Abstract
Cholangiocarcinoma represents a diverse group of epithelial cancers united by late diagnosis and poor outcomes. Specific diagnostic and therapeutic approaches are undertaken for cholangiocarcinomas of different anatomical locations (intrahepatic, perihilar, and distal). Mixed hepatocellular cholangiocarcinomas have emerged as a distinct subtype of primary liver cancer. Clinicians need to be aware of intrahepatic cholangiocarcinomas arising in cirrhosis and properly assess liver masses in this setting for cholangiocarcinoma. Management of biliary obstruction is obligatory in perihilar cholangiocarcinoma, and advanced cytological tests such as fluorescence in-situ hybridisation for aneusomy are helpful in the diagnosis. Liver transplantation is a curative option for selected patients with perihilar but not with intrahepatic or distal cholangiocarcinoma. International efforts of clinicians and scientists are helping to identify the genetic drivers of cholangiocarcinoma progression, which will unveil early diagnostic markers and direct development of individualised therapies.
Introduction
Cholangiocarcinoma is an epithelial cell malignancy arising from varying locations within the biliary tree showing markers of cholangiocyte differentiation. The most contemporary classification based on anatomical location includes intrahepatic, perihilar, and distal cholangiocarcinoma. Intrahepatic cholangiocarcinoma is defined as a cholangiocarcinoma located proximally to the second degree bile ducts (proximal and distal refers to the direction of bile flow such that the intrahepatic bile ducts are proximal to the common bile duct); within the liver, perihilar cholangiocarcinoma is localised to the area between the second degree bile ducts and the insertion of the cystic duct into the common bile duct; whereas distal cholangiocarcinoma is confined to the area between the origin of the cystic duct and ampulla of Vater.1 Most cholangiocarcinomas are well, moderately, and poorly differentiated adenocarcinomas with other histological subtypes encountered rarely.2,3 Surgical treatment is the preferred option for all subtypes, but, when contemplated, involvement of the vascular structures and lymph nodes needs to be considered. The highly desmoplastic nature of cholangiocarcinoma, its extensive support by a rich tumour microenvironment, and profound genetic heterogeneity, all contribute to its therapeutic resistance. Although surgery and curative liver transplantation are options for selected patients with perihilar cholangiocarcinoma, 5-year survival rates are very low. The chemotherapy regimen of gemcitabine and cisplatin is often used for inoperable disease. Locoregional therapies are used for intrahepatic cholangiocarcinoma, but conclusive evidence for efficacy is lacking. Understanding of cholangiocarcinoma biology, the oncogenic landscape of this disease, and its complex interaction with the tumour microenvironment could lead to optimum therapies with improvement in patient survival. In view of much recent interest in this disease, a review of recent medical advances for cholangiocarcinoma is both timely and topical. In this Seminar we focus mainly on intrahepatic and perihilar cholangiocarcinoma because progress has predominantly occurred in these subtypes (panel).
Epidemiology and risk factors
Perihilar disease represents about 50%, distal disease 40%, and intrahepatic disease less than 10% of cholangiocarcinoma cases.4 Mixed hepatocellular-cholangiocellular carcinomas, also called combined hepatocellular-cholangiocellular carcinomas according to the WHO classification, were only recently acknowledged as a distinct subtype of cholangiocarcinoma.2,5,6 According to scarce reports,5,7 mixed hepatocellular-cholangiocellular carcinomas represent less than 1% of all liver cancers. The incidence of intrahepatic cholangiocarcinoma seems to be increasing in many western countries, although this pattern is not universal.8,9 Age-adjusted rates of cholangiocarcinoma are reported to be highest in Hispanic and Asian populations (2.8–3.3 per 100 000) and lowest in non-Hispanic white people and black people (both 2.1 per 100 000).10–12 The disease has a slight male predominance (1.2–1.5 per 100 000 vs one per 100 000 population),12 with the exception of the female Hispanic population in whom intrahepatic cholangiocarcinoma rates are increased (1.5 per 100 000) compared with the male population (0.9 per 100 000).12 Cholangiocarcinoma is unusual in children. Cumulative cholangiocarcinoma mortality rates have increased by 39% because of increased disease incidence.12 Mortality rates are higher in men and boys (1.9 per 100 000) than in women and girls (1.5 per 100 000). Mortality rates from intrahepatic cholangiocarcinoma are highest in American Indian and Alaska Native groups (1.3 per 100 000) and Asian populations (1.4 per 100 000) and lowest in white people (0.8 per 100 000) and black people (0.7 per 100 000).9 Both increased recognition and incidence have contributed to rising interest in this cancer.13
Most cholangiocarcinomas arise de novo, and no risk factors are identified. Recently, cirrhosis and viral hepatitis C and B have been recognised as risk factors for cholangiocarcinoma, especially intrahepatic disease. The contribution of hepatitis C and hepatitis B in tumour development differs in western countries, where hepatitis C is more prevalent, versus Asian countries, where hepatitis B is endemic. In studies from the USA and Europe,14–17 hepatitis C was shown to be a risk factor for cholangiocarcinoma with the strongest association for intrahepatic cholangiocarcinoma. Studies from South Korea and China18–20 have shown more consistently hepatitis B as a risk factor for intrahepatic cholangiocarcinoma; a Japanese study confirmed findings from western countries where intrahepatic cholangiocarcinoma association was stronger with hepatitis C exposure than with hepatitis B.21 Association with cirrhosis of different causes was identified in almost all of these studies. Pathogenically, release of inflammatory cytokines, cell death coupled to increases in cell proliferation, as well as changes in the liver in fibrosis favour tumorigenesis. However, the presence of cirrhosis is not uniformly shown in all patients with viral hepatitis who develop cholangiocarcinoma.20 A meta-analysis22 of several case-control studies on risk factors for intrahepatic cholangiocarcinoma showed the following associations: cirrhosis had a combined odds ratio (OR) of 22.92 (95% CI 18.24–28.79), hepatitis C of 4.84 (2.41–9.71), and hepatitis B of 5.10 (2.91–8.95).
There is a well established association between primary sclerosing cholangitis, marked by chronic inflammation with liver injury and likely proliferation of the progenitor cells, and cholangiocarcinoma, especially perihilar disease. The lifetime incidence of cholangiocarcinoma in this patient population ranges between 5% and 10%.23–26 About 50% of patients with primary sclerosing cholangitis who develop cholangiocarcinoma are diagnosed with cholangiocarcinoma within 24 months of diagnosis of primary sclerosing cholangitis.23,27 The risk of cholangiocarcinoma is lower 2–10 years after the diagnosis of primary sclerosing cholangitis (7%).26 The mean age of cholangiocarcinoma diagnosis in patients with primary sclerosing cholangitis is the fourth decade of life23,28 compared with the seventh decade in the general population.10,17 Although various risk factors for cholangiocarcinoma in primary sclerosing cholangitis have been reported, none are sufficient to guide risk stratification for disease surveillance. Guidelines for cholangiocarcinoma surveillance in patients with primary sclerosing cholangitis have been published.25,29,30
Early age at diagnosis is also noted in patients with bile duct cystic disorders, including Caroli's disease.10,14,31 These patients develop cholangiocarcinoma at a mean age of 32 years with lifetime incidence ranging from 6% to 30%.18 Southeast Asia has a very high incidence (113 per 100 000)10 of cholangiocarcinoma that is due to high prevalence of hepatobiliary flukes, Opisthorchis viverrini and Clonorchis sinensis, which are risk factors for cholangiocarcinoma.32,33 This risk is probably increased by environmental and genetic factors.34 Hepatolithiasis, in which 7% of patients develop intrahepatic cholangiocarcinoma,2,35 and biliary-enteric drainage, predisposing patients to enteric bacteria bile duct colonisation and infections,36 are additional risk factors for cholangiocarcinoma. Several genetic polymorphisms have been identified that increase risk of development of cholangiocarcinoma. The genes implicated as risk factors can be classified into those encoding proteins participating in cell DNA repair (MTHFR, TYMS, GSTO1, and XRCC1), cellular protection against toxins (ABCC2, CYP1A2, and NAT2), or immunological surveillance (KLRK1, MICA, and PTGS2).10 The results from studies on the role of alcohol and smoking exposure have been inconsistent.10,22 The metabolic syndrome was associated with an increased risk of intra hepatic cholangiocarcinoma in the Surveillance and Epidemiology Results database analysis (OR 1.6, 1.32–1.83, p<0.0001).37 Consistent with these observations, the meta-analysis22 of US and Danish studies identified an association of intra hepatic cholangiocarcinoma with diabetes with an OR of 1.89 (95% CI 1.74–2.07) and obesity with an OR of 1.56 (1.26–1.94). Although obesity is a biologically plausible risk factor for cholangiocarcinoma development, too few data are available to definitely establish an association at this time.10,22
Molecular pathogenesis
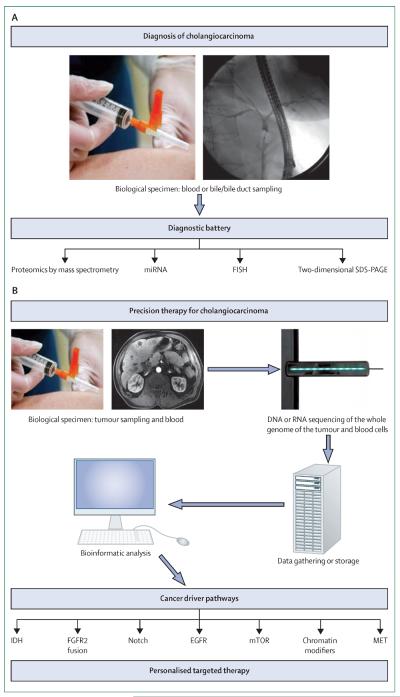
The era of individualised medicine and targeted therapies needs improved understanding of tumour biology and molecular pathogenesis. Carcinogenesis involves specific cell genome derangements.38 The genetic pathways contributing to the selective growth advantage of cancer cells can be organised into those governing cell fate and differentiation, proliferation, cell survival, and maintenance of genome integrity. Contemporary research techniques are allowing identification of several of these genetic changes in cholangiocarcinoma.39 However, misclassification of perihilar cholangiocarcinoma as intrahepatic cholangiocarcinoma in the early studies should be considered during interpretation of retrospective studies on molecular profiling. Further knowledge could identify driver mutations that can be successfully targeted resulting in improved patient survival. Unfortunately, curative therapies have been difficult to develop for solid tumours because of the extreme genetic heterogeneity between patients and rapid development of therapeutic resistance as the tumour genetically evolves. Several oncogenic pathways and drugs targeting these pathways have been identified (table). Several studies identifying genetic changes in cholangiocarcinoma have been published, but most of the data generated from the single studies need further validation. Hopefully, personalised or precision medicine is in the near future for the treatment of cholangiocarcinoma (figure 1).
Table.
Targetable cholangiocarcinoma signalling pathways with estimated frequency and corresponding molecular inhibitors
| Molecular inhibitors | |
|---|---|
| EGFR (RAS, RAF, MEK, ERK/MAPK), 14% | Erlotinib, cetuximab, irinotecan, panitumumab, lapatinib, sorafenib |
| VEGF, frequency unknown | Sorafenib, bevacizumab, erlotinib, cediranib, vandetanib |
| Her2/neu, 8% | Lapatinib |
| MET (PI3K, AKT, mTOR), 5% | Onartuzumab, tivantinib, crizotinib |
| mTOR, frequency unknown | Everolimus |
| MEK, frequency unknown | Selumetinib, trametinib |
| AKT, 1% | MK2206 |
| NFκB, frequency unknown | Bortezomib |
| PI3K/mTOR, 9% | GDC-0980 |
| PARP1/2, frequency unknown | Veliparib |
| MET/ROS/ALK, frequency unknown | Crizotinib |
| FGFR2 gene fusion, frequency unknown | PD173074, pazopanib |
| IDH1 and IDH2, 10–23% of intrahepatic cholangiocarcinomas | AGI-6780, AGI-5198 |
Table modified from Geynisman and colleagues.44
Figure 1. Integrative approach to (A) diagnosis and (B) individualised medicine in cholangiocarcinoma.
FISH=fluorescence in-situ hybridisation. SDS-PAGE=sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Cell survival signalling pathways
The Ras-MAPK pathway is one of the main signalling networks in cholangiocarcinoma biology and was reported in several studies. For example, Sia and colleagues40 used an integrative molecular analysis technique and correlated identified gene signatures with clinicopathological traits and patient outcomes for 119 cases of intrahepatic cholangiocarcinoma. The group described two distinct gene signature classes: a proliferation class and an inflammatory class. The proliferation class (62% of cases) was associated with copy number variations in several oncogenes, including but not restricted to KRAS and BRAF, as well as in genes from RAS, MAPK, and MET signalling networks. The proteins encoded by these genes are part of the signalling network in which the RAS-RAF-MEK-ERK signalling axis stimulates cell proliferation or the PI3K-AKT-mTOR signalling axis promotes cell survival. The inflammatory class showed activation of inflammatory pathways causing overexpression of cytokines and STAT3. The transcriptional factor STAT3 modulates cell growth and survival and has been implicated in carcinogenesis.41 These gene classes, particularly the proliferation class, in intrahepatic cholangiocarcinoma overlapped with those previously identified in hepatocellular carcinoma in which cell-cycle dysregulation, transforming growth factor β (TGFβ)/Wnt activation, α-fetoprotein positivity, and cholangiocarcinoma-like and cluster A classes were associated with poor outcomes. This finding implies cells of similar origin in both cancer subtypes or hepatocellular carcinoma cell dedifferentiation towards an adenocarcinoma phenotype. These data also emphasise that not all cancers have a proliferative signature. Besides uncontrollable cell proliferation, neoplastic transformation can also be accomplished by evasion of apoptosis, facilitation of cell migration (ie, metastatic potential), resistance to hypoxia, and increased vascularisation.
In another study,42 transcriptome profiling in 104 patients after cholangiocarcinoma resection in Europe, the USA, and Australia showed that KRAS mutations were associated with deregulation of epidermal growth factor (EGFR) and ERBB2 (also known as HER2) signalling network, which included MET. Derangement of genes participating in proteasomal activity was associated with poor prognosis. The therapeutic potential of tyrosine kinase inhibition in cholangiocarcinoma cell lines with activated EGFR and HER2 was also shown.42 Although EGFR might act as a hub for transmitting downstream signals to activate RAS-MAPK, JAK-STAT, and PI3K-AKT-mTOR pathways,43,44 the more likely situation is that cross-talk exists between various receptor tyrosine kinases.
206 somatic mutations were identified and examined with exome sequencing and comparison of eight liver-fluke-associated cholangiocarcinoma and matched normal tissue specimens in a study from Singapore.45 Among the common cancer-related genes, mutations in TP53 responsible for maintenance of genome integrity was most common (44%), followed by KRAS (17%), and SMAD4 (17%); SMAD4 contributes to the TGFβ signalling network, which is a key driver of metastatic cancer. Somatic mutations of genes involved in deactivation of histone modifiers, activation of G proteins, and loss of genome stability were present in 3.7–14.8% of cases in this study with many of the genes being newly implicated in oncogenesis (eg, KMT2C, ROBO2, RNF43, PEG3, and GNAS).45
Genetic changes in the tumour suppressive gene PTEN in combination with either activated AKT or mTOR were associated with poor patient outcomes in microarray analysis of 221 samples of extrahepatic cholangiocarcinoma.46 However, the correlation between these genetic changes and good outcomes was reported in 101 patients with intrahepatic cholangiocarcinoma in another study, in which mTOR and AKT activation was detected in more differentiated tumours.47 Novel fibroblast growth factor receptor 2 (FGFR2) rearrangements with gene fusion were identified in a subset of patients with cholangiocarcinoma and these mutations are targetable.48
Cell fate and differentiation
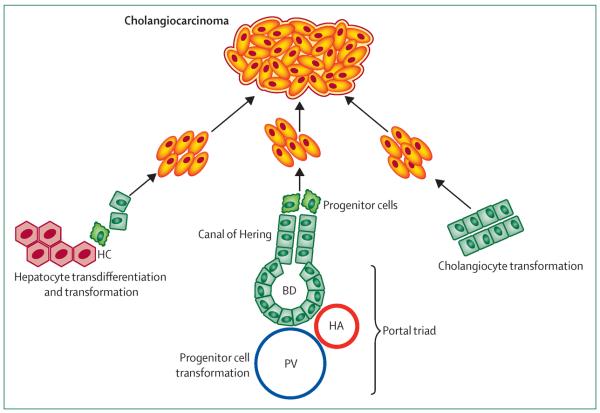
Notch signalling is vital in cell fate determination and regulates biliary duct formation.49 Its involvement in cholangiocarcinoma biology was reported in several studies. Notch pathway activation was implicated in conversion of mature adult hepatocytes into precursors of intrahepatic cholangiocarcinoma in two preclinical models involving cell fate tracing techniques.20,50 These studies challenge the theory that cholangiocarcinoma cells are derived from cholangiocytes, peribiliary glandular cells, or hepatic progenitor cells. They also emphasise the plasticity of liver cells regarding their differentiated state, and draw attention to transcriptome studies identifying overlap in hepatocellular carcinoma and cholangiocarcinoma signatures.40 A study51 in an animal model of diethylnitrosamine-induced hepatocellular carcinoma carcinogenesis showed the role of constitutive Notch2 activation in the development of poorly differentiated hepatocellular carcinoma with features of biliary epithelium (SOX9 positivity). These studies suggest that even differentiated liver cell subtypes are plastic and dominance of underlying oncogenic pathways can dictate cell histological features with variable malignant phenotypes (eg, hepatocellular or cholangiocellular carcinomas). Figure 2 shows the potential cells of origin for cholangiocarcinoma.
Figure 2. Potential cells of origin in intrahepatic cholangiocarcinoma.
PV=portal vein. HA=hepatic artery. BD=bile duct. HC=hepatic cell.
Experimental studies have also shown an important role for the Hh survival signalling pathway in cholangiocarcinoma52 with pathway inhibition being tumour suppressive in several studies.53 The mechanisms vary from inhibition of transcriptional activation and migration, to inhibition of miRNA expression.53–55 Interplay between Hh signalling and the myofibroblast-enriched cholangiocarcinoma microenvironment has also been identified: platelet-derived growth factor BB promotes tumour survival in an Hh-dependent manner in vitro and in an animal model.56
Isocitrate dehydrogenase (IDH) mutations and epigenetic changes
Genetic changes leading to survival advantages can also occur through epigenetic changes coupled to DNA coding changes. Hot-spot mutations of genes encoding IDH1 and IDH2 were recently reported by several groups to be fairly specific to intrahepatic cholangiocarcinoma in various gastrointestinal and biliary cancers (10–23%).57–59 These mutations are commonly identified in association with global DNA hypermethylation leading to multiple epigenetic changes.58 Identification of these new mutated genes is especially interesting because the product of enzymatic activity of IDH1 and IDH2, 2-hydroxyglutarate, can be detected in the serum and, therefore, potentially be used as a biomarker.60 Importantly, inhibition of IDH gain of function mutations has been reported, which reverses epigenetic methylation and promotes cancer cell differentiation.61,62 Cholangiocarcinoma would be a candidate for treatment with these inhibitors.
Cytotoxic and targeted therapies
A pragmatic practice standard was established by the ABC-2 study,63 in which 410 patients with advanced biliary tract cancer were randomly assigned to receive either gemcitabine and cisplatin in combination or gemcitabine alone. Patients receiving combination therapy had a median overall survival (OS) of 11.7 months versus 8.1 months in patients receiving gemcitabine alone (hazard ratio [HR] 0.64, 95% CI 0.52–0.80).63 Patients with gallbladder cancer and intrahepatic cholangiocarcinoma responded better to this regimen than did the rest of the trial population. The benefits of the combination therapy are, however, small and the number of patients low compared with other oncological trials. Therefore, these results should not preclude development of head-to-head trials of gemcitabine plus cisplatin versus promising therapies.44,64 More precise therapy might provide improved efficacy and safety profiles, and several of the signalling pathways involved in cholangiocarcinoma biology are possible targets (table).44
The number of clinical trials with targeted therapy alone or in combination with traditional chemotherapy is expanding. The single open-label randomised phase 3 trial65 with gemcitabine and oxaliplatin with or without erlotinib showed a small improvement in median progression-free survival in the subset of patients with cholangiocarcinoma receiving chemotherapy plus targeted therapy (5.9 months) versus chemotherapy alone (3.0 months; HR 0.73, 95% CI 0.53–1.00, p=0.049). Although sorafenib and lapatinib monotherapy was not effective, the combination of gemcitabine and oxaliplatin with cetuximab or bevacizumab is promising.66 Results of several phase 2 trials are pending. OS, instead of progression-free survival, should be the main endpoint in contemporary clinical trial designs.
Intrahepatic cholangiocarcinoma
Clinical classification and diagnosis
Intrahepatic cholangiocarcinoma can be classified morphologically by growth patterns as mass-forming, periductal-infiltrating, intraductal, superficial spreading, and undefined subtypes.2,67–69 The superficial spreading and intraductal subtypes are associated with the best prognosis and periductal and mass-forming subtypes with the worst. Intrahepatic cholangiocarcinoma presents as a malignant mass lesion usually in a noncirrhotic liver. However, when an intrahepatic lesion is noted in an imaging study in the setting of cirrhosis, the next diagnostic step is the differentiation between cholangiocarcinoma and hepatocellular carcinoma. Typical radiological features of cholangiocarcinoma include progressive contrast uptake throughout both arterial and venous phases of a cross-sectional imaging study.70 By contrast, hepatocellular carcinoma lesions are associated with hyperenhancement in the arterial phase and contrast washout in the venous phase of a contrast-enhanced imaging study. CT scan performance in intrahepatic cholangiocarcinoma was recently validated in a study71 in which intrahepatic lesions in patients with cirrhosis detected either on surveillance with ultrasound or incidentally (66% and 34%, respectively) were reassessed with a CT scan. All but one cholangiocarcinoma lesion showed typical heterogeneous contrast uptake due to a highly vascularised interface from peritumoural inflammation resulting in arterial enhancement of the tumour parenchymal margins, so-called rim enhancement. However, these classic features of intrahepatic cholangiocarcinoma were present in only 70% of cases in another study.72
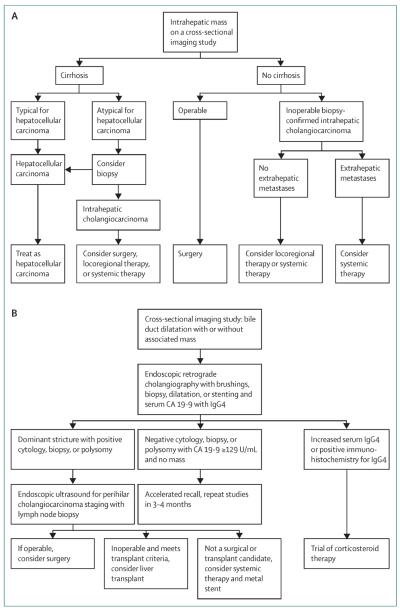
To further complicate this issue, liver cancer can contain both elements of cholangiocarcinoma and hepatocellular carcinoma in the same nodule, termed mixed hepatocellular-cholangiocellular carcinomas.5 Studies suggest that mixed hepatocellular-cholangio cellular carcinomas have a distinct appearance on cross-sectional imaging studies. A strong enhancing rim and irregular shape on gadoxetic acid-enhanced MRI favours mixed hepatocellular-cholangiocellular carcinoma, and lobulated shape, weak rim, and a target appearance favours a mass-forming intrahepatic cholangio carcinoma.73 The target appearance can also help to differentiate mixed hepatocellular-cholangiocellular carcinomas from atypical hypovascular hepatocellular carcinoma.74 The presence of liver capsule retraction and biliary dilatation in the vicinity of the intrahepatic lesion can also raise suspicion for a diagnosis of intrahepatic cholangiocarcinoma. A PET scan might be beneficial in assessment of metastatic disease,75 but many cholangiocarcinomas are PET negative with 18F-fluorodeoxyglucose.76 Contrast-enhanced ultrasound is associated with a very high misdiagnosis rate compared with MRI (52% vs 9%) and CT scans (52% vs 4%).77 Biopsy of the intrahepatic lesion is needed to differentiate hepatocellular carcinoma from cholangiocarcinoma to diagnose intrahepatic chol angiocarcinoma, especially if imaging studies do not show classic signs of hepatocellular carcinoma or if the distinction will change management (figure 3A).
Figure 3. Approach to management of (A) intrahepatic and (B) perihilar cholangiocarcinoma.
HCC=hepatocellular carcinoma. CA 19-9=carbohydrate antigen 19-9. Reproduced with modifications from reference 69 by permission of Elsevier.
Carbohydrate antigen 19-9 (CA 19-9) is a traditional serum biomarker used for cholangiocarcinoma diagnosis. In patients with primary sclerosing cholangitis the most reliable cutofffor intrahepatic cholangiocarcinoma is 129 U/mL, which provides sensitivity, specificity, and adjusted positive predictive values of 79%, 98%, and 57%, respectively.78,79 However, more than 30% of patients with primary sclerosing cholangitis with a CA 19-9 value higher than 129 U/mL do not have cholangiocarcinoma on long-term follow up,80,81 and alternative causes for this increase, including bacterial cholangitis, should be considered. CA 19-9 concentrations higher than 1000 U/mL are consistent with advanced disease often involving the peritoneum.80–82 When interpreting serum CA 19-9 concentrations one should also note whether patients who are negative for Lewis antigen (7% of general population) have undetectable serum CA 19-9 concentrations.83 A better-performing biomarker is needed.
Surgical resection and liver transplantation
Recommendations for treatment take into consideration the patient's surgical candidacy, biochemical characteristics, lesion size, presence of metastatic lesions, and vascular and lymphatic involvement. The tumour burden should be assessed with cross-sectional imaging studies of the chest and abdomen and potentially biopsy of the lymph nodes, when lymph nodes are larger than 2 cm. Curative surgical resection with negative tumour margins can be achieved in less than 30% of patients.4 The median survival time by intention-to-treat analysis of lesions considered to be surgically resectable on imaging studies is 36 months84 Positive tumour margins, lymph node metastases, cirrhosis, especially advanced cirrhosis with Child-Pugh score beyond A, and presence of portal hypertension are associated with poor outcomes in surgical cohorts.4,84,85 Contemporary studies do not support the option of liver transplantation for intrahepatic cholangiocarcinoma unlike for selected patients with perihilar cholangiocarcinoma. Indeed, even patients with mixed hepatocellular-cholangiocellular carcinomas have 1-year and 5-year cumulative risk of tumour recurrence of 42% and 65%, respectively, after liver transplantation.86
Palliative treatment with locoregional therapies
Like hepatocellular carcinoma, intrahepatic cholangiocarcinoma has a metastatic predilection for the liver and, therefore, locoregional therapy might be a reasonable palliative approach; the effectiveness of this option, however, has not been evaluated in high-quality randomised studies. Limitations of radiofrequency ablation are low effectiveness in lesions larger than 5 cm and technical complications in close proximity to the large vascular structures and liver capsule.87,88 Recurrence rates for intrahepatic cholangiocarcinoma are also quite high after radiofrequency ablation.87 Most studies examining transarterial chemoembolisation (TACE) are retrospective and do not use a standardised chemotherapeutic drug or schedule. However, data suggest acceptable tolerability and a potential survival benefit in patients receiving TACE (OS 12–15 months vs 3.3 months in the best supportive care group).89–92 TACE with use of drug-eluting beads might have similar effectiveness as systemic chemotherapy (OS 11.7 months and 11 months, respectively) and performs better than conventional TACE (OS 5.7 months).93 Safety and efficacy of selective intra-arterial radiotherapy with radioactive 90Y in an adjuvant setting was recently reported.94 The group reported a median OS of 22 months with no major toxicity-related events. In another report, 1-year survival after 90Y treatment was 56%.95 Contemporary stereotactic body radiotherapy in cholangiocarcinoma is associated with a high rate of treatment-related complications including acute radiation-induced liver dysfunction, biliary strictures, and gastrointestinal mucosa damage.96–98
Perihilar cholangiocarcinoma
Clinical classification and diagnosis
Perihilar cholangiocarcinomas are confined to the larger bile ducts in the hepatic hilum and are classified on the basis of morphological growth appearance in mass-forming exophytic and intraductal subtypes. Intraductal subtypes can be further subclassified as periductal infiltrating, the most common perihilar cholangiocarcinoma subtype, in addition to mass, and nodular perihilar cholangiocarcinoma subtypes. Intraductal papillary neoplasms are often well differentiated and have favourable prognosis, whereas presence of an invasive component predisposes to metastasis. The most recently described subtype is an intraductal tubulopapillary neoplasm, which has better prognosis than does exophytic perihilar cholangiocarcinoma.99 The acute onset of painless jaundice is a heralding presentation in 90% of patients with chol angiocarcinoma.3,100
Careful evaluation with cross-sectional imaging studies and endoscopic ultrasonography helps to delineate the tumour location, size, morphology, hepatic artery and portal vein involvement, volume of potential liver remnant, lymph node involvement, and presence of distant metastases.101 The number of studies dedicated to the performance of imaging techniques in perihilar cholangiocarcinoma is small and their quality modest.102 CT scan accuracy for the evaluation of the degree of bile duct involvement is 86% (95% CI 77–92) with sensitivity and specificity for portal vein involvement of 89% (80–94) and 92% (85–96), hepatic artery of 83% (63–94) and 93% (69–99), and lymph node involvement of 61% (28–86) and 88% (74–95), respectively.102 CT scans frequently do not detect peritoneal metastases.103 MRI, similar to CT, can detect proximal to stricture bile duct dilatation and perihilar mass, but magnetic resonance cholangiography (MRC) adds another dimension to the study, better delineating extent of the bile duct lesion (figure 4A, B, and D). MRI enhanced with MRC has 89% sensitivity and 76% accuracy.78 When treatment with liver transplantation is feasible, evaluation of perihilar cholangiocarcinoma with endoscopic ultrasonography should not be accompanied by tumour sampling because of the high risk of needle tract seeding, which precludes this potentially curative treatment.104,105 By contrast, fine-needle aspiration of lymph node tissue can be a valuable aid in the diagnosis of advanced perihilar chol angiocarcinoma. The role of CA 19-9 in the diagnosis of perihilar cholangiocarcinoma does not differ from that for intrahepatic disease. The serum concentration of IgG4 should be obtained to rule out IgG4-related cholangiopathy.106 However, serum IgG4 can also be increased in cholangiocarcinoma.107
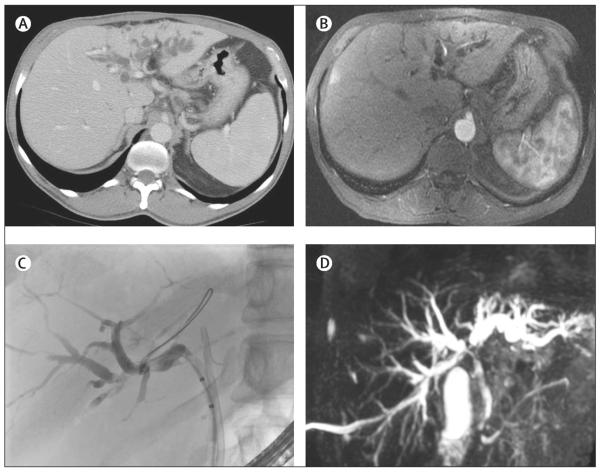
Figure 4. Imaging studies of a patient with perihilar cholangiocarcinoma of the left hepatic duct.
Note prominent left hepatic duct dilatation with obstruction of the left hepatic duct system on the CT scan (A), MRI (B), endoscopic retrograde cholangiography (C), and magnetic resonance cholangiography (D); (D) also shows bilateral obstruction of the biliary system at the right and left hepatic bile duct confluence.
Endoscopic retrograde cholangiography is an invaluable approach in the initial evaluation of the biliary tree (figure 4C) and as a first therapeutic step. Delineation of the biliary anatomy with MRI/MRC or CT scan, or both, before cholangiography should guide the endoscopic approach. Presence of a dominant stricture with or without upstream biliary duct dilatation is an indication for cytological evaluation through biliary brushings (figure 3B). The evaluation should be done with conventional cytological analysis and, if available, fluorescence in-situ hybridisation (FISH). Assessment of conventional cytology is compromised by inflammatory changes due to stenting and infection and the highly desmoplastic nature of perihilar cholangiocarcinomas. FISH analysis, which is based on detection of quantitative genetic chromosomal changes indicating aneusomy (chromosome pair imbalance), increases sensitivity of conventional cytology from 15% to 38–58%.69 Serial polysomy detected by FISH in patients with primary sclerosing cholangitis can identify a subgroup of patients at high risk of development of cholangiocarcinoma compared with patients without polysomy.108 FISH analysis can detect lesions up to 2.7 years before a tumour is apparent on imaging studies.108,109 Percutaneous transhepatic cholangiography (PTC) can assist in gaining access to strictures not amenable to endoscopic retrograde cholangiography. However, PTC is best used as an interim step with PTC-placed stent internalisation either simultaneously with the procedure or after tract maturation, in 2–4 weeks, to improve physiological bile flow and minimise patient discomfort.
Surgical treatment and liver transplantation
A new proposed surgical staging system has been designed to guide the surgical plan and selection of patients who might benefit from surgery.101 The surgery is rather complex and often necessitates lobar hepatic and bile duct resection, regional lymphadenopathy, and Roux-en-Y hepaticojejunostomy. The surgical techniques become more sophisticated, and have been aided by the incorporation of extended lobectomy, vascular reconstruction, and preoperative portal vein embolisation.110–112 This final procedure promotes hypertrophy of the uncompromised liver lobe and increases liver remnant volume. The success of the approach is often dependent on vascular anatomy.113 Portal vein ligation and in-situ splitting of the liver, referred to as associating liver partition and portal vein ligation for staged hepatectomy, promotes rapid liver regeneration and was newly introduced for the “small-for-size” setting.110 However, this technique is associated with substantial morbidity and mortality and needs further evaluation. Positive regional lymph nodes (ie, cystic, pericholedochal, hepatic arterial, portal, and posterior pancreaticoduodenal) are no longer an absolute contraindication to surgical resection but, understandably, are associated with less favourable outcomes compared with patients with negative lymph nodes.69,114
The role of biliary tract stenting immediately before surgery is still the subject of debate.115 In patients with inoperable disease, drainage of 50% or more of the liver parenchyma can improve patient survival,116,117 but bilateral biliary stents can also predispose to stent-related complications, including infectious cholangitis.118 Despite misperceptions, covered self-expandable metal stents do not preclude either further surgery or radiotherapy. Before the treatment plan is finalised, plastic biliary or covered self-expandable metal stents should be used. Covered stents prevent tumour ingrowth, but might migrate and are associated with increased rates of acute cholecystitis and pancreatitis.119–122 Placement of uncovered self-expandable metal stents, which can be dilated or stented in the future but cannot be removed, is a palliative option. As a rule of thumb, any patient with a biliary stent or stents in place and symptoms suggestive of acute infection should be promptly started on antibiotics providing coverage for Gram-negative microorganisms and be seen for stent re-evaluation and possible exchange.
Liver transplantation with neoadjuvant chemoradiation is the approach associated with the best outcomes in this lethal disease; however, only a few patients meet criteria for this option. The inclusion criteria include unresectable perihilar cholangiocarcinoma 3 cm or less in radial diameter without intrahepatic or extrahepatic metastases.123 The 5-year recurrence-free survival rate is similar to that for other widely accepted indications for liver transplantation at 68%.124 Patients with perihilar cholangiocarcinoma complicating primary sclerosing cholangitis should be treated, when possible, with liver transplantation, which will address the oncogenic field defect and underlying chronic progressive liver disease and negate potential complications from surgery in patients with advanced parenchymal liver disease (eg, portal hypertension). The notion of a neoplastic field defect in primary sclerosing cholangitis refers to the process in which chronic exposure of the biliary epithelium to oncogenic stimuli leads to field cancerisation. This idea is supported by frequent findings of synchronous biliary dysplastic lesions in liver explants of patients with primary sclerosing cholangitis who were diagnosed with cholangiocarcinoma.125
Treatment for advanced disease
When a patient is not eligible for surgery or liver transplantation, chemotherapy with a gemcitabine and cisplatin combination can be considered. However, in the ABC-02 trial63 the efficacy of this combination was not significantly different from that of gemcitabine alone in patients with perihilar cholangiocarcinoma; therefore, a practice standard of care has not been established for this subtype of cholangiocarcinoma. Proper preparation for chemotherapy includes biliary stenting. If palliative therapy is the goal of treatment and life expectancy is beyond 4–6 months, metal stents provide better durability, subject patients to less frequent invasive procedures, and are more cost-effective compared with plastic stents.126–128 Benefits of metal stents versus plastic stents and bilateral versus unilateral stents have been shown in maintenance of biliary patency.129 Metal stents have also been reported to improve survival compared with plastic stents (146 days and 49 days, respectively).130 Endoscopic intraductal radiofrequency ablation is another feasible palliative option with acceptable complication rates and is currently under development.131
Metastatic cholangiocarcinoma
The most common route for intrahepatic cholangiocarcinoma dissemination is intrahepatic involving the venous system. Spreading through the lymphatic system or along the biliary lumen is also reported. Perihilar cholangiocarcinoma usually metastasises through the lymphatic system. High tumour expression of VEGF is associated with intrahepatic metastases and EGFR overexpression with perineural invasion and lymphatic vessel invasion in intrahepatic cholangiocarcinoma.132 Stromal cells and their secreted extracellular proteins are crucial for establishing the metastatic niche.133 In an animal model of cholangiocarcinoma, a Smac mimetic was shown to prevent extrahepatic metastases.134 Presence of metastases in cholangiocarcinoma is one of the main determinants of therapy, and patients with metastatic disease should be considered for systemic chemotherapy with gemcitabine and cisplatin.
Future directions
Continued dissection of the molecular pathways driving cholangiocarcinoma progression will focus our efforts on an individualised medicine approach for this cancer when advanced or in the adjuvant setting. Recent work examining risk factors for the development of intrahepatic cholangiocarcinoma emphasises the association between metformin use and a reduction in incidence of this disease in patients with diabetes.135 This finding is biologically plausible because the mTOR signalling pathway, which is a target of metformin pharmacologically, is part of the cholangiocarcinoma oncogenic network. Thus, metformin use might be chemopreventive and calls for prospective studies, especially if a high-risk group can be identified (eg, a genetically high-risk population with primary sclerosing cholangitis). Another new direction is to approach tumour treatment in the context of its microenvironment. The stroma encompassing tumour can no longer be regarded as a barrier to tumour progression, but rather a crucial component governing tumour development and progression. Specifically, evidence is growing for the role of cancer-associated fibroblasts (CAFs) in tumour advancement, metastases, and chemoresistance.136 Tumour expression of α-SMA, the hallmark of CAFs, was negatively associated with survival of patients with intrahepatic cholangiocarcinoma. Active cross-talk between the tumour microenvironment and CAFs involves paracrine and autocrine signalling through modulation of growth factors and developmental (ie, Hedgehog) pathways.136 Targeting of CAFs could be an additional focus for development of new therapies and success of this approach was reported in a preclinical model using the BH3 mimetic, navitoclax.137 Further improvement of the currently available animal models of cholangiocarcinoma138 will be beneficial.
Identification of new tumour biomarkers in biological specimens is another important future direction. Genetic signatures for cholangiocarcinoma in serum, bile, or stool, similar to DNA stool testing for pancreatic cancer,139 need to be evaluated. Bile specimen examination with cytology and development of more specific diagnostic batteries using advanced technologies (ie, electrospray ionisation tandem mass spectrometry, two-dimensional gel electrophoresis, surface-enhanced laser desorption or ionisation, protein chips, and proteome analysis) might also be informative. These studies should be strengthened by elucidation of the role of the biomarkers in tumour biology (ie, the role of miRNAs in cholangiocarcinoma biology).140,141 The quality process for sample acquisition, processing, and interpretation needs to be standardised. In the near future we might be able to offer our patients an individualised therapy based on the driver mutation for their particular cancer and practise precision medicine.
Search strategy and selection criteria.
We searched PubMed for articles in English using the combination of keywords “cholangiocarcinoma” with “carcinogenesis”, “progression”, “pathophysiology”, “molecular pathogenesis”, “genetics”, “diagnosis”, “markers”, “imaging”, “treatment”, “chemotherapy”, “surgery”, “stent”, and “radiation”. The search included articles published from Jan 1, 1985, to May 31, 2013, and preferences were given to highly cited publications, articles published in the past 3 years, and articles published since the previous Seminar about cholangiocarcinoma in The Lancet in 2005. Owing to the very small number of randomised controlled trials in cholangiocarcinoma and limited space, review articles and centre experiences comprise a large number of references in this Seminar.
Panel: Key messages
Cholangiocarcinoma is anatomically classified as intrahepatic, perihilar, and distal
Mixed hepatocellular-cholangiocellular carcinoma is a subtype of intrahepatic neoplasm that shows markers of hepatocellular carcinoma and cholangiocarcinoma differentiation simultaneously and is associated with worse prognosis compared with hepatocellular carcinoma
Cirrhosis and hepatitis B and C are recently identified risk factors for intrahepatic cholangiocarcinoma
All intrahepatic lesions in cirrhosis should be investigated to rule out the possibility of intrahepatic cholangiocarcinoma
Fluorescence in-situ hybridisation improves performance of cytological evaluation of biliary brushings for the diagnosis of perihilar cholangiocarcinoma
Proliferative and inflammatory gene signature classes have been described in intrahepatic cholangiocarcinoma; FGFR2 gene fusion and IDH1 and IDH2 mutations are newly identified targetable derangements in cholangiocarcinoma
Surgical resection is a first-line therapy in patients with intrahepatic or perihilar cholangiocarcinoma who are good surgical candidates and have no evidence of disease progression beyond regional lymph nodes
Surgical techniques for perihilar cholangiocarcinoma are improved by extended resection, portal vein embolisation, and associating liver partition and portal vein ligation for staged hepatectomy
The best outcomes are observed in highly selected patients with perihilar cholangiocarcinoma treated with liver transplantation coupled with neoadjuvant chemoradiation
Locoregional therapies can be considered for intrahepatic cholangiocarcinoma
Gemcitabine and cisplatin combination is an acceptable standard of practice for advanced intrahepatic cholangiocarcinoma; for perihilar disease the effectiveness remains less proven
Elucidation of cholangiocarcinoma molecular pathogenesis could guide early diagnosis, prevention, and individualised treatment
Acknowledgments
This work was supported by US National Institutes of Health grants DK59427 (GJG) and T32 DK007198 (NR), and the Mayo Foundation. We thank Konstantinos Lazaridis for contributing to the discussion of cholangiocarcinoma treatment in the context of personalised medicine, and Courtney Hoover for secretarial support.
Footnotes
Contributors NR contributed to outline and drafting of the Seminar, critical revision, and important intellectual content. GJG contributed to the outline of the Seminar, critical revision for important intellectual content, and provided writing supervision.
Conflicts of interest We declare that we have no conflicts of interest.
References
- 1.Razumilava N, Gores GJ. Combination of gemcitabine and cisplatin for biliary tract cancer: a platform to build on. J Hepatol. 2011;54:577–78. doi: 10.1016/j.jhep.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010;2:419–27. doi: 10.4254/wjh.v2.i12.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–21. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komuta M, Govaere O, Vandecaveye V, et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876–88. doi: 10.1002/hep.25595. [DOI] [PubMed] [Google Scholar]
- 6.Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818–22. doi: 10.1038/sj.onc.1209558. [DOI] [PubMed] [Google Scholar]
- 7.Akiba J, Nakashima O, Hattori S, et al. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol. 2013;37:496–505. doi: 10.1097/PAS.0b013e31827332b0. [DOI] [PubMed] [Google Scholar]
- 8.Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–54. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 9.McLean L, Patel T. Racial and ethnic variations in the epidemiology of intrahepatic cholangiocarcinoma in the United States. Liver Int. 2006;26:1047–53. doi: 10.1111/j.1478-3231.2006.01350.x. [DOI] [PubMed] [Google Scholar]
- 10.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–84. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–25. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 12.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–44. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 13.Khan SA, Davidson BR, Goldin RD, et al. British Society of Gastroenterology Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657–69. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 14.Welzel TM, Mellemkjaer L, Gloria G, et al. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer. 2007;120:638–41. doi: 10.1002/ijc.22283. [DOI] [PubMed] [Google Scholar]
- 15.Donato F, Gelatti U, Tagger A, et al. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12:959–64. doi: 10.1023/a:1013747228572. [DOI] [PubMed] [Google Scholar]
- 16.El-Serag HB, Engels EA, Landgren O, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: a population-based study of U.S. veterans. Hepatology. 2009;49:116–23. doi: 10.1002/hep.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620–26. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 18.Lee TY, Lee SS, Jung SW, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol. 2008;103:1716–20. doi: 10.1111/j.1572-0241.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhou YM, Yin ZF, Yang JM, et al. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2008;14:632–35. doi: 10.3748/wjg.14.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–18. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto S, Kubo S, Hai S, et al. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci. 2004;95:592–95. doi: 10.1111/j.1349-7006.2004.tb02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25-year single-centre experience. Eur J Gastroenterol Hepatol. 2012;24:1051–58. doi: 10.1097/MEG.0b013e3283554bbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–27. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 25.Chapman R, Fevery J, Kalloo A, et al. American Association for the Study of Liver Diseases Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–78. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 26.Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158–64. doi: 10.1016/j.jhep.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Boberg KM, Bergquist A, Mitchell S, et al. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol. 2002;37:1205–11. doi: 10.1080/003655202760373434. [DOI] [PubMed] [Google Scholar]
- 28.Chalasani N, Baluyut A, Ismail A, et al. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology. 2000;31:7–11. doi: 10.1002/hep.510310103. [DOI] [PubMed] [Google Scholar]
- 29.European Association for the Study of the Liver EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–67. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011;54:1842–52. doi: 10.1002/hep.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Söreide K, Körner H, Havnen J, Söreide JA. Bile duct cysts in adults. Br J Surg. 2004;91:1538–48. doi: 10.1002/bjs.4815. [DOI] [PubMed] [Google Scholar]
- 32.Kaewpitoon N, Kaewpitoon SJ, Pengsaa P, Sripa B. Opisthorchis viverrini: the carcinogenic human liver fluke. World J Gastroenterol. 2008;14:666–74. doi: 10.3748/wjg.14.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin HR, Lee CU, Park HJ, et al. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996;25:933–40. doi: 10.1093/ije/25.5.933. [DOI] [PubMed] [Google Scholar]
- 34.Honjo S, Srivatanakul P, Sriplung H, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast Thailand. Int J Cancer. 2005;117:854–60. doi: 10.1002/ijc.21146. [DOI] [PubMed] [Google Scholar]
- 35.Huang MH, Chen CH, Yen CM, et al. Relation of hepatolithiasis to helminthic infestation. J Gastroenterol Hepatol. 2005;20:141–46. doi: 10.1111/j.1440-1746.2004.03523.x. [DOI] [PubMed] [Google Scholar]
- 36.Tocchi A, Mazzoni G, Liotta G, Lepre L, Cassini D, Miccini M. Late development of bile duct cancer in patients who had biliary-enteric drainage for benign disease: a follow-up study of more than 1000 patients. Ann Surg. 2001;234:210–14. doi: 10.1097/00000658-200108000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–71. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zabron A, Edwards RJ, Khan SA. The challenge of cholangiocarcinoma: dissecting the molecular mechanisms of an insidious cancer. Dis Model Mech. 2013;6:281–92. doi: 10.1242/dmm.010561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sia D, Hoshida Y, Villanueva A, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829–40. doi: 10.1053/j.gastro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30:1005–14. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–31. e15. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han W, Lo HW. Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett. 2012;318:124–34. doi: 10.1016/j.canlet.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geynisman DM, Catenacci DV. Toward personalized treatment of advanced biliary tract cancers. Discov Med. 2012;14:41–57. [PubMed] [Google Scholar]
- 45.Ong CK, Subimerb C, Pairojkul C, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet. 2012;44:690–93. doi: 10.1038/ng.2273. [DOI] [PubMed] [Google Scholar]
- 46.Chung JY, Hong SM, Choi BY, Cho H, Yu E, Hewitt SM. The expression of phospho-AKT, phospho-mTOR, and PTEN in extrahepatic cholangiocarcinoma. Clin Cancer Res. 2009;15:660–67. doi: 10.1158/1078-0432.CCR-08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee D, Do IG, Choi K, et al. The expression of phospho-AKT1 and phospho-MTOR is associated with a favorable prognosis independent of PTEN expression in intrahepatic cholangiocarcinomas. Mod Pathol. 2012;25:131–39. doi: 10.1038/modpathol.2011.133. [DOI] [PubMed] [Google Scholar]
- 48.Wu YM, Su F, Kalyana-Sundaram S, et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013;3:636–47. doi: 10.1158/2159-8290.CD-13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hofmann JJ, Zovein AC, Koh H, Radtke F, Weinmaster G, Iruela-Arispe ML. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137:4061–72. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan B, Malato Y, Calvisi DF, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–15. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dill MT, Tornillo L, Fritzius T, et al. Constitutive Notch2 signaling induces hepatic tumors in mice. Hepatology. 2013;57:1607–19. doi: 10.1002/hep.26165. [DOI] [PubMed] [Google Scholar]
- 52.Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–51. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 53.Jinawath A, Akiyama Y, Sripa B, Yuasa Y. Dual blockade of the Hedgehog and ERK1/2 pathways coordinately decreases proliferation and survival of cholangiocarcinoma cells. J Cancer Res Clin Oncol. 2007;133:271–78. doi: 10.1007/s00432-006-0166-9. [DOI] [PubMed] [Google Scholar]
- 54.El Khatib M, Kalnytska A, Palagani V, et al. Inhibition of hedgehog signaling attenuates carcinogenesis in vitro and increases necrosis of cholangiocellular carcinoma. Hepatology. 2013;57:1035–45. doi: 10.1002/hep.26147. [DOI] [PubMed] [Google Scholar]
- 55.Razumilava N, Bronk SF, Smoot RL, et al. miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and promotes apoptosis resistance in cholangiocarcinoma. Hepatology. 2012;55:465–75. doi: 10.1002/hep.24698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fingas CD, Bronk SF, Werneburg NW, et al. Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54:2076–88. doi: 10.1002/hep.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang P, Dong Q, Zhang C, et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene. 2013;32:3091–100. doi: 10.1038/onc.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kipp BR, Voss JS, Kerr SE, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol. 2012;43:1552–58. doi: 10.1016/j.humpath.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Reitman ZJ, Parsons DW, Yan H. IDH1 and IDH2: not your typical oncogenes. Cancer Cell. 2010;17:215–16. doi: 10.1016/j.ccr.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–30. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang F, Travins J, DeLaBarre B, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340:622–26. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]
- 63.Valle J, Wasan H, Palmer DH, et al. ABC-02 Trial Investigators Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 64.Hezel AF, Deshpande V, Zhu AX. Genetics of biliary tract cancers and emerging targeted therapies. J Clin Oncol. 2010;28:3531–40. doi: 10.1200/JCO.2009.27.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13:181–88. doi: 10.1016/S1470-2045(11)70301-1. [DOI] [PubMed] [Google Scholar]
- 66.Sia D, Tovar V, Moeini A, Llovet JM. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013;32:4861–70. doi: 10.1038/onc.2012.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10:288–91. doi: 10.1007/s00534-002-0732-8. [DOI] [PubMed] [Google Scholar]
- 68.Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–22. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11:13–21. e1. doi: 10.1016/j.cgh.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rimola J, Forner A, Reig M, et al. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology. 2009;50:791–98. doi: 10.1002/hep.23071. [DOI] [PubMed] [Google Scholar]
- 71.Iavarone M, Piscaglia F, Vavassori S, et al. Contrast enhanced CT-scan to diagnose intrahepatic cholangiocarcinoma in patients with cirrhosis. J Hepatol. 2013;58:1188–93. doi: 10.1016/j.jhep.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Kim SH, Lee CH, Kim BH, et al. Typical and atypical imaging findings of intrahepatic cholangiocarcinoma using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging. J Comput Assist Tomogr. 2012;36:704–09. doi: 10.1097/RCT.0b013e3182706562. [DOI] [PubMed] [Google Scholar]
- 73.Hwang J, Kim YK, Park MJ, et al. Differentiating combined hepatocellular and cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma using gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2012;36:881–89. doi: 10.1002/jmri.23728. [DOI] [PubMed] [Google Scholar]
- 74.Chong YS, Kim YK, Lee MW, et al. Differentiating mass-forming intrahepatic cholangiocarcinoma from atypical hepatocellular carcinoma using gadoxetic acid-enhanced MRI. Clin Radiol. 2012;67:766–73. doi: 10.1016/j.crad.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 75.Lan BY, Kwee SA, Wong LL. Positron emission tomography in hepatobiliary and pancreatic malignancies: a review. Am J Surg. 2012;204:232–41. doi: 10.1016/j.amjsurg.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90–97. doi: 10.1016/j.gassur.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Galassi M, Iavarone M, Rossi S, et al. Patterns of appearance and risk of misdiagnosis of intrahepatic cholangiocarcinoma in cirrhosis at contrast enhanced ultrasound. Liver Int. 2013;33:771–79. doi: 10.1111/liv.12124. [DOI] [PubMed] [Google Scholar]
- 78.Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106–17. doi: 10.1002/hep.22441. [DOI] [PubMed] [Google Scholar]
- 79.Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50:1734–40. doi: 10.1007/s10620-005-2927-8. [DOI] [PubMed] [Google Scholar]
- 80.Venkatesh PG, Navaneethan U, Shen B, McCullough AJ. Increased serum levels of carbohydrate antigen 19-9 and outcomes in primary sclerosing cholangitis patients without cholangiocarcinoma. Dig Dis Sci. 2013;58:850–57. doi: 10.1007/s10620-012-2401-3. [DOI] [PubMed] [Google Scholar]
- 81.Sinakos E, Saenger AK, Keach J, Kim WR, Lindor KD. Many patients with primary sclerosing cholangitis and increased serum levels of carbohydrate antigen 19-9 do not have cholangiocarcinoma. Clin Gastroenterol Hepatol. 2011;9:434–9. e1. doi: 10.1016/j.cgh.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 82.Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204–07. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 83.Nehls O, Gregor M, Klump B. Serum and bile markers for cholangiocarcinoma. Semin Liver Dis. 2004;24:139–54. doi: 10.1055/s-2004-828891. [DOI] [PubMed] [Google Scholar]
- 84.Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 85.Li YY, Li H, Lv P, et al. Prognostic value of cirrhosis for intrahepatic cholangiocarcinoma after surgical treatment. J Gastrointest Surg. 2011;15:608–13. doi: 10.1007/s11605-011-1419-8. [DOI] [PubMed] [Google Scholar]
- 86.Sapisochin G, Fidelman N, Roberts JP, Yao FY. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl. 2011;17:934–42. doi: 10.1002/lt.22307. [DOI] [PubMed] [Google Scholar]
- 87.Kim JH, Won HJ, Shin YM, Kim KA, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011;196:W205–9. doi: 10.2214/AJR.10.4937. [DOI] [PubMed] [Google Scholar]
- 88.Xu HX, Wang Y, Lu MD, Liu LN. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol. 2012;85:1078–84. doi: 10.1259/bjr/24563774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiefer MV, Albert M, McNally M, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer. 2011;117:1498–505. doi: 10.1002/cncr.25625. [DOI] [PubMed] [Google Scholar]
- 90.Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66:322–28. doi: 10.1016/j.crad.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 91.Vogl TJ, Naguib NN, Nour-Eldin NE, et al. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: results and prognostic factors governing treatment success. Int J Cancer. 2012;131:733–40. doi: 10.1002/ijc.26407. [DOI] [PubMed] [Google Scholar]
- 92.Shen WF, Zhong W, Liu Q, Sui CJ, Huang YQ, Yang JM. Adjuvant transcatheter arterial chemoembolization for intrahepatic cholangiocarcinoma after curative surgery: retrospective control study. World J Surg. 2011;35:2083–91. doi: 10.1007/s00268-011-1171-y. [DOI] [PubMed] [Google Scholar]
- 93.Kuhlmann JB, Euringer W, Spangenberg HC, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol. 2012;24:437–43. doi: 10.1097/MEG.0b013e3283502241. [DOI] [PubMed] [Google Scholar]
- 94.Hoffmann RT, Paprottka PM, Schön A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35:105–16. doi: 10.1007/s00270-011-0142-x. [DOI] [PubMed] [Google Scholar]
- 95.Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol. 2013;36:440–48. doi: 10.1007/s00270-012-0463-4. [DOI] [PubMed] [Google Scholar]
- 96.Kopek N, Holt MI, Hansen AT, Høyer M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol. 2010;94:47–52. doi: 10.1016/j.radonc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 97.Barney BM, Olivier KR, Miller RC, Haddock MG. Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol. 2012;7:67. doi: 10.1186/1748-717X-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Polistina FA, Guglielmi R, Baiocchi C, et al. Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience. Radiother Oncol. 2011;99:120–23. doi: 10.1016/j.radonc.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 99.Katabi N, Torres J, Klimstra DS. Intraductal tubular neoplasms of the bile ducts. Am J Surg Pathol. 2012;36:1647–55. doi: 10.1097/PAS.0b013e3182684d4f. [DOI] [PubMed] [Google Scholar]
- 100.Nagorney DM, Donohue JH, Farnell MB, Schleck CD, Ilstrup DM. Outcomes after curative resections of cholangiocarcinoma. Arch Surg. 1993;128:871–77. doi: 10.1001/archsurg.1993.01420200045008. [DOI] [PubMed] [Google Scholar]
- 101.Deoliveira ML, Schulick RD, Nimura Y, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363–71. doi: 10.1002/hep.24227. [DOI] [PubMed] [Google Scholar]
- 102.Ruys AT, van Beem BE, Engelbrecht MR, Bipat S, Stoker J, Van Gulik TM. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol. 2012;85:1255–62. doi: 10.1259/bjr/88405305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vilgrain V. Staging cholangiocarcinoma by imaging studies. HPB (Oxford) 2008;10:106–09. doi: 10.1080/13651820801992617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford) 2011;13:356–60. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levy MJ, Heimbach JK, Gores GJ. Endoscopic ultrasound staging of cholangiocarcinoma. Curr Opin Gastroenterol. 2012;28:244–52. doi: 10.1097/MOG.0b013e32835005bc. [DOI] [PubMed] [Google Scholar]
- 106.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–51. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 107.Oseini AM, Chaiteerakij R, Shire AM, et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology. 2011;54:940–48. doi: 10.1002/hep.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barr Fritcher EG, Kipp BR, Voss JS, et al. Primary sclerosing cholangitis patients with serial polysomy fluorescence in situ hybridization results are at increased risk of cholangiocarcinoma. Am J Gastroenterol. 2011;106:2023–28. doi: 10.1038/ajg.2011.272. [DOI] [PubMed] [Google Scholar]
- 109.Gonda TA, Glick MP, Sethi A, et al. Polysomy and p16 deletion by fluorescence in situ hybridization in the diagnosis of indeterminate biliary strictures. Gastrointest Endosc. 2012;75:74–79. doi: 10.1016/j.gie.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 110.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–14. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 111.Hemming AW, Mekeel K, Khanna A, Baquerizo A, Kim RD. Portal vein resection in management of hilar cholangiocarcinoma. J Am Coll Surg. 2011;212:604–13. doi: 10.1016/j.jamcollsurg.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 112.Hong YK, Choi SB, Lee KH, et al. The efficacy of portal vein embolization prior to right extended hemihepatectomy for hilar cholangiocellular carcinoma: a retrospective cohort study. Eur J Surg Oncol. 2011;37:237–44. doi: 10.1016/j.ejso.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 113.Mouly C, Fuks D, Browet F, et al. Feasibility of the Glissonian approach during right hepatectomy. HPB (Oxford) 2013;15:638–45. doi: 10.1111/hpb.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nagorney DM, Kendrick ML. Hepatic resection in the treatment of hilar cholangiocarcinoma. Adv Surg. 2006;40:159–71. doi: 10.1016/j.yasu.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 115.van der Gaag NA, Rauws EA, van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129–37. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 116.Deviere J, Baize M, de Toeuf J, Cremer M. Long-term follow-up of patients with hilar malignant stricture treated by endoscopic internal biliary drainage. Gastrointest Endosc. 1988;34:95–101. doi: 10.1016/s0016-5107(88)71271-7. [DOI] [PubMed] [Google Scholar]
- 117.Vienne A, Hobeika E, Gouya H, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. 2010;72:728–35. doi: 10.1016/j.gie.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 118.Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47:354–62. doi: 10.1016/s0016-5107(98)70218-4. [DOI] [PubMed] [Google Scholar]
- 119.Krokidis M, Fanelli F, Orgera G, Bezzi M, Passariello R, Hatzidakis A. Percutaneous treatment of malignant jaundice due to extrahepatic cholangiocarcinoma: covered Viabil stent versus uncovered Wallstents. Cardiovasc Intervent Radiol. 2010;33:97–106. doi: 10.1007/s00270-009-9604-9. [DOI] [PubMed] [Google Scholar]
- 120.Kullman E, Frozanpor F, Söderlund C, et al. Covered versus uncovered self-expandable nitinol stents in the palliative treatment of malignant distal biliary obstruction: results from a randomized, multicenter study. Gastrointest Endosc. 2010;72:915–23. doi: 10.1016/j.gie.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 121.Kahaleh M, Tokar J, Conaway MR, et al. Effi cacy and complications of covered Wallstents in malignant distal biliary obstruction. Gastrointest Endosc. 2005;61:528–33. doi: 10.1016/s0016-5107(04)02593-3. [DOI] [PubMed] [Google Scholar]
- 122.Isayama H, Komatsu Y, Tsujino T, et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729–34. doi: 10.1136/gut.2003.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl Int. 2010;23:692–97. doi: 10.1111/j.1432-2277.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- 124.Darwish Murad S, Kim WR, Therneau T, et al. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology. 2012;56:972–81. doi: 10.1002/hep.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lewis JT, Talwalkar JA, Rosen CB, Smyrk TC, Abraham SC. Precancerous bile duct pathology in end-stage primary sclerosing cholangitis, with and without cholangiocarcinoma. Am J Surg Pathol. 2010;34:27–34. doi: 10.1097/PAS.0b013e3181bc96f9. [DOI] [PubMed] [Google Scholar]
- 126.Raju RP, Jaganmohan SR, Ross WA, et al. Optimum palliation of inoperable hilar cholangiocarcinoma: comparative assessment of the efficacy of plastic and self-expanding metal stents. Dig Dis Sci. 2011;56:1557–64. doi: 10.1007/s10620-010-1550-5. [DOI] [PubMed] [Google Scholar]
- 127.Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63:986–95. doi: 10.1016/j.gie.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 128.Yeoh KG, Zimmerman MJ, Cunningham JT, Cotton PB. Comparative costs of metal versus plastic biliary stent strategies for malignant obstructive jaundice by decision analysis. Gastrointest Endosc. 1999;49:466–71. doi: 10.1016/s0016-5107(99)70044-1. [DOI] [PubMed] [Google Scholar]
- 129.Liberato MJ, Canena JM. Endoscopic stenting for hilar cholangiocarcinoma: efficacy of unilateral and bilateral placement of plastic and metal stents in a retrospective review of 480 patients. BMC Gastroenterol. 2012;12:103. doi: 10.1186/1471-230X-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sangchan A, Kongkasame W, Pugkhem A, Jenwitheesuk K, Mairiang P. Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: a randomized controlled trial. Gastrointest Endosc. 2012;76:93–99. doi: 10.1016/j.gie.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 131.Wadsworth CA, Westaby D, Khan SA. Endoscopic radiofrequency ablation for cholangiocarcinoma. Curr Opin Gastroenterol. 2013;29:305–11. doi: 10.1097/MOG.0b013e32835faacc. [DOI] [PubMed] [Google Scholar]
- 132.Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418–25. doi: 10.1038/sj.bjc.6604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Erez N. Cancer: angiogenic awakening. Nature. 2013;500:37–38. doi: 10.1038/nature12459. [DOI] [PubMed] [Google Scholar]
- 134.Fingas CD, Blechacz BR, Smoot RL, et al. A smac mimetic reduces TNF related apoptosis inducing ligand (TRAIL)-induced invasion and metastasis of cholangiocarcinoma cells. Hepatology. 2010;52:550–61. doi: 10.1002/hep.23729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chaiteerakij R, Yang JD, Harmsen WS, et al. Risk factors for intrahepatic cholangiocarcinoma: association between metformin use and reduced cancer risk. Hepatology. 2013;57:648–55. doi: 10.1002/hep.26092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2012;9:44–54. doi: 10.1038/nrgastro.2011.222. [DOI] [PubMed] [Google Scholar]
- 137.Mertens JC, Fingas CD, Christensen JD, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897–907. doi: 10.1158/0008-5472.CAN-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ko KS, Peng J, Yang H. Animal models of cholangiocarcinoma. Curr Opin Gastroenterol. 2013;29:312–18. doi: 10.1097/MOG.0b013e32835d6a3e. [DOI] [PubMed] [Google Scholar]
- 139.Kisiel JB, Yab TC, Taylor WR, et al. Stool DNA testing for the detection of pancreatic cancer: assessment of methylation marker candidates. Cancer. 2012;118:2623–31. doi: 10.1002/cncr.26558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ishida M, Selaru FM. miRNA-based therapeutic strategies. Curr Anesthesiol Rep. 2013;1:63–70. doi: 10.1007/s40139-012-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Takahashi K, Yan I, Wen HJ, Patel T. microRNAs in liver disease: from diagnostics to therapeutics. Clin Biochem. 2013;46:946–52. doi: 10.1016/j.clinbiochem.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]