Abstract
Boron neutron capture therapy (BNCT) of cancer depends on the selective delivery of a sufficient number of boron-10 (10B) atoms to individual tumor cells. Cell killing results from the 10B (n, α)7Li neutron capture and fission reactions that occur if a sufficient number of 10B atoms are localized in the tumor cells. Intranuclear 10B localization enhances the efficiency of cell killing via damage to the DNA. The net cellular content of 10B atoms reflects both bound and free pools of boron in individual tumor cells. The assessment of these pools, delivered by a boron delivery agent, currently cannot be made at subcellular scale resolution by clinically applicable techniques such as PET and MRI. In this study, secondary ion mass spectrometry (SIMS) based imaging instrument, a CAMECA IMS 3f ion microscope, capable of 500 nm spatial resolution was employed. Cryogenically prepared cultured human T98G glioblastoma cells were evaluated for boron uptake and retention of two delivery agents. The first, L-p-boronophenylalanine (BPA), has been used clinically for BNCT of high grade gliomas, recurrent tumors of the head and neck region and melanomas. The second, a boron analogue of an unnatural amino acid, 1-amino-3-borono-cyclopentanecarboxylic acid (cis-ABCPC), has been studied in rodent glioma and melanoma models by quantification of boron in the nucleus and cytoplasm of individual tumor cells. The bound and free pools of boron were assessed by exposure of cells to boron-free nutrient medium. Both BPA and cis-ABCPC delivered almost 70% of the pool of boron in the free or loosely bound form to the nucleus and cytoplasm of human glioblastoma cells. This free pool of boron could be easily mobilized out of the cell and was in some sort of equilibrium with extracellular boron. In the case of BPA, the intracellular free pool of boron also was affected by the presence of phenylalanine in the nutrient medium. This suggests that it might be advantageous if patients were placed on a low phenylalanine diet prior to the initiation of BNCT. Since BPA currently is used clinically for BNCT, our observations may have direct relevance to future clinical studies utilizing this agent and provides support for individualized treatment planning regimens rather than the use of fixed BPA infusion protocols.
Keywords: Boron neutron capture therapy, BNCT, SIMS, Boron imaging, Imaging mass spectrometry, Glioblastoma multiforme, Gliomas, Boronophenylalanine, Boronated unnatural amino acids, T98G human glioblastoma cells, Multi-nucleate giant glioblastoma cells, Phenylalanine, Aspartame
Introduction
High grade gliomas, and more specifically glioblastoma multiforme (GBM), are uniformly fatal and have no curative treatment. Patients with these tumors have a life expectancy of approximately 12-15 months, even with the current standard therapy consisting of surgery and radiation therapy with the concomitant administration of temozolomide. Primary brain tumors are responsible for over 12,000 deaths per year in the U.S. and are the second most common cause of cancer deaths in children under the age of 15 (Kohler et al., 2011).
Boron Neutron Capture Therapy (BNCT) is a binary modality that has the potential to treat high grade gliomas (Sweet, 1951; Barth et al., 1990; Hatanaka, 1991). The main requirements for this therapy are the selective targeting of tumor cells with sufficient quantities of 10B atoms (109/cell or ~ 20 μg/g) and neutron irradiation with either epithermal (E≅ 10,000 eV) or low-energy thermal neutrons (Eth < 0.4 eV) depending upon the depth of the tumor. BNCT is based on the neutron capture and fission reactions, [10B(n, α)7Li], that occur when 10B atoms captures the thermal neutrons and instantaneously decays to produce high linear energy transfer (LET) alpha particles (stripped down 4He atoms) and recoiling 7Li nuclei (Locher, 1936; Godwin et al., 1955). In tissues, these particles have short path lengths (5 μm for 7Li and 9 μm for 4He), approximately the width of a single cell. The average LET is high (7Li, 162 keV/μm; 4He, 196 keV/μm), which results in densely ionizing radiation restricted to the path of each particle. Cell killing is enhanced by localization of 10B in the nucleus, where high LET radiation has a greater probability of damaging the DNA (Gabel et al., 1987; Kobayashi and Kanda, 1987). BNCT potentially is capable of killing individual cancer cells while sparing the normal brain tissue. To minimize normal tissue injury, the quantity of boron in tumor cells should exceed that found in surrounding normal cells by at least a factor of three (Fairchild and Bond, 1985; Zamenhof et al., 1992) . Consequently, knowledge of the microdistribution of 10B atoms in cells and tissues is of critical importance for the success of BNCT.
The measurement of 10B atoms in individual tumor cells under clinical conditions has remained challenging since clinically applicable techniques of PET and MRI do not provide sufficient spatial resolution for boron quantification in single cells. The net content of 10B atoms inside a cell represents a combination of both bound and free pools of 10B. Therefore, a high ratio of the free to the bound pool of intracellular boron would render it susceptible to the loss of boron atoms from the cell interior between the time of administration of the drug and the initiation of BNCT. Therefore, a boron compound capable of delivering higher concentrations of the bound pool of boron to tumor cells would be highly advantageous for BNCT. It should be noted that subcellular scale measurements of boron cannot be made with confidence with bulk methods of boron determination on samples of nuclei and cytoplasm collected after fractionation of tumor cells. The free and loosely bound pools of boron would most likely be lost from their native subcellular locations in various steps used in fractionation of cells, such as the centrifugation of cells in liquid media. The use of cryogenic sampling and single cell imaging mass spectrometry techniques overcome these hurdles and provide powerful approaches for the assessment of boron concentrations at subcellular scale resolution (Chandra et al., 2002b; Arlinghouse et al., 2004; Yokoyama et al., 2007; Alkins et al., 2013).
In the present study, we have used a secondary ion mass spectrometry (SIMS) based imaging technique of ion microscopy (Chandra et al., 2000) on cryogenically prepared cells for the assessment of bound and mobile (free or loosely bound) pools of boron in the nucleus and cytoplasm of cultured human GBM cells following exposure to BPA, which has been used clinically (Barth et al., 2012), and a new experimental compound cis-ABCPC (Kabalka et al., 2009; Chandra et al., 2013). The CAMECA IMS-3f SIMS instrument used in the present study was capable of producing visual images of gradients of any element from H to U with ppm-to-ppb sensitivity and image lateral resolution of 500 nm. The technique has been standardized for quantitative subcellular scale analysis of boron isotopes for studies of BNCT drugs (Ausserer et al, 1989; Chandra et al, 2000). Our observations revealed that nearly 70% pool of boron delivered by either BPA or cis-ABCPC is present in the free or loosely bound form in the nucleus and cytoplasmic compartments of human glioblastoma cells. This free pool of boron could be easily mobilized out of the cell and was in some sort of equilibrium with the extracellular boron. In the case of BPA, the intracellular free pool of boron also was affected by the presence of phenylalanine in the nutrient medium. Our observations may have relevance for optimizing the regimens of administering BPA prior to initiating BNCT.
Materials & Methods
Cell growth and drug treatments
Cells from the T98G human glioblastoma cell line (ATCC CRL-1690, Manassas, VA) were propagated in Eagle’s MEM with non-essential amino acids, 1 mM sodium pyruvate, Earle’s BSS and 10% FBS (Stein, 1979). The cells were grown on the polished surface of high purity N-type semiconductor grade silicon wafer pieces. An electrically conducting cell growth substrate is required for SIMS analysis since the sample is held at 4,500 volts in the sample chamber of the instrument. The silicon pieces (~ 1 cm2) were cut from Si wafers and sterilized prior to cell seeding. Each 60 mm plastic petri dish used for cell growth contained 5 silicon substrates. The cells were seeded at a density of 250,000 cells per dish. Approximately 20,000 latex beads (11 μm diameter) also were added to each petri dish. The beads acted as spacers in the cryogenic sandwich-fracture method. The cells were grown to ~ 70% confluency prior to initiating the studies. Since mitochondria are known to cluster in a well defined perinuclear cytoplasmic region in T98G cells (Weller et al., 1997), which can be spatially resolved in SIMS boron images (Chandra et al., 2002a), in one experiment the cells were treated with rhodamine 123 for imaging of mitochondria (Johnson et al., 1980). The chemical structure of BPA is shown in Fig. 1. The 10B in BPA was over 95% abundant. A boron equivalent concentration of 110 μg/ml of L-p-BPA in the form of a fructose complex (Coderre et al., 1994) was used to increase its solubility in water. The BPA concentration was similar to that used in previous SIMS studies and was non-toxic to T98G cells (Chandra et al., 2002a,b; Chandra and Lorey II, 2007). The cells were subjected to various treatments: (i) 2 hr. 110 μg/ml boron equivalent of BPA, (ii) 6 hr. 110 μg/ml boron equivalent of BPA, (iii) 2 hr. 110 μg/ml boron equivalent of BPA followed by 2 hr. exposure to nutrient medium alone, (iv) 2 hr. 110 μg/ml boron equivalent of BPA followed by 2 hr. exposure to nutrient medium containing an equimolar amount of phenylalanine amino acid as present in the BPA treatment, (v) 6 hr. 110 μg/ml boron equivalent of BPA followed by 2 hr. exposure to nutrient medium alone, and (vi) 6 hr. 110 μg/ml boron equivalent of BPA followed by 2 hr. exposure to nutrient medium containing an equimolar amount of phenylalanine amino acid as present in the BPA treatment. The two and six hr. exposure treatments to BPA have relevance to clinical BNCT of high grade gliomas (Barth et al., 2012). The exposure of cells to the nutrient medium alone following the BPA treatments should provide information on boron-retention in the nuclei and cytoplasm of cells, which plausibly represents the bound pool of boron to the cell matrix components. The addition of phenylalanine to the nutrient medium following exposure to BPA should provide information on the interaction between it and the intracellular retention of BPA. This treatment could have relevance to clinical BNCT since phenylalanine intake can vary among individual patients depending on their dietary consumption of different types of foods and drinks. For example, the consumption of diet sodas and other food products containing the artificial sweetener aspartame, a dipeptide of aspartic acid and phenylalanine may lead to a significant load of phenylalanine as a metabolite (Magnuson et al., 2007).
Fig. 1.
Chemical structures of p-boronophenylalanine (BPA) and 1-amino-3-borono-cyclopentanecarboxylic acid (cis-ABCPC) shown in its cis-L-ABCPC and cis-D-ABCPC enantiomeric forms.
For studies of cis-ABCPC as a mixture of its L- and D- enantiomers (Fig. 1), the compound was purified by removing contaminating ammonium chloride (Barth et al., 2013). The cis-ABCPC contained naturally abundant boron isotopes, i.e. 20% 10B and 80% 11B. The cells were exposed to a 30 ppm boron equivalent of the test compound for 1, 2, 4, and 6 hr. by directly dissolving it in the nutrient medium. As shown later, SIMS analysis indicated that the 4 hr. treatment was optimal for boron uptake in cells. Therefore, the 4 hr. cis-ABCPC treatment of T98G cells was chosen to assess bound and free pools of boron in subcellular compartments. In this experiment the cells were treated with 30 ppm boron equivalent of cis-ABCPC for 4 hr., followed by an additional 2 hr. exposure to either the nutrient medium alone or medium containing 10 ppm boron equivalent of cis-ABCPC. The concentration of 10 ppm boron was chosen to simulate the residual blood-boron-concentration in patients that would not initiate a 10B(n, α)7Li capture reaction during neutron irradiation.
Cryogenic freeze-fracture sample preparation and optical and confocal microscopy imaging of fractured freeze-dried T98G human GBM cells
After the designated treatments, the cells were cryogenically prepared using our sandwich-fracture method (Chandra et al., 1986). Cells fractured at the apical membrane are essentially whole cells without the EF-leaflet of the plasma membrane (Chandra & Morrison, 1997). The cells were then freeze-dried at low temperatures ranging between −65 to −80 °C overnight in a Tis-U-Dry freeze-drier (FTS Systems, Inc., Stone Ridge, NY). The temperature of the sample stage of the freeze-drier was increased gradually to 30-40 °C to avoid sample rehydration during venting and transfer of samples from the freeze-drier. The freeze-drier was vented with dry nitrogen, and the samples were transferred to a desiccator and stored until light microscopy and SIMS measurements were made. An Olympus microscope with reflected light Nomarski optics was used for photographing the fractured freeze-dried cells. A Bio-Rad MRC 600/Zeiss Axiovert 10 confocal microscope was used to image Rhodamine 123 specific fluorescence in fractured freeze-dried cells for mitochondrial localization in individual cells.
Quantitative subcellular imaging analysis of boron isotopes with SIMS ion microscopy
A CAMECA IMS-3f SIMS ion microscope (CAMECA, Paris, France), capable of producing isotopic images with a lateral resolution of 500 nm, was used in the study (Chandra et al., 2000). This instrument was equipped with a primary beam mass filter and a 5f Hall Probe control chassis and has been extensively upgraded over its years of use. The control system of the instrument also has been upgraded with a Charles Evans and Associates model PC-1CS Computer Interface system and Windows-based computer. For this study, the instrument was operated in the positive secondary ion imaging mode with samples biased to +4500 V. An O2+ primary ion beam, accelerated to 10 keV, was focused and adjusted to a nominal beam current of 150-200 nA with a diameter of ~75 μm. The primary ion beam was raster scanned over a 250 μm2 region. A 60 μm contrast aperture was employed for imaging positive secondary ion images. The cells were coated with a thin layer of Au/Pd to enhance their electrical conductivity for SIMS analysis. In the positive secondary ion detection mode, the images of isotopes with masses 10, 11, 12, 23, 39, and 40 revealed the distribution of 10B, 11B, 12C, 23Na, 39K, and 40Ca, respectively. The SIMS matrix effects (mass interferences, sputter rate variations, and practical ion yield variations), were found to be negligible between the nucleus and the cytoplasm of fractured freeze-dried cells (Chandra et al., 1987). The 10B+ or 11B+ signals were not detectable in control cells not treated with boronated agents.
SIMS ion images representing isotopic distributions were recorded from the image detection assembly using a Photometrics CCD CoolSNAP HQ2 FireWire Digital Camera capable of 14 bits/pixel image digitization. The image data were transferred from the camera controller to the PC workstation via Nikon NIS-Elements Imaging Software for storage and digital image processing (Princeton Digital Corp., USA). The camera was operated in the 2 × 2 binning mode. SIMS image integration times varied according to their intensities. In general, 39K and 23Na images were recorded for 0.4 sec. The 10B, 11B, 12C, and 40Ca images were recorded for 2 min. The pixel-by-pixel image quantification of 10B+ from BPA and 11B+ from cis-ABCPC signals was achieved by using 12C+ carbon normalization approach and the relative-sensitivity-factors (RSF) of boron isotopes to the 12C+ cell (tissue) matrix signals (Ausserer et al., 1989; Chandra et al., 2000; Chandra, 2010). The absolute boron concentrations produced from the SIMS images were converted into estimated wet weight concentrations by assuming 85% water content in cells.
Statistical analysis
A Minitab Statistical Software was used for statistical analysis of the data using ANOVA. A p value of less than 0.05 was considered significant.
Results
Morphological evaluation of fractured, freeze-dried T98G human GBM cells
Morphological features in fractured freeze-dried T98G human GBM cells are illustrated in a reflected light Nomarski image (Fig. 2), which revealed a characteristic kidney shape nucleus (N) with discernible nucleoli in individual cells. The multinucleated giant cells often were found together with a characteristic perinuclear organelle-rich cytoplasmic region (PNC) in their cytoplasm (C). This PNC contained a high density of mitochondria, as revealed by fluorescence imaging of rhodamine 123 with CLSM in individual T98G cells (Fig. 3). This also was consistent with a previous transmission electron microscopic study of this cell line (Weller et al., 1997). Morphological characterization of the three subcellular compartments (nucleus, perinuclear cytoplasm, and the remaining cytoplasm) in T98G cells facilitated the recognition of boron gradients by SIMS imaging analysis.
Fig. 2.
Morphological evaluation of fractured freeze-dried T98G human glioblastoma cells. The nuclei (N), cytoplasm (C), and a characteristic organelle-rich perinuclear cytoplasmic region (PNC) is illustrated in individual cells. The glioblastoma cells often contained multiple nuclei.
Fig. 3.
Fluorescence imaging of rhodamine 123 with confocal laser scanning microscopy in T98G human glioblastoma cells revealed a higher density of mitochondria in the perinuclear cytoplasmic region (PNC) in comparison to the remaining cytoplasm (C). The nuclear regions (N) were devoid of mitochondria.
Subcellular SIMS imaging analysis of potassium, sodium, calcium and boron in T98G GBM cells treated with BPA
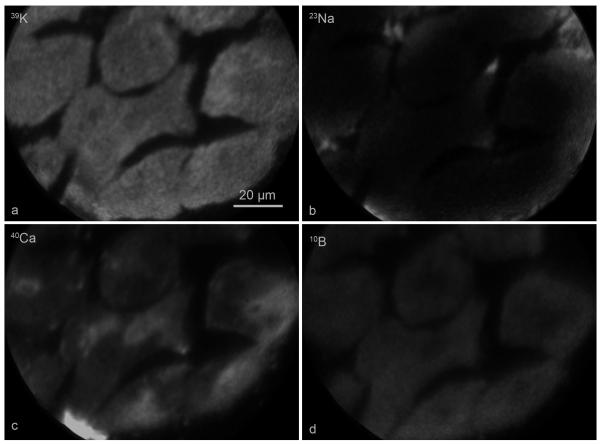
Figure 4 shows an example of the SIMS analysis of 39K, 23Na, 40Ca, and 10B in fractured, freeze-dried T98G GBM cells following a 6 hr treatment with a 110 ppm boron equivalent concentration of BPA. The level of brightness within an individual SIMS image is directly proportional to the isotopic concentration. The cells revealed physiologically relevant high 39K-low 23Na signals (K/Na ~10) indicative of the well preserved chemical composition in fast frozen, freeze-fractured, and freeze-dried cell matrix (Fig. 4a and 4b). Furthermore, the total calcium distribution illustrated in the 40Ca image in the same cells (Fig. 4c) clearly showed lower total calcium concentrations in kidney-shaped nuclei of cells compared to the cytoplasm, which contained calcium-sequestering membranous organelles such as the endoplasmic reticulum, Golgi, and mitochondria (Chandra et al., 1991, Chandra, 2005). The location of the nucleus in the calcium image provided a marker for localizing boron gradients across the nucleus and cytoplasm in each cell. The subcellular distribution of 10B from BPA revealed that it was distributed almost homogeneously between the nucleus and the cytoplasm with the exception of a distinctly lower concentration in the mitochondria-rich perinuclear cytoplasmic region in each cell (Fig. 4d). This pattern of boron distribution from BPA was consistent with previous SIMS studies using this cell line (Chandra et al., 2002a,b; Chandra and Lorey II, 2007). The significance of these observations and subcellular boron concentrations in various BPA treatments will be discussed later.
Fig. 4.
Subcellular SIMS imaging analysis of 39K, 23Na, 40Ca, and 10B from BPA in T98G human glioblastoma cells treated with 110 μg/g boron equivalent of 10BPA for 6 hr. SIMS images revealing the subcellular distributions of designated isotopes are shown in panels a-d. The image integration times for 39K and 23Na images were 0.4 s. The 40Ca and 10B images were integrated for 2 min each.
SIMS imaging analysis of multinucleated giant T98G GBM cells for subcellular boron distribution studies of BPA
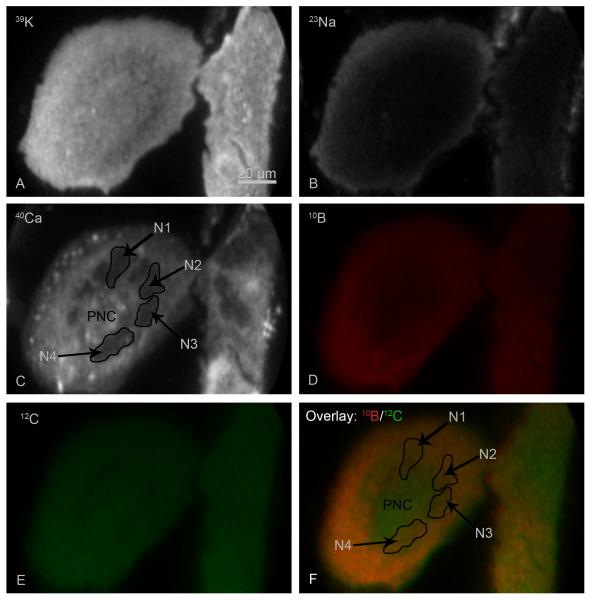
In the T98G GBM cell line, multinucleated giant cells often were present as a common feature of gliomas (Stein et al., 1979). However, these giant cells may have defective biochemical pathways (Fujita et al., 2004). Therefore, SIMS analysis of giant T98G cells was deemed important for subcellular boron uptake studies. Figure 5 shows an example of SIMS analysis of a multinucleated giant cell following a 2 hr BPA treatment (Fig. 5). In the 39K image the giant cell can be recognized as an elliptically shaped cell that was much bigger than other neighboring cells (Fig. 5a), having a transverse diameter of ~120 μm. Both the giant and the other T98G cells in the SIMS imaging field of view had high-K-and low-Na signals with a physiologically relevant K/Na ratio of approximately 10 (Fig. 5a and 5b). The SIMS 40Ca image revealed the presence of many dark nuclei with a lower concentration of total calcium signals in the giant cell, four of which were identified as N1-N4 (Fig. 5c). The multiple nuclei in the giant cell seemed to encapsulate the PNC in a circular pattern, and the remaining cytoplasm was contained on the opposite side of nuclei leading to the outer periphery of the cell. For a better display of boron gradients in the giant cell, the 10B image is shown in red (Fig. 5d) and the 12C cell matrix image, which was used for pixel-by-pixel boron quantification, is shown in green (Fig. 5e). For displaying the boron distribution across the three subcellular compartments in the giant cell, an overlay image of 10B/12C is shown in panel F (Fig. 5f). This approach compensated for primary ion beam density variations and microchannel plate response heterogeneity in SIMS images and is particularly useful for the display purposes of much larger and thicker cells such as the T98G giant cell shown here (Chandra et al., 2000; Chandra, 2010). The location of four nuclei in the giant cell is identified in the overlay image (Fig. 5f) in the identical location as in the 40Ca image. From the mixing of red and green colors, the formation of various intensities of yellow represents a qualitative display of boron concentrations across the giant cell. It is evident that the PNC contained lower concentrations of boron in comparison to multiple nuclei and remaining cytoplasm in peripheral regions of the giant cell. Quantitatively, the boron concentrations in nuclei of multinucleate giant cells were not significantly different from nuclei of other smaller T98G cells. This implies that pathophysiological processes, which resulted in the formation of giant cells, did not significantly affect the characteristic of boron uptake from BPA in T98G cell line. Therefore, giant cells were pooled along with other population of T98G cells in quantitative comparisons of boron concentrations in various treatments in the present study.
Fig. 5.
The SIMS imaging analysis of a multinucleated giant glioblastoma cell from a 2 hr treatment with 110 μg/g boron equivalent of 10BPA. SIMS images revealing the subcellular distribution of designated isotopes are shown in panels A-E. The 10B image (D) is shown in red and the 12C image in green (E). The overlay image of 10B/12C illustrates boron gradients in yellow, via mixing of red and green, in the giant cell (F). The position of 4 nuclei, designated as N1-N4, and the perinuclear region (PNC) in the giant cell is illustrated in the 40Ca (C) and the overlay image panel (F). The image integration times for 39K and 23Na images were 0.4 s. The 40Ca, 12C, and 10B images were integrated for 2 min each.
Quantitative comparisons of boron concentrations from SIMS studies of BPA for the evaluation of intracellular boron-uptake and retention in subcellular compartments
The boron concentrations from SIMS observations in the BPA study in three subcellular compartments of the nucleus, perinuclear mitochondria-rich cytoplasm, and the remaining cytoplasm in T98G human glioblastoma cells are summarized in Table 1. These observations on boron uptake and retention studies can be summarized as follows: (i) boron was distributed evenly throughout the cell with the exception of the perinuclear mitochondria-rich cytoplasmic region, which contained significantly less (p<0.05) boron than the nucleus or the remaining cytoplasm, (ii) a significant increase in boron uptake occurred in all three subcellular compartments between the 2 and 6 hr exposures to BPA, (iii) ~30% of the boron pool was retained in intracellular compartments of cells treated with BPA and then transferred to BPA-free nutrient medium for 2 hr (designated as retention in Table 1), and (iv) the boron retention was significantly (p<0.05) enhanced if the nutrient medium contained equimolar concentrations of phenylalanine as present in the BPA treatments. These observations indicate a highly dynamic nature of boron in the cell interior irrespective of 2 or 6 hr exposures to BPA. Approximately 70% of boron was easily mobilized (lost) from the cells upon exposure to nutrient medium not containing the BPA. Furthermore, this mobile or free pool of intracellular boron was present in some form of equilibrium with BPA or phenylalanine in the nutrient medium as it enhanced intracellular boron retention (Table 1). Since a short 2 or 6 hr long infusion of BPA have been used clinically for BNCT of high grade gliomas (Chanana et al., 1999; Sköld et al., 2010a,b; Barth et al., 2012), the observations discussed here may provide a better understanding of the dynamic nature of intracellular free and bound pools of boron within tumor cells.
Table 1.
Subcellular boron uptake and retention from BPA study in T98G human glioblastoma cells. The cells were treated with 110 μg/g boron equivalent of BPA for either 2 or 6 hr. duration. The boron retention studies were carried out in either boron-free nutrient medium or in the presence of equimolar concentration of the amino acid phenylalanine, as present in the BPA treatment. In each treatment more than 30 individual cells were analyzed in 5-7 SIMS imaging fields.
| Treatment | 10B Concentration (mean±SD, μg/g wet weight) | ||
|---|---|---|---|
| Nucleus | Mitochondria-Rich Perinuclear Cytoplasm |
Remaining Cytoplasm |
|
| 2 hr. BPA* | 252±31 | 190±26 | 269±36 |
| 2 hr. BPA followed by 2 hr. exposure to nutrient medium |
76±13 (30% retention) |
60±19 (31% retention) |
70±18 (26% retention) |
| 2 hr. BPA followed by 2 hr. exposure to nutrient medium containing equimolar concentration of phenylalanine as in BPA |
124±17 (49% retention) |
99±15 (52% retention) |
127±14 (47% retention) |
| 6 hr. BPA* | 374±88 | 265±72 | 379±71 |
| 6 hr. BPA followed by 2 hr. exposure to nutrient medium |
93±18 (25% retention) |
78±18 (29% retention) |
93±20 (25% retention) |
| 6 hr. BPA followed by 2 hr. exposure to nutrient medium containing equimolar concentration of phenylalanine as in BPA |
157±38 (42% retention) |
132±29 (50% retention) |
153±46 (40% retention) |
The subcellular boron concentrations were significantly (p < 0.05) different between the boron uptake and retention treatments. The presence of phenylalanine in the nutrient medium significantly (p < 0.05) enhanced the retention of boron from BPA in all three subcellular compartments of human glioblastoma cells.
Subcellular SIMS imaging studies of boron uptake and retention in cis-ABCPC treated T98G GBM cells
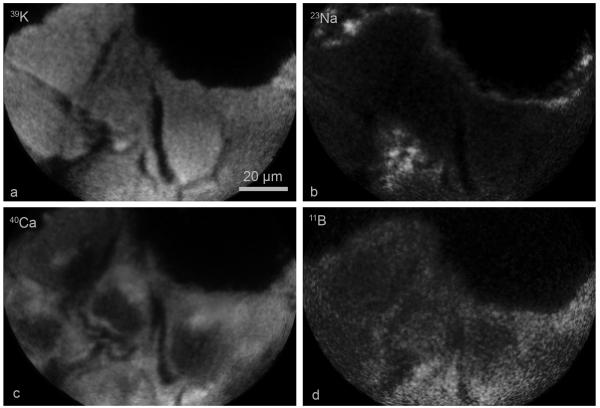
Figure 6 shows an example of the SIMS analysis of 39K, 23Na, 40Ca, and 11B in fractured freeze-dried T98G GBM cells from the 4 hr. treatment with 30 ppm boron equivalent concentration of cis-ABCPC. The cells had physiologically relevant high 39K-low 23Na signals (K/Na ~10) (Fig. 6a and 6b). The nucleus in each cell was identified in the 40Ca image as the darker low concentration region in comparison to the cell cytoplasm (Fig. 6c). The 11B distribution from cis-ABCPC in the same cells showed some degree of heterogeneity in individual tumor cells (Fig. 6d). Quantitative SIMS observations, summarized in Table 2, from the cis-ABCPC study indicate that there was no significant difference between the 1 and 2 hr exposure time for boron uptake. However, the uptake increased significantly (p < 0.05) and peaked at the 4 hr exposure, which was not significantly different from the 6 hr exposure (Table 1). These observations indicate the time-dependence of optimal boron uptake from cis-ABCPC in this cell line. At 4 or 6 hr exposures of cis-ABCPC, the boron concentration ratio for the cell nucleus to the nutrient medium was ~4:1 and 5:1 for the cell cytoplasm. The mitochondria-rich PNC lagged behind in boron uptake and revealed this ratio to be ~3:1 at 4 or 6 hr of exposure (Table 2).
Fig. 6.
Subcellular SIMS imaging analysis of 39K, 23Na, 40Ca, and 11B from cis-ABCPC in T98G human glioblastoma cells treated with 30 μg/g boron equivalent of cis-ABCPC for 4 hr. SIMS images revealing the subcellular distributions of designated isotopes are shown in panels a-d. The image integration times for 39K and 23Na images were 0.4 s. The 40Ca and 11B images were integrated for 2 min each.
Table 2.
Subcellular boron uptake and retention from cis-ABCPC study on T98G human glioblastoma cells. The cells were treated with 30 μg/g boron equivalent of cis-ABCPC for 1, 2, 4, and 6 hr. durations. The boron retention studies were carried out in either boron-free nutrient medium or in the nutrient medium containing 10 μg/g boron equivalent concentration of cis-ABCPC. In each treatment more than 25 cells were analyzed in 4-6 SIMS imaging fields.
| Treatment Boron Uptake* |
Boron Concentrations (Mean±SD, μg/g wet weight) | ||
|---|---|---|---|
| Nucleus | Mitochondria-rich Perinuclear Cytoplasm |
Remaining Cytoplasm | |
| 1 hr. | 68±14 | 59±11 | 90±22 |
| 2 hr. | 78±20 | 66±22 | 96±24 |
| 4 hr. | 120±27 | 90±21 | 150±29 |
| 6 hr. | 122±25 | 93±21 | 162±38 |
| Boron Retention | Boron Concentrations (Mean±SD, μg/g wet weight) | ||
| 4 hr. cis-ABCPC followed by 2 hr. exposure to Nutrient Medium |
36±6 (30% retention) |
30±8 (33% retention) |
41±9 (27% retention) |
| 4 hr. cis-ABCPC followed by 2 hr. exposure to nutrient medium containing 10 μg/g boron equivalent concentration of cis- ABCPC |
55±10 (46% retention) |
45±10 (50% retention) |
77±16 (51% retention) |
The 4 and 6 hr. long exposures of cis-ABCPC were significantly (p < 0.05) higher in boron uptake in all three subcellular compartments of human glioblastoma cells than either 1 or 2 hr. exposures.
Quantitative SIMS observations on boron retention following the 4 hr cis-ABCPC treatment indicated that ~30% of intracellular boron pool was retained intracellularly regardless of the subcellular compartment under study, within 2 hr exposure of cells to boron free nutrient medium (Table 2). However, if only 10 ppm boron equivalent of the test agent was left in the medium, then intracellular boron retention increased significantly (p < 0.05) to ~ 50% level. These observations indicate the presence of a large pool of intracellular free or loosely bound boron that is in some sort of equilibrium with extracellular cis-ABCPC in cultured GBM cells.
Discussion
In the present study we have evaluated quantitatively the bound and free pools of intracellular boron in the nucleus and cytoplasm of T98G GBM cells delivered by either BPA or cis-ABCPC. The net boron-10 content of a tumor cell represents the sum of both free and bound pools of boron. Consequently, it is important to characterize boronated compounds in delivering boron to these different intracellular pools and to understand the relationship between intra- and extracellular boron. BPA has been used clinically for BNCT of high grade gliomas, melanomas, and tumors of the head and neck region (Wittig et al., 2000; Barth et al., 2012). BNCT of high grade gliomas mainly has relied on pre-determined BPA infusion times for either 2 or 6 hr, followed by a lag period of ~2 hrs, which is needed to lower the blood boron concentrations to a safe level that would not initiate a 10B (n, α)7Li capture reaction during neutron irradiation. There is little information in the literature on factors affecting the net boron concentrations in tumor cells during clinical BNCT of high grade gliomas, since MRI and PET imaging do not offer the spatial resolution for subcellular scale boron measurements in individual tumor cells. Therefore, analysis of cultured human GBM cells under laboratory conditions by a single cell imaging technique provides an effective approach for understanding the boron uptake and retention characteristics of boron delivery agents.
SIMS observations on BPA revealed a highly dynamic nature of the intracellular boron pool in cultured GBM cells regardless of a 2 or 6 hr exposure to BPA (Table 1). These observations are consistent with a previous study on GS-9L glioma cell line, which demonstrated a large pool (~ 45%) of boron following a 6 hr exposure to BPA that could be mobilized from intracellular pools within 20 min of exposure to BPA-free nutrient medium (Bennett et al., 1994). Our observations indicate that there is some benefit to raising the net tumor cell boron concentrations with a longer exposure of 6 hr versus a 2 hr exposure to BPA, but it does not increase the ratio of bound-to-free pool of intracellular boron in GBM cells. In both cases, the bound pool of boron remained in the range of 25-30%, since the remainder of the boron pool could be mobilized from GBM cells (see retention data in Table 1). Furthermore, the free or loosely bound pool of intracellular boron, which was ~70% after a 2 hr and 75% after a 6 hr exposure of the total intracellular boron content, was in some sort of equilibrium with extracellular BPA or even phenylalanine in the nutrient medium (Table 1). The competing effects of the intracellular boron pool with phenylalanine may decrease both boron uptake and retention. Since higher dietary intake of phenylalanine would interfere with both boron uptake and retention from BPA, it would be advantageous if patients were placed on a low phenylalanine diet prior to the initiation of BNCT.
The observation that the majority of the boron pool from BPA in GBM cells was present as the free and/or loosely bound pool of intracellular boron, which can be decreased by extracellular phenylalanine and possibly by other competing amino acids, provides compelling support for individualized confirmation of tumor cell boron concentrations rather than using fixed protocols of either 2 hr or 6 hr infusion of BPA prior to BNCT. The development of PET imaging techniques with 18F-labeled BPA for monitoring boron concentrations in the tumor mass and the normal brain may provide a useful approach for individualized treatment planning for BNCT of high grade gliomas (Imahori et al., 1998: Nichols et al., 2002). However, this approach has a serious limitation of not having sufficient spatial resolution for the detection of individual infiltrating tumor cells in normal brain, which are protected by the blood-brain-barrier. Since infiltrating tumor cells are the main targets of BNCT, alternative approaches of boron-delivery or longer infusions could be employed in order to normalize the possible differences in boron concentrations between the tumor cells in the main tumor mass versus the infiltrating cells in the normal brain, such has been reported in rat models of high grade gliomas (Smith et al., 2001; Yokoyama et al., 2007; Alkins et al., 2013).
The cis-ABCPC delivered optimal intracellular boron within 4 hrs with a nucleus-to-nutrient medium boron ratio of 4:1 and 5:1 for cytoplasm of GBM cells (Table 2). In a recent study on cultured B16 melanoma cells with cis-ABCPC, a 7:1 boron ratio for nucleus-to-nutrient medium was observed after 4 hr. exposure (Chandra et al., 2013). These observations indicate cell type differences in boron uptake for cis-ABCPC. In the current study, the mitochondria-rich PNC in GBM cells lagged behind in boron accumulation in comparison to nucleus and cytoplasm. This plausibly may have been due to a diffusion barrier for cis-ABCPC provided by the mitochondrial double membrane system. Our observations also indicate that ~70% of the boron pool from cis-ABCPC was present as a free or loosely bound pool of boron in the nucleus, as it was released after exposure of cells to boron free nutrient medium (Table 2). This free pool of boron also was in some sort of equilibrium with the extracellular cis-ABCPC in the nutrient medium, as indicated by boron retention studies after the exposure of cells to 10 ppm boron equivalent amount of cis-ABCPC (Table 2). These observations, taken together, indicate a highly dynamic nature of boron pools delivered by cis-ABCPC to cell interiors of GBM cells in vitro.
In summary, both BPA and cis-ABCPC delivered nearly 70% of the pool of boron in the free or loosely bound form to the nucleus and cytoplasm of human GBM cells. This free pool could be mobilized easily out of the cell and was in some sort of equilibrium with the extracellular boron. In the case of BPA, the intracellular free boron pool also was affected by the presence of phenylalanine. Since BPA is the drug most widely used for clinical BNCT, our observations may have relevance to the development of improved regimens for the administration of BPA, especially the placement of patients on low phenylalanine diets prior to BNCT. This study provides support to individualized treatment planning rather than the use of fixed BPA infusion protocols of 2 or 6 hr infusions for BNCT of high grade gliomas.
Acknowledgments
This study was funded by a NIH grant 5R01CA129326. Cornell Microscopy and Imaging Facility (MIF), Department of Biomedical Engineering, is acknowledged for culturing the T98G human glioblastoma cells and the use of a Confocal Laser Scanning Microscope. Dr. Jeffrey Coderre, Massachusetts Institute of Technology, is gratefully acknowledged for providing us with the 10BPA-Fructose compound. The Cornell SIMS Laboratory, under the direction of S. Chandra, is affiliated with New York State Foundation for Science, Technology, and Innovation (NYSTAR). We thank Mrs. Heidi Bosworth for secretarial assistance in the preparation of this manuscript.
References
- Alkins RD, Broderoen PM, Sodhi RNS, Hynymen K. Enhancing drug delivery for neutron capture therapy of brain tumors with focused ultrasound. Neuro. Oncol. 2013;15:1225–1235. doi: 10.1093/neuonc/not052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlinghaus HF, Fartmann M, Kriegeskotte C, Dambasch S, Wittig A. Subcellular imaging of cell cultures and tissue for boron localization with laser-SNMS. Surf Interface Anal. 2004;36:698–701. [Google Scholar]
- Ausserer WA, Ling Y-C, Chandra S, Morrison GH. Quantitative imaging of boron, calcium, magnesium, potassium, and sodium in cultured cells. Anal. Chem. 1989;61:2690–2695. doi: 10.1021/ac00199a002. [DOI] [PubMed] [Google Scholar]
- Barth RF, Kabalka GW, Yang W, Huo T, Nakkula R, Shaikh AL, Haider SA, Chandra S. Evaluation of unnatural cyclic amino acids as boron delivery agents for treatment of melanomas and gliomas. Appl. Radiat. Isotopes. 2013 doi: 10.1016/j.apradiso.2013.11.133. DOI: 10.1016/j.apradiso.2013.11.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth RF, Soloway AH, Fairchild RG. Boron neutron capture therapy for cancer. Scientific American. 1990;263:100–103. 106–107. doi: 10.1038/scientificamerican1090-100. [DOI] [PubMed] [Google Scholar]
- Barth RF, Vicente MGH, Harling OK, Kiger WS, III, Riley KJ, Binns PJ, Wagner FM, Suzuki M, et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 2012;7:146. doi: 10.1186/1748-717X-7-146. doi: 10.1186/1748-717X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Mumford-Zisk J, Coderre JA, Morrison GH. Subcellular localization of p-boronophenylalanine-delivered boron-10 in the rat 9L gliosarcoma: cryogenic preparation in vitro and in vivo. Radiat. Res. 1994;140:72–78. [PubMed] [Google Scholar]
- Chanana AD, Capala J, Chadha M, Coderre JA, Diaz AZ, Elowitz EH, Iwai J, Joel DD, et al. Boron neutron capture therapy for glioblastoma multiforme: Interim results from the phase I/II dose-escalation studies. Neurosurg. 1999;44:1182–1193. doi: 10.1097/00006123-199906000-00013. [DOI] [PubMed] [Google Scholar]
- Chandra S. Quantitative imaging of subcellular calcium stores in mammalian LLC-PK1 epithelial cells undergoing mitosis by SIMS ion microscopy. Eur. J. Cell Biol. 2005;84:783–797. doi: 10.1016/j.ejcb.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Chandra S. Quantitative imaging of chemical composition in single cells by secondary ion mass spectrometry: cisplatin affects calcium stores in renal epithelial cells. Methods Mol. Biol. 2010;656:113–130. doi: 10.1007/978-1-60761-746-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Ausserer WA, Morrison GH. Evaluation of matrix effects in ion microscopic analysis of freeze-fractured, freeze-dried cultured cells. J. Microsc. 1987;148:223–239. doi: 10.1111/j.1365-2818.1987.tb02869.x. [DOI] [PubMed] [Google Scholar]
- Chandra S, Barth RF, Haider SA, Yang W, Huo T, Shaikh AL, Kabalka GW. Biodistribution and subcellular localization of an unnatural boron-containing amino Acid (Cis-ABCPC) by imaging secondary ion mass spectrometry for neutron capture therapy of melanomas and gliomas. PLoS ONE. 2013;8(9):e75377. doi: 10.1371/journal.pone.0075377. doi:10.1371/journal.pone.0075377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Kabalka GW, Lorey DR, II, Smith DR, Coderre JA. Imaging of fluorine and boron from fluorinated-boronophenylalanine in the same cell at organelle resolution by correlative SIMS ion microscopy and confocal laser scanning microscopy. Clin. Cancer Res. 2002a;8:2675–2683. [PubMed] [Google Scholar]
- Chandra S, Kable EPW, Morrison GH, Webb WW. Calcium sequestration in the Golgi apparatus of cultured mammalian cells revealed by laser scanning confocal microscopy and ion microscopy. J. Cell Sci. 1991;100:747–752. doi: 10.1242/jcs.100.4.747. [DOI] [PubMed] [Google Scholar]
- Chandra S, Lorey DR., II SIMS ion microscopy imaging of boronophenylalanine (BPA) and 13C15N-labeled phenylalanine in human glioblastoma cells: relevance of subcellular scale observations to BPA-mediated boron neutron capture therapy of cancer. Int. J. Mass Spect. 2007;260:90–101. [Google Scholar]
- Chandra S, Lorey DR, II, Smith DR. Quantitative subcellular secondary ion mass spectrometry (SIMS) imaging of boron-10 and boron-11 isotopes in the same cell delivered by two combined BNCT drugs: in vitro studies on human glioblastoma T98G cells. Radiat. Res. 2002b;157:700–710. doi: 10.1667/0033-7587(2002)157[0700:qssims]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Chandra S, Morrison GH. Evaluation of fracture planes and cell morphology in complimentary fractures of cultured cells in the frozen-hydrated state by field-emission secondary electron microscopy: feasibility for ion localization and fluorescence imaging studies. J. Microsc. 1997;186:232–245. doi: 10.1046/j.1365-2818.1997.2030763.x. [DOI] [PubMed] [Google Scholar]
- Chandra S, Morrison GH, Wolcott CC. Imaging intracellular elemental distribution and ion fluxes in cultured cells with ion microscopy: a freeze-fracture methodology. J. Microsc. 1986;144:15–37. doi: 10.1111/j.1365-2818.1986.tb04670.x. [DOI] [PubMed] [Google Scholar]
- Chandra S, Smith DR, Morrison GH. Subcellular imaging by dynamic SIMS ion microscopy. Anal. Chem. 2000;72:104A–114A. doi: 10.1021/ac002716i. [DOI] [PubMed] [Google Scholar]
- Coderre JA, Button TM, Micca PL, Fischer C, Nawrocky MM, Liu HB. Neutron capture therapy of the 9L rat gliosarcoma using the p-boronophenylalaninefructose complex. Int. J. Radiat. Oncol. Biol. Phys. 1994;30:643–652. doi: 10.1016/0360-3016(92)90951-d. [DOI] [PubMed] [Google Scholar]
- Fairchild RG, Bond BP. Current status of boron-10 neutron capture therapy: enhancement of tumor dose via beam filtration and dose rate, and the effects of these parameters on minimum boron content- A theoretical evaluation. Int. J. Radiat. Oncol. Biol. Phys. 1985;11:831–835. doi: 10.1016/0360-3016(85)90318-9. [DOI] [PubMed] [Google Scholar]
- Fujita M, Mizuno M, Nagasaka T, Wakayashi T, Maeda K, Ishii D, Arima T, Kawajiri A, et al. Aurora-B dysfunction of multinucleated giant cells in glioma detected by site-specific phosphorylated antibodies. J. Neurosurg. 2004;101:1012–1017. doi: 10.3171/jns.2004.101.6.1012. [DOI] [PubMed] [Google Scholar]
- Gabel D, Foster S, Fairchild RG. The Monte Carlo simulation of the biological effect of the 10B(n,α)7Li reaction in cells and tissue and its implications for boron neutron capture therapy. Radiat. Res. 1987;111:14–25. [PubMed] [Google Scholar]
- Godwin JT, Far LE, Sweet WH, Robertson JS. Pathological study of eight patients with glioblastoma multiforme treated by neutron-capture therapy using boron-10. Cancer. 1955;8:601–615. doi: 10.1002/1097-0142(1955)8:3<601::aid-cncr2820080326>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hatanaka H. In: Boron neutron capture therapy for tumors. Glioma, Karim ABMF, Lewis ER, editors. Springer; Berlin: 1991. pp. 233–249. [Google Scholar]
- Imahori Y, Ueda S, Ohmori Y, Kusuki T, Ono K, Fujii R, Ido T. Fluorine-18-labeled fluoroboronophenylalanine PET in patients with glioma. J. Nucl. Med. 1998;39:325–333. [PubMed] [Google Scholar]
- Johnson LV, Walsh ML, Chen LV. Localization of mitochondria in living cells with Rhodamine 123. Proc. Natl. Acad. Sci. (U.S.A.) 1980;77:990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabalka GW, Yao M-L, Marepally SR, Chandra S. Biological evaluation of boronated unnatural amino acids as new boron carriers. Appl. Radiat. Isotopes. 2009;67:S374–S379. doi: 10.1016/j.apradiso.2009.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Kanda K. Analytical calculation of boron-10 dosage in cell nucleus for neutron capture therapy. Radiat. Res. 1987;111:14–25. [PubMed] [Google Scholar]
- Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, Jemal A, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J. Natl. Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher GL. Biological effects and therapeutic possibilities of neutrons. Am. J. Roentgenol. Radium Ther. 1936;36:1–13. [Google Scholar]
- Magnuson BA, Burdock GA, Doull J, Kroes RM, Marsh GM, Pariza MW, Spencer PS, Waddell WJ, et al. Aspartame: a safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit. Rev. Toxicol. 2007;37:629–727. doi: 10.1080/10408440701516184. [DOI] [PubMed] [Google Scholar]
- Nichols TL, Kabalka GW, Miller LF, Khan MK, Smith GT. Improved treatment planning for boron neutron capture therapy for glioblastoma multiforme using fluorine-18 labeled boronophenylalanine and positron emission tomography. Med. Phys. 2002;29:2351–2358. doi: 10.1118/1.1507780. [DOI] [PubMed] [Google Scholar]
- Sköld K, Gorlia T, Pellettieri L, Giusti V, Stenstam BH, Hopewell JW. Boron neutron capture therapy for newly diagnosed glioblastoma multiforme: an assessment for clinical potential. Brit. J. Radiol. 2010a;83:596–603. doi: 10.1259/bjr/56953620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld K, Stenstam BH, Diaz AZ, Giusti V, Hopewell JW. Boron neutron capture therapy for glioblastoma multiforme: advantage of prolonged infusion of BPA-f. Acta Neurol. Scand. 2010b;122:58–62. doi: 10.1111/j.1600-0404.2009.01267.x. [DOI] [PubMed] [Google Scholar]
- Smith DR, Chandra S, Barth RF, Yang W, Joel DD, Coderre JA. Quantitative imaging and microlocalization of boron-10 in brain tumors and infiltrating tumor cells by SIMS ion microscopy: Relevance to neutron capture therapy. Cancer Res. 2001;61:8179–8187. [PubMed] [Google Scholar]
- Stein GH. T98G: an anchorage-independent human tumor cell line that exhibits stationary phase G1 arrest in vitro. J. Cell Physiol. 1979;99:43–54. doi: 10.1002/jcp.1040990107. [DOI] [PubMed] [Google Scholar]
- Sweet WH. The uses of nuclear disintegration in the diagnosis and treatment of brain tumor. N. Engl. J. Med. 1951;245:875–878. doi: 10.1056/NEJM195112062452301. [DOI] [PubMed] [Google Scholar]
- Weller M, Winter S, Schmidt C, Esser P, Fontana A, Dichgans J, Groscurth P. Topoisomerase-I inhibitors for human malignant glioma: differential modulation of p53, p21, bax and bcl-2 expression and of CD95-mediated apoptosis by camptothectin and blapachone. Int. J. Cancer. 1997;73:707–714. doi: 10.1002/(sici)1097-0215(19971127)73:5<707::aid-ijc16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Wittig A, Sauerwein WA, Coderre JA. Mechanisms of Transport of p-Borono-Phenylalanine through the Cell Membrane. Radiat. Res. 2000;153:173–180. doi: 10.1667/0033-7587(2000)153[0173:motopb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Miyatake S-I, Kajimoto Y, Kawabata S, Doi A, et al. Analysis of boron distribution in vivo for boron neutron capture therapy using two different boron compounds by secondary ion mass spectrometry. Radiat. Res. 2007;167:102–109. doi: 10.1667/RR0501.1. [DOI] [PubMed] [Google Scholar]
- Zamenhof RG, Kalend AM, Bloomer WD. BNCT: Looking for a few good molecules. J. Natl. Cancer Inst. 1992;84:1290–1291. doi: 10.1093/jnci/84.16.1290-a. [DOI] [PubMed] [Google Scholar]