Abstract
Efferent ductules are responsible for the transportation of sperm from the testis to the epididymis and their epithelium is responsible for the reabsorption of over 90% of the luminal fluid. The purpose of this research was to characterize the gross morphology and histology of efferent ductules in the male Golden Syrian hamster. The efferent ductules emerge from rete testis with a unique polarity at the apex or cephalic pole of the testis. The number of efferent ductules varied from 3 to 10 with an average of 6.0 and blind ending ducts were observed in approximately 56 % of the males. The ductules merged into a single common duct prior to entering the caput epididymidis. The proximal efferent ductule lumen was wider than the distal (conus and common ducts), consistent with reabsorption of most of the luminal fluid, as was morphology of the ductal epithelium. Nonciliated cells in the proximal region had prominent endocytic apparatuses, showing both coated pits and apical tubules in the apical cytoplasm. Large basolateral, intercellular spaces were also present in the epithelium of the proximal region. Distal nonciliated cells had an abundance of large endosomes and lysosomal granules. Localization of sodium/hydrogen exchanger-3 (NHE3; SLC9A3) and aquaporins 1 and 9 (AQP1, AQP9) along the microvillus border was also consistent with ion transport and fluid reabsorption by this epithelium. In comparison, the caput epididymidis epithelium expressed only AQP9 immunostaining. Another unusual feature of the hamster efferent ductules was the presence of glycogen aggregates in the basal cytoplasm of small groups of epithelial cells, but only in the proximal ducts near the rete testis. Androgen (AR), estrogen (ESR1 and ESR2) and vitamin D (VDR) receptors were also abundant in epithelial nuclei of proximal and distal efferent ductules. In comparison, caput epididymidis showed very little immunostaining for ESR1.
Keywords: Testis, rete testis, efferent ductules, epididymis, histology, ultrastructure, immunohistochemistry, aquaporin, androgen receptor, estrogen receptor, vitamin D receptor, NHE3
Introduction
In the 17th century De Graaf became the first to describe the ductuli efferentes, or efferent ducts of the testis. He described the tubules as being very thin and “coiled individually from side to side”. Since then, efferent ductules have been described in more than 20 different mammalian (Hess, 2002; Ilio & Hess, 1994) and numerous nonmammalian vertebrate species (Guerrero et al., 2004; Hernandez-Franyutti & Uribe, 2012; Hess et al., 1976; Holmes & Gist, 2004; Rheubert et al., 2010; Sever & Freeborn, 2012). Efferent ductules transport spermatozoa from rete testis to the epididymis. However, in addition to providing a conduit for sperm, the ductules play an important role in sperm maturation by isosmotic reabsorption of water, ions (Clulow et al., 1994; Clulow et al., 1998) and protein (Veeramachaneni et al., 1990), thereby increasing the concentration of sperm that enter the epididymis. Epithelial cells that line the ductal lumenare classified as either pseudostratified or simple columnar, consisting of nonciliated and ciliated cells and occasional intraepithelial lymphocytes (Hess, 2002; Ilio & Hess, 1994). A layer of smooth muscle and connective tissue surrounds the epithelium.
Considerable variation in histological appearance of the epithelium has been observed along the length of the ductules. One difference is a greater proportion of nonciliated cells in the proximal region and an increased proportion of ciliated cells in the conus and common ducts near the epididymis (Ilio & Hess, 1994). Another difference from proximal to distal is in the presence or absence of granules and vesicles previously identified as endosomes and lysosomes (Hermo et al., 1985; Hess, 2002; Robaire & Hermo, 1988). However, the granular component of the endocytic apparatus varies significantly between species, with larger mammals showing the highest variation (Ilio & Hess, 1994). Ciliated cells appear more consistent in structure, with an apical positioning of their nuclei, short microvilli between typical ciliary projections from basal bodies lining the apical surface and numerous mitochondria in the apical cytoplasm to support the high-energy requirement of motile cilia (Hess, 2002), which has also been shown in the hamster (Yokoyama & Chang, 1971).
Nonciliated cells are related embryologically to the derivation of proximal convoluted tubules of the kidney; therefore, it is not surprising that their ultrastructure is similar in appearance and consistent with their physiological function of fluid reabsorption. The efferent ductule epithelium is responsible for reabsorption of over 90% of the fluid that exits the testis, including water, low molecular weight solutes and proteins (Clulow et al., 1994; Clulow et al., 1998; Hansen et al., 2004) (Hermo et al., 1985; Igdoura et al., 1993). This important physiological function involves three major processes of active solute transport, passive paracellular diffusion and fluid phase endocytosis (Clulow et al., 1998; Hermo et al., 1985; Hermo & Morales, 1984; Hess, 2002; Robaire & Hermo, 1988). Morphologically, the apical cytoplasm contains a complex endocytic apparatus of tubular coated pits, apical tubules, endosomes, multivesicular bodies and secondary lysosomes (Hermo et al., 1985; Hermo et al., 1988), similar to structures reported in the Chinese hamster (Yokoyama & Chang, 1971). Hermo et al (1988) proposed a model in which the apical tubules are involved in both endosome activity and the recycling of membrane back to the apical plasmalemma.
This massive movement of water, which results in nearly 25-fold increase in the concentration of epididymal sperm (Clulow et al., 1994), is dependent on an active absorption of sodium and an apical expression of the Na(+)/H(+) exchanger (SLC9A3) and aquaporins (AQP) (Oliveira et al., 2005; Ruz et al., 2006) and basal localization of Na+-K+-ATPase (Ilio & Hess, 1992) and AQP-1 (Ruz et al., 2006). Consistent with this physiological activity, the basolateral plasma membranes show extensive interdigitations and often dilated intercellular spaces in the proximal ductules of some species (Goyal & Williams, 1988; Holmes & Gist, 2004; Ilio & Hess, 1992; Jones & Jurd, 1987; Pudney & Fawcett, 1984; Ramos & Dym, 1977; Sever & Freeborn, 2012).
The male reproductive tract function is dependent upon a complex expression of multiple nuclear steroid receptors (Hess et al., 2011; Robaire & Hamzeh, 2011; Robaire et al., 2006). In contrast to the androgen receptor’s (AR) importance in testis and epididymis, estrogen receptor (ESR1) plays a dominant role in the efferent ductules (Hess et al., 2011; Hess et al., 2002; Nie et al., 2002; Zhou et al., 2002), and also in contrast to AR, ESRs are constitutively expressed after castration (Oliveira et al., 2004). Although we have known since the 1980s that vitamin D and 17-β estradiol show intense nuclear labeling in epithelia of the efferent ductules and epididymal initial segment (Schleicher et al., 1984; Schleicher et al., 1989), only recent studies have begun to link the expression of their receptors with physiological functions in the upper male reproductive tract (Blomberg Jensen et al., 2010; Dornas et al., 2007; Hess et al., 2011; Mahmoudi et al., 2013; Oliveira et al., 2008; Oliveira & Oliveira, 2011). The lack of ESR1 or treatment with an antiestrogen disrupts efferent ductule development, alters epithelial morphology, and inhibits fluid reabsorption in the adult epithelium (Hess et al., 1997; Hess et al., 2001; Joseph et al., 2011). However, the balance of ESR1 and AR appears to be important, as some genes expressed in efferent ductules contain response elements for both receptors (Trepos-Pouplard et al., 2010). For example, Slc9a3 mRNA is reduced nearly 6-fold in ESR1 -/- mice and in mice treated with an antiestrogen compound (Joseph et al., 2010; Zhou et al., 2001), but following castration testosterone treatment alone increases the Slc9a3 message (Snyder et al., 2009).
Several studies have investigated the hamster efferent ductules (Flickinger et al., 1978; Montorzi & Labiano, 1970; Nagy, 1990; Vicentini et al., 1990; Yokoyama & Chang, 1971); however, gross morphological and immunohistochemical evaluations are lacking. An earlier study examined the ultrastructure of ciliated and nonciliated epithelial cells in the Chinese hamster efferent ducts (Yokoyama & Chang, 1971), but they did not distinguish between proximal and distal regions, which makes the study difficult to interpret. In the current study, efferent ducts were found to exit the hamster testis in a uniquely polarized manner from the cephalic pole and other unique observations were found, such as glycogen granules in the proximal ductules. Immunohistochemistry of the efferent ductule epithelium was compared to the caput epididymis. Numerous nuclear steroid receptors were present simultaneously in the epithelium of the efferent ductules, but some also displayed cytoplasmic staining.
Materials and Methods
Animals and Experimental Design
A total of 24 adult Golden Syrian hamsters were used in this study. The hamsters were maintained under a temperature of 22°C and a circadian light cycle of 16 hours light and 8 hours of darkness. Animals were fed ad libitum (Teklad Chow; Harlan Teklad, Madison, WI) with unlimited access to tap water. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Illinois and were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Gross Morphology
Sixteen hamsters used for gross morphology were deeply anesthetized by intraperitoneal injection of Sleepaway (0.1 ml; 26% sodium pentobarbital, 7.8% isopropyl alcohol; Fort Dodge Animal Health, Fort Dodge, IA). A midventral incision was made from the sternum to the umbilicus to expose the testes and epididymides, which were removed and incubated in calcium- and magnesium-free Hank’s Balanced Salt Solution (HBSS-CMF).
Excised tissues were dissected using a stereomicroscope and jeweler’s forceps, first removing the fat and connective tissue covering the reproductive tract. The efferent ductules were cut at the rete testis junction and the initial segment epididymis. Efferent ductules were transferred to a Petri dish containing HBSS-CMF and the remaining fat and connective tissue was removed. The partially dissected ductules were then placed in HBSS-CMF containing 1% trypsin-EDTA (Gibco – Invitrogen Corporation, Grand Island, NY) for 30 minutes at 4°C, followed by three washes in HBSS-CMF. Individual ductules were exposed further by dissection with forceps and iridectomy scissors, until the entire length of all ductules was revealed. The isolated ductules were fixed in 4% gluteraldehyde in 0.135 M phosphate buffer (pH 7.4). Digital images were obtained of the reproductive tracts and the efferent ductules using a SPOT Cooled Color Digital Camera (Diagnostic Instruments, Inc., Sterling Heights, MI).
Histology and electron microscopy
The male reproductive tract was fixed via vascular perfusion (Hess & Moore, 1993) and processed for histological, morphometric or immunohistochemical analysis (Guttroff et al., 1992; Oliveira et al., 2005). For bright-field light microscopy (morphometry) and transmission electron microscopy, 5 males were perfused with 4 % glutaraldehyde in 0.135 M phosphate buffer. For electron microscopy, glutaraldehyde fixed tissues were postfixed in 1% osmium tetroxide containing 1.5% potassium ferrocyanide and processed for epoxy embedment (Hess et al., 2000). Ultrathin sections were prepared and imaged with a Hitachi H600 electron microscope. For immunohistochemistry, 3 males were perfused with cold neutral buffered formalin (Zhou et al., 2002). Brightfield photomicrographs were imaged with a BX51 Olympus microscope (Olympus Corp., Melville, NY, Center Valley, PA, www.olympusamerica.com) and planapochromatic lenses.
Morphometry
Efferent ductules were dissected away from the surrounding tissue, dehydrated with ethanol, embedded in glycol methacrylate, sectioned at 2.5-μm thickness, stained with periodic acid-Schiff (PAS), counterstained with Mayer’s hematoxylin, and mounted for observation. To determine epithelial heights, images were captured using an Olympus BX51 microscope (Olympus, Melville, NY) and a digital camera (ProgRes C14, Jenoptik L.O.S. GmbH, Germany). Images were compiled using Adobe Photoshop (Adobe Systems, San Jose, CA) and analyzed with image J (version 1.29x, National Institutes of Health, U.S.A.). Five epithelial cell heights were determined in five distinct fields per hamster and reported as the mean of 25 measurements per male.
Immunohistochemistry
The efferent ductules and caput epididymidis were fixed with neutral buffered formalin and processed as for morphometry, but sectioned at 5-μm thickness, stained with the appropriate antibody, counterstained with Mayer’s hematoxylin (Sigma Diagnostics, St. Louis, MO) and mounted for observation. Immunohistochemical staining was performed for sodium/hydrogen exchanger-3 (NHE3; SLC9A3), aquaporins 1 and 9 (AQP1, AQP9), androgen receptor (AR), estrogen receptor-alpha (ESR1), estrogen receptor-beta (ESR2) and vitamin D receptor (VDR). Similar procedures were followed as previously reported (Oliveira et al., 2008; Oliveira et al., 2005; Zhou et al., 2002) (Picciarelli-Lima et al., 2006; Ruz et al., 2006; Zhou et al., 2001). All tissues were blocked in 10% normal goat serum and for ESR1 and AR the slides were exposed to avidin-biotin blocking solutions. Incubation of the primary antibodies was performed overnight (4°C). Primary antibody included the following: rabbit anti-rat NHE3 (SLC9A3; Chemicon International, Temecula, CA) diluted 1:1000; rabbit anti-rat AQP1 and AQP9 (Alpha Diagnostic International, San Antonio, TX), diluted 1:1000; mouse anti-human ESR1 (NCL-L-ER-6F11; Novocastra Laboratories LTD, Newcastle upon Tyne, UK) diluted 1:100; rabbit anti-rat ESR2 (PA1-310; Affinity BioReagents, Golden, CO) diluted 1:50; rabbit anti-rat AR (PG21-40, kindly provided by Dr. Gail Prins, University of Chicago, IL) diluted 1:300; rat anti-human VDR (MA1-710; Affinity BioReagents, Golden, CO) diluted 1:150. Tissues were then incubated with secondary antibodies at room temperature for one hour and antibody binding was visualized using the avidin-biotin complex (ABC Kit, Vector Laboratories, Burlingame, CA) and diaminobenzidine (DAB; Sigma Diagnostics, St. Louis, MO) and counterstained with Mayer’s hematoxylin. Antibody competition was previously performed for AR and ESR1 (Zhou et al., 2002). Negative controls were obtained by staining without the primary antibodies. Stained sections were visualized and images captured using an Olympus microscope model BX51 (Olympus, Melville, NY) and the ProgRes C14 digital camera (Jenoptik L.O.S. GmbH, Germany).
Results
Gross Morphology
In the Golden Syrian hamster, excurrent ducts were polarized, as the rete testis and attached efferent ductules exited the testis at the apex or cephalic pole (Fig. 1). There were three regions of efferent ductules, proximal nearest the rete testis, conus where the ductules merged and the common or single ductule that entered the capsule of the epididymis. The smaller efferent ductules were surrounded by adipose tissue and connected to a larger caput epididymidis. The caput region then formed a much thinner corpus, which flowed into a rather large cauda epididymidis (Fig. 1A). The number of efferent ducts varied from 3 to 10 with an average of 6.0 ±0.4 (Table 1). Blind ending ductules were present in approximately 56 % of the hamsters examined, and if present there was on average 1.3 ±0.2 blind-ending ductules per male.
Figure 1.
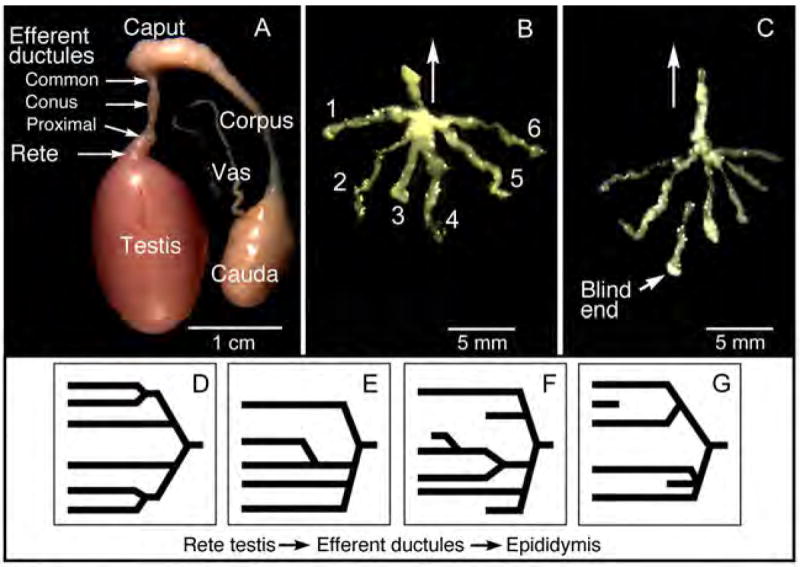
A) Gross morphology of the male hamster reproductive tract after dissection to remove surrounding connective tissues and fat. The rete testis exits under the capsule at the apex or cephalic pole of the testis, connecting to thin, delicate efferent ductules. Efferent ductules connect to the expanded caput epididymidis that connects to a very thin corpus and then a very large cauda epididymidis. A thin vas deferens extends from the cauda. B) Microdissection revealed six individual efferent ductules that merge into a common duct that flows toward the epididymis (arrow). The number of ductules ranged from 3 to 10 ducts per testis. C) In this sample, microdissection revealed a blind ending efferent ductule in the region nearest the rete testis. Arrow indicates direction of epididymis. (D-G) These drawings represent 4 different patterns observed of efferent ductules merging into a common duct.
Table 1.
The number of efferent ductules in Golden Syrian hamstera
| Mean | Minimum | Maximum | |
|---|---|---|---|
| Total Ducts | 6.0 | 3 | 10 |
| Normal Ducts | 5.4 | 3 | 10 |
| Blind Ending Ducts | 0.6 | 0 | 3 |
16 hamsters were used in the analysis, with 26 reproductive tracts successfully dissected
A great variety of branching patterns for the efferent ductules were observed (Fig. 1). The ductules were highly convoluted in the conus region, but eventually converged into one small, highly convoluted common duct that rapidly transitioned into the initial segment of the caput epididymis. Although in a few animals the efferent ductules had a symmetrical pattern of branching, most branching exhibited an asymmetrical alignment.
Light and Electron Microscopy
The proximal efferent ductule began abruptly after the rete testis exited the testicular capsule. The squamous or low cuboidal epithelium of the rete testis suddenly changed into a columnar epithelium with ciliated and nonciliated cells lining the proximal ducts (Fig. 2). The proximal ductules had a wide lumen that became progressively smaller in diameter in the common duct. Luminal sperm concentration was dilute in the proximal region, while the common duct contained a much higher concentration. In proximal regions, the epithelium was 35% taller than that of the common ductule (Table 2), but common and conus ductal epithelia contained PAS+ lysosomal-like granules that were more numerous and larger in size than in the proximal region (Figs. 2D-I). In the proximal epithelium, occasional groups of cells contained lipid-like droplets in the basal cytoplasm (Fig. 2G), similar to that reported in the rat (Ilio & Hess, 1994). Additionally, the proximal epithelium was unique in that small groups of cells near the rete testis junction, both ciliated and nonciliated, contained an abundance of glycogen granules that were PAS+ and electron dense (Fig. 2B-C, D).
Figure 2.
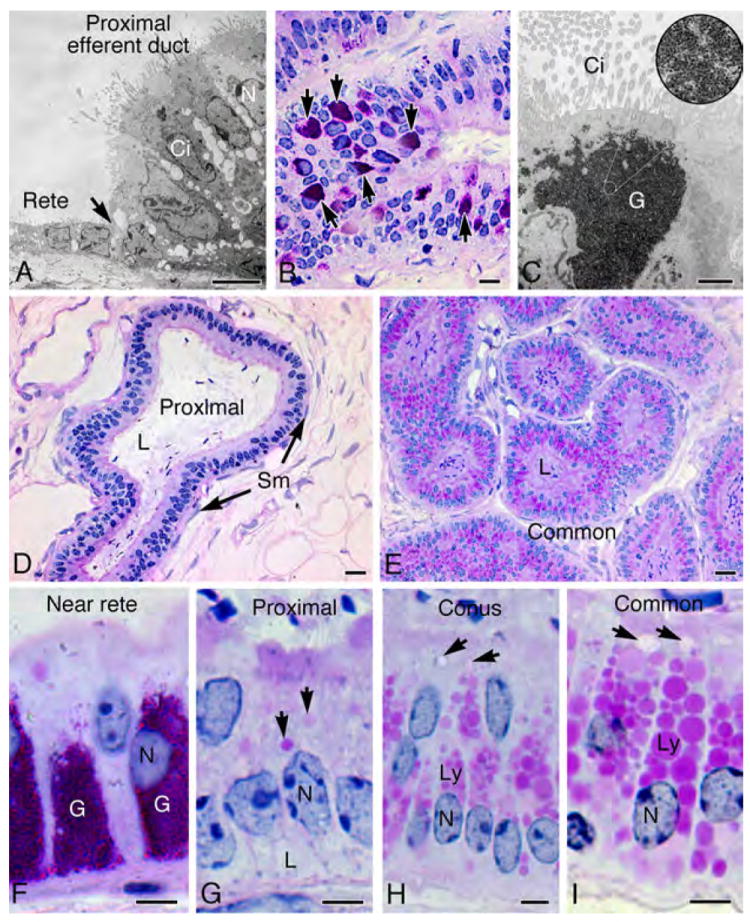
Morphology of efferent ductules in the Golden Syrian hamster. A) Transmission electron microscopy (TEM) of the epithelial junction (arrow) between rete testis and proximal efferent ductule shows cilia projecting into the lumen from the ciliated cell (Ci), while microvilli extend from the surface of the nonciliated cell (N). B) Proximal efferent ductule epithelium near the rete testis contained aggregates of PAS+ granules (arrows). C) TEM of a ciliated cell (Ci) shows aggregates of glycogen (G). At higher magnification in the circular inset photo, the rosettes or glycogen granules are resolved. D) The proximal efferent ductule has a wider lumen (L) than the more distal ducts. E) The common efferent ductule has a narrow lumen (L) and the epithelium contains numerous PAS+ lysosomal granules in the apical cytoplasm. F) Higher magnification of proximal efferent ductal epithelium near the rete testis illustrates an abundance of glycogen (PAS+) granules (G). N, nucleus. G) An area of proximal efferent ductule illustrates epithelial cells with smaller endosomes (arrows) in the apical cytoplasm and lipid-like vacuoles (shown by TEM in Fig. 3 to be large intercellular spaces) in the basal cytoplasm (L). N, nucleus. H) In the conus efferent ductules, nonciliated cells have an abundance of large lysosomal granules (Ly) in the apical cytoplasm and endosomes (arrows). N, nucleus. I) Nonciliated cells in the common efferent duct contain the largest and most numerous lysosomal granules (Ly) and also larger endosomes (arrows). N, nucleus. Bars for A, F-I=5μm; Bar for C=2 μm; Bars for B, D, E=20μm.
Table 2.
Morphometric analysis of efferent ductule epithelium in the Golden Syrian hamster
| Proximal Ductule | Common Ductule | |
|---|---|---|
| Epithelial Cell Height, (μm) | 43.6 ± 1.4* | 32.4 ± 2.5 |
| Number of epithelial Cells / 100 μm | 21.9 ± 0.6 * | 18.2 ± 0.5 |
P<0.01, comparing means from the proximal versus the common ductules(N=5).
Ultrastructure of the proximal efferent duct epithelium revealed prominent intercellular spaces, primarily in the basolateral regions (Fig. 3A-B). The apical cytoplasm of proximal nonciliated cells also had a few endosomes and lysosomes, consistent with light microscopy (Fig. 2), but an abundance of tubular coated pits originating between microvilli at the luminal surface, along with an apical tubular system (Fig. 3C). An occasional intraepithelial lymphocyte-like cell was seen resting near the basement membrane. These did not appear to be epithelial basal cells because their plasmalemma did not appear attached to the basal lamina (Fig. 3B).
Figure 3.
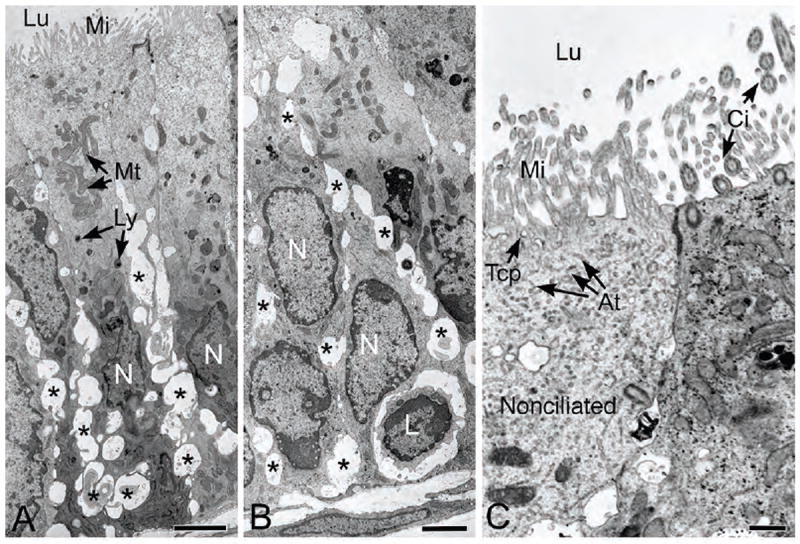
Transmission electron microscopy of the proximal efferent ductule epithelium. A-B) Numerous basolateral dilations (*) are present in spaces between the epithelial cells. The apical cytoplasm shows numerous mitochondria (Mt) and several small lysosomal-like granules (Ly). The nuclei (N) show considerable variation in shape. One small, lymphocyte-appearing cell (L) is noted near the basement membrane. Lu, lumen; Mi, microvilli. C) Higher magnification of the nonciliated cell (N) reveals initial components of the endocytic apparatus, tubular coated pits (Tcp) and apical tubules (At) beneath a prominent microvillus border (Mi) that lines the lumen (Lu). To the right of the nonciliated cell, cilia (Ci) extend into the lumen from a ciliated cell. Bars for A, B=2.5μm; Bar for C=0.5μm.
Immunohistochemistry
Some major proteins that are involved in the reabsorption of luminal fluid were localized by immunohistochemistry in the efferent ductules. Caput epididymal epithelium was stained for comparison (Table 3). NHE3 has the primary responsibility for Na+/H+ exchange at the luminal surface and thereby mediates autoregulated fluid reabsorption (Clulow et al., 1998). NHE3 was present on the apical border of nonciliated cells of the efferent ductules, but absent in the ciliated cells (Fig. 4A-C). Staining intensity for NHE3 was higher in the proximal compared to the distal (conus and common) efferent ductules. In contrast, caput epididymidis epithelium was negative. AQP1 staining was intense among the microvilli and cilia of the proximal and distal efferent ducts; however, in the proximal region, but not distal, cytoplasmic staining was observed, as well as basolateral membranes (Fig. 4D-F). AQP9 staining of efferent ductules was more intense on microvilli of nonciliated cells in the proximal than in the distal region and cilia appeared to be negative (Fig. 4G-I). In caput epididymidis, AQP1 was negative, except for intense staining of the blood vessels; however, AQP9 stained intensely the entire layer of long microvilli (Fig. 4F, I).
Table 3.
Histological and immunohistochemical features of efferent ductules and caput epididymidis epithelium in the Golden Syrian hamster1
| Location in Reproductive Tract | |||
|---|---|---|---|
| Cellular Feature | Proximal Efferent Ductule | Distal Efferent Ductule2 | Caput Epididymidis3 |
| Glycogen | +/- | - | - |
| PAS+ lysosomes | + | +++ | - |
| NHE3 | ++ | ++ | - |
| AQP1 | +++ | ++ | - |
| AQP9 | ++ | ++ | +++ |
| AR | +++ | +++ | +++ |
| ESR1 | +++ | +++ | -/+ |
| ESR2 | ++ | ++ | ++ |
| VDR | ++/- | ++/- | ++ |
Staining intensity scores were as follows: Staining intensity scores were as follows: -, negative; +, weak staining; ++, moderate staining; +++, strong staining; +/-, some cells were negative; -/+, some cells were positive
Conus and common efferent ductules
Photos are shown only for the immunohistochemistry
Figure 4.
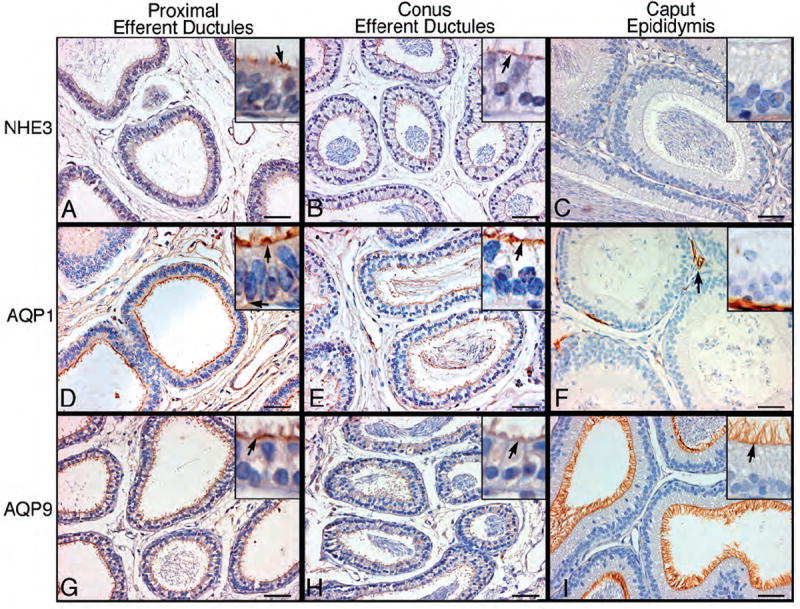
Immunohistochemistry for sodium/hydrogen exchanger-3 (NHE3), aquaporins-1 (AQP1) and -9 (AQP9) in proximal and conus efferent ductules and caput epididymidis. Arrows point to strongly positive areas of staining. A-C) NHE3 staining was intense on the apical border of nonciliated cells of the proximal efferent ductules, but absent in the ciliated cells. Conus efferent ducts show less intense staining for NHE3, but remain specific for nonciliated cells. Caput epididymidis epithelium was negative for NHE3. D-F) AQP1 staining was intense among the microvilli and cilia of the proximal efferent ducts. Cytoplasmic staining was also observed, as well as along the basolateral membranes. AQP1 staining in the conus region was intense along the microvillus and ciliary borders, but cytoplasmic staining was absent. In the caput epididymidis, AQP1 staining was observed only in capillaries. G-I) AQP9 staining was intense among the microvilli and cilia of the proximal efferent ducts. In the conus ductules, AQP9 showed less intense staining compared to the proximal region. In the caput epididymidis, AQP9 staining was intense along the entire layer of the long microvilli. Bars=50 μm.
AR immunostaining was strong in nuclei of ciliated and nonciliated epithelial cells of proximal and distal (conus and common) efferent ductules and in all epithelial cells of the caput epididymis (Fig. 5A-C). Nuclear ESR1 staining was equally intense in all regions of the efferent ductules, but there appeared to be a low level of staining in cytoplasm of epithelial cells in the proximal ductules. In contrast, apical cell nuclei of the caput epididymidis were negative for ESR1, while principal and basal cells showed occasional low levels of immunostaining (Fig. 5D-F). ESR2 staining was equally distributed among epithelial cell nuclei of all regions of the efferent ductules and the caput epididymidis, but with less intensity in the efferent ducts compared to ESR1 (Fig. 5G-I). VDR staining in efferent ductule epithelium was less consistent than for the other steroid hormone receptors, but staining was observed in all epithelial cell nuclei of the caput epididymidis (Fig. 5J-L). In efferent ducts, most ciliated cells were negative for VDR, while most nonciliated cells were positive.
Figure 5.
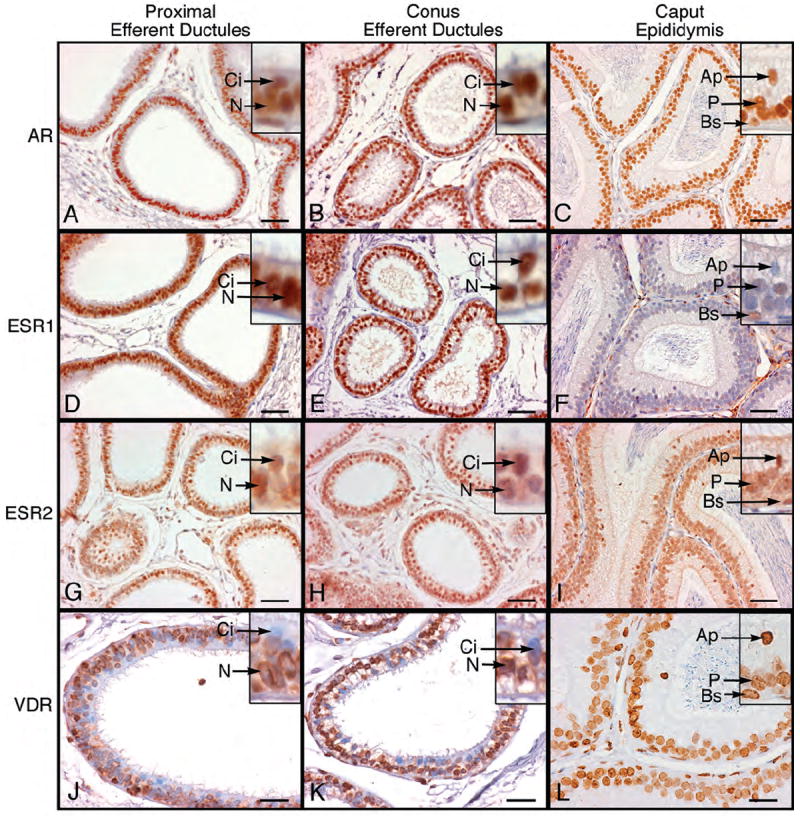
Immunohistochemistry for androgen receptor (AR), estrogen receptor-α (ESR1), estrogen receptor-β (ESR2) and vitamin D3 receptor (VDR) in proximal and conus efferent ductules and caput epididymidis. A-C) AR was localized as intense nuclear staining in ciliated (Ci) and nonciliated (N) epithelial cells of the efferent ductules and in apical (Ap), principal (P) and basal (Bs) epithelial cells of caput epididymidis. D-F) ESR1 appeared as intense nuclear staining in ciliated (Ci) and nonciliated (N) epithelial cells of the efferent ductules. The cytoplasm also showed immunostaining as well. In caput epididymidis the apical nuclei (Ap) were negative and principal cells (P) showed occasional weak staining, while basal cell nuclei (Bs) had moderate staining. G-I) ESR2 stained ubiquitously all nuclei throughout the efferent ductules and caput epididymidis. Ci, ciliated; N, nonciliated; Ap, apical; P, principal; Bs, basal. J-L) VDR staining was moderate to intense in nuclei of nonciliated (N) epithelial cells of the proximal and conus efferent ductules and in cytoplasm of the proximal ductules. In the caput epididymidis, apical (Ap), principal (P) and basal cells (Bs) were positive for VDR. Bars for A-I=50 μm; Bars for J-L=25 μm.
Discussion
This study in the Golden Syrian hamster revealed a rather unusual anatomical arrangement of the rete testis/efferent ductules, as the ductules exited from the apex or cephalic end of the testicular capsule. Efferent ducts in the mouse and rat exit the testis eccentrically or off-center (Ilio & Hess, 1994), but never directly from the apex. The rete testis gave rise to an average of 6 efferent ductules per male reproductive tract, similar to the rat (Guttroff et al., 1992), with a minimum of 3 ducts and a maximum of 10 being observed. Others have reported an average of 5 ductules in the hamster (Mason & Shaver, 1952; Montorzi & Burgos, 1967; Montorzi & Labiano, 1970). Also similar to rodents, blind ending efferent ductules were common in the hamster. More than half of the hamsters examined had at least one blind ending efferent duct, which results from abnormal development of the ductules from the mesonephros (Guttroff et al., 1992; Ilio & Hess, 1994).
In the conus region, the efferent ductules began to merge into a single duct, following patterns of considerable variation. Efferent ductules enter the epididymis using one of only two basic designs; either as a single ductule that collects rete testis fluid in a funnel-like manner through the anastomosis of the efferent ductules, as seen in smaller mammals (Hess, 2002) oras parallel coils of efferent ductules that form multiple entries into the head of the epididymis, as observed in larger mammals (Hemeida et al., 1978; Ilio & Hess, 1994). Efferent ductules of the hamster merge into a single duct, similar to other rodents (Guttroff et al., 1992).
Efferent ductules are a key participant in the transportation of spermatozoa from testis to the epididymis. However, their major function is the reabsorption of over 90% of the testicular fluid entering their lumen (Clulow et al., 1994; Clulow et al., 1998; Hansen et al., 2004), at a rate that is greater than in the proximal convoluted tubules of the kidney (Jones & Jurd, 1987). Reabsorption is dependent upon the following: (a) Na+/H+ exchange and the expression of an apical NHE3 antiport (Clulow et al., 1998; Hansen et al., 1999; Hess et al., 1997; Zhou et al., 2001); (b) active endocytosis (Hermo et al., 1985; Hermo & Morales, 1984; Hermo et al., 1988; Hess, 2002); and (c) expression of water channels (Hermo et al., 2004; Hermo & Smith, 2011; Oliveira et al., 2005; Ruz et al., 2006). Morphological features of hamster efferent ductal epithelium (Table 3) are consistent with this major function, as components of an active endocytic apparatus (Hermo et al., 1985; Hermo et al., 1988) were abundant in the apical cytoplasm of nonciliated cells, similar to a previous report in the Chinese hamster (Yokoyama & Chang, 1971). NHE3, AQP1 and AQP9 were prominent along the microvillus border of efferent ductules. In contrast, only AQP9 was found lining the lumen of the caput epididymidis.
A thorough review of APQs in the male reproductive tract has been published (Badran & Hermo, 2002; Hermo & Smith, 2011). In the rat and mouse efferent ductules, AQP-1 was present on microvilli of nonciliated cells but also on cilia of ciliated cells, while absent from the epididymal epithelium (Badran & Hermo, 2002; Ruz et al., 2006), similar to that seen in the hamster. Its presence on ductal cilia is not understood, but recent studies have linked the sensory function of the cilium with cell signaling pathways and the expression of AQPs in kidney tubules (Marion et al., 2011; Saigusa et al., 2012). In nonciliated cells, AQP-1 was also found along the basolateral plasmalemma (Badran & Hermo, 2002; Hermo & Smith, 2011), where Na+/K+-ATPase (Ilio & Hess, 1992) would couple its presence to allow for rapid movement of water into expanded intercellular channels along basolateral regions of the epithelium (Jones & Jurd, 1987). AQP-1 was also present in the apical cytoplasm of efferent ducts, consistent with that reported on the membranes of endosomes in rats and mice (Badran & Hermo, 2002). AQP-9 staining in the hamster was similar to that seen in other species, with exclusive staining of microvilli and more intensity in the caput epididymis (Badran & Hermo, 2002; Hermo et al., 2008; Hermo & Smith, 2011; Oliveira et al., 2005).
Since the mid 1990’s, it has been well recognized that, in addition to androgens acting through their nuclear receptor (AR) to regulate male reproductive tract function (Robaire & Hamzeh, 2011; Robaire & Viger, 1995), estrogen and its receptor (ESR1) also have a major role, especially in the regulation of fluid reabsorption in the efferent ductules (Hess et al., 1997). In the hamster, nuclear localizations of AR and ESR1 were intense in the epithelium that lined all regions of the efferent ductules, which is similar to other rodents (Joseph et al., 2011; Oliveira et al., 2004; Zhou et al., 2002). In contrast, caput epididymal epithelium was stained extensively with AR but showed a very weak staining for ESR1 in some principal and basal cells, as previously reported (Joseph et al., 2011). ESR1 staining in the epididymis shows tremendous species variability (Hess et al., 2011; Joseph et al., 2011), with more recent studies supporting the hypothesis that cytoplasmic and apical membrane receptors are also present and contributing to efferent ductule function (Hess et al., 2011; Sinkevicius et al., 2008; Weiss et al., 2008). Hamster efferent ductal epithelium also showed moderate cytoplasmic staining for ESR1, which in the past may have been ignored, but based upon newer studies (Hess et al., 2011) should receive special attention in the future. As in other species (Hess, 2003; Hess et al., 2011), ESR2 immunostaining was also present in all epithelial cells in these ducts of the hamster, as in all species studied to date (Hess & Carnes, 2004).
A link between estrogen and vitamin D has been demonstrated in various animal models (Iguchi et al., 1999; Kinuta et al., 2000; Liel et al., 1999); therefore, the discovery of high concentrations of ESR1 in efferent ductules (Hess et al., 1997; Oliveira et al., 2004) led to the investigation of VDR in this region of the male reproductive tract. Although there are only a few studies reporting VDR in the epididymis (Blomberg Jensen et al., 2013; Blomberg Jensen et al., 2010; Dornas et al., 2007; Schleicher et al., 1989), its expression in efferent ductules and epididymis is similar across species, including the hamster. In the rooster, epididymal lithiasis was associated with increases in the level of 17β-estradiol and expression of ESR2, but decreases in vitamin D3, testosterone and ESR1 (Mahecha et al., 2002; Oliveira et al., 2011). The role of hormonal imbalances and VDR expression in calcium homeostasis in the male reproductive tract needs to be investigated, as testicular microlithiasis has been observed in men (Menchinelli et al., 1999; Sakamoto et al., 2006; Serter et al., 2006; Thomas et al., 2000), but potential involvement of the efferent ductules and epididymis were never examined.
Finally, the proximal efferent ductules of the hamster exhibited an unusual abundance of glycogen in the basal cytoplasm of small groups of epithelial cells, always near the rete testis junction. Glycogen aggregates are rarely found in normal ductal epithelia. However, they have been reported in efferent ductules of the common shrew and guinea pig, and as in hamsters, the glycogen-rich cells were always located only in the rete testis area (Fawcett & Dym, 1974; Suzuki & Racey, 1984). Glycogen accumulations have been noted in epithelia of mesonephric tubules and ductules (Martino & Zamboni, 1966; Pelliniemi et al., 1983; Tiedemann, 1971) and are common in developmental tissues (Winkler & Wille, 1998). Its role in normal efferent ductule tissue is not known, but its presence in this species may be associated with the potential for hibernation in hamsters and subsequent reproductive tract regression and recrudescence. In the ESR1 knockout and antiestrogen-treated mice, glycogen has been associated with abnormal morphology of the efferent ductule epithelium, which exhibited underdevelopment or regression (Hess et al., 2000; Lee et al., 2000). Others have reported glycogen in neoplastic cells of epididymis and in tumors of the rete testis (Jones et al., 1997; Kimura et al., 1998; Torikata, 1994).
In conclusion, the hamster excurrent ducts of the testis exhibited a unique positional exit from the testicular capsule at the apex, compared to medial to off-center in most other species. The efferent ductules were similar to other rodents, except for two morphological features: glycogen granules accumulated in the basal cytoplasm of some proximal epithelial cells and nonciliated epithelial cells of the conus and common ducts contain very large PAS+ lysosomal granules. The presence of NHE3 and aquaporins was consistent with fluid reabsorption activity of the efferent ductules. Nuclear steroid receptors were prominent in the efferent ductules, as in other species, but the caput epididymis lacked ESR1 staining in most epithelial cells.
Acknowledgments
Support was provided by NIEHS Training Grant NIH T32 ES07326 and subproject [CIG-02-76] provided by CICCR, a program of CONRAD, Eastern Virginia Medical School. The views expressed by the authors do not necessarily reflect the views of CONRAD or CICCR.
Contributor Information
James Ford, Jr., Dept. of Comparative Biosciences, University of Illinois, Urbana, IL 61802-6199.
Kay Carnes, Dept. of Comparative Biosciences, University of Illinois, Urbana, IL 61802-6199.
Rex A. Hess, Dept. of Comparative Biosciences, University of Illinois, Urbana, IL 61802-6199
References Cited
- Badran HH, Hermo LS. Expression and regulation of aquaporins 1, 8, and 9 in the testis, efferent ducts, and epididymis of adult rats and during postnatal development. J Androl. 2002;23:358–373. [PubMed] [Google Scholar]
- Blomberg Jensen M, Lieben L, Nielsen JE, Willems A, Jorgensen A, Juul A, et al. Characterization of the testicular, epididymal and endocrine phenotypes in the Leuven Vdr-deficient mouse model: Targeting estrogen signalling. Mol Cell Endocrinol. 2013 doi: 10.1016/j.mce.2013.06.036. [DOI] [PubMed] [Google Scholar]
- Blomberg Jensen M, Nielsen JE, Jorgensen A, Rajpert-De Meyts E, Kristensen DM, Jorgensen N, et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod. 2010;25:1303–1311. doi: 10.1093/humrep/deq024. [DOI] [PubMed] [Google Scholar]
- Clulow J, Jones RC, Hansen LA. Micropuncture and cannulation studies of fluid composition and transport in the ductuli efferentes testis of the rat: comparisons with the homologous metanephric proximal tubule. Exp Physiol. 1994;79:915–928. doi: 10.1113/expphysiol.1994.sp003817. [DOI] [PubMed] [Google Scholar]
- Clulow J, Jones RC, Hansen LA, Man SY. Fluid and electrolyte reabsorption in the ductuli efferentes testis. J Reprod Fertil Suppl. 1998;53:1–14. [PubMed] [Google Scholar]
- Dornas RA, Oliveira AG, Kalapothakis E, Hess RA, Mahecha GA, Oliveira CA. Distribution of vitamin D3 receptor in the epididymal region of roosters (Gallus domesticus) is cell and segment specific. Gen Comp Endocrinol. 2007;150:414–418. doi: 10.1016/j.ygcen.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Dym M. A glycogen-rich segment of the tubuli recti and proximal portion of the rete testis in the guinea-pig. J Reprod Fertil. 1974;38:401–409. doi: 10.1530/jrf.0.0380401. [DOI] [PubMed] [Google Scholar]
- Flickinger JA, Howards SS, English HF. Ultrastructural differences in the efferent ducts and several regions of the epididymis of the hamster. Am J Anat. 1978;152:557–586. doi: 10.1002/aja.1001520409. [DOI] [PubMed] [Google Scholar]
- Goyal HO, Williams CS. The ductuli efferentes of the goat: a morphological study. Anat Rec. 1988;220:58–67. doi: 10.1002/ar.1092200108. [DOI] [PubMed] [Google Scholar]
- Guerrero SM, Calderon ML, de Perez GR, Ramirez Pinilla MP. Morphology of the male reproductive duct system of Caiman crocodilus (Crocodylia, Alligatoridae) Ann Anat. 2004;186:235–245. doi: 10.1016/s0940-9602(04)80009-8. [DOI] [PubMed] [Google Scholar]
- Guttroff RF, Cooke PS, Hess RA. Blind-ending tubules and branching patterns of the rat ductuli efferentes. Anat Rec. 1992;232:423–431. doi: 10.1002/ar.1092320311. [DOI] [PubMed] [Google Scholar]
- Hansen LA, Clulow J, Jones RC. The role of Na+-H+ exchange in fluid and solute transport in the rat efferent ducts. Exp Physiol. 1999;84:521–527. [PubMed] [Google Scholar]
- Hansen LA, Dacheux F, Man SY, Clulow J, Jones RC. Fluid reabsorption by the ductuli efferentes testis of the rat is dependent on both sodium and chlorine. Biol Reprod. 2004;71:410–416. doi: 10.1095/biolreprod.104.027490. [DOI] [PubMed] [Google Scholar]
- Hemeida NA, Sack WO, McEntee K. Ductuli efferentes in the epididymis of boar, goat, ram, bull, and stallion. Am J Vet Res. 1978;39:1892–1900. [PubMed] [Google Scholar]
- Hermo L, Clermont Y, Morales C. Fluid-phase and adsorptive endocytosis in ciliated epithelial cells of the rat ductuli efferentes. Anat Rec. 1985;211:285–294. doi: 10.1002/ar.1092110309. [DOI] [PubMed] [Google Scholar]
- Hermo L, Krzeczunowicz D, Ruz R. Cell specificity of aquaporins 0, 3, and 10 expressed in the testis, efferent ducts, and epididymis of adult rats. J Androl. 2004;25:494–505. doi: 10.1002/j.1939-4640.2004.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Hermo L, Morales C. Endocytosis in nonciliated epithelial cells of the ductuli efferentes in the rat. Am J Anat. 1984;171:59–74. doi: 10.1002/aja.1001710106. [DOI] [PubMed] [Google Scholar]
- Hermo L, Schellenberg M, Liu LY, Dayanandan B, Zhang T, Mandato CA, et al. Membrane domain specificity in the spatial distribution of aquaporins 5, 7, 9, and 11 in efferent ducts and epididymis of rats. J Histochem Cytochem. 2008;56:1121–1135. doi: 10.1369/jhc.2008.951947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermo L, Smith CE. Thirsty business: cell, region, and membrane specificity of aquaporins in the testis, efferent ducts, and epididymis and factors regulating their expression. J Androl. 2011;32:565–575. doi: 10.2164/jandrol.110.012831. [DOI] [PubMed] [Google Scholar]
- Hermo L, Spier N, Nadler NJ. Role of apical tubules in endocytosis in nonciliated cells of the ductuli efferentes of the rat: a kinetic analysis. Am J Anat. 1988;182:107–119. doi: 10.1002/aja.1001820202. [DOI] [PubMed] [Google Scholar]
- Hernandez-Franyutti A, Uribe MC. Seasonal spermatogenic cycle and morphology of germ cells in the viviparous lizard Mabuya brachypoda (Squamata, Scincidae) J Morphol. 2012 doi: 10.1002/jmor.20050. [DOI] [PubMed] [Google Scholar]
- Hess RA. The Efferent Ductules: Structure and Functions. In: Robaire B, Hinton B, editors. The Epididymis: from Molecules to Clinical Practice. Kluwer Academic/Plenum Publishers; New York: 2002. pp. 49–80. [Google Scholar]
- Hess RA. Estrogen in the adult male reproductive tract: A review. Reprod Biol Endocrinol. 2003;1:52. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, et al. A role for oestrogens in the male reproductive system. Nature. 1997;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lubahn DB, Zhou Q, Bouma J. Morphologic changes in efferent ductules and epididymis in estrogen receptor-alpha knockout mice. J Androl. 2000;21:107–121. [PubMed] [Google Scholar]
- Hess RA, Carnes K. The role of estrogen in testis and the male reproductive tract: a review and species comparison. Anim Reprod. 2004;1:5–30. [Google Scholar]
- Hess RA, Fernandes SA, Gomes GR, Oliveira CA, Lazari MF, Porto CS. Estrogen and its receptors in efferent ductules and epididymis. J Androl. 2011;32:600–613. doi: 10.2164/jandrol.110.012872. [DOI] [PubMed] [Google Scholar]
- Hess RA, Gist DH, Bunick D, Lubahn DB, Farrell A, Bahr J, et al. Estrogen receptor (alpha and beta) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl. 1997;18:602–611. [PubMed] [Google Scholar]
- Hess RA, Moore BJ. Histological Methods for the Evaluation of the Testis. In: Chapin RE, Heindel JJ, editors. Methods in Reproductive Toxicology. Academic Press; San Diego, CA: 1993. pp. 52–85. [Google Scholar]
- Hess RA, Thurston RJ, Biellier HV. Morphology of the epididymal region and ductus deferens of the turkey (Meleagris gallopavo) J Anat. 1976;122:241–252. [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Zhou Q, Nie R. The Role of Estrogens in the Endocrine and Paracrine Regulation of the Efferent Ductules, Epididymis and Vas deferens. In: Robaire B, Hinton BT, editors. The Epididymis: from Molecules to Clinical Practice. Kluwer Academic/Plenum Publishers; New York: 2002. pp. 317–338. [Google Scholar]
- Hess RA, Zhou Q, Nie R, Oliveira C, Cho H, Nakai M, et al. Estrogens and epididymal function. Reproduction Fertility and Development. 2001;13:273–283. doi: 10.1071/rd00100. [DOI] [PubMed] [Google Scholar]
- Holmes HJ, Gist DH. Excurrent duct system of the male turtle Chrysemys picta. J Morphol. 2004;261:312–322. doi: 10.1002/jmor.10251. [DOI] [PubMed] [Google Scholar]
- Igdoura SA, Hermo L, Rosenthal A, Morales CR. Nonciliated cells of the rat efferent ducts endocytose testicular sulfated glycoprotein-1 (SGP-1) and synthesize SGP-1 derived saposins. Anat Rec. 1993;235:411–424. doi: 10.1002/ar.1092350310. [DOI] [PubMed] [Google Scholar]
- Iguchi M, Takamura C, Umekawa T, Kurita T, Kohri K. Inhibitory effects of female sex hormones on urinary stone formation in rats. Kidney Int. 1999;56:479–485. doi: 10.1046/j.1523-1755.1999.00586.x. [DOI] [PubMed] [Google Scholar]
- Ilio KY, Hess RA. Localization and activity of Na+, K+-ATPase in the ductuli efferentes of the rat. Anat Rec. 1992;234:190–200. doi: 10.1002/ar.1092340206. [DOI] [PubMed] [Google Scholar]
- Ilio KY, Hess RA. Structure and function of the ductuli efferentes: a review. Microsc Res Tech. 1994;29:432–467. doi: 10.1002/jemt.1070290604. [DOI] [PubMed] [Google Scholar]
- Jones MA, Young RH, Scully RE. Adenocarcinoma of the epididymis: a report of four cases and review of the literature. Am J Surg Pathol. 1997;21:1474–1480. doi: 10.1097/00000478-199712000-00010. [DOI] [PubMed] [Google Scholar]
- Jones RC, Jurd KM. Structural differentiation and fluid reabsorption in the ductuli efferentes testis of the rat. Aust J Biol Sci. 1987;40:79–90. [PubMed] [Google Scholar]
- Joseph A, Shur BD, Hess RA. Estrogen, efferent ductules, and the epididymis. Biol Reprod. 2011;84:207–217. doi: 10.1095/biolreprod.110.087353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A, Shur BD, Ko C, Chambon P, Hess RA. Epididymal Hypo-Osmolality Induces Abnormal Sperm Morphology and Function in the Estrogen Receptor Alpha Knockout Mouse. Biol Reprod. 2010;82:958–967. doi: 10.1095/biolreprod.109.080366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Wakasugi E, Uematsu K, Miyazato H, Furuta T, Teramura K, et al. Pagetoid spread of intratubular germ cell neoplasia into rete testis forming tumor-like mass. Rinsho Byori. 1998;46:186–189. [PubMed] [Google Scholar]
- Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000;141:1317–1324. doi: 10.1210/endo.141.4.7403. [DOI] [PubMed] [Google Scholar]
- Lee KH, Hess RA, Bahr JM, Lubahn DB, Taylor J, Bunick D. Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biol Reprod. 2000;63:1873–1880. doi: 10.1095/biolreprod63.6.1873. [DOI] [PubMed] [Google Scholar]
- Liel Y, Shany S, Smirnoff P, Schwartz B. Estrogen increases 1,25-dihydroxy vitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology. 1999;140:280–285. doi: 10.1210/endo.140.1.6408. [DOI] [PubMed] [Google Scholar]
- Mahecha GA, Oliveira CA, Balzuweit K, Hess RA. Epididymal lithiasis in roosters and efferent ductule and testicular damage. Reproduction. 2002;124:821–834. [PubMed] [Google Scholar]
- Mahmoudi AR, Zarnani AH, Jeddi-Tehrani M, Katouzian L, Tavakoli M, Soltanghoraei H, et al. Distribution of vitamin D receptor and 1alpha-hydroxylase in male mouse reproductive tract. Reprod Sci. 2013;20:426–436. doi: 10.1177/1933719112459235. [DOI] [PubMed] [Google Scholar]
- Marion V, Schlicht D, Mockel A, Caillard S, Imhoff O, Stoetzel C, et al. Bardet-Biedl syndrome highlights the major role of the primary cilium in efficient water reabsorption. Kidney Int. 2011;79:1013–1025. doi: 10.1038/ki.2010.538. [DOI] [PubMed] [Google Scholar]
- Martino Cd, Zamboni L. A morphologic study of the mesonephros of the human embryo. J Ultrastruct Res. 1966;16:399–427. doi: 10.1016/s0022-5320(66)80072-2. [DOI] [PubMed] [Google Scholar]
- Mason KE, Shaver SL. Some functions of the caput epididymis. Ann N Y Acad Sci. 1952;55:585–593. doi: 10.1111/j.1749-6632.1952.tb26578.x. [DOI] [PubMed] [Google Scholar]
- Menchinelli P, De Giovanni L, Belli P, Manasia P, Weir JM, Ronzoni G. Unilateral testicular microlithiasis. Arch Ital Urol Androl. 1999;71:199–200. [PubMed] [Google Scholar]
- Montorzi NM, Burgos MH. Uptake of colloidal particles by cells of the ductuli efferentes of the hamster. Z Zellforsch. 1967;83:58–69. doi: 10.1007/BF00334740. [DOI] [PubMed] [Google Scholar]
- Montorzi NM, Labiano SA. Uptake of water, sodium and glucose by the ductuli efferentes of the hamster “in vitro”. Acta Physiol Lat Am. 1970;20:135–139. [PubMed] [Google Scholar]
- Nagy F. On the ultrastructure of the male reproductive tract in the Siberian hamster Phodopus sungorus campbelli. I. The ductuli efferentes. J Submicrosc Cytol Pathol. 1990;22:615–625. [PubMed] [Google Scholar]
- Nie R, Zhou Q, Jassim E, Saunders PT, Hess RA. Differential expression of estrogen receptors alpha and beta in the reproductive tracts of adult male dogs and cats. Biol Reprod. 2002;66:1161–1168. doi: 10.1095/biolreprod66.4.1161. [DOI] [PubMed] [Google Scholar]
- Oliveira AG, Dornas RA, Kalapothakis E, Hess RA, Mahecha GA, Oliveira CA. Vitamin D3 and androgen receptors in testis and epididymal region of roosters (Gallus domesticus) as affected by epididymal lithiasis. Anim Reprod Sci. 2008;109:343–355. doi: 10.1016/j.anireprosci.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Oliveira AG, Dornas RA, Praes LC, Hess RA, Mahecha GA, Oliveira CA. Roosters affected by epididymal lithiasis present local alteration in vitamin D3, testosterone and estradiol levels as well as estrogen receptor 2 (beta) expression. Reproduction. 2011;142:439–446. doi: 10.1530/REP-11-0131. [DOI] [PubMed] [Google Scholar]
- Oliveira AG, Oliveira CA. Epididymal lithiasis in roosters: in the middle of the way there was a stone. Life Sci. 2011;89:588–594. doi: 10.1016/j.lfs.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Oliveira CA, Carnes K, Franca LR, Hermo L, Hess RA. Aquaporin-1 and -9 are differentially regulated by estrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol Cell. 2005;97:385–395. doi: 10.1042/BC20040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CA, Mahecha GA, Carnes K, Prins GS, Saunders PT, Franca LR, et al. Differential hormonal regulation of estrogen receptors ER alpha and ER beta and androgen receptor expression in rat efferent ductules. Reproduction. 2004;128:73–86. doi: 10.1530/rep.1.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelliniemi LJ, Kellokumpu-Lehtinen P, Hoffer AP. Glycogen accumulations in differentiating mesonephric ducts and tubuli in male human embryos. Anat Embryol. 1983;168:445–453. doi: 10.1007/BF00304280. [DOI] [PubMed] [Google Scholar]
- Picciarelli-Lima P, Oliveira AG, Reis AM, Kalapothakis E, Mahecha GA, Hess RA, et al. Effects of 3-beta-diol, an androgen metabolite with intrinsic estrogen-like effects, in modulating the aquaporin-9 expression in the rat efferent ductules. Reprod Biol Endocrinol. 2006;4:51. doi: 10.1186/1477-7827-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudney J, Fawcett DW. Seasonal changes in fine structure of the ductuli efferentes of the ground squirrel Citellus lateralis Say. Anat Rec. 1984;208:383–399. doi: 10.1002/ar.1092080309. [DOI] [PubMed] [Google Scholar]
- Ramos AS, Jr, Dym M. Ultrastructure of the ductuli efferentes in monkeys. Biol Reprod. 1977;17:339–349. doi: 10.1095/biolreprod17.3.339. [DOI] [PubMed] [Google Scholar]
- Rheubert JL, Sever DM, Geheber AD, Siegel DS. Proximal testicular ducts of the Mediterranean gecko (Hemidactylus turcicus) Anat Rec (Hoboken) 2010;293:2176–2192. doi: 10.1002/ar.21282. [DOI] [PubMed] [Google Scholar]
- Robaire B, Hamzeh M. Androgen action in the epididymis. J Androl. 2011;32:592–599. doi: 10.2164/jandrol.111.014266. [DOI] [PubMed] [Google Scholar]
- Robaire B, Hermo L. Efferent ducts, epididymis, and vas deferens: structure, functions, and their regulation. In: Knobil E, Neill J, editors. The Physiology of Reproduction. Vol. 1. Raven Press; New York: 1988. pp. 999–1080. [Google Scholar]
- Robaire B, Hinton BT, Orgebin-Crist M-C. The Epididymis. In: Neill JD, editor. Kobil and Neill’s Physiology of Reproduction. Vol. 1. Elsevier, Inc.; St. Louis, MO: 2006. pp. 1071–1148. [Google Scholar]
- Robaire B, Viger RS. Regulation of epididymal epithelial cell functions. Biol Reprod. 1995;52:226–236. doi: 10.1095/biolreprod52.2.226. [DOI] [PubMed] [Google Scholar]
- Ruz R, Gregory M, Smith CE, Cyr DG, Lubahn DB, Hess RA, et al. Expression of aquaporins in the efferent ductules, sperm counts, and sperm motility in estrogen receptor-alpha deficient mice fed lab chow versus casein. Mol Reprod Dev. 2006;73:226–237. doi: 10.1002/mrd.20390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigusa T, Reichert R, Guare J, Siroky BJ, Gooz M, Steele S, et al. Collecting duct cells that lack normal cilia have mislocalized vasopressin-2 receptors. Am J Physiol Renal Physiol. 2012;302:F801–808. doi: 10.1152/ajprenal.00253.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Shichizyou T, Saito K, Okumura T, Ogawa Y, Yoshida H, et al. Testicular microlithiasis identified ultrasonographically in Japanese adult patients: prevalence and associated conditions. Urology. 2006;68:636–641. doi: 10.1016/j.urology.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Schleicher G, Drews U, Stumpf WE, Sar M. Differential distribution of dihydrotestosterone and estradiol binding sites in the epididymis of the mouse. An autoradiographic study. Histochemistry. 1984;81:139–147. doi: 10.1007/BF00490107. [DOI] [PubMed] [Google Scholar]
- Schleicher G, Privette TH, Stumpf WE. Distribution of soltriol [1,25(OH)2-vitamin D3] binding sites in male sex organs of the mouse: an autoradiographic study. J Histochem Cytochem. 1989;37:1083–1086. doi: 10.1177/37.7.2543697. [DOI] [PubMed] [Google Scholar]
- Serter S, Gumus B, Unlu M, Tuncyurek O, Tarhan S, Ayyildiz V, et al. Prevalence of testicular microlithiasis in an asymptomatic population. Scand J Urol Nephrol. 2006;40:212–214. doi: 10.1080/00365590600589641. [DOI] [PubMed] [Google Scholar]
- Sever DM, Freeborn LR. Observations on the anterior testicular ducts in snakes with emphasis on sea snakes and ultrastructure in the yellow-bellied sea snake, Pelamis platurus. J Morphol. 2012;273:324–336. doi: 10.1002/jmor.11025. [DOI] [PubMed] [Google Scholar]
- Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, et al. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149:2970–2979. doi: 10.1210/en.2007-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Small CL, Li Y, Griswold MD. Regulation of gene expression by estrogen and testosterone in the proximal mouse reproductive tract. Biol Reprod. 2009;81:707–716. doi: 10.1095/biolreprod.109.079053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki F, Racey PA. Light and electron microscopical observations on the male excurrent duct system of the common shrew (Sorex araneus) J Reprod Fertil. 1984;70:419–428. doi: 10.1530/jrf.0.0700419. [DOI] [PubMed] [Google Scholar]
- Thomas K, Wood SJ, Thompson AJ, Pilling D, Lewis-Jones DI. The incidence and significance of testicular microlithiasis in a subfertile population. Br J Radiol. 2000;73:494–497. doi: 10.1259/bjr.73.869.10884745. [DOI] [PubMed] [Google Scholar]
- Tiedemann K. Fine structure of the epithelia of the Wolffian duct and of the vas deferens in fetal sheep. Z Zellforsch Mikrosk Anat. 1971;113:230–248. [PubMed] [Google Scholar]
- Torikata C. Papillary cystadenoma of the epididymis. An ultrastructural and immunohistochemical study. J Submicrosc Cytol Pathol. 1994;26:387–393. [PubMed] [Google Scholar]
- Trepos-Pouplard M, Lardenois A, Staub C, Guitton N, Dorval-Coiffec I, Pineau C, et al. Proteome analysis and genome-wide regulatory motif prediction identify novel potentially sex-hormone regulated proteins in rat efferent ducts. Int J Androl. 2010;33:661–674. doi: 10.1111/j.1365-2605.2009.01006.x. [DOI] [PubMed] [Google Scholar]
- Veeramachaneni DN, Amann RP, Palmer JS, Hinton BT. Proteins in luminal fluid of the ram excurrent ducts: changes in composition and evidence for differential endocytosis. J Androl. 1990;11:140–154. [PubMed] [Google Scholar]
- Vicentini CA, Orsi AM, Gregorio EA. Fine structure of the ductuli efferentes of the hamster (Mesocricetus auratus) Gegenbaurs Morphol Jahrb. 1990;136:111–118. [PubMed] [Google Scholar]
- Weiss J, Bernhardt ML, Laronda MM, Hurley LA, Glidewell-Kenney C, Pillai S, et al. Estrogen actions in the male reproductive system involve estrogen response element-independent pathways. Endocrinology. 2008;149:6198–6206. doi: 10.1210/en.2008-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler F, Wille KH. Early fetal development of the small intestine mucosa in cattle (Bos primigenius taurus) Anat Histol Embryol. 1998;27:335–343. doi: 10.1111/j.1439-0264.1998.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Chang JP. An ultracytochemical and ultrastructural study of epithelial cells in the ductuli efferentes of Chinese hamster. J Histochem Cytochem. 1971;19:766–774. doi: 10.1177/19.12.766. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, et al. Estrogen action and male fertility: Roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci U S A. 2001;98:14132–14137. doi: 10.1073/pnas.241245898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl. 2002;23:870–881. [PubMed] [Google Scholar]


