous-actin in the pellets into actin monomers. The pellet was then further fractioned to insoluble and soluble forms by centrifugation at 14 000 r.p.m. for 1 min. The insoluble portion containing the cell membrane was solubilized in RIPA buffer. Equal amounts (2 μg of protein for each sample) of each fraction were subjected to SDS–PAGE and analyzed by western Blot with an anti-actin antibody provided in the kit.
Results
HYD1 blocks laminin-5-dependent migration and invasion
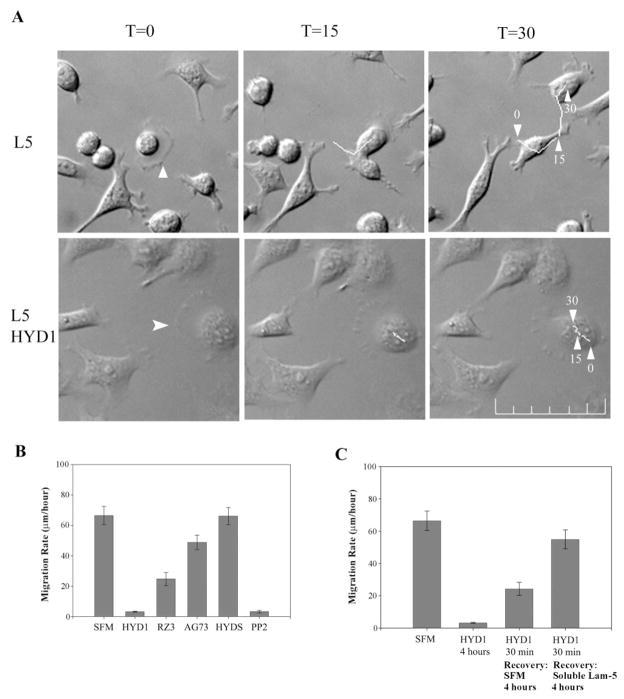
Effect of the peptides, HYD1 and RZ3, on laminin-5-mediated cell migration and invasion was investigated. Video microscopy was used to measure random haptotaxis and a modified Boyden chamber assay was used to assess the effects on invasion toward laminin-5. In order to analyze the effect of the peptides independent of disrupting adhesive contacts with the native ligand, cells were allowed to adhere for 1 h prior to treatment with peptides in serum-free media. Treatment with HYD1 completely blocked random haptotaxis on laminin-5 that was quantified for a 4-h period (Figure 1A and B and Supplementary Video). A scrambled variant of HYD1, HYDS, was inactive. The activity of HYD1 was a post-ligand-occupancy event since there was no loss of adhesion and the cells remain spread over the course of the video. In addition, membrane surfaces were observed to remain active after peptide treatment and cell division occurred normally. The effect of HYD1 was reversible following peptide wash-out (Figure 1C). Adding soluble laminin-5 after peptide wash-out enabled a faster recovery of migration over a 4-h period as compared to adding serum-free media, indicating that the cells retain the capacity to respond to a pro-migratory stimulus. The inhibitory activity for HYD1 was quantitatively comparable to PP2, a Src family kinase inhibitor previously published to inhibit migration of prostate carcinoma cell lines (33) (Figure 1B). The morphology of cells treated with HYD1 as compared to PP2 suggest a distinct mechanism of action. HYD1 treated cells remain spread and have active membrane surfaces compared with the non-spread, rounded morphology of cells treated with PP2 (Supplementary Video). RZ3 reduced the rate of random haptotaxis (Figure 1B); however, its inability to remain soluble may contribute to the activity since many cells were trapped in an insoluble peptide web (Supplementary Video). In addition, AG73, a cell adhesion peptide derived from the alpha globular domain of laminin-1 (22) had no migration-blocking activity in this assay.
Fig. 1.
HYD1 blocks cell migration on laminin-5. (A) PC3N cells were placed on laminin-5 for 1 h followed by the addition of serum-free media or serum-free media containing HYD1 (75 μg/ml). Videos were started ~30 min post-treatment. Time-lapse images were taken at start of video T = 0, 15 and 30 min. White arrowhead in T = 0 frame marks the cell followed during analysis at T = 30. Scale bar = 100 μm. (B) Cells were added as described in (A) and treated with 75 μg/ml of the peptides HYD1, RZ3, AG73 or HYDS in serum-free media or serum-free media alone (SFM). The Src inhibitor, PP2, was used at a concentration of 10 μM. Videos were started 30 min post-treatment and images were taken every 5 min for 4 h. (C) The effect of HYD1 is reversible. Cells were added as described in (A). Cells were treated with HYD1 for the entire course of the video (4 h). Alternatively, cells were treated for 30 min, the peptide was washed-out, and the cells were exposed to serum-free media alone (SFM) or serum-free media containing soluble laminin-5 and allowed to recover over the 4-h period. Migration rates were quantified as described in Materials and methods. Error bars are standard error from the mean migration rate of 15 cells/experiment.
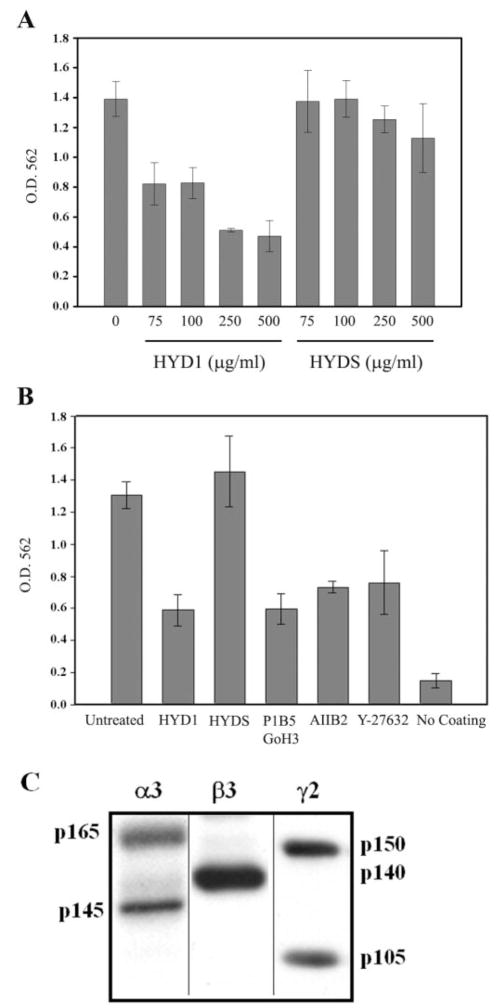
HYD1 also was functional in inhibiting invasion toward immobilized laminin-5. The activity of HYD1 to inhibit invasion was concentration dependent with ~60% inhibition at a concentration of 250 μg/ml (Figure 2A). The scrambled variant, HYDS, was inactive. The HYD1 peptide was able to inhibit invasion to the same extent as other known inhibitors of invasion such as the Rho-kinase inhibitor, Y-27632 (34), as well as integrin-function blocking antibodies to α6, α3 and β1 (Figure 2B). The composition of the laminin-5 preparation used in these experiments is shown in Figure 2C. Taken together, these data indicate that HYD1 can block random haptotaxis on laminin-5 and inhibit cellular invasion toward laminin-5.
Fig. 2.
HYD1 inhibits laminin-5-dependent cellular invasion. (A) Concentration-dependent inhibition of PC3N cell invasion toward laminin-5. Cells were added into the upper chamber of laminin-5-coated transwells for 1 h prior to treatment with 75, 100, 250 or 500 (μg/ml) HYD1 or HYDS in serum-free media as described in Materials and methods. (B) Comparison of HYD1 activity to other known inhibitors of invasion. Cells were added as in (A) and followed by treatment with 250 μg/ml HYD1 or HYDS in serum-free media. The Rho-kinase inhibitor, Y-27632, was used at a concentration of 25 μM in serum-free media. Integrin function-blocking antibodies P1B5 (α3), GoH3 (α6) and AIIB2 (β1) were used at a concentration of 10 μg/ml and were incubated with cells for 15 min at room temperature prior to adding the cells into transwells. Error bars are standard deviation from the mean. (C) Laminin subchain composition of laminin-5 matrix material. The concentrated conditioned media from HaCaT cells were analyzed by 6% PAGE, transferred to PVDF membranes and probed for the α3 chain (BM165, mouse monoclonal antibody), the β3 chain (BM140, mouse monoclonal antibody) or the γ2 chain (GB3, goat monoclonal antibody).
HYD1 induces actin cytoskeletal remodeling and cortactin-mediated actin dynamics at the cell membrane
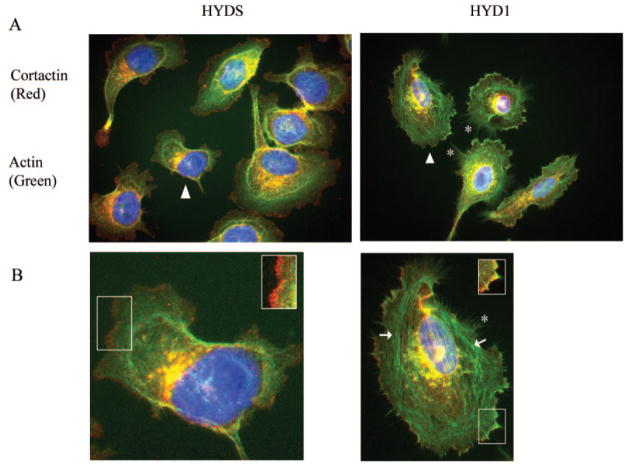
A critical component of cell motility dynamics is the regulation of the actin cytoskeleton (35,36). Given the immediate loss of motility after HYD1 treatment we investigated the concurrent changes in actin organization. Cells treated for 30 min with HYD1 displayed increased filamentous actin and an accumulation of actin at the cell membrane with associated microspikes (Figure 3A and B; asterisks). This robust change in the cytoskeletal architecture is not seen with treatment of the scrambled peptide, HYDS. Interestingly, although HYD1 generated the formation of stress fibers (Figure 3B; arrows), the peptide did not increase Rho activity (data not shown) suggesting Rho-independent signaling events are responsible for the phenotype.
Fig. 3.
HYD1 induces cytoskeletal reorganization on laminin-5. (A) HYD1 treatment causes global actin reorganization. PC3N cells were placed on laminin-5-coated coverslips for 1 h followed by addition of HYD1 or HYDS (75 μg/ml) in serum-free media for 30 min. Coverslips were fixed and stained for actin (green) and cortactin (red) as described in Materials and methods. Asterisks designate points of microspike formation at the cell membrane. Closed arrowhead marks the cell enlarged in (B). (B) HYD1 induces colocalization of cortactin with actin at the cell membrane. The cells marked in (A) were enlarged using Photoshop 7.0 software. The insets were created by using the auto level function in Photoshop on the boxed region of the cell membrane. Asterisks designate points of microspike formation at the cell membrane. Closed arrows designate areas containing stress fibers.
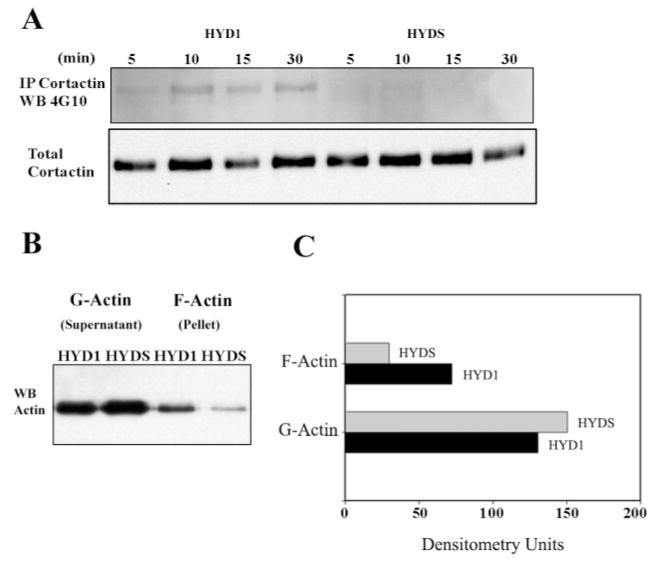
Cortactin is a cortical actin binding protein that localizes at sites of dynamic membrane activity (37). Given the accumulation of actin and actin microspikes at the cell membrane following HYD1 treatment, we hypothesized that this phenotype may be mediated through cortactin. We show that cortactin is involved in HYD1 induced accumulation of filamentous actin at the cell membrane by its colocalization with actin at the cell membrane in cells treated with HYD1 for 30 min (Figure 3B; inset). In addition, HYD1 induced an increase in tyrosine phosphorylation of cortactin over time (Figure 4A). This is consistent with a concomitant increase in filamentous actin in the cell membrane fraction following treatment with HYD1 for 30 min (Figure 4B and C). These data show that HYD1 stimulates cytoskeletal reorganization mediated in part by signaling through cortactin.
Fig. 4.
HYD1 induces phosphorylation of cortactin and alters membrane actin dynamics. (A) HYD1 induces phosphorylation of cortactin. PC3N cells were placed on laminin-5 for 1 h followed by treatment with HYD1 or HYDS (75 μg/ml) in serum-free media for 5, 10, 15 or 30 min before lysis. The phosphorylation state of cortactin was analyzed after immunoprecipitation with the phosphotyrosine antibody 4G10. (B) HYD1 induces F-actin assembly at the cell membrane. PC3N cells were added as described in (A) and treated with HYD1 or HYDS for 30 min. The distribution of F-actin in the membrane compared with the globular pool of actin was analyzed as described in Materials and methods. (C) Desitometry plot of results shown in B.
HYD1 interacts with α6 and α3 integrins and activates integrin-associated signaling
Given that cell migration on laminin-5 is mediated by α6 and α3 integins (11,38–41), we determined whether either peptide interacted with α6 or α3 integrin subunits or the collagen receptor, α2 integrin. To define receptor specificity, each peptide was incubated with membrane preparations from DU-145H prostate carcinoma cells and the resulting precipitate was analyzed for integrin content. HYD1 interacted with complexes containing full-length α6 and β3 integrins but not the α2 integrin (Figure 5). RZ3 interacted with α3 integrin to a greater extent than α6 integrin as judged by the amount of integrin retrieved. HYD1 was superior to RZ3 in retrieving the α6 and α3 subunits. The scrambled derivative, HYDS, does not interact with any tested integrin alpha subunits (Figure 5). Further, since the DU-145H cells contain both the α6β1 and α6β4 integrins, we determined whether the HYD1 peptide specifically retrieved α6β1 complexes. Depletion of β1 integrin containing integrins from the lysate removed the opportunity for HYD1 to bind the α6 integrin (Figure 5), indicating that the peptide interacts with the α6β1 heterodimer.
Fig. 5.
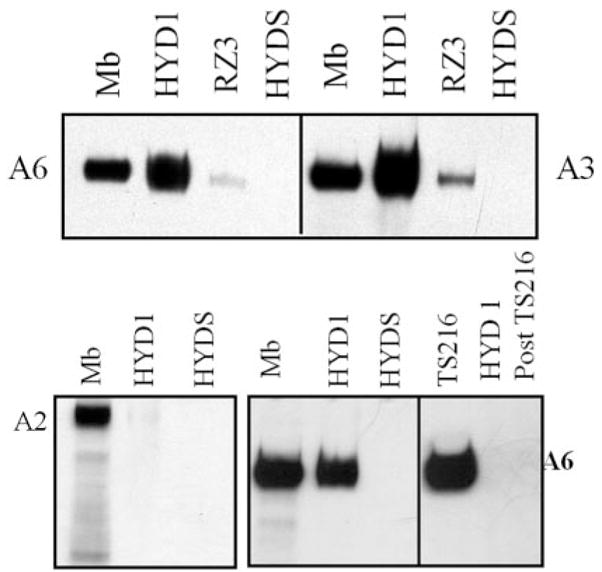
HYD1 interacts with α6 and α3 integrins. Affinity precipitation reactions were performed with HYD1, RZ3 or HYDS peptides on DU-145H cell membrane fractions as described in Materials and methods. The precipitates were analyzed for α6, α3 or α2 integrin by SDS–PAGE electrophoresis followed by a western blot with AA6A antibody (α6 specific), Antibody 1920 (α3 specific or α2 specific). Mb (membrane lysate) indicates the starting material. Depletion of the membrane lysate of β1 containing integrins by TS216 (β1 integrin specific) will retrieve the α6 integrin. No α6 integrin can be recovered by HYD1 if the lysate has been pre-cleared of β1 containing integrin (HYD1 post TS2/16).
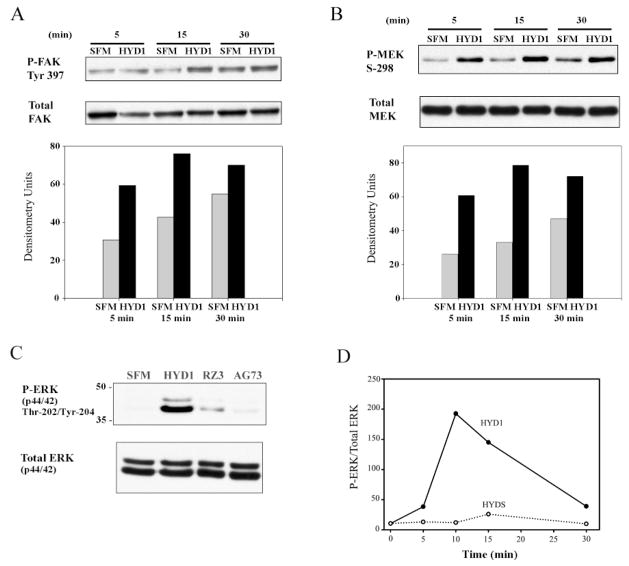
Upon engagement with the ECM, integrins are signal transduction receptors with the capability of activating many intracellular protein kinases and influencing a wide array of cellular processes (12). One kinase linked to integrin activation and cell motility is FAK, and phosphorylation of FAK on tyrosine 397 is indicative of FAK activation (42,43). Phosphorylation of MEK on serine 298 is an adhesion-dependent phosphorylation regulated by PAK (44). Using these two signals as biochemical markers for integrin activation, we determined whether HYD1 or RZ3 could act as signaling ligands to PC3N cells attached to BSA-coated plates. After 30 min of exposure, HYD1 induced both activation of FAK and phosphorylation of MEK on serine 298 (lane 2 in Figure 6A and B). RZ3 and the scrambled derivative, HYDS, and AG73 showed no activity compared to cells treated with serum-free media alone.
Fig. 6.
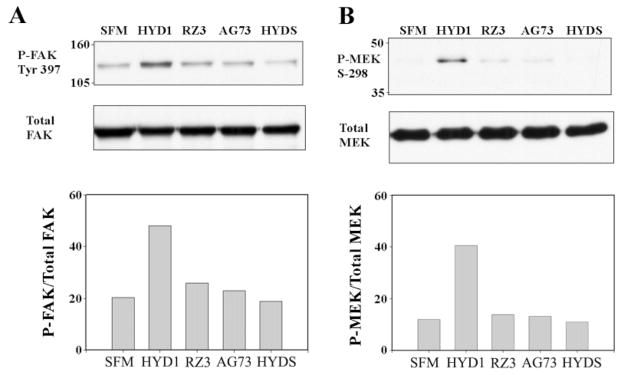
HYD1 activates integrin-associated signaling. PC3N cells were attached to BSA-coated plates in the presence of serum-free media for 1 h followed by the addition of peptides (75 μg/ml) in serum-free media or serum-free media (SFM) alone for 30 min. Cell lysates were analyzed for phosphorylation of FAK Tyr-397 (A) and phosphorylation of MEK Serine 298 (B). Blots were stripped and reprobed for total FAK and MEK and the amount of activity was normalized by densitometry.
HYD1 enhances signaling on laminin-5 resulting in transient activation of ERK
Cellular haptotaxis involves the dynamic regulation of signaling and cytoskeletal components. Ultimately, signals elicited from integrin interaction with the ECM drive the dynamic adhesive and cytoskeletal remodeling necessary for movement (2). The anti-migratory activity of HYD1 coupled with its ability to reorganize the cytoskeleton prompted us to analyze the immediate effect on laminin-5-dependent pro-migratory signals. As mentioned previously, cell migration on laminin-5 is coupled to the activity of several kinases including FAK and PAK (5,11). After 5 min of treatment, HYD1 increased the amount of activated FAK and elevated phosphorylation of MEK on serine 298, which is mediated by PAK (44), compared with the baseline levels generated by haptotaxis on laminin-5 (Figure 7A and B; lanes 1 and 2). Both of these signals had the greatest difference above baseline at 15 min post-treatment (Figure 7A and B; lanes 3 and 4). At this time-point, given that PAK phosphorylation of MEK on serine 298 primes the MAPK pathway for activation, (44,45) we investigated the activation of ERK in response to the peptides. HYD1 induced activation of ERK (Figure 7C) and a time course revealed that this response was maximal at 10 min post-treatment and returned to baseline levels after 30 min (Figure 7D). Taken together these data demonstrate that HYD1 disrupts the normal pro-migratory signaling dynamic on laminin-5 and results in a transient activation of ERK.
Fig. 7.
HYD1 temporally enhances activation of FAK and phosphorylation of MEK on laminin-5 resulting in transient activation of ERK. PC3N cells were placed on laminin-5-coated tissue culture plates in the presence of serum-free media for 1 h followed by the addition of HYD1 (75 μg/ml) in serum-free media or serum-free media (SFM) alone for 5, 15 and 30 min. (A) Effect of HYD1 on FAK activation (Tyr-397). (B) Effect of HYD1 on MEK phosphorylation (Serine 298). (C) Effect of HYD1, RZ3 and AG73 on ERK activation at 15 min post-treatment. (D) Time course of ERK activation following treatment with 75 μg/ml of HYD1 or HYDS. Blots were stripped and reprobed for total FAK, MEK and ERK. The amount of activity was normalized by densitometry.
Discussion
We have identified a D-amino acid peptide, HYD1, which inhibits tumor cell migration on laminin-5. HYD1 interacts with both α6 and α3-containing integrin complexes but not the α2 integrin. Depletion of the β1 containing integrins from the lysate will remove all α6 integrin that is capable of interacting with HYD1. Since the DU-145H cells express both α6β1 and α6β4 integrins, these data indicate that α6β1 integrin and not α6β4 integrin complexes interact with HYD1. Both α6 and α3 containing integrins are receptors for laminin-5 (46) and although α3β1 has been documented as the primary integrin receptor involved in mediating migration on laminin-5 by use of function-blocking antibodies to α3 (11,38), evidence also suggests that α6 integrins play a major regulatory role in motility on laminin-5 (39–41). The receptor specificity mediating the direct effects on motility, signaling and the cytoskeleton will require further investigation.
It is well known that integrin function-blocking antibodies or RGD-based peptidomimetics can inhibit cell invasion by blockage of integrin/ECM interaction. HYD1 is novel in that its activity is not dependent on interrupting integrin/laminin-5 interactions. This suggests that the effects are induced through binding to available integrin complexes on the surface rather than integrin complexes pre-engaged with the ECM. Alternatively, our previous study (29) suggested that HYD1 interaction was independent of the calcium-binding motif. Therefore, the peptide may affect both non-engaged and pre-engaged integrin complexes. The post-ligand occupancy activity of HYD1 is reinforced by the fact that cells remained attached and spread on laminin-5 after peptide treatment. The inhibitory effect of the peptide was reversible by removing the peptide and adding soluble laminin-5. This suggests that the migration-blocking activity is not a lethal event and can be rescued by the normal ligand.
Several peptide sequences that bind α6β1 have been isolated from the laminin α1 chain (22,47) as well as a peptide from CCN1, (48) a protein that is involved in angiogenesis. Screening of a phage display library (49) has also isolated α6β1 peptide ligand mimetics. These peptides do not reveal a consensus-binding motif and do not overlap with the sequence of HYD1. In addition, since HYD1 is a D-amino acid peptide it is structurally distinct from other L-amino acid ligand mimetics and therefore not a good candidate for comparative purposes. The peptide sequence was the result of a proposed hybrid D-amino acid containing sequence called HYD1. The sequence was postulated from the analysis of several adhesion competent D-amino acid sequences that were identified in our previous study. HYD1 does not match any known amino acid sequences within existing proteins because HYD1 is comprised of D-amino acids. The diversity of the ligand sequences for α6β1 may allude to the fact that integrins are involved in a multitude of biological processes and specific ligands could interact with distinct binding sites on integrins to give the appropriate biological response.
Studies have shown that migration rates of cells are dependent on the organization of the actin cytoskeleton and the presence of long, centrally distributed stress fibers correlates with reduced migration rates (9,10,50). HYD1 generates this type of cytoskeletal morphology within 30 min, producing a global increase in filamentous actin both at the membrane and throughout the cytoplasm. Cortactin, a regulator of membrane actin dynamics, is also shown to be tyrosine phosphorylated in response to the peptide and colocalized at the cell membrane with filamentous actin. Although the full significance of cortactin phosphorylation has yet to be determined, tyrosine phosphorylation is partially mediated by Src in response to cell matrix interactions (51). This suggests that the cytoskeletal remodeling events at the cell membrane produced by HYD1 are activated in part by integrin-mediated signaling through cortactin.
Interestingly, HYD1 induced cytoskeletal reorganization occurred independent of increased RhoA activity (data not shown); however, RhoA effectors can be modulated by other signaling pathways such as the MAPK pathway (2,52). Therefore, HYD1 may elicit changes in cytoskeletal architecture directly through RhoA effectors rather than increasing RhoA activity. Alternatively, it has been shown in keratinocytes that ablation of α3β1 created a cytoskeletal reorganization event that resulted in enhanced stress fiber formation (53). The interaction of HYD1 with α6 and α3 integrins is consistent with the idea that the cytoskeletal effects could be a consequence of inhibiting integrin function independent of modulating actin dynamics through cellular signals.
Laminin-5-dependent motility depends on the activity of multiple cell signaling molecules including FAK, PAK, PI3K and the MAP-kinase pathway and studies have shown that inhibition of these mediators can reduce migration (10,11,33). It is somewhat surprising then that HYD1 could block migration yet stimulate signaling through these pathways. However, studies with disintegrins have displayed similar phenomena. For example, it has been shown that contortrostatin, a snake venom disintegrin that inhibits cell motility, induces the tyrosine phosphorylation of FAK, p130CAS and activation of ERK2 (54–57). Disintegrins have also generated actin cytoskeletal reorganization that was dependent on tyrosine kinase activity (58). Although HYD1 does not contain a conserved disintegrin domain, the similarity of activity is remarkable.
In summary, we have identified an integrin-targeting peptide, HYD1, that blocks tumor cell migration on laminin-5. Loss of cell migration involved intense cytoskeletal remodeling events mediated in part by cortactin. HYD1 interacts with full-length α6 and α3 integrins and disrupts the pro-migratory signaling dynamic induced from laminin-5 haptotaxis. Therefore, HYD1 uncouples the induction of pro-migratory signals with the appropriate cytoskeletal response necessary for motility. The dramatic effect of HYD1 on cell migration and invasion prompts for further study to determine whether it can efficiently block invasion and metastasis in vivo. In addition, we are determining the minimal element for activity in this peptide by deletion analysis. The minimal element of HYD1 may provide insight into novel compounds for drug development. HYD1 is novel because it does not have to compete with the native ligand for activity, suggesting that integrin functions can be regulated independently from adhesion to the extracellular matrix. This study may provide new insights into development of antagonists of specific integrin functions to target cancer metastasis.
Acknowledgments
We would like to thank Doug Cromey, M. S., Southwest Environmental Health Services Center Cellular Imaging Core for his help and expertise with time-lapse video microscopy and analysis. This work was supported by grants CA 23074, CA 75152 and CA 56666.
Abbreviations
- ECM
extracellular matrix
- ERK
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- MEK
mitogen-activated protein kinase kinase
- PAK
p21-activated kinase
- PI3K
phosphoinositide 3-OH kinase
Footnotes
Conflict of Interest Statement: None declared.
For Permissions, please email: journals.permissions@oxfordjournals.org
Supplementary material
Supplementary material can be found at: http://www.carcin.oxfordjournals.org/
References
- 1.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 2.Carragher NO, Frame MC. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14:241–249. doi: 10.1016/j.tcb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 4.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 5.Zhou H, Kramer RH. Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. J Biol Chem. 2005;280:10624–10635. doi: 10.1074/jbc.M411900200. [DOI] [PubMed] [Google Scholar]
- 6.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 7.Rabinovitz I, Nagle RB, Cress AE. Integrin alpha 6 expression in human prostate carcinoma cells is associated with a migratory and invasive phenotype in vitro and in vivo. Clin Exp Metastasis. 1995;13:481–491. doi: 10.1007/BF00118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmelz M, Cress AE, Scott KM, Burger F, Cui H, Sallam K, McDaniel KM, Dalkin BL, Nagle RB. Different phenotypes in human prostate cancer: alpha6 or alpha3 integrin in cell-extracellular adhesion sites. Neoplasia. 2002;4:243–254. doi: 10.1038/sj.neo.7900223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu J, Sumida Y, Sanzen N, Sekiguchi K. Laminin-10/11 and fibronectin differentially regulate integrin-dependent Rho and Rac activation via p130(Cas)-CrkII-DOCK180 pathway. J Biol Chem. 2001;276:27090–27097. doi: 10.1074/jbc.M102284200. [DOI] [PubMed] [Google Scholar]
- 10.Kariya Y, Miyazaki K. The basement membrane protein laminin-5 acts as a soluble cell motility factor. Exp Cell Res. 2004;297:508–520. doi: 10.1016/j.yexcr.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 11.Shang M, Koshikawa N, Schenk S, Quaranta V. The LG3 module of laminin-5 harbors a binding site for integrin alpha3beta1 that promotes cell adhesion, spreading, and migration. J Biol Chem. 2001;276:33045–33053. doi: 10.1074/jbc.M100798200. [DOI] [PubMed] [Google Scholar]
- 12.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 13.Hay ED. Cell Biology of the Extracelluar Matrix. Plenum Press; New York: 1991. [Google Scholar]
- 14.Lee EC, Lotz MM, Steele GD, Jr, Mercurio AM. The integrin alpha 6 beta 4 is a laminin receptor. J Cell Biol. 1992;117:671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercurio AM, Shaw LM. Laminin binding proteins. Bioessays. 1991;13:469–473. doi: 10.1002/bies.950130907. [DOI] [PubMed] [Google Scholar]
- 16.Cress AE, Rabinovitz I, Zhu W, Nagle RB. The alpha 6 beta 1 and alpha 6 beta 4 integrins in human prostate cancer progression. Cancer Metastasis Rev. 1995;14:219–228. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- 17.Friedrichs K, Ruiz P, Franke F, Gille I, Terpe HJ, Imhof BA. High expression level of alpha 6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res. 1995;55:901–906. [PubMed] [Google Scholar]
- 18.Carey TE, Laurikainen L, Ptok A, Reinke T, Linder K, Nair TS, Marcelo C. Culture conditions affect expression of the alpha 6 beta 4 integrin associated with aggressive behavior in head and neck cancer. Adv Exp Med Biol. 1992;320:69–79. doi: 10.1007/978-1-4615-3468-6_10. [DOI] [PubMed] [Google Scholar]
- 19.Putz E, Witter K, Offner S, Stosiek P, Zippelius A, Johnson J, Zahn R, Riethmuller G, Pantel K. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. Cancer Res. 1999;59:241–248. [PubMed] [Google Scholar]
- 20.Hibino S, Shibuya M, Engbring JA, Mochizuki M, Nomizu M, Kleinman HK. Identification of an active site on the laminin alpha5 chain globular domain that binds to CD44 and inhibits malignancy. Cancer Res. 2004;64:4810–4816. doi: 10.1158/0008-5472.CAN-04-0129. [DOI] [PubMed] [Google Scholar]
- 21.Ponce ML, Hibino S, Lebioda AM, Mochizuki M, Nomizu M, Kleinman HK. Identification of a potent peptide antagonist to an active laminin-1 sequence that blocks angiogenesis and tumor growth. Cancer Res. 2003;63:5060–5064. [PubMed] [Google Scholar]
- 22.Nomizu M, Kim WH, Yamamura K, Utani A, Song SY, Otaka A, Roller PP, Kleinman HK, Yamada Y. Identification of cell binding sites in the laminin alpha 1 chain carboxyl-terminal globular domain by systematic screening of synthetic peptides. J Biol Chem. 1995;270:20583–20590. doi: 10.1074/jbc.270.35.20583. [DOI] [PubMed] [Google Scholar]
- 23.Nakahara H, Mueller SC, Nomizu M, Yamada Y, Yeh Y, Chen WT. Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J Biol Chem. 1998;273:9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- 24.Bresalier RS, Schwartz B, Kim YS, Duh QY, Kleinman HK, Sullam PM. The laminin alpha 1 chain Ile-Lys-Val-Ala-Val (IKVAV)-containing peptide promotes liver colonization by human colon cancer cells. Cancer Res. 1995;55:2476–2480. [PubMed] [Google Scholar]
- 25.Skubitz AP, Letourneau PC, Wayner E, Furcht LT. Synthetic peptides from the carboxy-terminal globular domain of the A chain of laminin: their ability to promote cell adhesion and neurite outgrowth, and interact with heparin and the beta 1 integrin subunit. J Cell Biol. 1991;115:1137–1148. doi: 10.1083/jcb.115.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song SY, Nomizu M, Yamada Y, Kleinman HK. Liver metastasis formation by laminin-1 peptide (LQVQLSIR)-adhesion selected B16-F10 melanoma cells. Int J Cancer. 1997;71:436–441. doi: 10.1002/(sici)1097-0215(19970502)71:3<436::aid-ijc22>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 27.Iwamoto Y, Robey FA, Graf J, Sasaki M, Kleinman HK, Yamada Y, Martin GR. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238:1132–1134. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- 28.Pennington ME, Lam KS, Cress AE. The use of a combinatorial library method to isolate human tumor cell adhesion peptides. Mol Divers. 1996;2:19–28. doi: 10.1007/BF01718696. [DOI] [PubMed] [Google Scholar]
- 29.DeRoock IB, Pennington ME, Sroka TC, Lam KS, Bowden GT, Bair EL, Cress AE. Synthetic peptides inhibit adhesion of human tumor cells to extracellular matrix proteins. Cancer Res. 2001;61:3308–3313. [PubMed] [Google Scholar]
- 30.Lam KS, Lebl M, Krchnak V. The “One-Bead-One-Compound” combinatorial library method. Chem Rev. 1997;97:411–448. doi: 10.1021/cr9600114. [DOI] [PubMed] [Google Scholar]
- 31.Tran NL, Nagle RB, Cress AE, Heimark RL. N-Cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion withStromal cells. Am J Pathol. 1999;155:787–798. doi: 10.1016/S0002-9440(10)65177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breitkreutz D, Schoop VM, Mirancea N, Baur M, Stark HJ, Fusenig NE. Epidermal differentiation and basement membrane formation by HaCaT cells in surface transplants. Eur J Cell Biol. 1998;75:273–286. doi: 10.1016/S0171-9335(98)80123-4. [DOI] [PubMed] [Google Scholar]
- 33.Slack JK, Adams RB, Rovin JD, Bissonette EA, Stoker CE, Parsons JT. Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene. 2001;20:1152–1163. doi: 10.1038/sj.onc.1204208. [DOI] [PubMed] [Google Scholar]
- 34.Somlyo AV, Bradshaw D, Ramos S, Murphy C, Myers CE, Somlyo AP. Rho-kinase inhibitor retards migration and in vivodissemination of human prostate cancer cells. Biochem Biophys Res Commun. 2000;269:652–659. doi: 10.1006/bbrc.2000.2343. [DOI] [PubMed] [Google Scholar]
- 35.Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 36.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 37.Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–6434. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 38.Plopper GE, Domanico SZ, Cirulli V, Kiosses WB, Quaranta V. Migration of breast epithelial cells on laminin-5: differential role of integrins in normal and transformed cell types. Breast Cancer Res Treat. 1998;51:57–69. doi: 10.1023/a:1006086218174. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Ma Z, Zhu J, Li K, Wang S, Bie P, Dong J. Laminin-5 promotes cell motility by regulating the function of the integrin alpha6beta1 in pancreatic cancer. Carcinogenesis. 2004;25:2283. doi: 10.1093/carcin/bgh319. [DOI] [PubMed] [Google Scholar]
- 40.Mercurio AM, Rabinovitz I, Shaw LM. The alpha 6 beta 4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001;13:541–545. doi: 10.1016/s0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- 41.Russell AJ, Fincher EF, Millman L, Smith R, Vela V, Waterman EA, Dey CN, Guide S, Weaver VM, Marinkovich MP. Alpha 6 beta 4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha 3 beta 1 integrin. J Cell Sci. 2003;116:3543–3556. doi: 10.1242/jcs.00663. [DOI] [PubMed] [Google Scholar]
- 42.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 43.Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB, Marshall MS, Weber MJ, Parsons JT, Catling AD. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J Cell Biol. 2003;162:281–291. doi: 10.1083/jcb.200212141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slack-Davis JK, Parsons JT. Emerging views of integrin signaling: implications for prostate cancer. J Cell Biochem. 2004;91:41–46. doi: 10.1002/jcb.10665. [DOI] [PubMed] [Google Scholar]
- 46.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 47.Nakahara H, Nomizu M, Akiyama SK, Yamada Y, Yeh Y, Chen WT. A mechanism for regulation of melanoma invasion. Ligation of alpha6beta1 integrin by laminin G peptides. J Biol Chem. 1996;271:27221–27224. doi: 10.1074/jbc.271.44.27221. [DOI] [PubMed] [Google Scholar]
- 48.Leu SJ, Liu Y, Chen N, Chen CC, Lam SC, Lau LF. Identification of a novel integrin alpha 6 beta 1 binding site in the angiogenic inducer CCN1 (CYR61) J Biol Chem. 2003;278:33801–33808. doi: 10.1074/jbc.M305862200. [DOI] [PubMed] [Google Scholar]
- 49.Murayama O, Nishida H, Sekiguchi K. Novel peptide ligands for integrin alpha 6 beta 1 selected from a phage display library. J Biochem (Tokyo) 1996;120:445–451. doi: 10.1093/oxfordjournals.jbchem.a021431. [DOI] [PubMed] [Google Scholar]
- 50.Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol. 1996;134:1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vuori K, Ruoslahti E. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J Biol Chem. 1995;270:22259–22262. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- 52.Fincham VJ, James M, Frame MC, Winder SJ. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 2000;19:2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hodivala-Dilke KM, DiPersio CM, Kreidberg JA, Hynes RO. Novel roles for alpha3beta1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J Cell Biol. 1998;142:1357–1369. doi: 10.1083/jcb.142.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ritter MR, Zhou Q, Markland FS., Jr Contortrostatin, a homodimeric disintegrin, actively disrupts focal adhesion and cytoskeletal structure and inhibits cell motility through a novel mechanism. Cell Commun Adhes. 2001;8:71–86. doi: 10.3109/15419060109080708. [DOI] [PubMed] [Google Scholar]
- 55.Ritter MR, Markland FS., Jr Contortrostatin activates ERK2 and tyrosine phosphorylation events via distinct pathways. Biochem Biophys Res Commun. 2000;274:142–148. doi: 10.1006/bbrc.2000.3111. [DOI] [PubMed] [Google Scholar]
- 56.Schmitmeier S, Markland FS, Ritter MR, Sawcer DE, Chen TC. Functional effect of contortrostatin, a snake venom disintegrin, on human glioma cell invasion in vitro. Cell Commun Adhes. 2003;10:1–16. doi: 10.1080/15419060302062. [DOI] [PubMed] [Google Scholar]
- 57.Ritter MR, Zhou Q, Markland FS., Jr Contortrostatin, a snake venom disintegrin, induces alphavbeta3-mediated tyrosine phosphorylation of CAS and FAK in tumor cells. J Cell Biochem. 2000;79:28–37. [PubMed] [Google Scholar]
- 58.Coelho AL, De Freitas MS, Mariano-Oliveira A, Rapozo DC, Pinto LF, Niewiarowski S, Zingali RB, Marcinkiewicz C, Barja-Fidalgo C. RGD- and MLD-disintegrins, jarastatin and EC3, activate integrin-mediated signaling modulating the human neutrophils chemotaxis, apoptosis and IL-8 gene expression. Exp Cell Res. 2004;292:371–384. doi: 10.1016/j.yexcr.2003.09.013. [DOI] [PubMed] [Google Scholar]