Abstract
Background
Phosphate binders are an important therapeutic option for managing hyperphosphatemia in hemodialysis patients. Whether sevelamer confers a survival advantage over calcium acetate is unclear.
Study Design
Observational cohort study using US Renal Data System (USRDS) data linked to Medicare Part D prescription drug data.
Setting & Participants
Medicare-enrolled, elderly, incident hemodialysis patients initiating calcium acetate or sevelamer between July 1, 2006, and March 31, 2011.
Predictor
Prescription for sevelamer (hydrochloride or carbonate) or calcium acetate.
Outcomes & measurements
All-cause and cardiovascular-related mortality, hospital admissions and hospital days assessed from Medicare Parts A, B, and D claims and other USRDS data.
Results
The sevelamer and calcium acetate groups included 16,916 and 18,335 patients, respectively. After multivariable adjustment, all-cause (21.9 versus 21.8 deaths per 100 patient-years; adjusted HR, 0.97; 95% CI, 0.94-1.03) and cardiovascular (8.7 versus 8.6 deaths per 100 patient-years; HR, 0.99; 95% CI, 0.93-1.04) mortality did not differ significantly between the sevelamer group and calcium acetate group (referent). Mortality results in propensity-score matched cohorts showed a significantly lower risk of death in sevelamer than in calcium acetate patients (HR, 0.94; 95% CI, 0.91-0.98). Mortality results from additional analyses including only patients with low-income subsidy status were consistent with results from analyses including patients with and without low-income subsidy status. There were no significant differences between the sevelamer and calcium acetate groups for all-cause and cardiovascular-related first hospitalization, multiple hospitalizations, and hospital days.
Limitations
Results may not be applicable to younger patients; information about laboratory data and over-the-counter calcium-containing binders was lacking.
Conclusions
Relative to treatment with calcium acetate, treatment with sevelamer was associated with similar or slightly lower risk of death and similar risk of hospitalization in elderly incident hemodialysis patients.
Index words: Medicare Part D, hyperphosphatemia, phosphate binders, dialysis, calcium acetate, sevelamer, ESRD, mortality, cardiovascular disease, elderly, coronary calcification, comparative effectiveness
Hyperphosphatemia is associated with cardiovascular disease and increased mortality in dialysis patients. Because dietary phosphorus restriction alone is insufficient to maintain serum phosphorus in the recommended range for most dialysis patients, phosphate binders are the mainstay of hyperphosphatemia treatment. Among US dialysis patients enrolled in Medicare Part D in 2010, phosphate binding agents were the most frequently prescribed medication class, accounting for over $400 million in net Medicare Part D costs.1
The most commonly used phosphate binding agents are sevelamer (carbonate and hydrochloride) and calcium acetate; sevelamer is available only as a trade name product and is much costlier than calcium acetate. Although these agents exhibit similar phosphorus-reducing ability when used in equipotent doses,2;3 their effects on clinical outcomes may not be similar. Sevelamer decreases the risk of coronary calcification in hemodialysis patients compared with calcium-based phosphate binders in some but not all studies,4-7 and it exhibits pleiotropic effects.8-11 These effects have fueled suggestions that sevelamer may have a comparative advantage over calcium acetate. Results from randomized clinical trials have been mixed. The randomized, open-label, parallel design Dialysis Clinical Outcomes Revisited (DCOR; n = 2103) trial showed no significant difference in mortality risk between sevelamer and calcium-based phosphate binders in the overall cohort of prevalent hemodialysis patients, but a subgroup analysis showed decreased mortality risk in patients aged 65 years or older.12 The Renagel in New Dialysis (RIND) trial (n = 127) showed that sevelamer was significantly associated with survival compared with calcium-based phosphate binders in incident hemodialysis patients, but mortality was a secondary endpoint in this small trial.13 Given the large difference in expense between sevelamer and calcium acetate, the substantial economic burden of these drugs on Medicare, and the signal of a positive mortality benefit with sevelamer in incident hemodialysis patients in the small RIND study and in older prevalent patients in the DCOR study, there is a compelling need to assess comparative clinical outcomes associated with these agents in a large group of older incident hemodialysis patients.
Our objectives were to examine the association of sevelamer and calcium acetate with mortality and hospitalizations in elderly hemodialysis patients with Medicare Part D benefits and to examine the effectt of adherence to phosphate binder therapy on the association between phosphate binder type and mortality. Because sevelamer and calcium acetate reduce phosphorus to a similar degree when given in equipotent doses,2;3 any differential effect on mortality is probably not mediated through phosphorus control. Because sevelamer decreases risk of coronary calcification and exhibits pleiotropic effects, we hypothesized that hemodialysis patients receiving sevelamer would exhibit better outcomes than patients receiving calcium acetate. We also hypothesized that adherent sevelamer patients would exhibit better survival than adherent calcium acetate patients.
Methods
Data Source
We used the US Renal Data System (USRDS) database linked with Medicare Part D (prescription drug) data to examine mortality and hospitalizations in elderly patients with prescription claims for sevelamer (hydrochloride or carbonate) or calcium acetate. The USRDS database contains data on patients with end-stage renal disease (ESRD) in the United States. As most ESRD patients have Medicare coverage, the USRDS database includes Medicare enrollment history, death dates and causes, and Medicare Parts A and B claims including information on hospitalizations, clinic visits, medical procedures, and costs. Medicare Part D data contain enrollment information and prescription drug event data for ESRD patients with Part D benefits.
Study Population
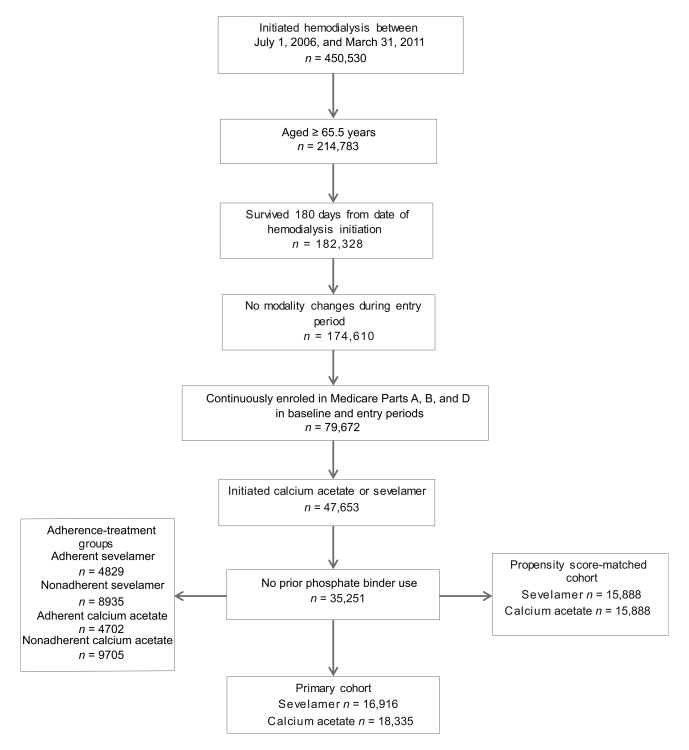
Figure 1 illustrates the study cohorts. Included patients initiated hemodialysis between July 1, 2006, and March 31, 2011, and met the following criteria: (1) were aged 65.5 years or older on the first ESRD service date, (2) survived ≥ 180 days following the first ESRD service date (the entry period), (3) received hemodialysis treatment during the entry period, (4) were continuously enrolled in Medicare Parts A, B, and D between 180 days before and 180 days after the first ESRD service date, (5) received one or more prescriptions for, sevelamer carbonate, sevelamer hydrochloride, or calcium acetate during the entry period. We excluded patients who had been exposed to a prescription phosphate-binding agent (sevelamer, calcium acetate, lanthanum) within 180 days before hemodialysis initiation.
Figure 1. Cohorts creation flow chart.
Phosphate Binder Exposure, Study Endpoint, Covariates
Using these exclusion and inclusion criteria, we identified new users of sevelamer (carbonate or hydrochloride) and of calcium acetate, defined by a filled prescription for a study medication during the entry period.
Follow-up began the day after the index phosphate binder agent was dispensed, and continued for each cohort member through the earliest of date of event (death or hospitalization), kidney transplantation, loss of Medicare Part D coverage, switch from index phosphate binder to a different phosphate binder, or December 31, 2011. The main outcomes of interest were all-cause and cardiovascular mortality. Cardiovascular mortality was identified using the methodology used by the USRDS.1 All-cause and cardiovascular hospitalizations were determined using the USRDS hospitalization data. Baseline covariates are listed in Table 1. Vascular access and hematocrit information was obtained from the ESRD Medical Evidence Report (Centers for Medicare & Medicaid Services [CMS] form CMS-2728) and claims. Exposure to other medications was defined as at least one prescription record for each medication in the 90 days preceding the index phosphate binder prescription. Baseline comorbid conditions were ascertained from claims in the 6 months preceding the index phosphate binder prescription; we required one inpatient claim or two outpatient claims for each comorbid condition.14;15
Table 1. Characteristics of patients treated with calcium acetate or with sevelamer.
| Characteristic | Main Cohort (n = 35,251) | Propensity Score-Matched Cohort (n = 31,776) | |||||
|---|---|---|---|---|---|---|---|
| Sevelamer | Calcium | P | Standardized Difference (%)a | Sevelamer | Calcium | Standardized Difference (%)a | |
| No. of participants | 16,916 | 18,335 | 15,888 | 15,888 | |||
| Age (y) | 75.6 ±6.6 | 75.4 ±6.5 | <0.001 | 4.1 | 75.6 ±6.6 | 75.5 ±6.6 | 1.7 |
| Female sex | 55.3 | 50.7 | <0.001 | 9.1 | 53.9 | 53.5 | 0.8 |
| Race | <0.001 | 4.1 | 1.5 | ||||
| Black | 26.4 | 24.6 | 25.9 | 25.2 | |||
| White | 67.2 | 69.3 | 67.7 | 68.6 | |||
| Asian | 5.7 | 5.2 | 5.8 | 5.3 | |||
| Other | 0.7 | 1.0 | 0.7 | 1.0 | |||
| Hispanic ethnicity | 13.1 | 16.1 | <0.001 | 8.4 | 13.7 | 13.5 | 0.7 |
| Diabetes as primary cause of ESRD | 45.9 | 47.7 | 0.001 | 3.8 | 46.5 | 46.5 | 0.0 |
| Dialysis vintage (d) | 59 [4-176] | 43 [3-156] | <0.001 | 20.1 | 55 [4-156] | 52 [4-159] | 1.5 |
| Vascular access via catheterb | 68.5 | 72.7 | <0.001 | 9.2 | 69.9 | 70.4 | 1.1 |
| Hematocrit (%)b | 32.1 [24.0-40.5] | 31.5 [23.7-39.9] | <0.001 | 3.7 | 32.1 [24.0-40.2] | 31.8 [24.0-39.9] | 2.3 |
| Medication usec | |||||||
| Vitamin D | 37.8 | 30.8 | <0.001 | 14.8 | 35.5 | 34.8 | 1.6 |
| β blockers | 64.3 | 65.5 | 0.01 | 2.7 | 64.6 | 65.3 | 1.4 |
| ACEi/ARBs/renin inhibitors | 41.1 | 42.1 | 0.05 | 2.1 | 41.3 | 41.7 | 0.7 |
| Dihydropyridine CCB | 48.7 | 50.1 | 0.01 | 2.8 | 48.9 | 49.4 | 0.9 |
| Non-dihydropyridine CCB | 9.8 | 9.6 | 0.5 | 0.7 | 9.8 | 9.7 | 0.5 |
| Statin | 48.3 | 46.1 | <0.001 | 4.5 | 47.8 | 47.8 | 0.1 |
| Cinacalcet | 3.2 | 2.0 | <0.001 | 7.8 | 2.4 | 2.3 | 1.0 |
| ESAd | 51.9 | 44.9 | <0.001 | 6.2 | 49.9 | 49.2 | 1.3 |
| Hospitalizede | 70.7 | 71.0 | 0.6 | 0.6 | 70.7 | 70.5 | 0.4 |
| Comorbid conditionsf | |||||||
| Atherosclerotic heart disease | 37.7 | 36.6 | 0.02 | 2.4 | 37.6 | 37.5 | 0.1 |
| Congestive heart failure | 45.0 | 43.0 | <0.001 | 4.1 | 44.7 | 43.7 | 1.9 |
| CVA/TIA | 13.2 | 12.3 | 0.01 | 2.6 | 12.9 | 12.7 | 0.6 |
| PVD | 24.1 | 22.3 | <0.001 | 4.3 | 23.7 | 23.1 | 1.4 |
| Other cardiac disease | 28.6 | 27.0 | 0.001 | 3.7 | 28.1 | 28.1 | 0.0 |
| COPD | 20.5 | 19.8 | 0.1 | 1.7 | 20.3 | 20.5 | 0.3 |
| GI disease | 8.4 | 7.8 | 0.05 | 2.1 | 8.4 | 8.0 | 1.4 |
| Liver disease | 3.8 | 3.5 | 0.1 | 1.6 | 3.7 | 3.7 | 0.0 |
| Dysrhythmia | 29.4 | 27.4 | <0.001 | 4.5 | 28.7 | 28.8 | 0.0 |
| Cancer | 8.5 | 8.0 | 0.1 | 1.6 | 8.5 | 8.0 | 1.8 |
| Diabetes | 48.5 | 46.8 | 0.001 | 3.4 | 48.1 | 47.4 | 1.5 |
Note: Values for categorical variables are given as percentages; values for continuous variables are given as mean ± standard deviation or median [5th-95th percentile].
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; TIA, transient ischemic attack; ESA, erythropoiesis-stimulating agent; ESRD, end-stage renal disease; GI, gastrointestinal; PVD, peripheral vascular disease.
For each variable, standardized difference was obtained by dividing absolute difference between mean of sevelamer group and mean of calcium group by an estimate of pooled standard deviation of variable. A standardized difference <10% signifies little or no imbalance between sevelamer and calcium patients for that variable.
Most recent value before first phosphate binder use.
At least one record of medication prescription in medication class required in 90 days before first phosphate binder prescription.
Record of ESA in 180 days preceding first phosphate binder prescription.
Any hospitalization in 180 days preceding first phosphate binder prescription.
Comorbidity determined in 180 days preceding first phosphate binder prescription.
Statistical Analysis
For categorical baseline characteristics and comorbidity variables, groups were compared using chi-squared tests. Continuous parameters were compared using t-tests and Wilcoxon rank-sum tests. Survival was compared using Kaplan-Meier estimates.
Mortality and first hospitalization risks were assessed using Cox proportional hazards models; multiple hospitalization analyses were performed using the Andersen-Gill repeated-event model with robust variance.16 The proportionality assumption of the Cox model was checked by including time-dependent covariates (generated by creating interactions of the explanatory variables and a log function of survival time) in the model. A Poisson regression model was used for hospital days analyses, with Pearson adjustment for over-dispersion. We controlled for potential confounders in two ways: (1) including potential confounders as covariates in multivariable adjusted regression models and (2) using a propensity score matching strategy. Covariates included in multivariable adjusted models were age, sex, race, primary cause of ESRD, cumulative hemodialysis vintage at index study drug prescription, vascular access type, hematocrit, exposure to other medications, previous hospitalization, and comorbid conditions.
For the propensity score matching strategy, we calculated propensity scores using a logistic regression model to estimate the predicted probability of exposure to sevelamer.17;18 Covariates related to the phosphate binder prescription were included in the regression model used to compute the propensity score. Each sevelamer patient was matched to a calcium acetate patient on the propensity score using a greedy algorithm with a 5-digit match.19
We sought to identify adherent and non-adherent calcium acetate and sevelamer users and to examine the hazards of mortality in these four exposure groups. We queried prescription records for phosphate binders in the 6 months following the index binder fill for patients included in the main study cohort and computed proportion of days covered (calculated as the proportion of days in the measurement period covered by a prescription for either sevelamer or calcium acetate20) as an adherence measure. The measurement period was defined as the number of outpatient days in the 6-month interval beginning the first day of qualifying phosphate binder use.
We identified four exposure groups based on all combinations of adherence status (proportion of days covered ≤80%, >80%) and type of phosphate binder (sevelamer, calcium acetate). We then examined the multivariable adjusted mortality risks in these groups using Cox proportional hazard regression.
The Medicare Part D database does not contain information on use of non-prescription calcium-based phosphate binders (such as the antacid marketed as TUMS). However, Medicare Part D beneficiaries who qualify for low-income subsidy (LIS) status have little or no copayment/coinsurance for prescriptions, and hence have no incentive to obtain medications outside the Medicare Part D system. Hence, we performed an additional mortality analysis using a subset of patients enrolled in Medicare Part D with LIS status. We performed all statistical analyses using SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics
The main (unmatched) study cohort included 35,251 hemodialysis patients with a mean age at baseline of 75.5 years. Patients treated with sevelamer were slightly older, more likely to be female and black, and more likely to have used erythropoiesis-stimulating agents, intravenous vitamin D, statins, and cinacalcet (Table 1). After propensity score matching, the propensity score-matched cohort included 31,776 patients (Table 1). The propensity score matching technique resulted in cohorts that were more closely matched at baseline than the unmatched cohorts.
All-Cause and Cardiovascular-Related Mortality
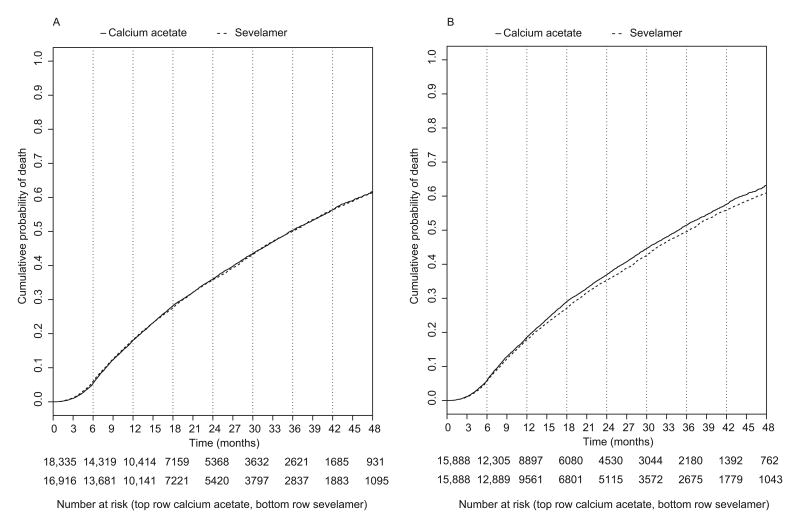
In the entire unmatched cohort, mean lengths of follow-up for sevelamer and calcium acetate patients were 1.6 and 1.5 years, respectively. During the follow-up period, 891 patients were censored for Part D disenrollment, 11,549 patients switched phosphate binder agents, 327 patients underwent kidney transplantation, and 12,069 patients died (6018 and 6051 deaths in the sevelamer and calcium acetate groups, respectively). Figure 2A shows a Kaplan-Meier plot of all-cause mortality in the main cohort and Figure 2B in the propensity score-matched cohort. In the main cohort, cumulative probabilities of death in the sevelamer versus calcium acetate groups were 16.4% versus 18.0% at 12 months, respectively, and 35.8% versus 36.0% at 24 months, respectively. The all-cause mortality rate for sevelamer patients was not significantly different from the rate for calcium acetate patients in unadjusted and multivariable adjusted Cox models (21.9 versus 21.8 deaths per 100 patient-years; unadjusted hazard ratio [HR] of 0.99 [95% confidence interval (CI), 0.96-1.03] and multivariable adjusted HR of 0.97 [95% CI, 0.94-1.03]; Table 2). However, in the propensity-score matched cohort, sevelamer was associated with a significantly lower risk of death compared with calcium acetate (HR, 0.94; 95% CI, 0.91-0.98). Cardiovascular mortality was not significantly different for sevelamer and calcium acetate patients (8.7 versus 8.6 deaths per 100 patient-years; unadjusted HR of 1.01 [95% CI, 0.95-1.06], multivariable adjusted HR of 0.99 [95% CI, 0.93-1.04], and propensity score-matched cohort HR of 0.95 [95% CI, 0.90-1.01]). We validated that the proportionality assumption of the Cox model was met.
Figure 2.
A. Cumulative probability of all-cause mortality in the main (unmatched) cohort. 2B. Cumulative probability of all-cause mortality in the propensity score-matched cohort.
Table 2. HRs for mortality comparing patients treated with sevelamer versus calcium acetate.
| HR (95% CI) | P | |
|---|---|---|
| All-cause mortality | ||
| Unadjusted | 0.99 (0.96-1.03) | 0.8 |
| Multivariable-adjusted a | 0.97 (0.94-1.03) | 0.1 |
| Propensity-score matched | 0.94 (0.91- 0.98) | 0.002 |
| Cardiovascular mortality | ||
| Unadjusted | 1.01 (0.95-1.06) | 0.8 |
| Multivariable-adjusted a | 0.99 (0.93-1.04) | 0.6 |
| Propensity-score matched | 0.95 (0.90-1.01) | 0.1 |
CI, confidence interval.HR, hazard ratio
Adjusted for age; sex; race; ethnicity; cause of end-stage renal disease; dialysis vintage; hematocrit value; vascular access type; baseline treatment with erythropoiesis-stimulating agents, vitamin D, β blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers/renin inhibitors, calcium channel blockers, statin, and cinacalcet; baseline hospitalizations; and baseline comorbid conditions.
We performed an alternative analysis using the unmatched cohort and adherence information. High adherence was slightly more prevalent among sevelamer than among calcium acetate patients (35.1% versus 32.6%, respectively; P < 0.001). All-cause mortality rates did not differ between adherent sevelamer and adherent calcium acetate patients (Table 3). However, the all-cause mortality risk was significantly lower for adherent than for non-adherent sevelamer and calcium acetate patients. Results were similar in the propensity score-matched cohort (Table S1, available as online supplementary material). A subgroup analysis restricted to a subset of patients enrolled in Medicare Part D with LIS status revealed HRs similar to those obtained for the primary cohorts (Table S2).
Table 3. HRs for all-cause mortality in the unmatched cohort for specified phosphate binder and adherence category.
| Comparison Groups | HR (95% CI) | P |
|---|---|---|
| Adherent calcium vs. non-adherent calcium | 0.88 (0.83-0.93) | < 0.001 |
| Adherent sevelamer vs. non-adherent sevelamer | 0.94 (0.89-0.99) | 0.03 |
| Adherent sevelamer vs. adherent calcium | 1.02 (0.95-1.09) | 0.6 |
Note: The HRs were obtained from models adjusted for age; sex; race; ethnicity; cause of end-stage renal disease; dialysis vintage; hematocrit value, vascular access type; baseline treatment with erythropoiesis-stimulating agents, vitamin D, β blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers/renin inhibitors, calcium channel blockers, statin, and cinacalcet; baseline hospitalizations; and baseline comorbid conditions.
CI, confidence interval; HR, hazard ratio
All-Cause and Cardiovascular Hospitalization and Hospital Days
Results for hospital admissions and hospital days analyses are shown in Table 4. During the follow-up period, we identified 34,787 and 34,992 hospitalizations in the sevelamer and calcium acetate groups, respectively. The rates of first hospital admissions were 0.70 and 0.71 per patient-year, respectively, and the rates of multiple admissions were 1.29 and 1.28 per patient-year, respectively. We found no significant differences between patients in the sevelamer and calcium acetate groups for all-cause and cardiovascular-related first hospitalization, multiple hospitalizations, or hospital days.
Table 4. First hospitalization, multiple hospitalizations, and hospital days.
| All-Cause Hospitalization | Cardiovascular-Related Hospitalization | ||||||
|---|---|---|---|---|---|---|---|
| Sevelamer | Calcium | P | Sevelamer | Calcium | P | ||
| Hospitalized | 58.4% | 56.7% | 18.0% | 17.8% | |||
| First hospitalization | |||||||
| Mean patient-y of F/U | 0.83 | 0.80 | 0.83 | 0.80 | |||
| First hospitalization rate (/patient-y) | 0.70 | 0.71 | 0.22 | 0.22 | |||
| Unadjusted RR | 1.00 (0.98-1.03) | Reference | 0.8 | 0.98 (0.93-1.03) | Reference | 0.4 | |
| Multivariable-adjusted RR a | 0.99 (0.96-1.02) | Reference | 0.6 | 0.97 | 0.93-1.02 | Reference | 0.3 |
| PSM cohort RR b | 0.99 (0.97-1.02) | Reference | 0.7 | 0.98 | 0.93-1.03 | Reference | 0.4 |
| Multiple hospitalizations | |||||||
| Mean patient-y of F/U | 1.59 | 1.49 | 1.59 | 1.49 | |||
| Multiple hospitalization rate (/patient-y) | 1.29 | 1.28 | 0.39 | 0.39 | |||
| Unadjusted RR | 1.03 (0.99-1.06) | Reference | 0.07 | 1.02 | 0.98-1.07 | Reference | 0.3 |
| Multivariable adjusted RR a | 1.01 (0.99-1.04) | Reference | 0.4 | 1.01 | 0.97-1.05 | Reference | 0.6 |
| PSM cohort RR b | 1.00 (0.97-1.07) | Reference | 0.8 | 1.00 | 0.96-1.05 | Reference | 0.9 |
| Hospital days | |||||||
| Mean patient-y of F/U | 1.62 | 1.52 | 1.62 | 1.52 | |||
| Rate for hospital days (/patient-y) | 8.4 | 8.4 | 2.1 | 2.1 | |||
| Unadjusted RR | 1.01 (0.96-1.05) | Reference | 0.8 | 1.00 | 0.95-1.07 | Reference | 0.9 |
| Multivariable-adjusted RR a | 0.99 (0.95-1.03) | Reference | 0.5 | 0.99 | 0.93-1.05 | Reference | 0.6 |
| PSM cohort RR b | 0.98 (0.94-1.03) | Reference | 0.4 | 0.99 | 0.93-1.05 | Reference | 0.7 |
Values in parentheses are 95% confidence intervals.
PSM, propensity score-matched; F/U, follow-up; RR. relative risk
Adjusted for age; sex; race; ethnicity; cause of end-stage renal disease; dialysis vintage; hematocrit value; vascular access type; baseline treatment with erythropoiesis-stimulating agents, vitamin D, β blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers/renin inhibitors, calcium channel blockers, statin, and cinacalcet; baseline hospitalizations; and baseline comorbid conditions.
Analysis was restricted to patients included in the PSM cohort.
Discussion
To our knowledge, this is the first large observational study using Medicare Part D data to evaluate clinical outcomes associated with two medication classes in dialysis patients. Although clinical trials are the gold standard for assessing comparative effectiveness of therapeutic interventions, adequately powered randomized comparative clinical trials of medications are rare in dialysis patients. Observational studies have thus become an increasingly important source of evidence to support prescribing decisions and treating patients on dialysis.
To date, only one relatively large comparative trial of phosphate binders has been conducted in dialysis patients (DCOR).12 The DCOR trial randomized prevalent hemodialysis patients to sevelamer hydrochloride or calcium-based phosphate binders, and found no significant difference in survival (HR for all-cause mortality, sevelamer versus calcium, 0.93; 95% CI, 0.79-1.10), but a subgroup analysis showed decreased mortality risk in patients aged 65 years or older.12 A secondary analysis of the DCOR trial using claims data (with more complete death data) also found no significant difference (adjusted HR for all-cause mortality, sevelamer versus calcium, 1.01; 95% CI, 0.89-1.16).21 However, an analysis of incident patients in the much smaller RIND trial showed that sevelamer was significantly associated with better survival than calcium-based phosphate binders (adjusted HR for all-cause mortality, sevelamer versus calcium, 0.32; 95% CI, 0.13-0.81) and that baseline coronary artery calcification (CAC) score was a significant predictor of mortality.13 Thus, data from the RIND study suggested that sevelamer may reduce mortality risk in incident dialysis patients and the DCOR trial suggested that mortality risk may be lower in older prevalent dialysis patients with sevelamer. Currently, the evidence is not sufficient to recommend sevelamer over a calcium-based phosphate binder for phosphorus control in older dialysis patients.22
On the basis of sevelamer's pleiotropic effects and evidence from RIND, we hypothesized that sevelamer may have a comparative clinical advantage over calcium acetate in a large sample of elderly incident hemodialysis patients. After multivariate adjustment, we found no significant differences in all-cause or cardiovascular mortality and hospitalizations between incident hemodialysis patients who were prescribed sevelamer (hydrochloride or carbonate) or calcium acetate. In the propensity score-matched cohort, we found that incident hemodialysis patients who were prescribed sevelamer (hydrochloride or carbonate) had a slightly but significantly lower risk of all-cause mortality than patients who were prescribed calcium acetate. However, the point estimates from the traditional multivariable adjustment technique (HR, 0.97) and the propensity score-matched cohorts (HR, 0.94) were very similar. Restricting the cohort to patients with Medicare Part D with LIS status yielded similar results. These findings suggest that sevelamer confers a small survival benefit among elderly incident hemodialysis patients.
Sevelamer may exhibit a survival advantage over calcium-based phosphate binders through two possible mechanisms. First, the strong association of CAC with mortality is well known.23-25 An interesting finding from the RIND trial was the strong relationship between baseline CAC score and all-cause mortality.13 Since sevelamer caused little or no increase in CAC and calcium-based agents increased CAC, it follows that sevelamer might show survival benefit over calcium acetate in incident patients. Furthermore, one might expect that if sevelamer's differential effects were exerted mainly through CAC, cardiovascular mortality may drive any differences seen in all-cause mortality. Second, sevelamer has certain pleiotropic effects in addition to its phosphate-binding activity.8-11 Such effects include lipid lowering, improved endothelial function, increased fetuin A levels, and anti-inflammatory activity; these actions may contribute positively to reducing both cardiovascular and non-cardiovascular causes of death. However, there was no evidence from either the multivariable adjustment or the propensity score approach suggesting that sevelamer treatment was associated with lower cardiovascular mortality or all-cause or cardiovascular hospitalization.
Adherent patients would be more likely than non-adherent patients to experience additional positive and negative effects of being treated with calcium acetate or sevelamer. One would expect that the positive effects of sevelamer (reduced CAC, pleiotropic effects) and the negative effect of calcium acetate (increased CAC) would manifest in adherent sevelamer and adherent calcium acetate patients respectively, leading to a net positive survival advantage for adherent sevelamer patients over adherent calcium acetate patients. However, results of the alternative analysis comparing survival in four adherence-treatment cohorts revealed that survival did not differ for adherent sevelamer and adherent calcium acetate patients. Simpson and colleagues26 found evidence to support adherence to either drug therapy or placebo reducing mortality, and adherence to drug therapy possibly being a surrogate for healthy behaviors. Our findings suggest that while adherence to phosphate binder therapy in general is associated with a survival advantage, the comparative effect of sevelamer and calcium acetate on mortality in incident hemodialysis patients cannot be explained by differential adherence to therapy.
Our study has several strengths. To our knowledge, this is the largest observational study to date to examine the association of sevelamer versus a calcium-based phosphate binder and mortality and hospitalization endpoints. We included more than 35,000 hemodialysis patients across the United States.12;13 Using Medicare Part D data enabled us to evaluate a large number of incident hemodialysis patients who had not been exposed to phosphate binders in the past. The incident-user design is advantageous in several respects, including more similar patient characteristics in each cohort (patient characteristics are measured before treatment initiation, avoiding adjustment for intermediate variables possibly in the causal pathway), accurate reflection of time-to-event from drug initiation, and more meaningful propensity scores that predict incident medication use.27 We restricted our analysis to older Medicare Part D–enrolled patients to determine whether patients had received phosphate binders before dialysis initiation. About 70% of older US hemodialysis patients are enrolled in Medicare Part D1; our results represent a large proportion of the older hemodialysis population. Our adherence measure accounts for patients who reduced or stopped taking their phosphate binders. Furthermore, we assessed confounding and selection bias by using a propensity score-matching technique and conducting subgroup analyses to test our hypothesis and the robustness of our findings.
Several limitations should also be considered. This was an observational study and associations should not be interpreted as causal. We included only hemodialysis patients aged 65.5 years or older and enrolled in Medicare Part D. Thus, our results may not be applicable to younger patients or to patients not enrolled in Medicare Part D. Medicare Part D enrollees without LIS status who reach the coverage gap may choose to purchase their more expensive brand-name medications (e.g., sevelamer) outside of Part D benefits or through another program, switch phosphate binders to a less costly alternative (calcium carbonate or calcium acetate), reduce doses or stop taking their phosphate binders, or receive their medication from patient assistance programs. Thus, some non-LIS patients in our cohorts who appeared to have stopped taking their phosphate binders may have received them from other sources, and would likely have been misclassified as non-adherent. This misclassification would be expected to bias our adherence results toward the null. However, a sensitivity analysis in LIS patients, who have no incentive to obtain medications outside their Part D plans, produced results similar to results in the entire cohort. Despite our use of different analytic techniques and alternative and subgroup analyses, factors we could not collect, such as baseline CAC scores and use of over-the-counter calcium-containing products, may have affected the results. However, combination use of sevelamer and calcium-containing binders is low in prevalent dialysis patients and would be expected to be even lower in incident patients.28 Death causes available from the USRDS database are not adjudicated, but this would not affect our all-cause mortality results.
As the USRDS database does not include laboratory data, we had no access to baseline phosphorus and calcium values, or to phosphorus control information. The DCOR and Calcium Acetate Renagel Evaluation (CARE) studies showed that serum phosphorus was about the same (DCOR) or lower (CARE) and calcium higher (both) with calcium binder compared with sevelamer treatment.12;29 However, a previous epidemiologic study showed that treatment with phosphate binders, in general, was independently associated with improved survival, and results were independent of baseline and follow-up phosphate levels.30 In addition, a recent randomized placebo-controlled study evaluating effects of calcium acetate, sevelamer carbonate, and lanthanum carbonate, or placebo, with moderate chronic kidney disease (glomerular filtration rate, 20-45 mL/min/1.73 m2) demonstrated small changes in serum phosphorus that did not reflect relatively large reductions in urine phosphorus, suggesting that serum phosphorus concentration may be a relatively poor marker of phosphate binder adherence or efficacy.31 Whether these results reflect an incident dialysis population with some residual kidney function is unclear. As it is common for phosphate binder doses to be titrated up or down based on phosphorus levels, the starting dose of the index phosphate binder may change over the course of the period during which adherence was measured.
To our knowledge, this study constitutes the first large observational study using Medicare Part D data to evaluate phosphate binding medication use in hemodialysis patients. Despite study limitations, the Medicare Part D data allow for robust evaluation of medication therapy in a vulnerable patient population often excluded from clinical trials. Our results suggest that sevelamer treatment is associated with similar or modestly improved survival compared with calcium acetate treatment, but the evidence for improved survival was weakened by results of a sensitivity analysis showing that adherence did not confer a survival advantage to sevelamer patients. Sevelamer treatment was not associated with decreased hospitalization.
Supplementary Material
Table S1: HRs for all-cause mortality in propensity score-matched cohort for specified phosphate binder and adherence category.
Table S2: HRs for mortality in patients who received low income subsidy and were treated with sevelamer vs calcium acetate.
Acknowledgments
The authors thank USRDS colleagues Beth Forrest for regulatory assistance, Dana Knopic, AAS, for manuscript preparation, and Nan Booth, MSW, MPH, ELS, for manuscript editing.
Support: This study was performed as a deliverable under Contract No. HHSN267200715002C (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Note: The supplementary material accompanying this article (doi: _______) is available at www.ajkd.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Renal Data System. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2012. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [Google Scholar]
- 2.Bleyer AJ, Burke SK, Dillon M, et al. A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis. 1999;33(4):694–701. doi: 10.1016/s0272-6386(99)70221-0. [DOI] [PubMed] [Google Scholar]
- 3.Hervas JG, Prados D, Cerezo S. Treatment of hyperphosphatemia with sevelamer hydrochloride in hemodialysis patients: a comparison with calcium acetate. Kidney Int. 2003;63(Suppl 85):S69–S72. doi: 10.1046/j.1523-1755.63.s85.17.x. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62(1):245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 5.Raggi P, Bommer J, Chertow GM. Valvular calcification in hemodialysis patients randomized to calcium-based phosphorus binders or sevelamer. J Heart Valve Dis. 2004;13(1):134–141. [PubMed] [Google Scholar]
- 6.Braun J, Asmus HG, Holzer H, et al. Long-term comparison of a calcium-free phosphate binder and calcium carbonate--phosphorus metabolism and cardiovascular calcification. Clin Nephrol. 2004;62(2):104–115. doi: 10.5414/cnp62104. [DOI] [PubMed] [Google Scholar]
- 7.Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68(4):1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 8.Chertow GM, Burke SK, Dillon MA, Slatopolsky E. Long-term effects of sevelamer hydrochloride on the calcium × phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant. 1999;14(12):2907–2914. doi: 10.1093/ndt/14.12.2907. [DOI] [PubMed] [Google Scholar]
- 9.Peres AT, Dalboni MA, Canziani ME, et al. Effect of phosphate binders on oxidative stress and inflammation markers in hemodialysis patients. Hemodial Int. 2009;13(3):271–277. doi: 10.1111/j.1542-4758.2009.00369.x. [DOI] [PubMed] [Google Scholar]
- 10.Lin YF, Chien CT, Kan WC, et al. Pleiotropic effects of sevelamer beyond phosphate binding in end-stage renal disease patients: a randomized, open-label, parallel-group study. Clin Drug Investig. 2011;31(4):257–267. doi: 10.2165/11539120-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Caglar K, Yilmaz MI, Saglam M, et al. Short-term treatment with sevelamer increases serum fetuin-a concentration and improves endothelial dysfunction in chronic kidney disease stage 4 patients. Clin J Am Soc Nephrol. 2008;3(1):61–68. doi: 10.2215/CJN.02810707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suki WN, Zabaneh R, Cangiano JL, et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 2007;72(9):1130–1137. doi: 10.1038/sj.ki.5002466. [DOI] [PubMed] [Google Scholar]
- 13.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71(5):438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 14.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14(6):270–277. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 15.McBean AM, Li S, Gilbertson DT, Collins AJ. Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups: whites, blacks, hispanics, and asians. Diabetes Care. 2004;27(10):2317–2324. doi: 10.2337/diacare.27.10.2317. [DOI] [PubMed] [Google Scholar]
- 16.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer-Verlag; 2000. Multiple events per subject; pp. 169–229. [Google Scholar]
- 17.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 18.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98(3):253–259. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. Proceedings of the Twenty-Sixth Annual SAS Users Group International Conference; [Accessed April 29, 2013]. http://www2.sas.com/proceedings/sugi26/p214-26.pdf. [Google Scholar]
- 20.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10(1):3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 21.St Peter WL, Liu J, Weinhandl E, Fan Q. A comparison of sevelamer and calcium-based phosphate binders on mortality, hospitalization, and morbidity in hemodialysis: a secondary analysis of the Dialysis Clinical Outcomes Revisited (DCOR) randomized trial using claims data. Am J Kidney Dis. 2008;51(3):445–454. doi: 10.1053/j.ajkd.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes(KDIGO) CKD-MBD Work Group. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int. 2009;76(Suppl 113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 23.Shantouf RS, Budoff MJ, Ahmadi N, et al. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am J Nephrol. 2010;31(5):419–425. doi: 10.1159/000294405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49(18):1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 25.Shimoyama Y, Tsuruta Y, Niwa T. Coronary artery calcification score is associated with mortality in Japanese hemodialysis patients. J Ren Nutr. 2012;22(1):139–142. doi: 10.1053/j.jrn.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson ES, Bartman BA, Briesacher BA, et al. Effective Health Care Program Research Report No 32. Rockville, MD: Agency for Healthcare Research and Quality; 2012. [Accessed April 29, 2013]. The incident user design in comparative effectiveness research. http://effectivehealthcare.ahrq.gov/ehc/products/442/1086/Decide32_IncidentUserDesign_FinalReport_20120515.pdf. [Google Scholar]
- 28.U.S. Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States. 2011. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 29.Qunibi WY, Hootkins RE, McDowell LL, et al. Treatment of hyperphosphatemia in hemodialysis patients: The Calcium Acetate Renagel Evaluation (CARE Study) Kidney Int. 2004;65(5):1914–1926. doi: 10.1111/j.1523-1755.2004.00590.x. [DOI] [PubMed] [Google Scholar]
- 30.Isakova T, Gutierrez OM, Chang Y, et al. Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol. 2009;20(2):388–396. doi: 10.1681/ASN.2008060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Block GA, Wheeler DC, Persky MS, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23(8):1407–1415. doi: 10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: HRs for all-cause mortality in propensity score-matched cohort for specified phosphate binder and adherence category.
Table S2: HRs for mortality in patients who received low income subsidy and were treated with sevelamer vs calcium acetate.