Abstract
Objective
The purpose of this study was to examine the timing of diagnostic and therapeutic services in cochlear implant recipients from a rural Appalachian region with healthcare disparity.
Study design
Retrospective analysis
Methods
Cochlear implant recipients from a tertiary referral center born with severe congenital sensorineural hearing loss were examined. Rural status and Appalachian status of their county of origin were recorded. A log-rank test was used to examine differences in the distributions of time to definitive diagnosis of hearing loss, initial amplification fitting, and cochlear implantation in these children. Correlation analysis of the rural status of each county and the timing of services was assessed.
Results
53 children born with congenital hearing loss were included in the study (36 from rural counties and 17 from urban/suburban counties). The distribution of weeks after birth to diagnosis (p=0.006), amplification (p=0.030), and cochlear implantation (p=0.002) was delayed in rural children compared with urban children. An analysis factoring in the effect of implementation of mandatory infant hearing screening in 2000 demonstrated a similar delay in rural children for weeks to diagnosis (p=0.028), amplification (0.087) and cochlear implantation (p<0.0001).
Conclusions
Children with severe hearing loss in very rural areas, such as Appalachia, may have significant delays in diagnostic and rehabilitative services. Further investigation is warranted to assess causative factors in delays of cochlear implantation and to develop interventions to promote timely diagnosis and care.
Keywords: Health disparity, Congenital hearing loss, Rural healthcare
INTRODUCTION
Pediatric hearing loss is a common problem with an incidence of approximately 1.4 per 1000 infants screened at birth.1 The importance of addressing early hearing loss in a timely manner cannot be overstated; as children with congenital hearing loss may have difficulty with receptive and expressive language development throughout childhood when compared with normal hearing peers.2 Delayed diagnosis and/or intervention for infants with hearing loss frequently result in language, cognitive, and social development deficits.3 Children with hearing loss early in life are more likely to have more difficulties in socialization, lower self-esteem, and have a higher incidence of behavioral problems.4–6
Children with hearing loss face a complicated diagnostic and therapeutic process, which needs to be accessed in a timely manner to prevent the linguistic, educational and social complications of hearing loss. Non-adherence to recommendations may involve socioeconomic factors, and access to healthcare facilities, and other issues, all of which may delay timely care.7–9 Children from rural regions often have limitations in access to care that affect their health;10 however, scant research has been conducted in the area of pediatric hearing healthcare delays and disparities. Although approximately 20% of the U.S. population resides in rural areas,11 the relationship of rural residence with timing of congenital hearing loss diagnosis and treatment has not been adequately assessed. To rectify this omission and address health disparities, this study aims to assess the timing of hearing loss diagnosis and intervention services in pediatric cochlear implant recipients from a very rural Appalachian region.
METHODS
Institutional review board (protocol 11-0872-P3H) approval was obtained prior to initiation of the study. We performed a retrospective analysis of children diagnosed with congenital sensorineural hearing loss who subsequently received cochlear implants. Clinical and demographic data from the records of children (<18 years old) with cochlear implants from the University of Kentucky and the Lexington Hearing and Speech Center (LHSC) were analyzed. These collaborative institutions have provided comprehensive diagnostic and treatment of hearing loss in children since 1992 and are geographically positioned adjacent to the Appalachian region of Kentucky (Eastern portion of the state). They serve as the primary cochlear implant center for Eastern and Central Kentucky. Inclusion criteria included pediatric cochlear implant recipients with failed infant hearing who were diagnosed with infantile severe congenital hearing loss. Children with known acquired hearing loss after birth and those with progressive hearing loss were excluded from the study. After failed newborn hearing screening, follow-up audiological testing confirmed severe sensorineural hearing loss and these children underwent a hearing aid trial. All subjects subsequently underwent cochlear implantation after hearing aid trial and all care was provided at this collaborative center. Data that we collected included date of birth, county or origin at time of birth based on ZIP code, date of diagnosis of hearing loss, date of hearing aid amplification, and date of cochlear implantation. Children were separated, based on the county of origin, to a urban/suburban region group or a rural region group based on rural status of each county of origin using the Beale codes of 2003 (United States Department of Agriculture Rural-Urban Continuum Coding system).12 This numerical scale has 9 classifications with the most urban county being 1 with a metro population of 1 million or more and 9 being the most rural code indicating that the county is completely rural or less than 2500 urban population and not adjacent to a metro area. Beale codes 1–3 are considered urban/suburban and codes 6–9 are considered rural. Based on the county of origin, we also recorded the designation of each county into the Appalachian region or non-Appalachian region, which is arbitrarily based on the Appalachian Region Commission classification.13
Descriptive statistical analysis included mean time to diagnosis, amplification, and cochlear implantation and compared for urban versus rural groups for the entire dataset. Similar analyses were performed on children born before and after 2000. All dates were recorded in weeks after birth. Differences in these variables in urban versus rural patients was examined using the Welch’s two-sample t-test. We used a log-rank test to examine differences in the distributions of time to diagnosis, amplification, and cochlear implantation between urban and rural children. The corresponding Kaplan-Meier curves are also provided to visualize these distributional differences. A similar analysis was performed adjusting for the variable of implementation of infant screening (born after 2000). Distance from the ZIP code of origin to the diagnostic/CI center was also recorded. Pearson’s correlation coefficient analysis was performed on this data to determine the relationship of distance to the timing of diagnosis, amplification, and cochlear implantation. Data were managed using an Excel spreadsheet (Microsoft, Redmond, WA, USA), and statistical analyses were performed with Stata (StataCorp, College Station, TX, USA) and R (R Development Core Team, Vienna, Austria).
RESULTS
We identified 70 pediatric cochlear implant recipients born between 1992 and 2010, of which, 53 children met inclusion criteria (38 children were born after 2000). All subjects received cochlear implants between 1993 and 2011. The sample included 32 females and 21 males.. There were 17 children from the suburban or urban counties and 36 from rural counties. The children from the urban/suburban counties were from Central Kentucky counties with Beale codes of 2 (classifying them as urban). These counties are all outside the Appalachian region and the average distance of travel to the implant center was 6.5 miles (range of 5–21 miles). There were 36 children from rural counties (primarily of Central and Eastern Kentucky) with an average Beale code of 6.7 (range 4–9) and 25 of these children were from Appalachian counties. The average distance of travel for these children to the implant center was 85.7 miles (range of 29–199 miles) with the Appalachian children within this group having an average travel distance of 98.2 miles.
The mean timing of diagnosis, hearing aid amplification, and cochlear implantation for all subjects is displayed in Table 1, including categorization based on birth before or after implementation of mandatory infant hearing screening. There was a significant delay in diagnosis in rural children compared with urban children (p=0.011). This difference was also found with a log-rank test (p=0.006). When controlling for the variable of birth after 2000, the distribution of age of diagnosis on the log-rank test still exhibited a delay in diagnosis for rural children (p=0.028) (Figure 1). Within the rural group, those from Appalachia were diagnosed at a mean age of 80 weeks after birth, which is over 6 times longer than the recommended age of diagnosis of 13 weeks after birth. Delayed amplification was also seen in rural children compared with urban children (p=0.031) and further demonstrated using a log-rank test for entire dataset (p=0.030). When controlling for year 2000 birth variable, we identified a trend toward delayed amplification in rural children, which did not reach statistical significance (p=0.087) (Figure 2). In the rural group, those from Appalachia were amplified at a mean age of 90 weeks after birth. There was a significant delay in the timing of cochlear implantation in rural children compared with urban children (p=0.017), which was demonstrated in the log-rank analysis (p=0.002). When controlling for the variable of birth after 2000, the distribution of age of diagnosis on the log-rank test still exhibited a delay in diagnosis for rural children (p<0.0001) (Figure 3). In the rural group, those from Appalachia were implanted at a mean age of 249 weeks after birth. Aside from the rural and urban comparisons, when analyzing all children in the dataset, the effect of implementation of infant hearing screening hastened tended to hasten the timing of cochlear implantation from a mean age of 360.3 weeks after birth to 146.5 weeks after birth (p=0.008).
Table 1.
Mean timing of diagnosis and treatment in urban and rural cochlear implant recipients
| All Subjects (53) | Born Post-2000 (38) | Born Pre-2000 (15) | ||
|---|---|---|---|---|
| Mean | Mean | Mean | ||
| Age of Diagnosis (weeks) | Urban | 33.8 | 34.5 | 28.3 |
| Rural | 82.7 | 65.2 | 119.6 | |
| Age of Amplification (weeks) | Urban | 47.4 | 47.7 | 45.7 |
| Rural | 88.2 | 68.4 | 127.8 | |
| Age of Implantation (weeks) | Urban | 124.3 | 126.4 | 108.7 |
| Rural | 242.7 | 159.5 | 402.2 |
Figure 1.

Kaplan Meier Analysis of Time (weeks after birth) to Diagnosis of Congenital Hearing Loss adjusting for the implementation of infant hearing screening in 2000 (p=0.028).
Figure 2.

Kaplan Meier Analysis of Time (weeks after birth) to Hearing Aid Amplification adjusting for the implementation of infant hearing screening in 2000 (p=0.087).
Figure 3.

Kaplan Meier Analysis of Time (weeks after birth) to Cochlear Implantation adjusting for the implementation of infant hearing screening in 2000 (p<0.0001).
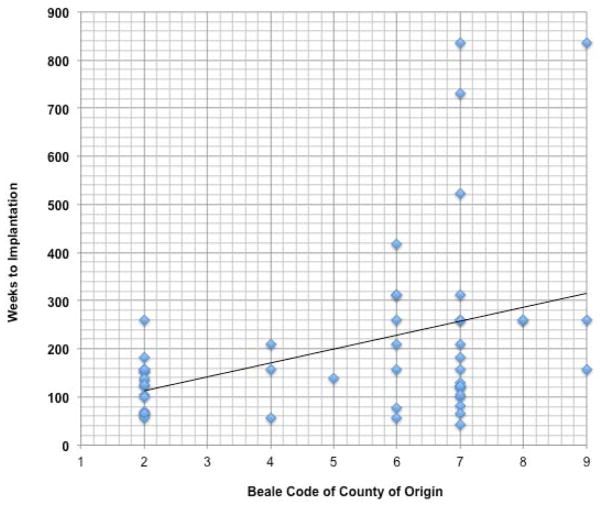
Pearson’s correlation analysis was performed on the distance to the hearing facility, county of residence Beale code, and the diagnostic and therapeutic time point variables. We found that there was a strong linear relationship between distance from the implant center and Beale code of the county of origin (r=0.704, p<0.001). We also identified a weak linear relationship between the Beale code of the county of origin and the timing of diagnosis (r=0.312, p=0.0307), timing of hearing aid amplification (r=0.288, p=0.049), and the timing of cochlear implantation (r=0.400, p=0.004) (Figure 4). When examining only the children born after 2000, the correlations between the Beale code of the county of origin and the timing of diagnosis (r=0.2588, p=0.1275), timing of hearing aid amplification (r=0.2161, p=0.2124), and the timing of cochlear implantation (r=0.20167, p=0.2177) did not reach statistical significance.
Figure 4.
Correlation Analysis of Beale code of County of Origin to Timing of Cochlear Implantation (r=0.4, p=0.004).
DISCUSSION
The National Institutes of Health,14 Joint Committee on Infant Hearing (JCIH),15–17 and the American Academy of Pediatrics18 have provided recommendations for timing of infant hearing testing and subsequent treatment. It is vital to identify hearing loss early as this tends to result in timely intervention,19,20 which subsequently improves language expression and school performance.21–24 For children who are severely hearing impaired, early intervention with cochlear implantation is crucial to stimulate cortical auditory system development25–27 and timing of implantation is the primary predictor of language development in these children.28,29 Although there is no universally accepted age of implantation, it is accepted that earlier implantation, such as under 3 years of age, result in better language development.28
This study demonstrates a delay in the timing of hearing diagnostic and therapeutic services in cochlea implant recipients that highlight health inequities in a very rural region. These findings are complementary with another report documenting the delay in diagnosis of congenital hearing loss in children from Appalachian rural areas.30 In both of these reports, rural children were diagnosed, on average, after age 1 while urban children were diagnosed near the 6 months age range. Certainly the implementation of mandatory infant hearing screening has expedited the process of diagnosis and treatment of infant hearing loss across the country; however, there is still evidence of delayed care in this rural region. Interestingly, there was a short period of time between the timing of diagnosis and the timing of hearing aid amplification in the rural children compared with those from urban counties. The reason for this is unclear; however, we hypothesize that when a child (such as those from the rural region) has been delayed in diagnosis there is a greater sense of urgency in quickly providing amplification once they are diagnosed, especially if the child is over the age of 1 and has accompanying language development delays. The rural regions of the state have limited hearing healthcare services that provide care for children and health disparities are even more extreme in Appalachia. Rural children have unmet dental care in this region.31 Children in the Appalachian region are also at a higher risk of traumatic death compared with other Kentucky children due to barriers in access to care and lack of specialized care.32 When adjusting for age, health insurance status, household income, parental employment, and ethnicity, children from rural regions are more likely to have significantly higher odds ratios of unmet medical needs, problems getting dental care, and having at least 1 emergency department visit per year than children from urban areas.10 The delivery of care in rural areas is an important issue considering the sizeable U.S. rural population11 and since the diagnostic and therapeutic delays present in this population may occur in other rural regions.
The factors behind the observed rural diagnostic and therapeutic delays are likely multifactorial but cannot be determined with this retrospective study. The lack of consistent reporting of parental and infant demographic information from clinical charts prevents further analysis of causation of these findings; therefore, only a correlation relationship can be made. Nevertheless, we speculate that limitations in services and difficulty in accessing these services play a role in hearing healthcare delays. Fewer physicians, clinics, and hospitals exist in the Appalachian and other rural areas to provide care, and additionally the geographic isolation and lack of public transportation may make reaching health services very difficult.33 The cochlear implant recipients from the rural region of this study have to travel an average of 85.7 miles to a cochlear implant center and this may be a significant barrier to timely delivered care. Culturally, residents from Appalachia and other extreme rural areas are said to possess a strong sense of self-reliance and reluctance to leave the area even for needed medical services.34 Conclusions about hearing healthcare timing based on the Beale classification and/or Appalachian residence alone may oversimplify the complex cultural and economic differences in populations of children with congenital hearing loss. Additional factors, including socioeconomic, racial differences, education, health insurance disparities, must be considered when examining rural health disparities.35 Patients from Appalachia have poorer health in general as they are faced with barriers to adequate healthcare.33 The majority of rural counties in this region are distressed36 and the median household income for residents in very rural counties is $24,609 and the percentage of rural Kentucky citizens not in the labor force is 48.5%. The urban household income is $42,148 and 35.7% of urban/suburban Kentucky citizens are not in the labor force.37 Children from low-income families tend to have poorer language development38 and Niparko et al found that family income above $50,000 was associated with better language performance in pediatric cochlear implant recipients.39 Educational disparities may also play a role in health behaviors in rural regions as 35.3% of residents of the very rural regions of this study have not obtained a high school degree contrasted with 20.3% in urban residents.37 In addition, the percentage of uninsured in rural regions is concerning as the very rural region in this study has a 17% uninsured rate while the urban region has a 12.42% rate.37 Certainly, the urban and rural populations in this study are not homogenous and further investigation is warranted to examine factors that directly affect hearing healthcare timing.
This study is also limited by a modest sample size and the retrospective nature of data collection; however, we have demonstrated significant delays in hearing healthcare in pediatric cochlear recipients from a very rural region. This was also demonstrated when controlling for the implementation of mandatory infant hearing screening. Also, incomplete documentation from the medical record limited further causative factor data collection and subsequent analysis. Racial and cultural factors were not addressed in this study; however, these are likely to play a role in hearing healthcare timing. There may be similar healthcare disparities in urban communities that are related to racial differences and the effect on race on intervention timing also needs further investigation. This study serves as a first and key step in future prospective studies to investigate hearing healthcare timing and causative factors that impact timing and language outcomes in regions of healthcare inequities.
CONCLUSIONS
We found that children with congenital hearing loss requiring cochlear implantation from a rural region are delayed in diagnosis and treatment when compared to children from an urban area. Furthermore, there is a weak linear correlation between the timing of pediatric cochlear implantation and the county of origin rural status. Further assessment of factors causing this delay is warranted and interventions should be developed that improve access to timely care for those that live in rural regions.
Acknowledgments
The authors would like to thank Marcey Ansley and Tara Guinn of the Lexington Hearing and Speech Center for their collaboration with data collection for this study and their ongoing commitment to provide timely hearing healthcare for children throughout Kentucky.
This publication was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grants UL1TR000117 and KL2 RR033171. This was also supported by National Institute of Deafness and Other Communication Disorders (1U24-DC012079-01) and National Institutes of Health Loan Repayment Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Level of Evidence: 3b
Conflicts of Interest and Source of Funding: This publication was supported by the National Institutes of Health (8 KL2 TR000116-02), the National Institute of Deafness and Other Communication Disorders (1U24-DC012079-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
BIBLIOGRAPHY
- 1.Centers for Disease Control and Prevention. National Center on Birth Defects and Developmental Disabilities. [Accessed March 27, 2011];Hearing loss. at: http://www.cdc.gov/ncbddd/dd/ddhi.htm.
- 2.National Information Center for Children and Youth with Disabilities. Deaf and Hearing Loss. Washington, DC: 2004. Publication FS3. [Google Scholar]
- 3.U.S. Preventive Services Task Force. Universal screening for hearing loss in newborns: U.S. Preventive Services Task Force Recommendation Statement. Pediatrics. 2008;122:143–148. doi: 10.1542/peds.2007-2210. [DOI] [PubMed] [Google Scholar]
- 4.Davis A, Hind S. The impact of hearing impairment: a global health problem. Int J Pediatr Otorhinolaryngol. 1999;49(suppl 1):S51–S54. doi: 10.1016/s0165-5876(99)00213-x. [DOI] [PubMed] [Google Scholar]
- 5.Van Eldik TT. Behavior problems with deaf Dutch boys. Am Ann Deaf. 1994;139(4):394–399. doi: 10.1353/aad.2012.0280. [DOI] [PubMed] [Google Scholar]
- 6.Vostanis P, Hayes M, Feu MD, Warren J. Detection of behavioural and emotional problems in deaf children and adolescents: comparison of two rating scales. Child Care Health Dev. 1997;23(3):233–246. doi: 10.1111/j.1365-2214.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 7.Brach C, Lewit EM, VanLandeghem K, et al. Who’s enrolled in the State Children’s Health Insurance Program (SCHIP)? An overview of findings from the Child Health Insurance Research Initiative (CHIRI) Pediatrics. 2003;112(6 pt 2) [PubMed] [Google Scholar]
- 8.Liu CL, Zaslavsky AM, Ganz ML, Perrin J, Gortmaker S, McCormick MC. Continuity of health insurance coverage for children with special health care needs. Matern Child Health J. 2005;9(4):363–375. doi: 10.1007/s10995-005-0019-1. [DOI] [PubMed] [Google Scholar]
- 9.Sommers BD. From Medicaid to uninsured: drop-out among children in public insurance programs. Health Serv Res. 2005;40(1):59–78. doi: 10.1111/j.1475-6773.2005.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeVoe JE, Krois L, Stenger R. Do children in rural areas still have different access to health care? Results from a statewide survey of Oregon’s food stamp population. J Rural Health. 2009 Winter;25(1):1–7. doi: 10.1111/j.1748-0361.2009.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Census 2000 Population Statistics. Office of Planning, Environment & Realty. [Accessed 3/11/13.];United States Department of Transportation Federal Highway Administration. http://www.fhwa.dot.gov/planning/census_issues/archives/metropolitan_planning/cps2k.cfm.
- 12.Rural-Urban Continuum Codes. USDA Economic Research Service. United States Department of Agriculture; [Accessed 2/25/13.]. http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx. [Google Scholar]
- 13.Kentucky. Appalachian Regional Commission; [Accessed 11/22/2013.]. http://www.arc.gov/appalachian_region/Kentucky.asp. [Google Scholar]
- 14.U.S. National Institutes of Health, Office of Medical Applications Research, U.S. National Institute on Deafness and Other Communication Disorders. Consensus development conference on early identification of hearing impairment in infants and children. Vol. 11. Bethesda, Md: National Institutes of Health; 1993. pp. 1–24. [Google Scholar]
- 15.Joint Committee on Infant Hearing of the American Academy of Pediatrics. Supplement to the JCIH 2007 position statement: principles and guidelines for early intervention after confirmation that a child is deaf or hard of hearing. Pediatrics. 2013;131:e1324–49. doi: 10.1542/peds.2013-0008. [DOI] [PubMed] [Google Scholar]
- 16.Joint Committee on Infant Hearing. Year 2000 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2000;106:798–817. doi: 10.1542/peds.106.4.798. [DOI] [PubMed] [Google Scholar]
- 17.Joint Committee on Infant Hearing. Year 2007 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. Pediatrics. 2007;120:898–921. doi: 10.1542/peds.2007-2333. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics Joint Committee on Infant Hearing. Joint Committee on Infant Hearing 1994 position statement. Pediatrics. 1995;95:152–6. [PubMed] [Google Scholar]
- 19.Apuzzo ML, Yoshinaga-Itano C. Early identification of infants with significant hearing loss and the Minnesota Child Development Inventory. Semin Hear. 1995;16:124–137. [Google Scholar]
- 20.Moeller MP. Early intervention and language development in children who are deaf and hard of hearing. Pediatrics. 2000;106:E43. doi: 10.1542/peds.106.3.e43. [DOI] [PubMed] [Google Scholar]
- 21.Yoshinaga-Itano C. Efficacy of early identification and early intervention. Semin Hear. 1995;16:115–123. [Google Scholar]
- 22.Yoshinaga-Itano C. Levels of evidence: universal newborn hearing screening (UNHS) and early hearing detection and intervention systems (EHDI) J Commun Disord. 2004;37:451–465. doi: 10.1016/j.jcomdis.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and later-identified children with hearing loss. Pediatrics. 1998;102:1161–1171. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]
- 24.Yoshinaga-Itano C, Coulter D, Thomson V. The Colorado Newborn Hearing Screening Project: effects on speech and language development for children with hearing loss. J Perinatol. 2000;20(8 pt 2):S132–S137. doi: 10.1038/sj.jp.7200438. [DOI] [PubMed] [Google Scholar]
- 25.Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23:532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Ryugo D, Limb C, Redd E. Brain plasticity. In: Niparko JK, editor. Cochlear Implants: Principles and Practices. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 33–56. [Google Scholar]
- 27.Gordon KA, Valero J, Papsin BC. Abnormal timing delays in auditory brainstem responses evoked by bilateral cochlear implant use in children. Otol Neurotol. 2008;29:193–198. doi: 10.1097/mao.0b013e318162514c. [DOI] [PubMed] [Google Scholar]
- 28.Nicholas JG, Geers AE. Effects of early auditory experience on the spoken language of deaf children at 3 years of age. Ear Hear. 2006;27:286–298. doi: 10.1097/01.aud.0000215973.76912.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirk KI, Miyamoto RT, Lento CL, Ying E, et al. Effects of age at implantation in young children. Ann Otol Rhinol Laryngol Suppl. 2002;189:69–73. doi: 10.1177/00034894021110s515. [DOI] [PubMed] [Google Scholar]
- 30.Bush M, Bianchi K, Lester C, Shinn J, Gal T, Fardo D, Schoenberg N. Delays in Diagnosis of Congenital Hearing Loss in Rural Children. Journal of Pediatrics. 2013 Oct 30; doi: 10.1016/j.jpeds.2013.09.047. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiener RC, Wiener MA. Unmet dental and orthodontic need of children with special healthcare needs in West Virginia. Rural Remote Health. 2012;12:2069. [PubMed] [Google Scholar]
- 32.Svenson JE, Spurlock C, Nypaver M. Factors associated with the higher traumatic death rate among rural children. Ann Emerg Med. 1996;27(5):625–32. doi: 10.1016/s0196-0644(96)70167-1. [DOI] [PubMed] [Google Scholar]
- 33.Shell R, Tudiver F. Barriers to cancer screening by rural Appalachian primary care providers. J Rural Health. 2004;20:368–373. doi: 10.1111/j.1748-0361.2004.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 34.Couto RA. Appalachia. Sowing Seeds in the Mountans. Community-based coalitions for cancer prevention and control. Bethesda, MD: NIH; 1994. pp. 1–343. NIH publication No. 94-3779. [Google Scholar]
- 35.Hartley D. Rural health disparities, population health, and rural culture. American Journal of Public health. 2004;1994:1675–1678. doi: 10.2105/ajph.94.10.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.County Economic Status and Distressed Areas in Appalachia. Appalachian Regional Commission; [Accessed 4/18/13.]. http://www.arc.gov/appalachian_region/CountyEconomicStatusandDistressedAreasinAppalachia.asp. [Google Scholar]
- 37.Davis AF. Kentucky’s Urban/Rural Landscape: What is driving the differences in wealth across Kentucky? [Accessed 2/22/2013.];Kentucky Annual Economic Report 2009. http://cber.uky.edu/Downloads/annrpt09.html.
- 38.Walker D, Greenwood C, Hart B, Carta J. Prediction of school outcomes based on early language production and socioeconomic factors. Child Dev. 1994;65(2 spec):606–621. [PubMed] [Google Scholar]
- 39.Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, et al. CDaCI Investigative Team. Spoken language development in children following cochlear implantation. JAMA. 2010;303:1498–1506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]