Abstract
Background
Schwann cells in the distal stump of transected nerve upregulate growth factors that support regeneration on a modality-specific basis. It is unclear, however, which of these preferentially support motor axon regeneration. Identification of these factors will require a model that can isolate growth factor effects to growing axons while reproducing the complex three-dimensional structure of peripheral nerve.
New Method
A two-compartment PDMS base is topped by a collagen-coated membrane that supports a spinal cord cross-section above one compartment. Fluorescent motoneurons in this section reinnervate a segment of peripheral nerve that directs axons through a watertight barrier to the second compartment, where nerve repair is performed.
Results
Motoneurons remain healthy for several weeks. The axons they project through the water-tight barrier survive transection and cross a nerve repair in substantial numbers to reinnervate an additional nerve segment. Fluidic isolation of the two compartments was confirmed with a dye leakage test, and the physiologic integrity of the system was tested by retrograde labeling of only those motor neurons to which tracer was exposed and by limitation of toxin effects to a single compartment.
Comparison with Existing Methods
Nerve repair cannot be modeled in monolayer cell culture. Our previous organotypic model accurately modeled nerve repair, but did not allow individual control of motoneuron and growth cone environments.
Conclusions
This model isolates treatment effects to growing axons while reproducing the complex three-dimensional structure of peripheral nerve. Additionally, it facilitates surgical manipulation of tissues and high-resolution imaging.
Keywords: regeneration, organotypic, spinal cord, peripheral nerve, YFP
1. Introduction
The role of pathway-derived growth factors in promoting motoneuron regeneration is poorly understood. Several growth factors are upregulated by denervated Schwann cells in the distal nerve stump soon after injury (Liu and Snider, 2001). Recently, anatomical and functional subsets of these Schwann cells have been characterized by unique growth factor profiles that have been found to support regeneration of sensory and motor axons on a modality-specific basis (Höke et al., 2006; Brushart et al., 2013). Although upregulation of several growth factors differs between sensory and motor nerve, it is not clear which of these factors could be responsible for the modality-specific support of motor axon regeneration. Identification of these factors will require a model that can both localize growth factor effects to growing axons and duplicate the complex three-dimensional architecture of peripheral nerve.
Growth factor effects on regeneration are isolated most easily in vitro. Cell culture devices such as the Campenot chamber and its microfluidic counterparts are able to isolate growth factor effects to the growing axon. However, the three-dimensional configuration of extracellular matrix components is especially difficult to model in vitro (Tucker et al., 2006). As a result, currently available techniques cannot reproduce the three dimensional structure of nerve, and thus cannot model nerve repair accurately (Campenot, 1977; Park et al., 2006; Yang et al., 2009).
Attempts to determine the role of pathway-derived growth factors in vivo are hampered by the complexity of the peri-axonal environment and by the paucity of relevant conditional knockout mice. Growth factors are produced not only by Schwann cells, but also by infiltrating macrophages, central glia, neurons that synapse on the regenerating motoneuron, and by the neuron itself. These growth factors can also have multiple effects that influence regeneration indirectly, such as promoting neuronal survival, signaling axonal injury to the neuron, and modulating Schwann cell behavior during Wallerian degeneration (Makwana and Raivich, 2005). Clearly, there is a need for a platform that selectively controls the growth factor environment within the three-dimensional structure of peripheral nerve. To address this need, our lab developed the first in vitro model of adult mammalian nerve repair in an organotypic co-culture system (Vyas et al., 2010). Organotypic cultures are prepared from nervous tissue without dissociation, and thus preserve the three dimensional cytoarchitecture within both spinal cord and peripheral nerve (Rothstein et al., 1993; Gähwiler et al., 1997). Additionally, organotypic culture of motoneurons overcomes the difficulties encountered when maintaining these cells in a monolayer environment (Kaal et al., 1997).
In our previously described in vitro model of nerve repair spinal cord sections from mice expressing yellow fluorescent protein (YFP) in their motoneurons were co-cultured with freshly-harvested segments of peripheral nerve (Vyas et al., 2010). To reconstruct ventral roots, these nerve segments were opposed to the ventral portion of the spinal cord section adjacent to the motor neuron pool to promote the ingrowth of YFP-expressing motor axons. After a week in culture, once the new ventral roots had been reinnervated, they were transected and nerve repair was performed by opposing their cut ends to freshly-harvested nerve grafts. As initially described, organotypic cultures were grown on a Transwell© collagen-coated insert within a 6-well plate. The height of the Transwell© enclosure compromised our ability to perform microsurgery on the cultured tissue and to achieve the working distances required for high resolution imaging. The Transwell© construct is designed to be imaged from below; image quality is degraded by the fluid and plastic beneath the membrane, and magnification is limited by the distance between lens and fluorescent tissue. Additionally, this construct did not permit selective manipulation of the nerve repair environment without simultaneously altering that of the parent neuron.
To overcome the physical limitations of the Transwell© construct, the walls of the membrane insert were shortened to increase mechanical access to the membrane for microsurgery and imaging. Fluidic isolation of motoneuron and regeneration compartments was obtained by replacing the 6-well plate with a low-profile two-compartment poly(dimethylsiloxane) (PDMS) base. Motor axons were conveyed from the motoneuron compartment into the nerve repair compartment through reconstructed ventral root that passed through a water-tight barrier. The result of these modifications is a biocompatible organotypic system that facilitates tissue manipulation and photography while permitting individual control of motoneuron and nerve repair environments. Growth factor effects can be studied in each compartment by adding growth factors or by blocking growth factor function with antibodies or siRNA. This model also has the potential to facilitate studies of Wallerian degeneration, myelination, and axonal pathfinding.
2. Materials and methods
2.1 Fabrication of compartmentalized culture system
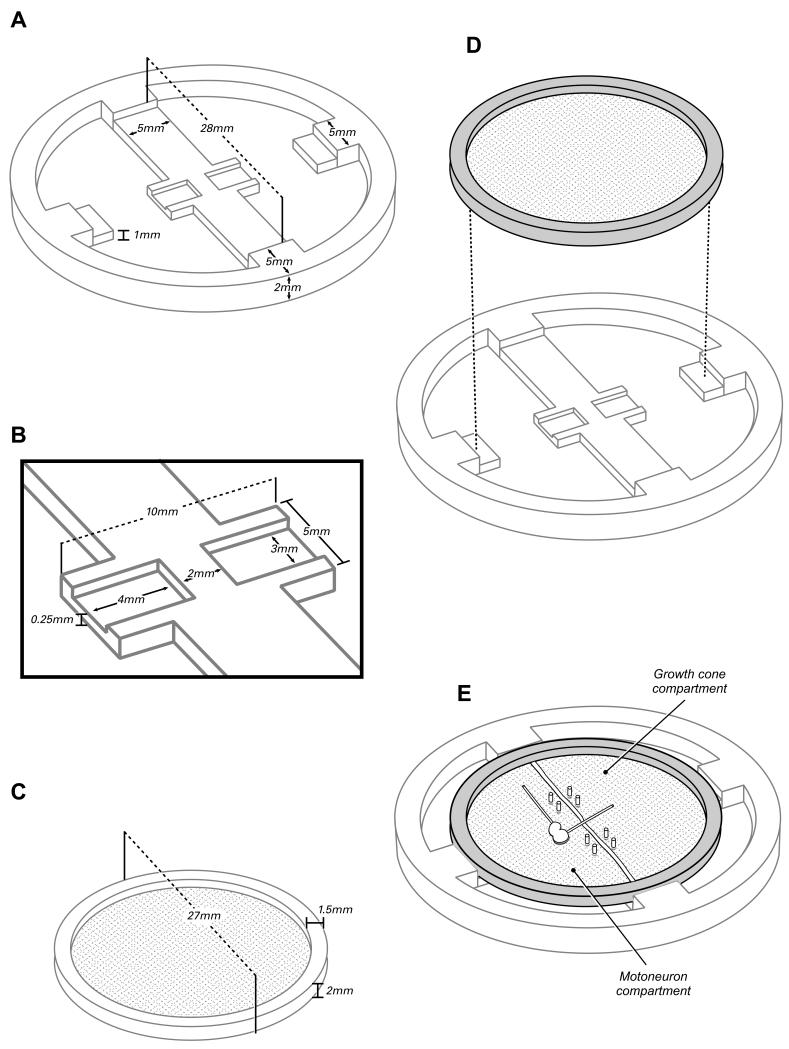
The completed device consists of two principle components: a PDMS reservoir for culture medium that is divided into two compartments by a central partition, and a superimposed Transwell© membrane (Corning, Acton, MA) that provides a surface for the growth of spinal cord and peripheral nerve co-cultures. The dimensions of these components are illustrated in Figure 1.
Figure 1.
The two-compartment organotypic chamber consists of a PDMS reservoir (A,B) and a modified Transwell© insert (C). The reservoir is bisected by a partition to create two fluidically isolated compartments. The Transwell© insert is seated within the insert so that the membrane surface contacts the surface of the PDMS partition and two addditional supports (D). To complete the fluidic isolation of the two compartments, the membrane is anchored to the partition with steel pins and bisected along the center axis of the partition (E). A thin bead of silicon grease is then placed to separate the cut edges of the membrane, insuring that no fluid can diffuse through the membrane from one compartment to the other. A spinal cord segment is placed in the motoneuron compartment; one reconstructed ventral root remains within that compartment, and the other is directed across the grease barrier into the growth cone compartment.
PDMS reservoirs are replicas from a master mold created by micromachining a negative relief of the device design into aluminum. Aluminum provides a robust surface for molding that is both reusable and economical. The dimensions of the PDMS reservoir are determined by the configuration of the Transwell© insert, the space required for media supply and exchange around the periphery of the insert, and the PDMS surface area required for adhesion to a glass substrate (Figure 1).The outer wall of the reservoir is circular with a 38 mm outer diameter and 33 mm inner diameter. The Transwell© is suspended over the media by the central partition, and by two steps placed at 90 degrees to the axis of the partition. The partition is 28 mm long and 1 mm tall, and bisects the device into two separate compartments. In order to provide adequate support for the membrane and to ensure bonding to the glass substrate, the partition is 5 mm wide along most of its length. At the center of the device, a 3mm-long segment of the partition narrows to 2mm wide to maximize media circulation to the nerve as it passes through the grease barrier between compartments (Figure 1B). To minimize sagging of the membrane beneath the spinal cord section, the central narrow segment of the partition is flanked by two 1mm-wide buttresses that extend perpendicular to its axis. A 0.25 mm thick film of PDMS connects the base of the buttresses to enhance their bonding to the glass beneath, and to promote the flow of media beneath the membrane so that bubbles do not form under the spinal cord explant.
The Transwell© membrane receives additional support from two 5mm-wide peripheral steps that are aligned on an axis at 90 degrees to that of the partition (Figure 1A). The lower stair step, which supports the Transwell© insert, is 1mm above the floor of the reservoir, and thus in the same plain as the surface of the partition; the upper step is the full height of the device, and limits horizontal movement of the Transwell©. The inner edge of the upper step extends 2.5 mm horizontally from the inner wall, creating a space 28 mm in diameter for the Transwell©. Once a Transwell© is placed into the device, the spaces around the Transwell© between the steps and the partition serve as access points for media exchange.
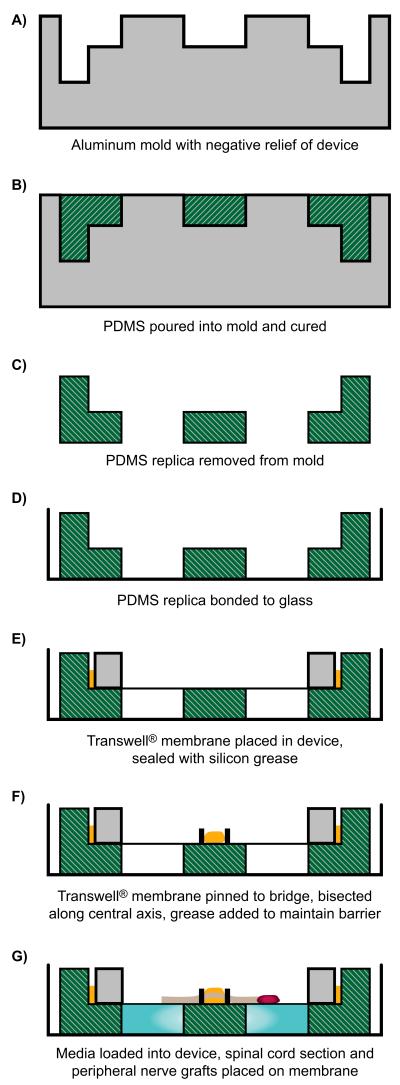
Cross-linked PDMS (sold as Sylgard 184, Dow Corning, Midland, MI) is used to make replicas of the reservoir using standard soft lithography techniques as described in detail elsewhere (Xia and Whitesides, 1998). Figure 2 summarizes the device process flow. Briefly, the base and crosslinker are mixed thoroughly in a ratio of 10:1, and placed under vacuum to remove bubbles. The PDMS is then poured into the aluminum master mold, leveled, and cured (cross-linked) at 85°C for at least 1 hour. Once removed from the mold, these replicas are cured for an additional 72 hours to minimize uncrosslinked components and impurities that could be toxic to the spinal cord cultures. The PDMS replicas are then cleaned with ethanol, dried, plasma bonded to 50 mm-diameter glass bottom dishes, and retreated with plasma once bonded to ensure sterility.
Figure 2.
Process flow for creating PDMS replicas from aluminium mold and assembling device, shown in cross-section through a plane perpendicular to that of the partition. Grey: aluminum mold; Green: PDMS base; Yellow: greese applied to anchor membrane (E) and to separate motoneuron and growth cone compartments (F): Red: spinal cord section.
The Transwell© insert normally consists of a 24 mm diameter PTFE membrane with 3 μm size pores that is stretched over the open end of a 27mm-diameter polycarbonate tube. In our preparation the tube is transected 1mm from the membrane surface, leaving a 1mm thick rim of plastic that serves as a stretcher for the Transwell© membrane (Figure 1 C). This modified Transwell© is placed atop the PDMS reservoir (Figure 1D), and anchored to the central PDMS partition with micropins fashioned from .2 mm wide steel wire (Fine Science Tools, Foster City, CA). Once anchored, the membrane is bisected with a #15 scalpel blade along the center axis of the partition to separate the two compartments, and a thin bead of silicon grease is interposed between the cut edges of the membrane to insure fluidic isolation (Figure 1 E).
2.2 Preparing Organotypic co-cultures
Each assembled device was filled with enough serum-containing culture medium (50% minimal essential medium (Gibco), 25% HBSS (Gibco), 25% heat-inactivated horse serum (Hyclone, Logan UT), 25 mM HEPES, 35 mM D-glucose, 2 mM glutamine, penicillin/streptomycin (Gibco), 70 ng/mL of GDNF (R&D Systems Inc, Minneapolis, MN) to fill the bottom of the device and wet the membrane, approximately 500 μL per compartment.
Spinal cords were obtained from mice expressing a green variant of yellow fluorescent protein (YFP) in sensory and motor neurons (Feng et al., 2000). Transgenic B6.Cg-Tg(Thy1-YFP)16Jrs/J animals (Jackson Laboratories, Bar Harbor, ME) were maintained as heterozygous breeders (line thy1-YFP-H). Spinal cord sections were obtained from day 3 or day 4 postnatal mouse pups using a modified version of described methods (Rothstein et al., 1993). Spinal cords were dissected from the pups, and their dura and root fragments removed. The lumbar spinal cord was cut into 350 μm-thick slices with a McIllwain tissue chopper (Ted Pella, Redding, CA). A single slice was placed in each device 2-3mm from the grease barrier with the motoneuron pools oriented closest to the barrier. The median and ulnar nerves of the sacrificed pup were harvested and their cut ends were each abutted to one of the two motor neuron pools in the spinal cord section to reconstruct ventral roots. One ventral root extended within the same compartment as the spinal cord parallel to the partition, while the other crossed the partition into the second compartment (Figure 1-E, Figure 3). Cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2. Medium was changed every other day, with the first two changes including 70 ng/ml of GDNF.
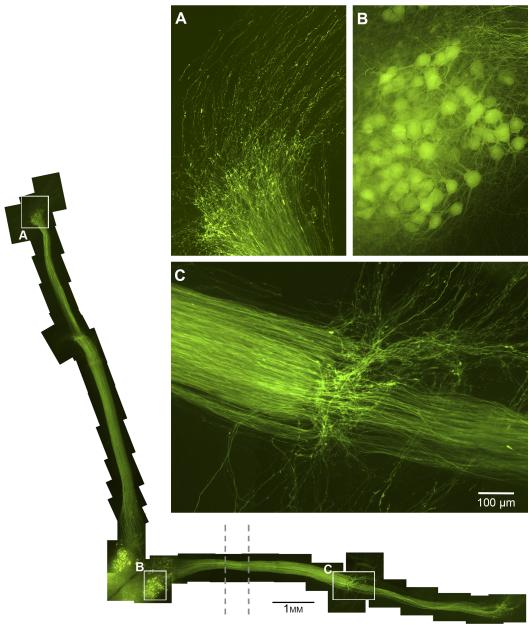
Figure 3.
Reconstruction of an organotypic preparation during nerve graft reinnervation (left and bottom). The images are from a representative 15-day culture. An initial 8 days was allowed for reinnervation of the ventral root, at which point the nerves were cut. Wildtype nerve grafts were abutted next to the cut ends and allowed to reinnervate with the YFP axons for another 7 days in culture. The dotted lines indicate the location of the grease barrier. The vertical axons are in the motoneuron compartment, and the horizontal axons cross the grease barrier to enter the regeneration compartment. A. Axons that have already traversed the vertical nerve graft are growing out the distal end of the graft onto the membrane surface. B.The motoneuron pool that projects axons across the barrier into the regeneration compartment remains healthy. C. The ventral root-graft interface. Many axons cross to reinnervate the graft, but those not directly opposed to distal nerve grow out on the membrane.
The compartment containing the spinal cord section is referred to as the motoneuron compartment, and the contralateral compartment is referred to as the growth cone or regeneration compartment. Applying a treatment to the motoneuron compartment will alter the environment of all motoneurons as well as the axons regenerating in that compartment, but will not change the environment in the regeneration compartment. Similarly, changing the environment in the regeneration compartment will not alter that of any motoneurons, or of the nerve regenerating in the motoneuron compartment, which serves as a control in each experiment.
Nerve repairs were performed five to seven days after the co-cultures had been established, by which time the ventral roots were re-populated with fluorescent axons. The reconstructed ventral roots were transected with microscissors 3-5 mm from the spinal cord. Median and ulnar nerves harvested from wildtype (C57BL) donor mice were then sharply transected and their cut surfaces opposed to those of the ventral roots (Figure 3). The axons in these grafts do not contain fluorescent protein, so all fluorescence in the grafts will represent regenerating axons.
2.3 Testing Fluidic Isolation
We sought to confirm the fluidic isolation of the two compartments. Trypan blue (Gibco, Grand Island, NY) was applied to the nerve repair compartment in cultures that had already been maintained for 3 weeks, with regular media exchange every other day. The stock 0.4% by w/v Trypan blue was diluted to 0.01%. The motor neuron compartment continued to contain culture media. Cultures (n=3) were monitored over an additional two days.
2.4 Testing Physiologic Integrity
Retrograde labelling was used to determine the relative ability of motoneurons to project axons through the grease barrier and into the regeneration compartment. The reinnervated ventral root in the regeneration compartment was transected sharply, and its cut end placed on a 1 mm square of stretched Parafilm. Crystals of Fluoro Ruby (Molecular Probes, Eugene, Oregon) were placed on the freshly-cut end of the graft for 30 minutes, after which the remaining tracer was carefully removed to prevent contamination of the medium with tracer.
We tested the physiologic integrity of the regeneration compartment by adding Nocodazole (Sigma Aldrich, St. Louis, MO) to inhibit microtubule polymerization. The Nocodazole was dissolved in dimethylsulfoxide (DMSO) (Quality Biological, Gaithersburg, MD) to a concentration of 33.3 mM, then further diluted in media to working concentrations. Based on previous studies, a 1μM concentration of Nocodazole was used initially. When this was found to have little differential effect, the nerves in both compartments were recut, and a 5 μM dose of Nocodazole was applied to the regeneration compartment. The media was replaced every other day in both compartments for 1 week (n=3).
To evaluate the permeability of cultured nerve to large molecules such as antibodies and growth factors, we added FITC-dextran (150 kDa; Sigma-Aldrich, St. Louis, MO) to the media at a concentration of 1 mg/ml for 24 hours. Nerve segments were then fixed for 2 hours in 4% paraformaldehyde in Sorensen’s buffer, stored overnight in 20% sucrose in Sorensen’s buffer, and sectioned longitudinally at 10u with a cryostat. Slides were then overlaid with coverslips using DPX (Sigms-Aldrich) to minimize fluorescent background.
2.5 Imaging
Real-time images were obtained with a Nikon Eclipse E600 fluorescent microscope equipped with a motorized stage, a spot CCD Camera, and an on-stage incubator that maintained required environmental culture conditions, including temperature, CO2 level, and humidity, to enable long-term monitoring and image acquisition. Using NIS-Elements Microscope Imaging Software, a stack of images was generated at progressive focal planes through the specimen. These stacked images were then flattened to a single image. Photoshop was used to adjust the contrast and brightness of individual flattened images and to create composites of several adjacent images.
3. Results
3.1 Viability of organotypic cultures in compartmentalized PDMS reservoir devices
Spinal cord and peripheral nerve are cultured on the upper surface of the membrane. They are nourished by underlying media that diffuses up through membrane pores, and have direct access to oxygen on their exposed upper surfaces. The media is replaced every two days through openings around the outer circumference of the device. Fluorescent images of a 15-day culture are shown in Figure 3. Motor neuron pools are densely populated with neurons that show no morphologic signs of degeneration (Figure 3-B), indicating adequate nourishment and oxygen supply. Similarly, motor axons in both compartments appear healthy in that they are smooth, continuous fibers with no beading or interruptions that would indicate degeneration. The portion of the reconstructed ventral root that conveys axons into the regeneration compartment does not receive direct nourishment, as it is isolated from underlying media by the grease barrier. Axons in this area appear healthy none the less, suggesting that longitudinal diffusion of media within the nerve is sufficient to maintain viability over short distances.
Reconstructed ventral roots are populated with fluorescent axons 5-7 days after the cultures are set up, an interval similar to that observed in single-compartment cultures (Vyas et al., 2010). Once this has occurred, nerve repairs are performed in both compartments by transecting the ventral roots and opposing nerve grafts from wild type mice to their cut surfaces (Figure 3 C). Reinnervation of the grafts was comparable in both compartments, as shown by the similar calibre of the reinnervated grafts (Figure 3). Grafts were reinnervated in 4-6 days, a slightly longer time than that the average 2-3 days observed in single-compartment cultures.
3.2 Fluidic Isolation
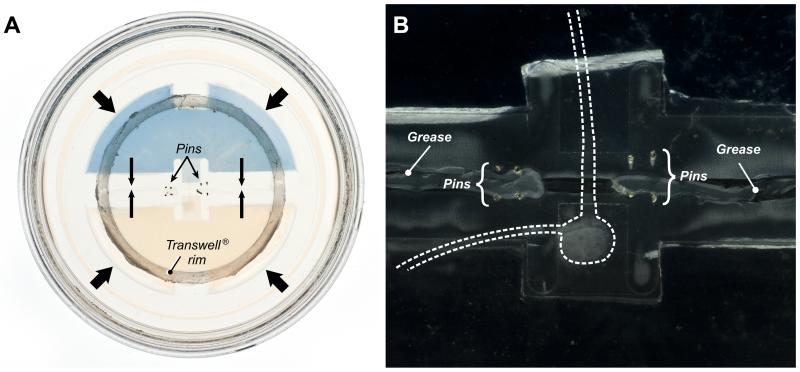
A critical attribute of the culture device is the ability to maintain fluidic isolation of the two compartments for the duration of an experiment. We reasoned that leakage was most likely to occur after the device had been handled several times for media exchange. We thus placed Trypan blue in either the nerve repair compartment or the motoneuron compartment of devices that had been maintained for 3 weeks. The devices were observed closely for an additional 48 hours, and no leakage was observed in either direction (Figure 4A).
Figure 4.
The extent and duration of fluidic isolation were tested by placing a solution of Trypan blue in the regeneration compartment. A. View of an entire device, showing confinement of Trypan blue to the regeneration compartment after 3 weeks, a duration longer than most of our experiments. B. Darkfield view of the same device at higher magnification to illustrate the location of the spinal cord segment and ventral roots in relation to the greese barrier and steel pins.
3.3 Physiologic integrity of the dual-chamber system
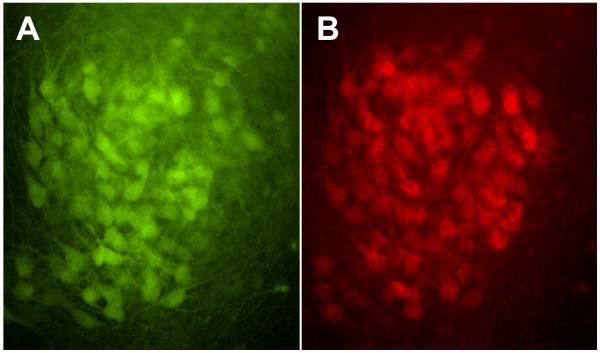
FluoroRuby was applied to axons in the regeneration compartment to label their cell bodies in the motoneuron compartment. This tracer is taken up by cut axons, is retrogradely transported through the nerve and across the barrier, and accumulates within parent motoneurons. During the process of regeneration, individual motoneurons may generate multiple collateral sprouts (Redett et al., 2005). A small number of motoneurons could thus fully reinnervate the reconstructed ventral roots. In the dual-chamber system, all YFP-positive motoneurons in the pool that projected to the regeneration compartment were also labelled with Fluoro-Ruby, indicating that they had all projected axons across the barrier (Figure 5). Furthermore, retrograde labelling was seen only in the motoneuron pool, indicating that other neuron types had not projected axons into the graft.
Figure 5.
Fluorescent images of the motoneuron pool used to populate the regeneration compartment. All YFP-positive motoneurons in the pool (A) are also labeled with Fluoro-ruby (B). Axons in the regeneration compartment are thus linked to the entire motoneuron pool, rather than to a subset of neurons that might differ in some way from the others. Close inspection reveals that not all motoneurons in the pool expressed YFP, as occasional motoneurons are labeled with Fluoro-Ruby alone.
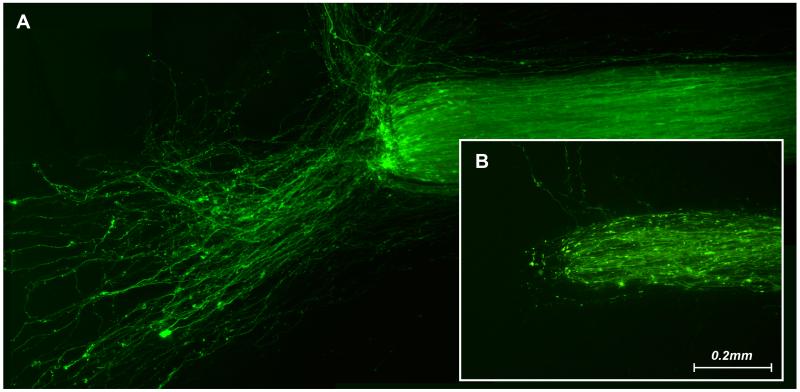
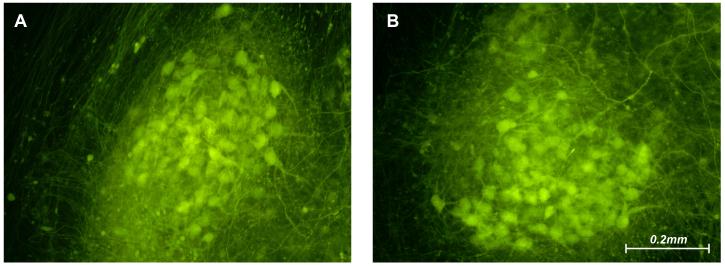
To test the physiologic integrity of the two compartments, we blocked microtubule polymerization in the regeneration compartment with 5 μM Nocodazole. The representative images in Figure 6 were taken after one week of Nocodazole exposure. Axons in the untreated motoneuron compartment regenerated vigorously (Figure 6A), while those in the treated regeneration compartment were degenerating (Figure 6B). Isolation of the treatment effect to the regeneration compartment was confirmed by the healthy appearance of both motoneuron pools (Figure 7).
Figure 6.
The ends of reconstructed ventral roots that had been transected 1 week previously. Nocodazole was added to the growth cone compartment at the time of nerve transection. In the untreated motoneuron compartment robust regeneration is evident, with many axons growing out on the membrane surface (A). In the treated growth cone compartment, however, axons are degenerating (B).
Figure 7.
Motoneuron pools corresponding to the reconstructed ventral roots illustrated in Figure 6. Motoneurons are abundant and healthy-appearing in both pools, indicating that Nocodazole has not leaked from the regeneration compartment into the motoneuron compartment.
Cultured peripheral nerve that had been exposed to 150 kDa FITC-dextran at a concentration of 1 mg/ml for 24 hours was uniformly fluorescent when viewed through a FITC filter, confirming that large molecules could access the endoneurial space.
4. Discussion
In our dual-chamber organotypic culture platform, YFP-expressing motoneurons in one chamber project their axons into a second chamber through a three-dimensional segment of peripheral nerve. Nerve repair is then performed in the second chamber by transecting the axon-bearing nerve and joining it to an additional nerve segment. In this system, the environment of either growth cone or motoneuron can be manipulated individually to localize signaling events that contribute to regeneration. The consequences of these manipulations can then be monitored by repeated fluorescence imaging of the YFP-positive axons as they regenerate.
Our construct is the three-dimensional descendant of the Campenot Chamber, a system for isolating neuronal cell bodies from their regenerating axons in monolayer cell culture (Campenot, 1977). In this on-slide device, axons elongate beneath a grease barrier that separates neuronal and growth cone compartments by following grooves scratched onto the glass surface. The Campenot Chamber was used extensively in early studies of growth factor signaling. In cultures of NGF-sensitive sympathetic neurons, for instance, Campenot demonstrated that NGF promoted neurite outgrowth when applied to the growth cone, but not when applied to the neuronal cell body (Campenot, 1982). Adult DRG neurons could also be studied because of their relative ease of culture, but difficulties in culturing motoneurons precluded their use with this device (Campenot, 1977; Kimpinski et al., 1997).
More recently, it has become possible to isolate neurons from their axonal growth cones by controlling the flow of medium rather than by imposing a physical barrier between them (Taylor et al., 2005). In these microfluidic devices, two fluid-filled chambers are connected by microchannels that direct axon growth. Because of the high resistance to fluid flow inherent in such minute channels, a small but sustained flow of medium is sufficient to counteract diffusion in the opposite direction, effectively preventing a substance added to the downstream chamber from entering the upstream chamber. These types of devices and their derivatives have been useful in a variety of neurobiology studies, including examination of local toxic effects, such as that of paclitaxel on sensory axons, as well as those of focal injury (Hosmane et al., 2011; Hosmane et al., 2010; Pearce and Williams, 2006; Taylor and Jeon, 2010; Yang et al., 2009). These devices also range from those that support multiple parallel experiments in a single device to those that allow for single axon injury (Hosmane et al., 2010; Kim et al., 2009). Although monolayer cell culture techniques that isolate neurons from their growth cones have been critical to our understanding of intracellular processes, they cannot model the complex three-dimensional interactions necessary for peripheral nerve regeneration. Furthermore, these techniques are difficult to apply to motoneurons because of their need for trophic support from surrounding glia (Elliott, 1999; Gingras et al., 2008).
Organotypic co-culture of spinal cord and peripheral nerve overcomes many of the shortcomings of monolayer techniques. Motoneurons that retain contact with glia and other motoneurons in spinal cord slices support robust regeneration and survive for up to three months (Rothstein et al., 1993). Similarly, peripheral nerve survives in culture, and maintains the three-dimensional relationship of Schwann cells and basal lamina critical to the support of axon regeneration (Crang and Blakemore, 1986; Ide et al., 1983). In our previous work, we co-cultured these two tissues and were able to innervate segments of peripheral nerve with motor axons (Vyas et al., 2010). The ability to contain these axons within the confines of a peripheral nerve segment, and thus independent of the membrane surface, suggested the possibility of using nerve as a flexible conduit to direct axons from one compartment to another.
Our initial model of organotypic nerve repair utilized the Transwell© culture system (Vyas et al., 2010). In the current model, a shortened Transwell© insert is supported by a two-chamber PDMS reservoir. These modifications enhance the quality of imaging, facilitate surgical manipulation of the tissues, and create separate motoneuron and regeneration compartments. In the Transwell© system, cultures are imaged from below, through both the plastic bottom of the dish and 1mm of culture medium. Reducing the height of the insert facilitates high resolution imaging from above the membrane, eliminating the distortion caused by plastic and medium and allowing the microscope objective to get closer to the tissue. Without the high walls surrounding the insert it is easier to manipulate tissues on the membrane with microsurgical instruments.
The greatest challenge in modifying our original model was to develop a partition that would simultaneously provide fluidic isolation of two compartments yet support the transfer of living axons from one chamber to the other. After trying several configurations, we succeeded by bisecting the membrane along the axis of the partition and separating the edges with a thin barrier of silicon grease. The consequences of modifying the membrane in this way included the need to pin the membrane to the partition and support it with lateral buttresses to keep it taut beneath the spinal cord and nerve segments.
As a result of these modifications, we have developed the first two-compartment organotypic spinal cord and peripheral nerve co-culture platform. We have demonstrated that motoneurons remain viable within this system, and extend their axons from one compartment to the other. Furthermore, we have confirmed the physiologic integrity of the system by applying nocadazole to inhibit axonal regrowth in one compartment without affecting axons or motoneuron cell bodies in the other compartment. With these advances, we can now isolate growth factor effects to regenerating motor axons without influencing their parent neurons. This can be done by adding the growth factor of choice to the regeneration compartment, or by perturbing growth factor function with antibodies or specific inhibitors. Additionally, work is ongoing in our laboratory to combine spinal cord and DRG cultures to produce mixed nerve for studies of regeneration specificity.
5. Conclusions
We have developed a two-compartment culture device for the study of motor axon regeneration after nerve repair. The device consists of a two-compartment PDMS base to which a collagen-coated membrane is attached. We have demonstrated that such a device can be used to support viable spinal cord and peripheral nerve co-cultures, to perform nerve repair in vitro, and to maintain fluidic isolation between motoneuron and growth cone compartments. This device is ideal for the study of growth factor effects on axons regenerating within the three-dimensional structure of peripheral nerve.
Highlights.
We describe the first two-compartment organotypic model of peripheral nerve repair.
Motor axons regenerate through three-dimensional peripheral nerve segments that can be transected and repaired.
The environment of regenerating axons can be modified without altering that of parent motoneurons.
This model is ideal for studying the effects of pathway-derived growth factors on regenerating motor axons.
Acknowledgements
The authors would like to acknowledge NIH R01 NS034484, the Institutute of Nanobiotechnology, and the Robert Packard Center for funding this project. Large composites were assembled by Mr. Norman Barker, and other illustrations were prepared with the help of Ms. Kate Kiefe.
Abbreviations
- GDNF
Glial cell-line derived neurotrophic factor
- DMSO
Dimethylsulfoxide
- DRG
Dorsal root ganglion
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- NGF
Nerve growth factor
- PDMS
Polydimethylsiloxane
- YFP
Yellow fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brushart TM, Aspalter M, Griffin JW, Redett R, Hameed H, Zhou C, Wright M, Vyas A, Hoke A. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Experimental Neurology. 2013;247:272–81. doi: 10.1016/j.expneurol.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. Development of sympathetic neurons in compartmentalized cultures: I. Local control of neurite growth by nerve growth factor. Developmental biology. 1982;93:1–12. doi: 10.1016/0012-1606(82)90232-9. [DOI] [PubMed] [Google Scholar]
- Campenot RB. Local control of neurite development by nerve growth factor. Proceedings of the National Academy of Sciences. 1977;74:4516–19. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A, Blakemore W. Observations on Wallerian degeneration in explant cultures of cat sciatic nerve. Journal of neurocytology. 1986;15:471–82. doi: 10.1007/BF01611730. [DOI] [PubMed] [Google Scholar]
- Elliott JL. Experimental models of amyotrophic lateral sclerosis. Neurobiology of Disease. 1999;6:310–20. doi: 10.1006/nbdi.1999.0266. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging Neuronal Subsets in Transgenic Mice Expressing Multiple Spectral Variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Gähwiler B, Capogna M, Debanne D, McKinney R, Thompson S. Organotypic slice cultures: a technique has come of age. Trends in neurosciences. 1997;20:471–7. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Gingras M, Beaulieu M-M, Gagnon V, Durham H, Berthod F. In Vitro study of axonal migration and myelination of motor neurons in a three-dimensional tissue-engineered model. Glia. 2008;56:354–64. doi: 10.1002/glia.20617. [DOI] [PubMed] [Google Scholar]
- Höke A, Redett R, Hameed H, Jari R, Zhou C, Li Z, Griffin J, Brushart T. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. The Journal of neuroscience. 2006;26:9646–55. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmane S, Fournier A, Wright R, Rajbhandari L, Siddique R, Yang IH, Ramesh K, Venkatesan A, Thakor N. Valve-based microfluidic compression platform: single axon injury and regrowth. Lab Chip. 2011;11:3888–95. doi: 10.1039/c1lc20549h. [DOI] [PubMed] [Google Scholar]
- Hosmane S, Yang IH, Ruffin A, Thakor N, Venkatesan A. Circular compartmentalized microfluidic platform: Study of axon-glia interactions. Lab Chip. 2010;10:741–7. doi: 10.1039/b918640a. [DOI] [PubMed] [Google Scholar]
- Ide C, Tohyama K, Yokota R, Nitatori T, Onodera S. Schwann cell basal lamina and nerve regeneration. Brain research. 1983;288:61–75. doi: 10.1016/0006-8993(83)90081-1. [DOI] [PubMed] [Google Scholar]
- Kaal E, Joosten E, Bär P. Prevention of apoptotic motoneuron death in vitro by neurotrophins and muscle extract. Neurochemistry international. 1997;31:193–201. doi: 10.1016/s0197-0186(96)00148-9. [DOI] [PubMed] [Google Scholar]
- Kim Y, Karthikeyan K, Chirvi S, Davé DP. Neuro-optical microfluidic platform to study injury and regeneration of single axons. Lab Chip. 2009;9:2576–81. doi: 10.1039/b903720a. [DOI] [PubMed] [Google Scholar]
- Kimpinski K, Campenot R, Mearow K. Effects of the neurotrophins nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor (BDNF) on neurite growth from adult sensory neurons in compartmented cultures. Journal of neurobiology. 1997;33:395–410. doi: 10.1002/(sici)1097-4695(199710)33:4<395::aid-neu5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Liu R, Snider W. Different signaling pathways mediate regenerative versus developmental sensory axon growth. Journal of Neuroscience. 2001;21:164. doi: 10.1523/JNEUROSCI.21-17-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makwana M, Raivich G. Molecular mechanisms in successful peripheral regeneration. Febs Journal. 2005;272:2628–38. doi: 10.1111/j.1742-4658.2005.04699.x. [DOI] [PubMed] [Google Scholar]
- Park JW, Vahidi B, Taylor AM, Rhee SW, Jeon NL. Microfluidic culture platform for neuroscience research. Nature protocols. 2006;1:2128–36. doi: 10.1038/nprot.2006.316. [DOI] [PubMed] [Google Scholar]
- Pearce TM, Williams JC. Microtechnology: meet neurobiology. Lab Chip. 2006;7:30–40. doi: 10.1039/b612856b. [DOI] [PubMed] [Google Scholar]
- Redett R, Jari R, Crawford T, Chen Y-G, Rohde C, Brushart TM. Peripheral pathways regulate motoneuron collateral dynamics. The Journal of neuroscience. 2005;25:9406–12. doi: 10.1523/JNEUROSCI.3105-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein J, Jin L, Dykes-Hoberg M, Kuncl R. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:6591–5. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Blurton-Jones M, Rhee S, Cribbs D, Cotman C, Jeon N. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nature methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Jeon NL. Micro-scale and microfluidic devices for neurobiology. Current opinion in neurobiology. 2010;20:640–7. doi: 10.1016/j.conb.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Tucker BA, Rahimtula M, Mearow KM. Laminin and growth factor receptor activation stimulates differential growth responses in subpopulations of adult DRG neurons. Eur J Neurosci. 2006;24:676–90. doi: 10.1111/j.1460-9568.2006.04963.x. [DOI] [PubMed] [Google Scholar]
- Vyas A, Li Z, Aspalter M, Feiner J, Hoke A, Zhou C, O’Daly A, Abdullah M, Rohde C, Brushart TM. An< i> in vitro</i> model of adult mammalian nerve repair. Experimental neurology. 2010;223:112–8. doi: 10.1016/j.expneurol.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Whitesides GM. Soft lithography. Annual review of materials science. 1998;28:153–84. [Google Scholar]
- Yang IH, Siddique R, Hosmane S, Thakor N, Höke A. Compartmentalized microfluidic culture platform to study mechanism of paclitaxel-induced axonal degeneration. Experimental neurology. 2009;218:124–8. doi: 10.1016/j.expneurol.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]