Abstract
Background
Kidney damage and reduced kidney function are potent risk factors for heart failure (HF), but existing studies are limited to assessing albuminuria or estimated glomerular filtration rate (eGFR). We evaluated the associations of urinary biomarkers of kidney tubular injury (interleukin 18 [IL-18] and kidney injury molecule 1 [KIM-1]) with future risk of HF.
Study Design
Retrospective cohort study.
Setting & Participants
2921 participants without HF in the Health, Aging, and Body Composition (Health ABC) cohort.
Predictors
Ratios of urine KIM-1, IL-18, and albumin to creatinine (KIM-1:Cr, IL-18:Cr, and ACR, respectively).
Outcomes
Incident HF over a median follow-up of 12 years.
Results
Median values of each marker at baseline were 812 (IQR, 497–1235) pg/mg for KIM-1:Cr, 31 (IQR, 19–56) pg/mg for IL-18:Cr, and 8 (IQR, 5–19) mg/g for ACR. 596 persons developed HF during follow-up. The top quartile of KIM-1:Cr was associated with risk of incident HF after adjustment for baseline eGFR, HF risk factors, and ACR (HR, 1.32; 95% CI, 1.02–1.70) in adjusted multivariate proportional hazards models. The top quartile of IL-18:Cr was also associated with HF in a model adjusted for risk factors and eGFR (HR, 1.35; 95% CI, 1.05–1.73), but was attenuated by adjustment for ACR (HR, 1.15; 95% CI, 0.89–1.48). The top quartile of ACR had a stronger adjusted association with HF (HR, 1.96; 95% CI, 1.53–2.51).
Limitations
Generalizability to other populations is uncertain.
Conclusions
Higher urine concentrations of KIM-1 were independently associated with incident HF risk, although the associations of higher ACR were of stronger magnitude.
Keywords: IL-18, KIM-1, cystatin C, heart failure, CKD, risk marker, cardiovascular disease (CVD), albuminuria, kidney tubular injury
Chronic kidney disease (CKD), defined by an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2 or urine albumin-creatinine ratio (ACR) > 30 mg/g is a major public health problem with 23.2 million people affected in the United States.1 Lower eGFR is an established, independent risk factor for cardiovascular disease (CVD), in particular for heart failure (HF).2 Albuminuria is an established marker of glomerular injury, and the elevated ACR is independently associated with risks of CKD-related adverse outcomes, including HF.3–5 Therefore, both glomerular injury and dysfunction appear pathophysiologically linked to HF.
Kidney tubule health is not part of the routine assessment of CKD, but may also be important in the pathogenesis of HF. Novel markers of kidney tubular injury have been developed that can detect and quantify early tubular damage, which in turn has been shown to predict the onset of CKD.6–8 As a harbinger of decreased homeostatic reserve in the kidney, these markers of kidney tubule damage may predict reduced capacity to regulate volume status that would result in clinical HF. To gain a broader understanding of the association between early kidney disease and HF, we sought to evaluate and compare associations of both glomerular and tubular injury markers with incident HF. In a population of community dwelling older adults, we hypothesized that both markers of kidney tubular injury (urine kidney injury molecule 1 [KIM-1] and interleukin 18 [IL-18]) and ACR would be independently associated with risk of incident HF.
Methods
Design and Participants
The Health, Aging, and Body Composition (Health ABC) Study is an National Institute on Aging (NIA)–sponsored cohort study that enrolled 3,075 well-functioning men and women aged 70–79 years from 2 clinical sites in Memphis, Tennessee and Pittsburgh, Pennsylvania. Additionally, since older adults experience the highest prevalence of CKD and HF, Health ABC presents a unique opportunity to study risk factors in this population because Health ABC was mostly composed of well-functioning older adults without HF at baseline. Participant eligibility required self-reported lack of difficulty walking a quarter mile, climbing 10 steps, performing basic activities of daily living, the absence of life-threatening illness, and plans to remain in the geographic area for at least 3 years. Baseline examinations occurred during 1997–1998 and participants underwent a 1-day evaluation that included medical history, physical activity assessment, physical examination, and radiographic tests. Those with prevalent HF or missing HF data at baseline, without baseline cystatin C measurements, and without baseline urine samples were excluded from this study (n=158). Prevalent HF at baseline was based on ICD-9 CM codes as defined by the Centers for Medicare & Medicaid Services from 1995–1998, self-reported history of HF, and use of selected medications.9 The results represent an average median follow up of 12.0 (interquartile range [IQR], 7.0–13.2) years with the last clinic visit occurring on the year-16 visit (2012–2013). The study was approved by the institutional review boards at the University of Tennessee Health Science Center and the University of Pittsburgh. In addition, the present study was approved by the University of California, San Francisco; San Francisco VA Medical Center; and Tufts University committees on human research.10,11
Urinary Markers
Primary predictors in this study were urine concentrations of albumin, KIM-1, and IL-18, all of which were measured concurrently from previously frozen stored urine samples and indexed by the concurrent urine creatinine concentrations (denoted as ACR, KIM-1:Cr, and IL-18:Cr, respectively). All urine biomarkers were measured at the Cincinnati Children’s Hospital Medical Center Biomarker Laboratory. Albumin and creatinine were measured by immunoturbidimetry and colorimetric enzyme assay, respectively, using a Siemens Dimension Xpand plus HM clinical analyzer (Siemens, Munich, Germany). The KIM-1 enzyme-linked immunosorbent assay (ELISA) was constructed using commercially available reagents (R&D Systems Inc, Minneapolis, MN).12 IL-18 was measured using a commercially available ELISA kit (Medical & Biological Laboratories Co Ltd, Nagoya, Japan). Coefficients of variation for the urine measures were albumin, 5.9%; creatinine, 4.1%; IL-18, 7.2%; and KIM-1, 5.2%.
Candidate Covariates
Other characteristics that were covariates for multivariable analyses included demographic characteristics, socioeconomic factors, and traditional risk factors for HF, all of which were measured at baseline. The following characteristics were included as covariates in all multivariable models: age, sex, race, education level, income, diabetes (use of hypoglycemic agents, self-report, fasting plasma glucose ≥126 mg/dL, or an oral glucose tolerance test ≥200 mg/dL), systolic blood pressure, hypertension (self-report plus use of antihypertensive medications) fasting HDL (Johnson & Johnson Vitros 950 analyzer) and LDL (calculated using the Friedewald equation)13 cholesterol, body mass index (BMI), waist circumference, prevalent cardiovascular disease (coronary heart disease, myocardial infarction, angina, coronary artery bypass), C-reactive protein (measured in duplicate by ELISA kits from R&D Systems Inc [Minneapolis, MN])14, current smoking (defined as current versus former or never), alcohol (defined as ≥1 versus <1 drink/d), and serum albumin (measured by a colorimetric technique on a Johnson & Johnson Vitros 950 analyzer)15.
An additional covariate was the baseline kidney function, assessed by serum cystatin C–based glomerular filtration rate estimates. Cystatin C measurements were measured by means of a particle-enhanced immunonephelometric assay (N Latex Cystatin C)16 using a BNII nephelometer (both Dade Behring Inc, Deerfield, IL). We estimated GFR using the 2012 CKD-EPI (CKD Epidemiology Collaboration) cystatin C equation, which includes age and sex.17
Incident Heart Failure Outcome
During semi-annual telephone interviews and annual clinical visits, participants were asked to report any hospitalization and were directly asked about interim events. Medical records from all reported overnight hospitalizations were examined. Incident HF was defined as the first overnight hospitalization for decompensated HF. HF criteria required at least a diagnosis from a physician and treatment for HF (prescription for a diuretic agent and either digitalis or a vasodilator). Events were confirmed through medical record review by a panel of clinicians based upon symptoms, signs, chest radiograph results, and echocardiogram findings, as previously described.18
Statistical Analyses
Baseline characteristics of participants by quartile of KIM-1:Cr, IL-18:Cr, and ACR were compared using t-test or Chi-squared test where appropriate. Spearman correlation coefficients were calculated among the three urine injury markers. To evaluate the form of association between each urine injury marker and HF risk, we then used natural piecewise-cubic splines and placed the specified interior knots at the quartiles of the distributions of KIM-1:Cr, IL-18:Cr, and ACR, respectively. We also dichotomized ACR at 30 mg/g because that is the clinical cut-point.
We then used Cox proportional hazards models to investigate associations of KIM-1:Cr, IL-18:Cr, and ACR with time to incident HF. Plots of the Schoenfeld residuals and formal tests suggested no violation of the proportional hazards assumption. Each biomarker was modeled categorically by quartile. Covariates were selected a priori based on biological plausibility. Models were nested and adjusted in stages for: (1) age, gender, race, site and education status; (2) HF risk factors (diabetes, hypertension, systolic blood pressure, smoking, prevalent coronary heart disease, albumin, C-reactive protein, and eGFR); and (3) ACR. Analyses of ACR with HF were conducted similarly with adjustment for the tubular biomarkers at the final stage. These analyses were then repeated after stratifying by black versus white race. In an additional sensitivity analysis, to account for the informative censoring of intervening deaths, we utilized the Fine-Grey model to account for competing risk. We evaluated the impact of KIM-1 and IL-18 on HF prediction by the C statistic in the multivariable model that included significant covariates and ACR. We tested for interaction of the biomarkers by race using multiplicative interaction terms. We used SPSS statistical software (version 16.0.2, SPSS Inc., Chicago, IL) and S-Plus (version 8.0, TIBCO, Seattle, WA) for these analyses.
Results
Among 2921 Health ABC participants meeting inclusion criteria, 596 developed HF during a median follow up of 12.0 (IQR, 7.0–13.2) years. Age and sex appeared similar among urine biomarker quartiles although there appeared to be a higher proportion of blacks in the lower quartiles of KIM-1:Cr and IL-18:Cr and the higher quartiles of ACR. Participants with the highest quartiles of KIM-1 and ACR were more likely to have chronic conditions such as diabetes mellitus, obstructive lung disease, hypertension, coronary artery disease, and peripheral artery disease. These conditions did not appear to be differentially distributed amongst the quartiles of IL-18:Cr. Baseline eGFR appeared similar among the quartiles of urine biomarkers (Table 1 for KIM-1:Cr and IL-18:Cr; Table S1 for ACR, provided as online supplementary material). The markers of tubular injury, KIM-1:Cr and IL-18:Cr, were significantly correlated with each other (ρ=0.185), and urine ACR was significantly correlated with KIM-1:Cr (ρ= 0.166) and IL-18:Cr (ρ =0.176).
Table 1.
Characteristics by quartiles of KIM-1 and IL-18
| All | KIM-1:Cr | IL-18:Cr | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1: <497 pg/mL |
Q2: 497–814 pg/mL |
Q3: 815–1,239 pg/mL |
Q4: ≥1240 pg/mL |
p for trend | Q1: <19 pg/mL |
Q2: 19–31 pg/mL |
Q3: 32–55 pg/mL |
Q4: ≥56 pg/mL |
p for trend |
||
| No. of participants | 2917 | 729 | 734 | 729 | 725 | 728 | 727 | 728 | 734 | ||
| Age (y) | 74 (3) | 73 (3) | 73 (3) | 74 (3) | 74 (3) | <0.001 | 74 (3) | 74 (3) | 74 (3) | 74 (3) | 0.6 |
| Male sex | 48% | 55% | 47% | 44% | 47% | 0.001 | 69% | 59% | 41% | 24% | <0.001 |
| Black race | 41% | 62% | 53% | 31% | 20% | <0.001 | 47% | 40% | 39% | 38% | 0.001 |
| Site | 0.006 | 0.005 | |||||||||
| Memphis | 50% | 49% | 47% | 52% | 55% | 48% | 48% | 50% | 55% | ||
| Pittsburgh | 50% | 51% | 54% | 48% | 45% | 52% | 52% | 50% | 45% | ||
| Education | <0.001 | 0.9 | |||||||||
| < HS | 25% | 34% | 28% | 21% | 21% | 28% | 26% | 23% | 24% | ||
| HS Graduate | 33% | 28% | 33% | 34% | 34% | 29% | 30% | 36% | 36% | ||
| Postsecondary | 42% | 38% | 40% | 45% | 45% | 43% | 44% | 41% | 40% | ||
| Diabetes | 24% | 21% | 24% | 20% | 29% | 0.009 | 24% | 23% | 23% | 24% | 0.9 |
| Hypertension | 66% | 68% | 63% | 63% | 68% | 0.8 | 67% | 64% | 66% | 66% | 0.8 |
| Systolic BP (mm Hg) | 136 (21) | 136 (21) | 135 (21) | 136 (20) | 136 (22) | 0.8 | 135 (21) | 135 (21) | 137 (20) | 136 (22) | 0.2 |
| Diastolic BP (mm Hg) | 71 (12) | 72 (12) | 72 (12) | 71 (11) | 71 (12) | <0.001 | 72 (11) | 72 (12) | 72 (12) | 70 (12) | 0.007 |
| Smoking | 0.3 | 0.6 | |||||||||
| Never | 44% | 46% | 45% | 46% | 39% | 39% | 38% | 48% | 52% | ||
| Former | 45% | 46% | 46% | 44% | 48% | 50% | 52% | 42% | 38% | ||
| Current | 11% | 10% | 9% | 10% | 13% | 11% | 10% | 10% | 11% | ||
| Alcohol | 0.7 | <0.001 | |||||||||
| Never | 28% | 29% | 29% | 30% | 25% | 24% | 24% | 31% | 33% | ||
| Former | 50% | 47% | 50% | 49% | 54% | 53% | 52% | 47% | 48% | ||
| Current | 22% | 24% | 21% | 21% | 21% | 22% | 24% | 22% | 19% | ||
| Prevalent CHD | 20% | 18% | 20% | 19% | 24% | 0.02 | 23% | 22% | 18% | 18% | 0.003 |
| Prevalent CBVD | 8% | 9% | 7% | 8% | 7% | 0.6 | 9% | 8% | 7% | 7% | 0.2 |
| Prevalent PAD | 5% | 4% | 5% | 4% | 6% | 0.2 | 5% | 6% | 4% | 4% | 0.05 |
| COPD | 20% | 19% | 18% | 18% | 25% | 0.02 | 20% | 19% | 23% | 19% | 0.7 |
| BMI (kg/m2) | 27.3 (4.8) | 27.8 (4.8) | 27.5 (4.7) | 27.3 (4.9) | 26.9 (4.7) | <0.001 | 27.5 (4.6) | 27.3 (4.3) | 27.3 (4.8) | 27.3 (5.3) | 0.3 |
| CRP (mg/L) | 1.66 [0.99–3.10] | 1.63 [0.97–2.95] | 1.67 [0.99–3.10] | 1.59 [0.98–2.87] | 1.78 [1.01–3.48] | 0.2 | 1.50 [0.91–2.81] | 1.57 [0.97–2.82] | 1.79 [1.03–3.43] | 1.85 [1.09–3.34] | 0.002 |
| Albumin (g/dL) | 3.98 (0.31) | 4.02 (0.32) | 3.97 (0.30) | 3.96 (0.30) | 3.96 (0.32) | <0.001 | 3.98 (0.32) | 3.99 (0.32) | 3.99 (0.30) | 3.96 (0.32) | 0.3 |
| Cholesterol | |||||||||||
| Total (mg/dL) | 203 (38) | 203 (37) | 205 (38) | 204 (38) | 201 (40) | 0.4 | 200 (38) | 202 (38) | 203 (38) | 207 (40) | <0.001 |
| LDL (mg/dL) | 122 (35) | 123 (33) | 123 (35) | 122 (34) | 119 (37) | 0.04 | 122 (34) | 123 (33) | 120 (35) | 122 (37) | 0.3 |
| HDL (mg/dL) | 54 (17) | 55 (17) | 56 (18) | 54 (17) | 53 (17) | 0.04 | 52 (16) | 52 (16) | 55 (17) | 58 (18) | <0.001 |
| Triglycerides (mg/dL) | 118 [88–163] | 113 [84–154] | 113 [85–151] | 124 [92–169] | 123 [92–175] | <0.001 | 113 [84–154] | 117 [89–161] | 120 [90–166] | 123 [90–168] | 0.03 |
| eGFRcys | 79 (21) | 80 (19) | 81 (20) | 80 (21) | 76 (22) | <0.001 | 77 (19) | 78 (21) | 79 (20) | 83 (23) | <0.001 |
Note: Unless otherwise indicated, values for categorical variables are given as percentages; values for continuous variables are given as mean ± standard deviation or median [interquartile range]. Conversion factors for units: HDL, LDL, and total cholesterol in mg/dL to mmol/L, ×0.02586; triglycerides in mg/dL to mmol/L, ×0.01129.
BMI, body mass index; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; Cr, creatinine; CRP, C-reactive protein; HS, high school; BP, blood pressure; CBVS, cerebrovascular disease; PAD, peripheral artery disease; eGFRcys, cystatin C–based estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; KIM-1, kidney injury molecule 1; IL-18, interleukin 18; Q, quartile
In spline analyses, KIM-1:Cr appeared to be associated with HF above approximately 1000 pg/mg, IL-18:Cr showed no consistent association with HF, and ACR appeared to have a linear association with HF when on a logarithmic scale (Figure 1). In demographic models, the highest quartile of KIM-1:Cr (> 1240 pg/mg) was associated with a 2-fold risk of incident HF relative to the lowest quartile, which was attenuated but remained significantly associated with higher risk of HF even after adjustment for HF risk factors and ACR. In contrast, the top quartile of IL-18:Cr was associated with an approximate 35% increased risk of HF, which persisted after adjusting for HF risk factors, but not ACR. In contrast, ACR was more strongly and linearly associated with incident HF with the third and fourth quartiles having significantly higher risk compared with the first quartile in the demographic and fully adjusted models. For comparison, the highest quartile was associated with approximately a 2-fold higher risk of HF compared to the lowest quartile (Table 2). We repeated analyses using the Fine-Grey model to account for competing risk, and we found that results were essentially unchanged. The associations (hazard ratios [HRs]) of the high quartiles of KIM-1:Cr, IL-18:Cr, and ACR with incident HF were 1.33 (95% CI, 1.03–1.72), 1.21 (95% CI, 0.92–1.59), and 1.78 (95% CI, 1.38–2.30), respectively. When added to the fully adjusted multivariable model that contains ACR, neither KIM-1:Cr nor IL-18:Cr significantly changed the C statistic (p of 0.8 and 0.4, respectively).
Figure 1.
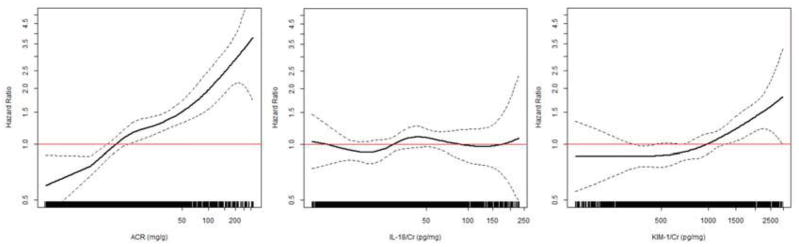
Association of baseline KIM-1/Cr, IL-18/Cr, and ACR with incident heart failure.
Solid lines denote the HR of incident heart failure (with dotted 95% CI bounds) calculated from unadjusted generalized additive models. Each line on the x-axis represents one study participant. The lowest and highest 2.5% of urine biomarker concentrations are truncated.
Table 2.
Association of KIM-1, IL-18 and ACR with incident heart failure
| No. of Participants | No. of Heart Failure Events | Rate/y of incident Heart Failure | Demographic*-adjusted HR (95% CI)* | Multivariable**-adjusted HR (95% CI) | Multivariable**+ACR–adjusted HR (95% CI) | |
|---|---|---|---|---|---|---|
| KIM-1:Cr | ||||||
| By quartile | ||||||
| Q1: <497 pg/mg | 729 | 129 | 1.72 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2: 497–814 pg/mg | 734 | 138 | 1.87 | 1.15 (0.90, 1.48) | 1.07 (0.83, 1.38) | 1.04 0.81, 1.34) |
| Q3: 815–1239 pg/mg | 729 | 145 | 1.96 | 1.40 (1.08, 1.80) | 1.33 (1.03, 1.71) | 1.25 0.97, 1.60) |
| Q4: ≥1240 pg/mg | 725 | 184 | 2.69 | 1.99 (1.54, 2.55) | 1.50 (1.17, 1.93) | 1.32 (1.02, 1.70) |
| IL-18:Cr | ||||||
| By quartile | ||||||
| Q1: <19 pg/mg | 728 | 144 | 1.98 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2: 19–31 pg/mg | 727 | 144 | 1.97 | 1.06 (0.83, 1.34) | 1.06 (0.84, 1.35) | 1.02 0.80, 1.30) |
| Q3: 32–55 pg/mg | 728 | 155 | 2.15 | 1.27 (1.00, 1.62) | 1.26 (0.99, 1.61) | 1.19 0.93, 1.52) |
| Q4: ≥56 pg/mg | 734 | 153 | 2.08 | 1.35 (1.05, 1.73) | 1.35 (1.05, 1.73) | 1.15 (0.89, 1.48) |
| ACR | ||||||
| By quartile | ||||||
| Q1: <4.57 mg/g | 743 | 115 | 1.44 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference)† |
| Q2: 4.57–8.28 mg/g | 738 | 124 | 1.60 | 1.17 (0.90, 1.52) | 1.17 (0.90, 1.52) | 1.13 0.87, 1.48) |
| Q3: 8.29–20.33 mg/g | 735 | 149 | 2.02 | 1.46 (1.14, 1.88) | 1.48 (1.14, 1.91) | 1.42 1.09, 1.83) |
| Q4: ≥20.34 mg/g | 701 | 208 | 3.48 | 2.44 (1.92, 3.09) | 1.96 (1.53, 2.51) | 1.84 (1.43, 2.38) |
| By clinical cut-points | ||||||
| <30 mg/g | 2395 | 431 | 1.74 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| >30 mg/g | 522 | 165 | 3.81 | 2.19 (1.82, 2.64) | 1.79 (1.47, 2.18) | 1.70 (1.39, 2.08) |
ACR, albumin-creatinine ratio; CI, confidence interval; Cr, creatinine; HR, hazard ratio; KIM-1, kidney injury molecule 1; IL-18, interleukin 18; Q, quartile
Adjusted for age, gender, race, site and education
Further adjusted for diabetes, hypertension, systolic blood pressure, smoking, prevalent coronary heart disease, albumin, C-reactive protein, and estimated glomerular filtration rate.
further adjusted for KIM-1 and IL-18.
We next stratified these analyses by race. Among whites and blacks, the associations of KIM-1:Cr and IL-18:Cr with incident HF appeared similar (p for interaction 0.3 and 0.9, respectively), although among blacks KIM-1:Cr appeared modestly stronger and IL-18:Cr moderately weaker (Table 3). The association of the high quartile of ACR with HF appeared somewhat stronger in Blacks compared with Whites (p for interaction 0.1); when dichotomized at 30 mg/g, the association of higher ACR with HF risk also appeared stronger in blacks (HR, 2.10; 95% CI, 1.50–2.79) compared with whites (HR, 1.50; 95% CI, 1.13–1.99).
Table 3.
Association of KIM-1, IL-18, and ACR with incident heart failure, stratified by race
| White* | Black* | |
|---|---|---|
| KIM-1:Cr | ||
| Q1: <497 pg/mg | 1.00 (reference) | 1.00 (reference) |
| Q2: 497–814 pg/mg | 1.29 (0.87, 1.90) | 0.90 (0.63, 1.26) |
| Q3: 815–1239 pg/mg | 1.36 (0.95, 1.96) | 1.30 (0.89, 1.89) |
| Q4: ≥1240 pg/mg | 1.47 (1.04, 2.09) | 1.62 (1.09, 2.40) |
| IL-18:Cr | ||
| Q1: <19 pg/mg | 1.00 (reference) | 1.00 (reference) |
| Q2: 19–31 pg/mg | 1.03 (0.74, 1.42) | 1.11 (0.77, 1.59) |
| Q3: 32–55 pg/mg | 1.32 (0.95, 1.82) | 1.20 (0.83, 1.74) |
| Q4: ≥56 pg/mg | 1.42 (1.01, 2.00) | 1.25 (0.85, 1.82) |
| ACR | ||
| Q1: <4.57 mg/g | 1.00 (reference) | 1.00 (reference) |
| Q2: 4.57–8.28 mg/g | 1.07 (0.77, 1.48) | 1.30 (0.83, 2.02) |
| Q3: 8.29–20.33 mg/g | 1.46 (1.06, 2.01) | 1.39 (0.90, 2.15) |
| Q4: ≥20.34 mg/g | 1.67 (1.20, 2.32) | 2.32 (1.57, 3.43) |
Note: Values are multivariable-adjusted hazard ratio (95% confidence interval). Adjusted for age, gender, race, education, diabetes, hypertension, systolic blood pressure, smoking, prevalent coronary heart disease, albumin, C-reactive protein, and estimated glomerular filtration rate).
ACR, albumin-creatinine ratio; Cr, creatinine; KIM-1, kidney injury molecule 1; IL-18, interleukin 18; Q, quartile
P values for interaction: 0.25 [KIM-1], 0.89 [IL-18], and 0.11 [ACR])
Discussion
In this large cohort of community-dwelling older adults without baseline HF, the top quartiles of urine KIM-1:Cr and IL-18:Cr were each associated with incident HF. For KIM-1:Cr, this association persisted even after adjustment for ACR, whereas for IL-18:Cr it did not. Associations of ACR with HF risk were substantially stronger than those of the tubular injury markers. Overall, these results indicate that although kidney tubular injury has a quantifiable and independent association with HF risk, glomerular injury appears to have stronger links to HF risk within a relatively healthy population of community-dwelling older adults.
To our knowledge, this is the first study that compares urine ACR and urine markers of tubular injury with risk of incident HF. Both IL-18 and KIM-1 are specific to the proximal tubule and have been implicated in ischemia-reperfusion injury to the kidney.19–24 Markers of tubular injury are predictive of clinical acute kidney injury, which is defined subsequently by elevations in serum creatinine.25–27 Also, in prior studies KIM-1 has been reported to predict allograft rejection after kidney transplantation,28 and IL-18 to predict severity of CKD in patients with nephrotic syndrome.29,30 Another study compared albuminuria and markers of tubular injury for long-term allograft failure in a cohort of patients who received a kidney transplant. Similar to our findings, while both albuminuria and KIM-1 were significantly associated with long-term allograft failure, albuminuria had stronger associations than KIM-1.31
The results of this study mirror those of other investigations demonstrating that kidney tubular injury markers have associations with longitudinal risk of disease among ambulatory adults. We previously found in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort that the top decile of KIM-1 was associated with incident CKD stage 3.8 Participants in both cohorts were relatively healthy at baseline, raising the possibility of a low prevalence of detectable tubular injury leading to threshold associations with the respective outcomes.
The association between CKD and incident HF is well established.32 Since a marker of early kidney disease, urine ACR, has been linked to HF risk, we hypothesized that markers of tubular injury would also be associated with HF risk.4,5 While the novel finding of this study is the association of the tubular injury markers with incident HF risk, it is also of great interest that albuminuria has a much stronger association with HF risk than either tubular injury marker. While albuminuria is typically thought to represent glomerular leakage or damage, it is also a marker of systemic microvascular disease; this microvascular pathway, which is likely characterized by endothelial dysfunction and injury,33–35 could be a pathway that leads to the simultaneous development of HF and albuminuria. Another possibility is that kidney disease, represented by both glomerular and tubular injury, is causally related to the onset of HF, but albuminuria was a more sensitive indicator of incipient kidney disease than the tubular injury markers. Although an elevated ACR predominantly represents glomerular damage, tubular damage may also promote albuminuria since amounts of albumin are filtered and taken up by the proximal tubule and degraded, so the findings may not be completely specific to glomerular injury. Thus, an important finding in our study is that KIM-1:Cr remained significantly associated with HF independent of ACR, and that the correlation of the two markers was relatively weak.
Albuminuria is an established predictor of CKD and its complications. The association between elevated ACR and incident HF is consistent with previous studies in the general population, demonstrating that elevated ACR is independently associated with HF, even when accounting for eGFR. 4,5 Previous studies have also shown that KIM-1 and other markers of tubular injury are associated with worse outcomes in patients with HF. In another study of 90 patients with HF, urinary KIM-1 and N-acetyl-β-D-glucosaminidase (NAG), both markers of tubulointerstitial damage, were associated with increased HF-related hospitalizations or death after adjusting for eGFR and ACR.36
There are multiple possibilities for why ACR had a stronger association with HF than the tubular injury markers. One possibility is that glomerular injury, as captured by ACR, may be more related to HF risk than tubular injury. A second is that ACR is a marker of microvascular disease, which is likely in the pathway linking kidney disease to HF risk. In addition, ACR may be more strongly associated with HF than KIM-1 because it captures a mix of both tubular and glomerular dysfunction, thus explaining why it somewhat attenuates the associations of the tubular markers with HF.
Strengths of our study include the large sample size, the measures of multiple urine biomarkers and ACR concurrently, the sex and bi-racial design of the Health ABC cohort, and the power with 596 HF events enabling the detection of relatively small associations of tubular injury markers with incident HF risk. Our study also has important limitations. We studied a relatively healthy older population who may have low amounts of tubular injury, hampering our ability to detect associations of tubular injury with incident HF. Additionally, protease inhibitors were not used during biomarker storage, thus potentially biasing our results towards the null. The available measurements do not allow us to differentiate between reduced and preserved ejection fraction HF. Another limitation relates to the generalizability of this study – we exclusively studied older persons of black and white race. Generalizability to younger persons and other race/ethnicities is uncertain. Additionally, since the definition of HF required inpatient hospitalization, the generalizability of our findings may be limited to a more advanced spectrum of disease.
In conclusion, to our knowledge, this is the first study to indicate that markers of tubular injury, urine KIM-1 and IL-18, are associated with incident HF risk in community-living individuals. However, the marker of predominantly glomerular injury, urine ACR, appeared to be more strongly associated with incident HF risk than the markers of tubular injury. Based on these findings, future studies might investigate the contribution of kidney tubular injury to other CKD-related outcomes in community-living populations.
Supplementary Material
Acknowledgments
The Health ABC Study Principal Investigators and Coordinators are as follows: Anne B. Newman, MD, MPH, Piera Kost, Diane Ives, University of Pittsburgh, Pittsburgh; Suzanne Satterfield, MD, DrPH, Frances A. Tylavsky, DrPH, Jan Elam, University of Tennessee, Memphis; Stephen B. Kritchevsky, PhD, Wake Forest University, Winston-Salem, North Carolina; Steven R. Cummings, MD, Michael C. Nevitt, PhD, Susan M. Rubin, MPH, University of California, San Francisco; Tamara B. Harris, MD, Melissa E. Garcia, MPH, NIA, Bethesda, Maryland.
Support: Drs Shlipak and Sarnak were supported by NIA grant 5R01AG027002-07. Dr Driver was supported by UCSF-CTSI grant TL1 TR000144. NIA contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grant R01-AG028050; and National Institute of Nursing Research grant R01-NR012459 provided further support. The study sponsors had no role in study design; collection, analysis, and interpretation of the data; writing the report; and the decision to submit the report for publication.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Table S1: Characteristics by quartiles of ACR.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Descriptive Text for Online Delivery of Supplementary Material
Supplementary Table S1 (PDF).
Characteristics by quartiles of ACR.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003 Nov;42(5):1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 3.Mahmoodi BK, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. 2012 Nov 10;380(9854):1649–1661. doi: 10.1016/S0140-6736(12)61272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blecker S, Matsushita K, Kottgen A, et al. High-normal albuminuria and risk of heart failure in the community. Am J Kidney Dis. 2011 Jul;58(1):47–55. doi: 10.1053/j.ajkd.2011.02.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waheed S, Matsushita K, Sang Y, et al. Combined association of albuminuria and cystatin C-based estimated GFR with mortality, coronary heart disease, and heart failure outcomes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2012 Aug;60(2):207–216. doi: 10.1053/j.ajkd.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012 May;23(5):905–914. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011 Sep;22(9):1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peralta CA, Katz R, Bonventre JV, et al. Associations of Urinary Levels of Kidney Injury Molecule 1 (KIM-1) and Neutrophil Gelatinase-Associated Lipocalin (NGAL) With Kidney Function Decline in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2012 Dec;60(6):904–911. doi: 10.1053/j.ajkd.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalogeropoulos A, Georgiopoulou V, Psaty BM, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010 May 11;55(19):2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ix JH, Wassel CL, Kanaya AM, et al. Fetuin-A and incident diabetes mellitus in older persons. JAMA. 2008 Jul 9;300(2):182–188. doi: 10.1001/jama.300.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiramoto JS, Katz R, Ix JH, et al. Sex differences in the prevalence and clinical outcomes of subclinical peripheral artery disease in the Health, Aging, and Body Composition (Health ABC) study. Vascular. 2013 Mar 19; doi: 10.1177/1708538113476023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5(2):128–134. doi: 10.7150/ijbs.5.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- 14.Koster A, Stenholm S, Alley DE, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity. 2010 Dec;18(12):2354–2361. doi: 10.1038/oby.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, et al. Serum albumin concentration and heart failure risk The Health, Aging, and Body Composition Study. Am Heart J. 2010 Aug;160(2):279–285. doi: 10.1016/j.ahj.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999 Feb;59(1):1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 17.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012 Jul 5;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005 Nov 28;165(21):2460–2466. doi: 10.1001/archinte.165.21.2460. [DOI] [PubMed] [Google Scholar]
- 19.Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care. 2010 Dec;16(6):556–561. doi: 10.1097/MCC.0b013e32834008d3. [DOI] [PubMed] [Google Scholar]
- 20.Carlsson AC, Larsson A, Helmersson-Karlqvist J, et al. Urinary kidney injury molecule 1 and incidence of heart failure in elderly men. European journal of heart failure. 2013 Apr;15(4):441–446. doi: 10.1093/eurjhf/hfs187. [DOI] [PubMed] [Google Scholar]
- 21.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004 Mar;43(3):405–414. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 22.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002 Jul;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 23.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998 Feb 13;273(7):4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 24.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004 Mar;286(3):F552–563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 25.Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011 Sep;22(9):1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006 Jul;70(1):199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 27.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005 Oct;16(10):3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 28.Szeto CC, Kwan BC, Lai KB, et al. Urinary expression of kidney injury markers in renal transplant recipients. Clin J Am Soc Nephrol. 2010 Dec;5(12):2329–2337. doi: 10.2215/CJN.01910310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilis-Pstrusinska K, Medynska A, Zwolinska D, Wawro A. Interleukin-18 in urine and serum of children with idiopathic nephrotic syndrome. Kidney Blood Press Res. 2008;31(2):122–126. doi: 10.1159/000124284. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto K, Kanmatsuse K. Elevated interleukin-18 levels in the urine of nephrotic patients. Nephron. 2001 Aug;88(4):334–339. doi: 10.1159/000046017. [DOI] [PubMed] [Google Scholar]
- 31.Nauta FL, Bakker SJ, van Oeveren W, et al. Albuminuria, proteinuria, and novel urine biomarkers as predictors of long-term allograft outcomes in kidney transplant recipients. Am J Kidney Dis. 2011 May;57(5):733–743. doi: 10.1053/j.ajkd.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Sarnak MJ, Katz R, Stehman-Breen CO, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005 Apr 5;142(7):497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 33.Shearman CP, Gosling P. Microalbuminuria and vascular permeability. Lancet. 1988 Oct 15;2(8616):906–907. doi: 10.1016/s0140-6736(88)92504-4. [DOI] [PubMed] [Google Scholar]
- 34.Vlachou E, Gosling P, Moiemen NS. Microalbuminuria: a marker of systemic endothelial dysfunction during burn excision. Burns. 2008 Mar;34(2):241–246. doi: 10.1016/j.burns.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Gosling P, Shearman CP, Gwynn BR, Simms MH, Bainbridge ET. Microproteinuria: response to operation. Br Med J (Clin Res Ed) 1988 Jan 30;296(6618):338–339. doi: 10.1136/bmj.296.6618.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damman K, Van Veldhuisen DJ, Navis G, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010 Aug;96(16):1297–1302. doi: 10.1136/hrt.2010.194878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


