Abstract
We tested the hypothesis that dopamine D1 and D2 receptor gene (DRD1 and DRD2, respectively) polymorphisms and the development of working memory skills can interact to influence symptom change over 10 years in children with attention-deficit/hyperactivity disorder (ADHD). Specifically, we examined whether improvements in working memory maintenance and manipulation from childhood to early adulthood predicted the reduction of ADHD symptoms as a function of allelic variation in DRD1 and DRD2. Participants were 76 7–11-year-old children with ADHD who were genotyped and prospectively followed for almost 10 years. ADHD symptoms were rated using the Attention Problems scale on the Child Behavior Checklist, and verbal working memory maintenance and manipulation, measured by Digit Span forward and backward, respectively, were assessed at baseline and follow-up. After correction for multiple testing, improvements in working memory manipulation, not maintenance, predicted reduction of symptomatology over development and was moderated by major allele homozygosity in two DRD1 polymorphisms (rs4532 and rs265978) previously linked with variation in D1 receptor expression. Depending on genetic background, developmental factors including age-dependent variation in DRD1 penetrance may facilitate the link between improvements in higher-order working memory and the remission of symptoms in individuals with childhood-diagnosed ADHD. Furthermore, the current findings suggest that DRD1 might contribute minimally to the emergence of symptoms and cognitive difficulties associated with ADHD in childhood, but may act as a modifier gene of these clinical features and outcome during later development for those with ADHD.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) symptoms typically emerge early in childhood and then either partially remit or persist during development. Approximately 30–50% of childhood cases remit by adolescence (Halperin, Trampush, Miller, Marks & Newcorn, 2008), and as many as 85% remit by the age of 25 years if full DSM-IV diagnostic criteria are applied (Faraone, Biederman & Mick, 2006). Nevertheless, most DSM-defined remitters remain symptomatic and many continue to have considerable impairment. Because of such marked diagnostic heterogeneity, it has been argued that ‘ADHD’ may be better conceptualized as representing the tail-end of a normally distributed cluster of dimensional traits that exist in the general population (Asherson, 2004). In support of this idea, at least one twin study has shown that genetic liability for ADHD is indeed continuously distributed throughout the general population (Chen, Zhou, Sham, Franke, Kuntsi, Campbell et al., 2008). Moreover, while childhood-onset ADHD is strongly heritable (Faraone, Perlis, Doyle, Smoller, Goralnick, Holmgren & Sklar, 2005), unique genetic factors seem to contribute to the stability of ADHD symptoms during development (Kuntsi, Rijsdijk, Ronald, Asherson & Plomin, 2005). However, specific genes that influence the developmental course of ADHD have not been identified.
Emerging evidence implicates impairments in working memory as a core feature of ADHD, which renders working memory as a potentially useful intermediate ADHD phenotype that may modulate the association between genes and clinical symptomatology (Castellanos & Tannock, 2002). Two key components of working memory are maintenance and manipulation; the ability to manipulate information in working memory is differentially weaker in individuals with ADHD relative to pure maintenance of information in an online cognitive state (Hale, Hoeppner & Fiorello, 2002). Manipulation is also weaker in unaffected siblings of children diagnosed with ADHD relative to typically developing children, further supporting its potential usefulness as an intermediate ADHD phenotype (Rommelse, Altink, Oosterlaan, Buschgens, Buitelaar & Sergeant, 2008).
Optimal working memory functions within a narrow range of dopamine neurotransmission (i.e. the classic inverted U-shaped curve) regulated by the G protein-coupled family of dopamine receptors (Williams & Castner, 2006). Dopamine D1 receptors are known to modulate the persistent neuronal activity that is needed to continuously update information in working memory (Paspalas & Goldman-Rakic, 2005). Dopamine D2 receptors also modulate components of working memory (Wang, Vijayraghavan & Goldman-Rakic, 2004), although to a lesser extent than D1 receptors (McNab, Varrone, Farde, Jucaite, Bystritsky, Forssberg & Klingberg, 2009). It is thus important to know the degree to which genetic variation influences the differential roles that D1 and D2 receptors play in working memory. A functional dopamine D1 receptor gene (DRD1) polymorphism (rs686) that affects D1 expression has been identified (Huang, Ma, Payne, Beuten, Dupont & Li, 2008), as have three functional dopamine D2 receptor gene (DRD2) polymorphisms (rs12364283, rs2283265 and rs1076560) (Zhang, Bertolino, Fazio, Blasi, Rampino, Romano, Lee, Xiao, Papp, Wang & Sadée, 2007). However, only the DRD2 variants have at this point been associated with working memory (Zhang et al., 2007).
The adult brain emerges from an organized yet dynamic ‘blueprint’ during the course of development (Andersen, 2003). Higher-order cognitive skills such as working memory are the last to fully develop as maturation of the frontal lobes is more delayed and variable relative to other brain regions (Andersen, 2003; Halperin & Schulz, 2006). Within the prefrontal cortex, developmental improvements in working memory manipulation relative to maintenance are associated with increased recruitment of cells within dorsolateral prefrontal cortex but not ventrolateral prefrontal regions (Crone, Wendelken, Donohue, van Leijenhorst & Bunge, 2006). Moreover, in rats, D1 receptor density is dynamic during development in that it peaks during adolescence and then decreases in adulthood (Andersen, Thompson, Rutstein, Hostetter & Teicher, 2000). DRD1 gene expression also varies in human prefrontal cortex in a similar way across development such that it peaks during late adolescence/early adulthood and then declines during later adulthood (Colantuoni, Lipska, Ye, Hyde, Tao, Leek, Colantuoni, Elkahloun, Herman, Weinberger & Kleinman, 2011). Clinically, we have reported that working memory deficits were only present in adolescents and young adults with childhood-diagnosed ADHD if they continued to meet criteria for the disorder (Halperin et al., 2008). More recently, we showed a related link between higher-order neurocognitive development and symptom remission in preschoolers with ADHD (Rajendran, Trampush, Rindskopf, Marks, O’Neill & Halperin, 2013). Adaptations in working memory may thus underlie some of the symptom heterogeneity in ADHD over time, and may be a viable endophenotype that is sensitive to both dopamine receptor function and developmental factors. It has been hypothesized that developmental changes in dopaminergic networks in the prefrontal cortex may play a central role in the trajectory of ADHD, but have a minimal role in the initial onset of symptoms (Halperin & Schulz, 2006).
This study examined whether changes in working memory maintenance and manipulation differentially correlated with the stability of ADHD symptoms over a 10-year period as a function of DRD1 and DRD2 polymorphisms in a prospectively followed sample of youth with childhood-diagnosed ADHD. We reasoned that searching for a main effect of genotype on cognition and/or symptoms would yield an incomplete story; therefore, our analytical strategy incorporated higher-order interactions of genotype × cognition on clinical symptoms and development in the context of a targeted neurobiologic system important for working memory. We did not posit differential improvements in working memory based solely on dopamine receptor genotype, but rather we hypothesized that improvements in working memory would be linked to fewer symptoms over time depending on genetic background and developmental stage. The motivation to carry out this study was based on the aforementioned cumulative evidence showing links between (i) symptom recovery in ADHD and working memory during later development (Halperin et al., 2008), (ii) dopamine D1 receptors in the prefrontal cortex being critical to working memory (Paspalas & Goldman-Rakic, 2005), and (iii) the prefrontal cortex being one of the last cortical regions to mature during development (Andersen, 2003; Halperin & Schulz, 2006). It was hypothesized that improvements in working memory manipulation during development would be linked with the diminution of ADHD symptoms but that the relationship would vary by genetic background, particularly DRD1 variants given the importance of D1 receptor function on working memory. To our knowledge, no prior studies have examined the prospective and interactive contributions of working memory maintenance and manipulation, DRD1 and DRD2 variants, and development on the stability of symptomatology in ADHD.
Method
Sample
The current study included 76 adolescents and young adults (mean age of 18.4 years, age range of 16–26 years) who were initially diagnosed with ADHD in childhood in the 1990s (mean age of 9.0 years, age range of 7–11 years), and then re-evaluated and genotyped at follow-up. This sample was drawn from a larger cohort of 98 ADHD probands who were successfully recruited for a longitudinal study and evaluated on average 9.3 years after initially being diagnosed with ADHD in childhood (Halperin et al., 2008). Among the 22 participants who did not take part in the current study, most were not included in this analysis because of genotyping problems or they did not consent to participate in the genetics protocol. There were no significant differences between the group of 76 who did participate and the 22 who did not participate on factors such as age at baseline or follow-up, socioeconomic status, IQ, or symptom severity (all p > .05). This predominantly inner-city cohort was racially and ethnically diverse (27.4% African-American, 36.3% Hispanic, 24.7% Caucasian and 11.6% mixed/other race), and of low to lower-middle socioeconomic status. Full details of the sample have been published previously (Halperin et al., 2008).
Childhood evaluation (‘baseline’)
Initial childhood screening included teacher reports on the Inattention/Overactivity scale of the IOWA Conners Rating Scale (Pelham, Milich, Murphy & Murphy, 1989), parent reports on the Child Behavior Checklist (CBCL; Achenbach, 1991), and structured interview of a parent using the Diagnostic Interview Schedule for Children (Shaffer, Fisher, Dulcan, Davies, Piacentini, Schwab-Stone, Lahey, Bourdon, Jensen, Bird, Canino & Regier, 1996). The attention problems subscale from the CBCL was used as the primary clinical measure for this study. Participants recruited prior to 1994 were diagnosed using DSM-III-R criteria for ADHD and tested using the Wechsler Intelligence Scale for Children, Revised Edition (WISC-R) (Wechsler, 1974); those recruited after 1994 were evaluated using DSM-IV criteria and tested using the third edition of the WISC (WISC-III) (Wechsler, 1991). Subgroups recruited before and after 1994 did not differ significantly on any parent or teacher rating. Psychosis, autism, neurological disorders, or IQ below 70 was exclusionary at baseline.
Adolescence/young adulthood evaluation (‘follow-up’)
At follow-up, participants and their parent(s) were interviewed using the Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime (KSADS-PL) to evaluate ADHD and other Axis-I psychiatric disorders (Kaufman, Birmaher, Brent, Rao, Flynn, Moreci, Williamson & Ryan, 1997). The Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) (Wechsler, 1997) was administered to all participants. Table 1 provides descriptive characteristics of the sample at baseline and follow-up.
Table 1.
Demographic and descriptive characteristics
| Baseline (n = 76) | Follow-up (n = 76) | |
|---|---|---|
| Age (years), mean (SD) | 9.0 (1.3) | 18.4 (1.7) |
| Age range (years) | 7.0–11.6 | 15.5–26.3a |
| Male, no. (%) | 67 (88) | – |
| Racial/ethnic minority, no. (%) | 57 (75) | – |
| Wechsler FSIQ, mean (SD) | 94.0 (15.0) | 92.9 (14.8) |
| CBCL Externalizing, mean (SD) | 69.7 (11.2) | 60.7 (12.9) |
| CBCL Internalizing, mean (SD) | 65.1 (12.0) | 56.8 (13.4) |
| CBCL Attention Problems, mean (SD) | 72.4 (10.2) | 60.6 (9.0) |
FSIQ, Full-scale intelligence quotient; CBCL, Child Behavior Checklist; SD, standard deviation.
Note that 75 of the 76 probands were 16–22 years old at follow-up.
Written informed consent was obtained from a parent/legal guardian of those < 18 years old and assent was obtained from all minors. Informed consent was obtained from participants ≥ 18 years old. All participants were compensated for their time and travel. The Institutional Review Boards of the participating institutions approved all procedures.
Neurocognitive and behavioral measures
Digit span forward
At baseline, the WISC-R/WISC-III included the Digit Span subtest. The forward component of digit span was used to assess working memory maintenance. Digit span forward is administered by orally presenting a series of numbers that the examinee has to verbally repeat. The longest series of digits that one could recall was used as the primary variable of interest. The WISC-R digit span forward section included pairs of seven items that ranged from 3 to 9 digits in length. The WISC-III digit span forward retained all seven WISC-R forward item pairs; however, a 2-digit series was added at the beginning (i.e. eight items that ranged from 2 to 9 digits in length) (Wechsler, 1991). Since no child had a longest digit span forward of two, this difference did not affect our measure of working memory maintenance.
Digit span backward
The backward component of digit span is a simple yet robust measure of working memory manipulation, testing the maximum number series an individual can aurally attend to, store and then repeat verbally in reverse order. The WISC-III retained all seven WISC-R digit span backward items (2–8 digits in length).
At follow-up, participants were administered digit span from the WAIS-III. With the exception of the actual digits used, digit span forward and backward from the WISC-R/III and WAIS-III are identical in administration and scoring. The WISC-III and WAIS-III manuals provided normative data for maximum/longest digit span forward and backward from their respective standardization samples stratified by age. These data were used to generate age-corrected z-scores for each participant’s digit span forward and backward raw score.
CBCL Attention Problems (CBCL-AP)
Parents completed the CBCL at baseline and follow-up. The attention problems subscale, which served as our primary clinical outcome measure because questions and scoring procedures were similar at both time points, consists of several items reflecting difficulties with attention, motor activity, impulsivity and other ADHD-related behaviors. The CBCL-AP effectively discriminates ADHD cases from non-ADHD controls (Hudziak, Copeland, Stanger & Wadsworth, 2004), and correlates well with the clinical DSM-IV assessment of ADHD (Derks, Hudziak, Dolan, Ferdinand & Boomsma, 2006). Importantly, scores on the CBCL-AP have proven to be highly heritable and genetically stable over time such that genetic effects explain approximately 75% of the heritability developmentally from age 7 years and older (Rietveld, Hudziak, Bartels, Beijsterveldt & Boomsma, 2004). Raw scores on the CBCL were transformed to age-corrected T-scores.
Genetic marker selection
We genotyped four DRD1 and four DRD2 single nucleotide polymorphisms (SNPs). All SNPs were either functional, in strong linkage disequilibrium with a functional polymorphism, or were previously associated with ADHD-related phenotypes and/or working memory (see Table 2). No other genotyping was conducted.
Table 2.
DRD1 and DRD2 polymorphisms
| Gene | SNP | Location | Alleles | Minor (freq.) | Function |
|---|---|---|---|---|---|
| DRD1 | rs4532 | Exon 1 (5′UTR) | T/C | C (0.24) | D1 expression (direct or LD) |
| rs265978 | 3.7 kb downstream | T/C | C (0.32) | Tag SNP for rs686 | |
| rs265975 | 5.5 kb downstream | C/T | T (0.37) | Associated with ND | |
| rs265973 | 7 kb downstream | C/T | T (0.48) | Associated with ND | |
| DRD2 | rs12364283 | Promoter | A/G | G (0.07) | >D2 activity in PFC & striatum |
| rs2283265 | Intron 5 | C/A | A (0.13) | D2 splicing SNP; A = >D2L | |
| rs1076560 | Intron 6 | C/A | A (0.15) | D2 splicing SNP; A = >D2L | |
| rs1800497 | DRD2/ANKK1 | G/A | A (0.29) | Extrastriatal D2 binding |
Note: DRD1, dopamine D1 receptor gene; DRD2, dopamine D2 receptor gene; SNP, single nucleotide polymorphism; UTR, untranslated region; LD, linkage disequilibrium; kb, kilo base pairs; ND, nicotine dependence; PFC, prefrontal cortex; D2L, dopamine D2 receptor long isoform; ANKK1, ankyrin repeat and kinase domain containing 1 gene.
Genotyping
Buccal swabs were obtained from participants as a source of DNA. All SNP assays were obtained as Taqman Assays-On-Demand (Applied Biosystems) and genotyping was performed according to the manufacturer’s protocol. Genotype was determined using an ABI 7900HT available in the Mount Sinai Medical Center Quantitative PCR Shared Resource Facility.
Analytical strategy
Plink 1.07 (Purcell, Neale, Todd-Brown, Thomas, Ferreira, Bender, Maller, Sklar, de Bakker, Daly & Sham, 2007) was used to verify genetic data quality and test for departure from Hardy-Weinberg equilibrium. Pairwise linkage disequilibrium was evaluated with Haploview 4.1 (Barrett, Fry, Maller & Daly, 2005) and expressed as D′. Hierarchical linear regression conducted using SPSS 17 (Chicago, IL) was used to test whether specific dopamine SNPs moderated the relations between working memory change over time (i.e. independent variable) and improvements in ADHD symptoms (i.e. dependent variable). A statistical moderator affects the direction and/or strength of the independent variables relation on the dependent variable. Here, moderation would be supported by a significant SNP × digit span forward/backward interaction. It is important to note that the main effect significance of the independent and moderator variables is not directly relevant conceptually to testing the moderator hypothesis; can be misleading depending on how variables are coded; and therefore should not be interpreted (Baron & Kenny, 1986). Model-based plotting of the simple slopes showing the relationship between change in working memory and change in ADHD symptomatology as a function of genotype was conducted using IRSE [Interactions in Multiple Linear Regression with SPSS and Excel; http://www.urenz.ch/irse; (Meier, 2008)].
Each regression equation included (a) SNP, (b) digit span forward, (c) digit span backward, (d) SNP × digit span forward, and (e) SNP × digit span backward entered hierarchically in this specified order as predictors of change in CBCL-AP scores. This sequential approach allowed us to assess whether higher-order working memory manipulation interacted with genetic background above and beyond any lower-order working memory maintenance effects and interactions. Change scores for digit span forward, backward and attention problems were extracted as standardized residuals, obtained by predicting the follow-up score from the baseline score. Using the residual to quantify change negates the correlation between change score and baseline performance, facilitating the measurement of variability in change over time after what is predicted from baseline status (Glass & Hopkins, 1996). We applied a conservative Bonferroni correction threshold of p < .0063 for this analysis, which corrects for testing eight non-independent SNPs. Post-hoc probing of significant moderator effects required plotting and testing the ‘simple slopes’ of the relation between ADHD symptoms and working memory by individual genotypes for each SNP (Holmbeck, 2002). Models were adjusted for the number of years that had elapsed between the baseline and follow-up evaluations. CBCL attention problems T-scores were logarithmically transformed due to the skewness of the data at follow-up (Supplementary Figures S1–S3).
Results
Stability (Pearson r) from baseline to follow-up was .34 (p = .004) for CBCL attention problems, .52 (p < .001) for digit span forward and .37 (p = .002) for digit span backward. Thus, each of these measures reflects a trait with moderate stability over development, but also a fair degree of variability over time (Supplementary Table 1).
No SNP deviated significantly from Hardy-Weinberg equilibrium (all p > .05). Pairwise evaluation of linkage disequilibrium detected a haploblock within each gene. In DRD1 (Figure 1A), D′ between rs4532 and rs265978 was 1.0, with haplotype frequencies of 67.6% for T-T, 23.8% for C-C, and 8.6% for T-C. In DRD2 (Figure 1B), rs2283265, rs1076560 and rs1800497 (D′=1.0) comprised the haploblock, with haplotype frequencies of 71.7% for C-C-G, 13.8% for C-C-A, 13.2% for A-A-A, and 1.4% for C-A-A.
Figure 1.
Haploview-generated linkage disequilibrium patterns of the (a) four DRD1 and (b) four DRD2 single nucleotide variants examined in this study. D′ values (x100) are noted at the intersection of each SNP pair. ‘Decreased’ shades of red in each box represent decreasing linkage disequilibrium values.
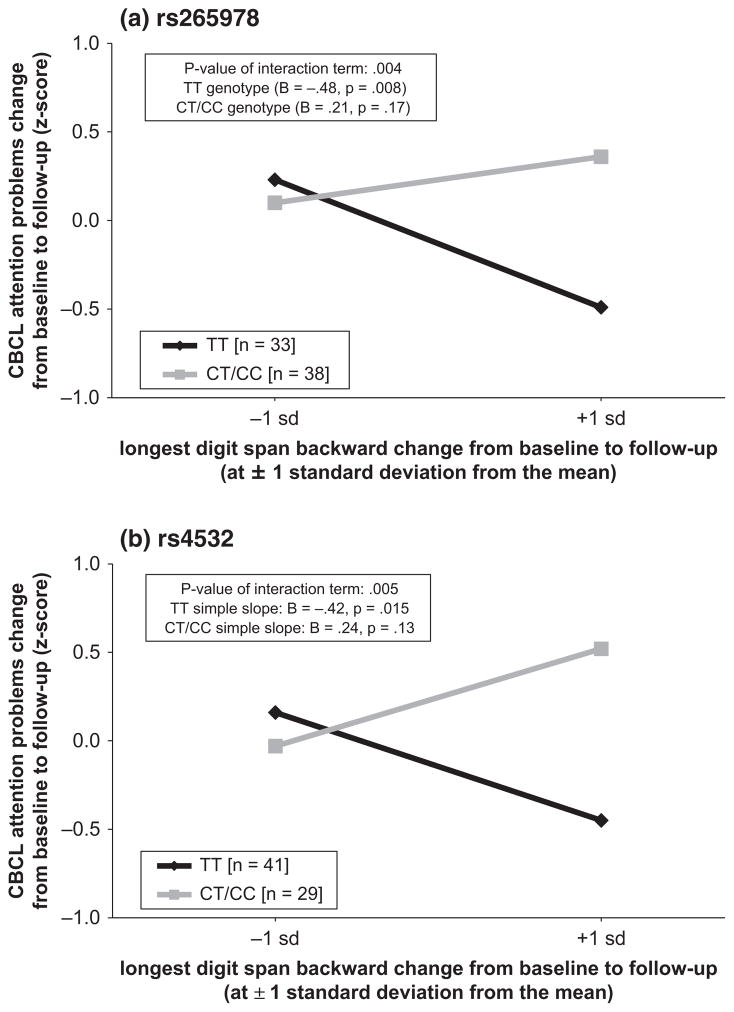
As detailed in Table 3, three DRD1 SNP × digit span backward interactions were detected at p < .05 that on average accounted for 10% of the variance in attention problem change from baseline to follow-up after controlling for all other variables in the respective model. Two of these interactions (SNPs rs4532 and rs265978) remained significant after Bonferroni correction and accounted for 11% of the variance in ADHD symptom change during development. As depicted in Figure 2, plotting the simple slopes revealed a strong link between improvements in digit span backward (i.e. working memory manipulation) and the remission of ADHD symptoms during the course of development in individuals who were homozygous for the major alleles of SNPs rs4532 and rs265978. By contrast, this link was not found in carriers of the minor allele of these two DRD1 SNPs. In other words, change in the ability to manipulate verbal information in working memory was unrelated to the magnitude of change in ADHD symptoms in minor allele carriers. Non-model-based plots of raw CBCL-AP and digit span backward change scores for each individual with regression slopes are plotted as function of rs4532 and rs265978 genotype and included in the Supplementary Materials (Figure S4a–b) for descriptive purposes.
Table 3.
Linear regression results of the developmental change analyses from baseline to follow-up. Shown is the change in variance accounted for (R2Δ) and level of significance at each step of the model building procedure after controlling for the number of years that had elapsed between the baseline and follow-up evaluations
| DRD1 SNPs Model/step | rs265973
|
rs265975
|
rs265978
|
rs4532
|
||||
|---|---|---|---|---|---|---|---|---|
| R2 Δ | p-value | R2 Δ | p-value | R2 Δ | p-value | R2 Δ | p-value | |
| 1. SNP | .015 | .290 | .012 | .358 | .015 | .305 | .027 | .170 |
| 2. Digit span forward | .001 | .786 | .000 | .978 | .001 | .767 | .001 | .766 |
| 3. Digit span backward | .007 | .480 | .004 | .611 | .007 | .501 | .005 | .554 |
| 4. SNP × digit span forward | .015 | .307 | .009 | .435 | .000 | .916 | .000 | .872 |
| 5. SNP × digit span backward | .084 | .013 | .001 | .835 | .117 | .004 | .109 | .005 |
| DRD2 SNPs Model/step | rs1800497
|
rs1076560
|
rs2283265
|
rs12364283
|
||||
|---|---|---|---|---|---|---|---|---|
| R2 Δ | p-value | R2Δ | p-value | R2 Δ | p-value | R2 Δ | p-value | |
| 1. SNP | .072 | .021 | .015 | .302 | .007 | .481 | .003 | .616 |
| 2. Digit span forward | .002 | .695 | .001 | .848 | .001 | .787 | .002 | .727 |
| 3. Digit span backward | .001 | .795 | .002 | .679 | .004 | .591 | .007 | .473 |
| 4. SNP × digit span forward | .065 | .025 | .013 | .339 | .020 | .225 | .001 | .844 |
| 5. SNP × digit span backward | .030 | .119 | .005 | .539 | .001 | .835 | .019 | .237 |
Values in bold are significant at a Bonferroni corrected p-value of .0063. DRD1, dopamine D1 receptor gene; DRD2, dopamine D2 receptor gene; SNP, single nucleotide polymorphism.
Figure 2.
Simple slope plots as a function of genotype for the two DRD1 SNPs (a) rs265978 and (b) rs4532 that evidenced a significant moderation effect. The y-axis spans the range of residualized CBCL attention problems change scores from baseline to follow-up. Negative scores are indicative of greater relative reduction in ADHD symptoms over time. The x-axis shows one standard deviation above and below the mean of the residualized digit span backward change score from baseline to follow-up. Positive scores are indicative of relative improvement in digit span backward performance over time (i.e. improvements in working memory manipulation). The lines represent the relationship between ADHD symptom reduction and working memory manipulation improvement as a function of genotype. As can be seen, symptoms significantly improved (p = .008–.015) in individuals with the common TT genotype if improvement in digit span backward was above average. Conversely, symptoms did not improve if digit span backward was below average, nor for carriers of the minor C allele irrespective of digit span backward score.
In contrast to the DRD1 findings, no DRD2 SNP × digit span backward interactions were significant. There was an rs1800497 × digit span forward interaction with p < .05, but this interaction did not survive correction (see Table 3).
Correlations with baseline and follow-up status
It was important to gauge whether the above developmental findings more closely reflected baseline or follow-up status. Therefore, an exploratory/post-hoc analysis of the baseline and follow-up data was conducted using the same statistical methods as above. We applied a conservative Bonferroni correction of p < .0031 to correct for 16 additional tests. No significant SNP × digit span interactions were detected in baseline analyses (Supplementary Table 2). By contrast, the two significant interactions between DRD1 SNPs rs4532 and rs265978 and digit span backward that emerged during development were also significant at follow-up after correction for multiple testing (Supplementary Table 3). Lastly, no DRD2 × digit span interactions were significant.
Potential confounding effects of race/ethnicity and stimulant treatment
Genetic heterogeneity due to racial/ethnic background and stimulant treatment for ADHD during development could have had an influence on the main results of the study. Therefore, to empirically garner information on this possibility, the developmental change models were re-analyzed with race/ethnicity and number of years an individual had been treated for ADHD with stimulant medication (ranging from no lifetime use to 12 years of treatment) included as covariates. The results of these secondary analyses are provided in Supplementary Tables S4 and S5. As can be seen in the tables, no appreciable changes in the results were detected. Thus, the sensitivity of the primary findings appears to be conserved in light of the potential influence of genetic heterogeneity and treatment history.
Discussion
These data support our hypothesis that the diminution of ADHD symptoms from childhood to late adolescence/early adulthood is linked to improvements in working memory during the same period of time in individuals with childhood-diagnosed ADHD with certain common genotypes of dopamine system genes. Specifically, the direction and strength of the relation between working memory and ADHD symptoms during development was specific to the higher-order manipulation component of working memory and moderated by DRD1 polymorphisms rs4532 and rs265978. Prediction of symptom remission (or lack thereof) over time by simultaneous improvements in working memory manipulation only occurred in individuals with two copies of the major/common allele at each of these SNPs. Conversely, ADHD symptoms and working memory manipulation were unrelated (i.e. ‘decoupled’) in minor allele carriers. Notably, the major alleles of rs4532 and rs265978 have been associated with increased D1 receptor expression (Huang et al., 2008). Developmental changes in ADHD symptoms were unrelated to changes in working memory maintenance regardless of DRD1 genotype. Furthermore, for DRD2, there were no significant associations between genotype, working memory manipulation and changes in ADHD symptoms. Finally, studying these associations developmentally was important, as no genetic effects were significant at baseline when the participants were 7–11 years old. Thus, it seems plausible that dopamine D1 receptor gene variants and the development of higher-order working memory skills interact to affect the trajectory of ADHD symptoms more so than the emergence of symptoms in early childhood (Halperin & Schulz, 2006). Conceptually, this suggests that DRD1 is a ‘modifier’ gene that influences clinical features of ADHD without altering susceptibility (Fanous & Kendler, 2005).
Dopamine D1 receptor expression is greater than D2 receptor expression in the prefrontal cortex (e.g. 20-fold increase of D1 relative to D2 in primates) (Lidow, Goldman-Rakic, Gallager & Rakic, 1991). Animal models of working memory have shown that pharmacologically enhancing D1 receptor function in prefrontal cortex can compensate for dopamine dysfunction and facilitate long-lasting recovery of cognitive and behavioral functions (Williams & Castner, 2006). There is indirect but strong evidence that the major alleles of rs4532 and rs265978 increase D1 expression via linkage disequilibrium with rs686, a known functional 3′ untranslated region (3′ UTR) DRD1 polymorphism that affects D1 receptor expression levels (Huang et al., 2008). As such, we speculate that in the current study, allelic variation moderated the developmental dynamics of DRD1 gene expression and function in a way that positively modified cognitive growth in a subgroup of ADHD probands to help them overcome some of their early difficulties with inattention, hyperactivity and impulsivity. Alternatively, our results could be reflecting the impact of DRD1 on changes in attentional control on working memory performance rather than vice versa. However, we have approached this study from the classic endophenotype model in which an intermediate cognitive phenotype (i.e. working memory) is hypothesized to contribute to the development of and recovery from a more complex psychiatric phenotype (i.e. ADHD). As such, we sequentially structured our analyses in the way laid out in the methods section and at no point made Digit Span the dependent variable and CBCL-AP a predictor variable. Therefore, we have no empirical data to support the inverse association (i.e. DRD1 facilitates improvement in attentional control to enhance working memory performance), though acknowledge that this association possibly exists to at least some degree.
Previous studies of DRD1 variants and ADHD have been inconsistent. For example, at least two studies indicated no association between rs4532 and ADHD (Kirley, Hawi, Daly, McCarron, Mullins, Millar, Waldman, Fitzgerald & Gill, 2002; Nyman, Ogdie, Loukola, Varilo, Taanila, Hurtig, Moilanen, Loo, McGough, Jarvelin, Smalley, Nelson & Peltonen, 2007), whereas others reported (Misener, Luca, Azeke, Crosbie, Waldman, Tannock, Roberts, Malone, Schachar, Ickowicz, Kennedy & Barr, 2004) and replicated (Luca, Laurin, Misener, Wigg, Anderson, Cate-Carter, Tannock, Humphries, Lovett & Barr, 2007) a significant association between the minor rs4532/C allele and inattention. The rs4532/C allele has also been associated with several ADHD-related traits including impulsive response/reaction time (Bobb, Addington, Sidransky, Gornick, Lerch, Greenstein, Clasen, Sharp, Inoff-Germain, Wavrant-De Vrièze, Arcos-Burgos, Straub, Hardy, Castellanos & Rapoport, 2005) and nicotine dependence (Huang et al., 2008; McClernon & Kollins, 2008). In the current study, rs4532/C, which is associated with a decrease in D1 expression, decoupled the relationship between improvements in working memory manipulation and the remission of ADHD symptoms during development. We speculate that lower D1 expression might hinder cognitive development in ADHD in a way that leads to a generalized, behaviorally dysregulated phenotype that is difficult to reliably predict from traditional measurement tools.
One of the most important aspects of these data is the developmental component and the examination of phenotypic change and variability over a decade. Our results suggest that the genetic factors that contribute to the emergence of ADHD in early childhood may not be the same factors that contribute to its course and outcome throughout adolescence and adulthood (Kuntsi et al., 2005). Our sample was initially recruited between the ages of 7 and 11, the approximate age range that the preponderance of molecular genetic studies of ADHD have likewise utilized (Faraone et al., 2005), with dopamine being the most extensively studied neurotransmitter system in this age group. Because the maturation of the anatomy and physiology of dopamine in general, and D1 receptor expression specifically in the cortex, is slower than other regions of the brain (Andersen et al., 2000), we did not expect to find significant dopamine gene effects at baseline and so focused on the developmental course of working memory and ADHD symptoms. For that reason, it is not surprising that dopamine gene associations with ADHD are difficult to replicate; had we not taken a developmental approach, we would have contributed yet another non-replication to this literature. Thus, it seems important that developmental factors be incorporated into future research of dopamine genes and ADHD.
Recently, data have become publicly available via BrainCloud on gene transcript expression in the dorsolateral prefrontal cortex of human postmortem brain samples across the lifespan (download available at http://braincloud.jhmi.edu/) (Colantuoni et al., 2011). We examined BrainCloud for the pattern of DRD1 expression across development. Similar to what has been described regarding DRD1 expression in rat prefrontal cortex (Andersen et al., 2000), there appears to be a nonlinear pattern of DRD1 expression in human dorsolateral prefrontal cortex that climbs in childhood and adolescence, peaks in early adulthood/early 20s, and then tails off over the course of later life (Figure S5 in Supplementary Materials). Even though this is an indirect observation, this pattern of DRD1 gene transcript expression across the lifespan could be relevant to the results of the current study given that our strongest effects did not emerge until late adolescence/early adulthood, and because the SNPs that we examined are putatively associated with differences in DRD1 expression (Huang et al., 2008).
Researchers who study intermediate cognitive and behavioral phenotypes in ADHD strive to reliably predict parent and teacher ratings of child behavior from performance on laboratory measures of cognitive functioning, and then link these associations to genes and biology. Unfortunately, it has been suggested that the investigation of endophenotypes for ADHD has been disappointing (Stevenson, Asherson, Hay, Levy, Swanson, Thapar & Willcutt, 2005). Several factors contribute to this lack of progress including incompatibility among labs in the tools that are used to measure behavioral variability. Hopefully our use of digit span, a working memory task that has been researched for over 100 years (Richardson, 2007), will increase the likelihood that others can replicate the current findings.
We examined several functional DRD2 polymorphisms. rs12364283 is located in a conserved suppressor region of DRD2 and affects expression in prefrontal cortex and striatum (Zhang et al., 2007). rs2283265 and rs1076560 are two frequent intronic SNPs that alter the expression of the short and long isoforms of DRD2, and are associated with differential neural activity patterns in prefrontal cortex and striatum during working memory (Zhang et al., 2007). Notably, rs1800497 is localized within the ankyrin repeat and kinase domain containing 1 gene (ANKK1) (Neville, Johnstone & Walton, 2004). However, despite residing 10 kilobases downstream from DRD2, rs1800497 has been shown to affect DRD2 messenger RNA translation and stability, and postsynaptic DRD2 density in striatum (Ritchie & Noble, 2003). Thus, we thought that the DRD2 SNPs investigated here might evidence associations with working memory and ADHD symptoms but none were detected. Of note, we did not look for epistasis within DRD1 or DRD2, nor between DRD1 and DRD2. Epitasis undoubtedly contributes to the development of a complex genetic disorder such as ADHD as well as to intermediate phenotypes such as working memory. Unfortunately, this study did not have the requisite large sample of subjects required to examine gene–gene interactions and it would have been statistically irresponsible to do so. Still, this is an important issue in psychiatric genetics to explore further in larger consortia datasets.
This study has limitations that could have influenced the results. First, despite considerable effort, it was not possible to follow up all individuals from the childhood sample and obtain their genetic information, although available data suggest that this subsample was representative of the original cohort (Halperin et al., 2008). Second, our modest sample size is potentially under-powered to detect genetic risk factors of small effect for an etiologically complex disorder like ADHD. Nevertheless, our ability to detect significance with our limited sample size suggests that the findings may represent robust associations. Conversely, the study lacks sufficient power to protect against making Type II errors. This could be relevant to the non-significant inverse relationship indicating that attention problems got worse even though digit span backward improved in minor allele carriers at rs4532 and rs265978, and relevant to other null findings as well. Third, this study did not have a non-ADHD comparison/control group that was followed from childhood; thus, it is not clear whether these results generalize beyond individuals with ADHD. Fourth, despite the fact that minority groups have been mostly underrepresented in molecular genetic studies of ADHD to date, population structure limitations (i.e. an ethnically/racially mixed population of inner-city individuals of lower SES) makes the results susceptible to population stratification effects. As noted, all results were analyzed with and without minority status as a covariate with no appreciable differences detected between the two sets of analyses. Lastly, it is important to acknowledge that Digit Span forward and backward are somewhat crude measures of working memory maintenance and manipulation, respectively. A comprehensive, multivariate approach to studying lower-order versus higher-order working memory might have been preferred. However, because of these limitations, this study focused on several polymorphisms for which there exists an expectation of association based on prior work. Nevertheless, replication in larger, independent samples of different racial/ethnic background is needed.
In sum, the present data showed that ADHD symptomatology improved in individuals whose higher-order working memory manipulation skills got better over time if they inherited two copies of the common allele of DRD1 SNPs rs4532 and rs265978. These SNPs tag SNP rs686, a DRD1 variant that changes D1 receptor expression by >25% (Huang et al., 2008). Conversely, carrying the minor allele decoupled the link between ADHD symptoms and working memory manipulation. Such work may help identify subgroups of children with ADHD who will preferentially respond to certain remediation strategies such as working memory training to alter long-term outcome. These findings also shed new light on DRD1 as a modifier gene that influences the trajectory of ADHD but not necessarily the onset of symptoms in childhood.
Supplementary Material
Table S1. Intercorrelations of digit span forward and digit span backward at baseline, change from baseline to follow-up, and follow-up.
Table S2. Baseline/childhood results.
Table S3. Follow-up/adolescence-adulthood results.
Table S4. Developmental change results controlling for race/ethnicity.
Table S5. Developmental change results controlling for history (number of years) of stimulant treatment.
Table S6. Summary statistics for CBCL attention problems, digit span forward, and digit span backward at baseline and follow-up as a function of DRD1 SNPs rs265978 and rs4532.
Figure S1 A–B. Histograms of CBCL attention problems at baseline. The top plot (A) represents the untransformed data and the bottom plot (B) represents the base-e logarithm transformed data.
Figure S2 A–B. Histograms of CBCL attention problems residual change scores. The top plot (A) represents the untransformed data and the bottom plot (B) represents the base-e logarithm transformed data.
Figure S3 A–B. Histograms of CBCL attention problems at follow-up. The top plot (A) represents the untransformed data and the bottom plot (B) represents the base-e logarithm transformed data.
Figure S4 A–B. Plots of the raw data from the main findings of the interaction between rs4532/rs265978 and digit span backward on ADHD symptoms.
This is the raw data from Figures 2a and 2b plotted as change scores for each individual with regression slopes as a function of rs4532 and rs265978 genotype. The data is provided to help capture a broader picture of the primary results obtained in Figures 2a and 2b in the main text. However, note that these plots do not take the covariates or SNP × digit span interactions into account, thus the plots should not be interpreted out of context.
Figure S5. Expression of DRD1 in human dorsolateral prefrontal cortex across the lifespan (from BrainCloud, Colantuoni et al., 2011).
DRD1 gene transcript expression in human postmortem dorsolateral prefrontal cortex (Brodmann area 46/9 in postnatal samples and the corresponding region of prefrontal cortex in fetal samples) evidences a nonlinear pattern of expression across the lifespan. DRD1 expression increases during prenatal life and continues to do so throughout childhood. DRD1 expression then appears to peak in early adulthood followed by a gradual decline in expression during the later stages of life. This is a sample of 269 healthy controls of mixed/race ethnicity. This dataset can be downloaded from (http://braincloud.jhmi.edu/). Additional details of the brain tissue collection process and subsequent tissue processing can be found in Colantuoni et al., 2011 and from the BrainCloud website.
Research highlights.
Using a longitudinal approach from childhood to adolescence/early adulthood, this study showed that improvement in higher-order working memory function paralleled improvement in symptomatology (e.g. inattention, hyperactivity and impulsivity) over time in subgroups of youth with ADHD.
This association was linked to two polymorphisms in the dopamine D1 receptor gene (DRD1).
The DRD1 by working memory interactions were only detectable in later developmental stages.
The current findings shed new light on DRD1 as a modifier gene that appears to influence the trajectory of ADHD over time but not necessarily the onset of symptoms in childhood.
Acknowledgments
This work was supported by a doctoral research grant from the Graduate Center of the City University of New York to Joey W. Trampush, and by grants from the National Institutes of Health (RO1 MH046448 and RO1 MH060698) to Jeffrey M. Halperin. We thank Dr. Joel Kleinman for assistance with BrainCloud. Joey W. Trampush is now an affiliate of the Division of Psychiatry Research, The Zucker Hillside Hospital, Glen Oaks, NY, and the Center for Psychiatric Neuroscience, The Feinstein Institute for Medical Research, Manhasset, NY, USA.
Footnotes
Disclosures
The authors declare no competing interests.
Additional Supporting Information may be found in the online version of this article
References
- Achenbach TM. Manual for the Child Behavior Checklist 4/18 and 1991 Profile. Burlington, VT: University of Vermont; 1991. [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews. 2003;27 (1–2):3–18. doi: 10.1016/S0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37 (2):167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Asherson P. Attention-Deficit Hyperactivity Disorder in the post-genomic era. European Child and Adolescent Psychiatry. 2004;13 (Suppl 1):I50–170. doi: 10.1007/s00787-004-1006-6. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51 (6):1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21 (2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bobb AJ, Addington AM, Sidransky E, Gornick MC, Lerch JP, Greenstein DK, Clasen LS, Sharp WS, Inoff-Germain G, Wavrant-De Vrièze F, Arcos-Burgos M, Straub RE, Hardy JA, Castellanos FX, Rapoport JL. Support for association between ADHD and two candidate genes: NET1 and DRD1. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2005;134B (1):67–72. doi: 10.1002/ajmg.b.30142. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews Neuroscience. 2002;3 (8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhou K, Sham P, Franke B, Kuntsi J, Campbell D, et al. DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2008;147B (8):1450–1460. doi: 10.1002/ajmg.b.30672. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Lipska BK, Ye T, Hyde TM, Tao R, Leek JT, Colantuoni EA, Elkahloun AG, Herman MM, Weinberger DR, Kleinman JE. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature. 2011;478 (7370):519–523. doi: 10.1038/nature10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103 (24):9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks EM, Hudziak JJ, Dolan CV, Ferdinand RF, Boomsma DI. The relations between DISC-IV DSM diagnoses of ADHD and multi-informant CBCL-AP syndrome scores. Comprehensive Psychiatry. 2006;47 (2):116–122. doi: 10.1016/j.comppsych.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Fanous AH, Kendler KS. Genetic heterogeneity, modifier genes, and quantitative phenotypes in psychiatric illness: searching for a framework. Molecular Psychiatry. 2005;10 (1):6–13. doi: 10.1038/sj.mp.4001571. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychological Medicine. 2006;36 (02):159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57 (11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Glass GV, Hopkins KD. Statistical methods in education and psychology. 3. Boston, MA: Pearson; 1996. pp. 1–674. [Google Scholar]
- Hale JB, Hoeppner JAB, Fiorello CA. Analyzing digit span components for assessment of attention processes. Journal of Psychoeducational Assessment. 2002;20 (2):128–143. doi: 10.1177/073428290202000202. [DOI] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin. 2006;132 (4):560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. Journal of Child Psychology and Psychiatry. 2008;49 (8):958–966. doi: 10.1111/j.1469-7610.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27 (1):87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Huang W, Ma JZ, Payne TJ, Beuten J, Dupont RT, Li MD. Significant association of DRD1 with nicotine dependence. Human Genetics. 2008;123 (2):133–140. doi: 10.1007/s00439-007-0453-9. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Copeland W, Stanger C, Wadsworth M. Screening for DSM-IV externalizing disorders with the Child Behavior Checklist: a receiver-operating characteristic analysis. Journal of Child Psychology and Psychiatry. 2004;45 (7):1299–1307. doi: 10.1111/j.1469-7610.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36 (7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kirley A, Hawi Z, Daly G, McCarron M, Mullins C, Millar N, Waldman I, Fitzgerald M, Gill M. Dopaminergic system genes in ADHD: toward a biological hypothesis. Neuropsychopharmacology. 2002;27 (4):607–619. doi: 10.1016/S0893-133X(02)00315-9. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Rijsdijk F, Ronald A, Asherson P, Plomin R. Genetic influences on the stability of attention-deficit/hyperactivity disorder symptoms from early to middle childhood. Biological Psychiatry. 2005;57 (6):647–654. doi: 10.1016/j.biopsych.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40 (3):657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- Luca P, Laurin N, Misener VL, Wigg KG, Anderson B, Cate-Carter T, Tannock R, Humphries T, Lovett MW, Barr CL. Association of the dopamine receptor D1 gene, DRD1, with inattention symptoms in families selected for reading problems. Molecular Psychiatry. 2007;12 (8):776–785. doi: 10.1038/sj.mp.4001972. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH. ADHD and smoking: from genes to brain to behavior. Annals of the New York Academy of Sciences. 2008;1141:131–147. doi: 10.1196/annals.1441.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323 (5915):800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Meier LL. IRSE. Interactions in Multiple Linear Regression with SPSS and Excel (Version 1.6) 2008 [Computer software and manual]. Retrieved 2 September 2012, from http://www.urenz.ch/irse.
- Misener VL, Luca P, Azeke O, Crosbie J, Waldman I, Tannock R, Roberts W, Malone M, Schachar R, Ickowicz A, Kennedy JL, Barr CL. Linkage of the dopamine receptor D1 gene to attention-deficit/hyperactivity disorder. Molecular Psychiatry. 2004;9 (5):500–509. doi: 10.1038/sj.mp.4001440. [DOI] [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Human Mutation. 2004;23 (6):540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Nyman ES, Ogdie MN, Loukola A, Varilo T, Taanila A, Hurtig T, Moilanen IK, Loo SK, McGough JJ, Jarvelin MR, Smalley SL, Nelson SF, Peltonen L. ADHD candidate gene study in a population-based birth cohort: association with DBH and DRD2. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46 (12):1614–1621. doi: 10.1097/chi.0b013e3181579682. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Presynaptic D1 dopamine receptors in primate prefrontal cortex: target-specific expression in the glutamatergic synapse. Journal of Neuroscience. 2005;25 (5):1260–1267. doi: 10.1523/JNEUROSCI.3436-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Jr, Milich R, Murphy DA, Murphy HA. Normative data on the IOWA Conners Teacher Rating Scale. Journal of Clinical Child Psychology. 1989;18 (3):259–262. doi: 10.1207/s15374424jccp1803_9. [DOI] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81 (3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran K, Trampush JW, Rindskopf D, Marks DJ, O’Neill S, Halperin JM. Association between variation in neuropsychological development and trajectory of ADHD severity in early childhood. American Journal of Psychiatry. 2013;170:1205–1211. doi: 10.1176/appi.ajp.2012.12101360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JTE. Measures of short-term memory: a historical review. Cortex. 2007;43 (5):635–650. doi: 10.1016/S0010-9452(08)70493-3. [DOI] [PubMed] [Google Scholar]
- Rietveld MJH, Hudziak JJ, Bartels M, Beijsterveldt CEM, Boomsma DI. Heritability of attention problems in children: longitudinal results from a study of twins, age 3 to 12. Journal of Child Psychology and Psychiatry. 2004;45 (3):577–588. doi: 10.1111/j.1469-7610.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochemical Research. 2003;28 (1):73–82. doi: 10.1023/A:1021648128758. [DOI] [PubMed] [Google Scholar]
- Rommelse NNJ, Altink ME, Oosterlaan J, Buschgens CJM, Buitelaar J, Sergeant JA. Support for an independent familial segregation of executive and intelligence endophenotypes in ADHD families. Psychological Medicine. 2008;38 (11):1595–1606. doi: 10.1017/S0033291708002869. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Dulcan MK, Davies M, Piacentini J, Schwab-Stone ME, Lahey BB, Bourdon K, Jensen PS, Bird HR, Canino G, Regier DA. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): description, acceptability, prevalence rates, and performance in the MECA Study. Methods for the Epidemiology of Child and Adolescent Mental Disorders Study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35 (7):865–877. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- Stevenson J, Asherson P, Hay D, Levy F, Swanson J, Thapar A, Willcutt E. Characterizing the ADHD phenotype for genetic studies. Developmental Science. 2005;8 (2):115–121. doi: 10.1111/j.1467-7687.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303 (5659):853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-Revised (WISC-R) San Antonio, TX: The Psychological Corporation; 1974. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: The Psychological Corporation; 1991. (WISC-III) [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. (WAIS-III) [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139 (1):263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee MLT, Xiao T, Papp A, Wang D, Sadée W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proceedings of the National Academy of Sciences of the United States of America. 2007;104 (51):20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Intercorrelations of digit span forward and digit span backward at baseline, change from baseline to follow-up, and follow-up.
Table S2. Baseline/childhood results.
Table S3. Follow-up/adolescence-adulthood results.
Table S4. Developmental change results controlling for race/ethnicity.
Table S5. Developmental change results controlling for history (number of years) of stimulant treatment.
Table S6. Summary statistics for CBCL attention problems, digit span forward, and digit span backward at baseline and follow-up as a function of DRD1 SNPs rs265978 and rs4532.
Figure S1 A–B. Histograms of CBCL attention problems at baseline. The top plot (A) represents the untransformed data and the bottom plot (B) represents the base-e logarithm transformed data.
Figure S2 A–B. Histograms of CBCL attention problems residual change scores. The top plot (A) represents the untransformed data and the bottom plot (B) represents the base-e logarithm transformed data.
Figure S3 A–B. Histograms of CBCL attention problems at follow-up. The top plot (A) represents the untransformed data and the bottom plot (B) represents the base-e logarithm transformed data.
Figure S4 A–B. Plots of the raw data from the main findings of the interaction between rs4532/rs265978 and digit span backward on ADHD symptoms.
This is the raw data from Figures 2a and 2b plotted as change scores for each individual with regression slopes as a function of rs4532 and rs265978 genotype. The data is provided to help capture a broader picture of the primary results obtained in Figures 2a and 2b in the main text. However, note that these plots do not take the covariates or SNP × digit span interactions into account, thus the plots should not be interpreted out of context.
Figure S5. Expression of DRD1 in human dorsolateral prefrontal cortex across the lifespan (from BrainCloud, Colantuoni et al., 2011).
DRD1 gene transcript expression in human postmortem dorsolateral prefrontal cortex (Brodmann area 46/9 in postnatal samples and the corresponding region of prefrontal cortex in fetal samples) evidences a nonlinear pattern of expression across the lifespan. DRD1 expression increases during prenatal life and continues to do so throughout childhood. DRD1 expression then appears to peak in early adulthood followed by a gradual decline in expression during the later stages of life. This is a sample of 269 healthy controls of mixed/race ethnicity. This dataset can be downloaded from (http://braincloud.jhmi.edu/). Additional details of the brain tissue collection process and subsequent tissue processing can be found in Colantuoni et al., 2011 and from the BrainCloud website.