Abstract
Contemporary vaccine development relies less on empirical methods of vaccine construction, and now employs a powerful array of precise engineering strategies to construct immunogenic live vaccines. In this review, we will survey various engineering techniques used to create attenuated vaccines, with an emphasis on recent advances and insights. We will further explore the adaptation of attenuated strains to create multivalent vaccine platforms for immunization against multiple unrelated pathogens. These carrier vaccines are engineered to deliver sufficient levels of protective antigens to appropriate lymphoid inductive sites to elicit both carrier-specific and foreign antigen-specific immunity. Although many of these technologies were originally developed for use in Salmonella vaccines, application of the essential logic of these approaches will be extended to development of other enteric vaccines where possible. A central theme driving our discussion will stress that the ultimate success of an engineered vaccine rests on achieving the proper balance between attenuation and immunogenicity. Achieving this balance will avoid over-activation of inflammatory responses, which results in unacceptable reactogenicity, but will retain sufficient metabolic fitness to enable the live vaccine to reach deep tissue inductive sites and trigger protective immunity. The breadth of examples presented herein will clearly demonstrate that genetic engineering offers the potential for rapidly propelling vaccine development forward into novel applications and therapies which will significantly expand the role of vaccines in public health.
Keywords: Salmonella, carrier, vaccine, foreign antigen, metabolic burden, over-attenuation
INTRODUCTION
The recent explosion in the availability of genomic sequences for a wide variety of pathogenic organisms, coupled with a rapid advance in powerful genetic engineering technologies, now offers the opportunity of efficiently developing highly immunogenic and protective vaccines against a wide variety of diseases. The pathogens against which these vaccines are developed may be of viral, bacterial, parasitic, or fungal origin, and the resulting vaccines can be engineered either for animal or human vaccination. In this review, we will focus on the engineering of live bacterial vaccines, and we will use the genus Salmonella to illustrate engineering strategies, which can in principle be applied to a variety of bacterial pathogens for which relevant molecular biology and pathogenicity data are available. A central theme of this review will be the importance of metabolic fitness and its impact on the immunogenicity and protective efficacy of live vaccines. The application of engineering technologies to pathogens without careful consideration of the balance between attenuation and immune responses can yield vaccine candidates that have excellent safety characteristics but have lost the capacity to reach immunological effector sites and consequently fail to induce protective immunity. Strategies that have been recently developed to address this critical balance between safety and immunogenicity will be emphasized within this context of metabolic fitness.
ENGINEERING of BACTERIA INTENDED AS HOMOLOGOUS VACCINES
Attenuating strategies targeting virulence and metabolism
It is relatively easy to weaken pathogens and engineer safe candidate attenuated vaccines. Given that these pathogens are exquisitely adapted to grow and replicate within their hosts, engineering disruptions in their intricate balance of metabolic and virulence mechanisms will certainly not require an inordinate amount of technical prowess to create attenuated strains. However, assuring safety while still achieving the immunogenicity and protective efficacy required with live vaccines has proven to be a much more challenging proposition for vaccine development. In cases where virulence factors such as toxins have been clearly defined, engineering deletions of such toxins has proven to be quite successful in creating effective vaccines. Complete deletion of virulence genes, rather than introduction of inactivating point mutations, is required to ensure that the likelihood of reversion of the vaccine candidate back to a wildtype pathogen is very low; to further reduce the possibility of reversion, introduction of one or more additional attenuating deletions is usually carried out as well. This early strategy for vaccine design was successfully applied by Tacket et al. almost a decade ago in the construction of an attenuated live cholera vaccine [1]. To accomplish this, the wildtype V. cholerae classical Inaba strain 569B was engineered for removal of both the catalytic subunit of cholera enterotoxin, as well as deletion of a putative hemolysin virulence factor. When tested in volunteers, this vaccine was found to be safe and highly immunogenic, with a protective efficacy of 91% against moderate to severe diarrhea and 80% against any diarrhea, after challenge with 105 colony forming units (CFUs) of fully virulent Vibrio cholerae.
In the case of Salmonella vaccines, attenuation of wildtype strains has focused both on deletion of virulence factors as well as disruption of metabolic pathways, and the two serovars of Salmonella with which most vaccine constructions have been carried out are Salmonella enterica serovars Typhimurium and Typhi. S. Typhimurium typically causes a self-limiting gastroenteritis in humans while S. Typhi is the etiologic agent of typhoid fever. In both serovars, virulence factors have been found to be chromosomally encoded within clusters called Salmonella Pathogenicity Islands (SPIs) which play critical roles in the manifestation of disease [2]. Much attention has been devoted in particular to two distinct pathogenicity islands that encode type III secretion systems (T3SS) that inject virulence proteins called effectors into target eukaryotic cells, disrupting normal host cellular functions and facilitating Salmonella invasion and systemic disease [3–5]. The SPI-1 T3SS externally targets eukaryotic host cells and injects effectors that trigger actin rearrangements to enhance uptake of Salmonella. Then using the SPI-2 T3SS, internalized Salmonella are able to inject additional effector proteins into the cytoplasm essential for bacterial intracellular survival and replication [6].
In a study reported by Hindle et al. [7], attenuated vaccine candidates from both S. Typhimurium (designated WT05) and S. Typhi (designated M01ZH09) were engineered such that delivery of all SPI-2 effectors was disrupted by deletion of a critical structural protein ssaV involved in the assembly of the effector injectisome apparatus. This deletion mutation was accompanied by a further deletion in aroC involved in the aromatic amino acid biosynthesis pathway, creating candidate vaccine strains which were then compared in a Phase 1 doseescalating clinical trial [7]. Both strains were shown to be safe, with negligible clinical symptoms and no vaccine organisms detected in the blood. The S. Typhi M01ZH09 vaccine was shed from the majority of volunteers for 3 days. However, the attenuated S. Typhimurium WT05 strain established an unacceptably persistent colonization of volunteers with shedding for up to 3 weeks, and this strain was not pursued further. When evaluated in Phase 2 clinical trials [8], single oral doses of M01ZH09 up to 1.7 × 1010 CFUs were found to be safe and immunogenic, with 97.4% of subjects responding to vaccination with either IgG or IgA responses to S. Typhi LPS, and 92.1% of those receiving a dose of 7.5 × 109 CFUs having a positive S. Typhi LPS-specific ELISPOT response.
Balancing safety and immunogenicity
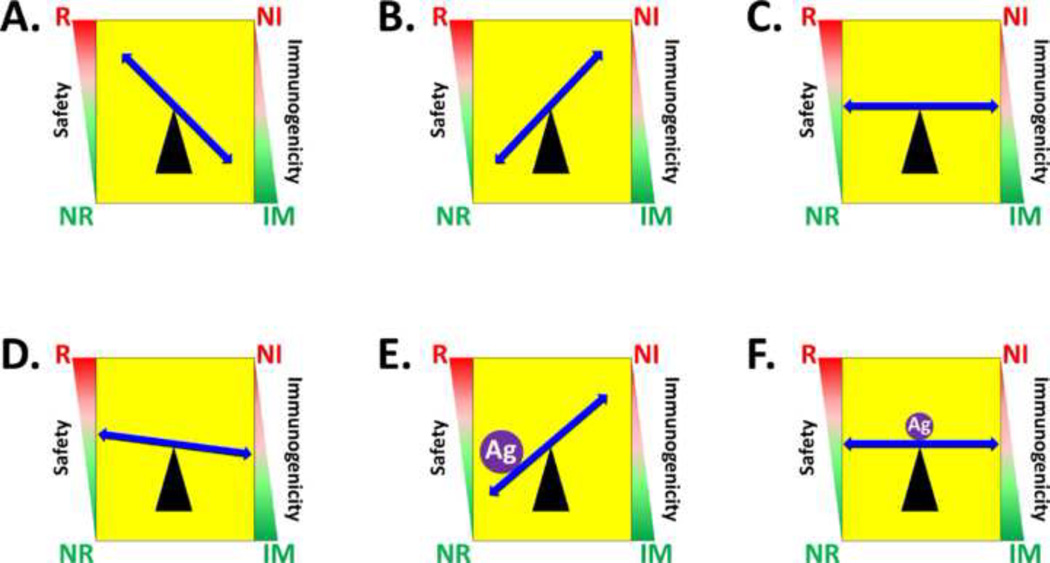
Live vaccines that are insufficiently attenuated elicit unacceptable clinically defined adverse events in vaccinees and are considered unacceptably reactogenic. As work with the attenuated M01ZH09 S. Typhi vaccine illustrates, attenuation strategies targeting both virulence determinants and metabolic factors can be quite effective for constructing safe and immunogenic live vaccines that perform well in clinical trials. However, care must be taken to ensure that metabolic attenuation strategies do not result in the over-attenuation of vaccines, with subsequent loss of immunogenicity resulting from the crippling of metabolic fitness of the live vaccine. Genetic inactivation of too many critical genes, or inappropriate selection of targets, can result in vaccine candidates that fail to colonize a host sufficiently to engage innate and acquired immunity, and elicit durable protection. We have previously reviewed the results of clinical trials conducted with attenuated S. Typhi candidate vaccines, and noticed a striking relationship between reactogenicity and immunogenicity [9], which we illustrate schematically in Figure 1. Fully virulent strains, as well as vaccine candidates, which are insufficiently attenuated elicit unacceptable clinical symptoms (i.e. highly reactogenic) but also tend to be highly immunogenic (Figure 1A). Vaccines that have been genetically engineered to minimize reactogenicity may become insufficiently immunogenic (Figure 1B). Ideally, the most promising live vaccines that perform well in clinical trials will achieve a delicate balance between reactogenicity and immunogenicity (Figure 1C).
Figure 1. Balancing reactogenicity and immunogenicity in the development of live vaccines.
Both the safety and immunogenicity of a live bacterial strain are schematically represented in the left and right sides of each panel as a gradient of values. For safety, the gradient extends from a region of unacceptable reactogenicity (red zone denoted by “R”) to a region of acceptable non-reactogenicity (green zone denoted by “NR”); similarly, for immunogenicity, the gradient extends from a region of minimal immunogenicity (red zone designated as non-immunogenic, “NI”) to the desired region of immunogenicity (green zone designated as immunogenic, “IM”). Panels A–C schematically represent construction of a properly attenuated vaccine strain; and Panels D–F represent construction of a carrier vaccine expressing additional protective antigens from unrelated pathogens. In this graphic, pathogenic organisms are represented by Panel A, over-attenuated vaccine strains engineered from pathogens are represented by Panel B, and properly engineered attenuated vaccines are represented by Panel C. Given that expression of a foreign antigen can elevate metabolic burden and increase attenuation, starting with a slightly reactogenic vaccine candidate (Panel D) and over-expressing a foreign antigen (purple filled circle labeled “Ag”) will tip the balance and result in a safe but non-immunogenic live vaccine (Panel E), while expression of sufficient levels of foreign antigen to elicit immunogenicity without over-attenuating the carrier strain will yield both a safe and immunogenic carrier vaccine (Panel F).
This concept is clearly illustrated by a series of attenuated S. Typhi candidate oral vaccines engineered from the wildtype strain CDC10-80, all carrying a deletion in the aroA gene critical to the aromatic amino acid biosynthesis pathway. When coupled with an additional mutation in aroD, the resulting ΔaroA ΔaroD strain proved to be insufficiently attenuated but highly immunogenic (Figure 1A) [10] . When the triple deletion mutant ΔaroA ΔaroD ΔhtrA was constructed (by further deletion of htrA encoding a heat-shock serine protease), safety improved at lower oral dosage levels but immunogenicity declined (Figure 1B); at higher oral doses which improved immunogenicity, reactogenicity (i.e. fever and bacteremia) was unacceptably high (Figure 1A) [10]. Combining ΔaroA with deletions in either purA (involved in the purine biosynthesis pathway), or phoP/phoQ (a two-component environmental regulatory system of virulence in Salmonella) dramatically reduced both reactogenicity and immunogenicity (Figure 1B) [11;12]. It was only when the phoP/phoQ deletion mutation alone was introduced into a different parent strain of S. Typhi (Ty2) that it became possible to balance reactogenicity with immunogenicity at high oral dosage levels (Figure 1C) to induce vaccine-specific immunity [13]. These observations clearly illustrate that the engineering of an attenuated live bacterial vaccine requires a carefully crafted balance between attenuation and immunogenicity that is not always attainable by deliberate engineering and may sometimes only be achieved by trial and error, with clinical trials ultimately determining the fate of vaccines that animal models can only suggest as promising candidates.
Over-attenuation and the subsequent failure of engineered strains to reach appropriate immune inductive sites was encountered by Kong et al. [14] with efforts to construct attenuated strains of S. Typhimurium by engineering modifications to lipopolysaccharide (LPS). LPS is the major component of the outer membrane of Salmonella, and is a key virulence determinant that confers protection against complement activation and killing by macrophages [15]. LPS is comprised of a lipid A membrane anchor, a core oligosaccharide, and the outer O-antigen which defines the various serovars of Salmonella. Since the enzymatic pathways involved in LPS synthesis are well characterized for Salmonella [16], Kong et al. undertook a systematic analysis of the effects of engineering truncations in O-antigen and core sugars of LPS on the virulence and immunogenicity of S. Typhimurium. All of the resulting mutants tested were shown to be avirulent in mice; the lethal dose of organisms resulting in death for 50% of a group of orally challenged mice (LD50) was determined to be >109 CFU for all engineered strains. A clear relationship emerged between the extent of truncating LPS and deep tissue colonization of mice, and it was determined that deletion of LPS into the core oligosaccharide region resulted in a severe drop in colonization while preservation of at least one sugar residue at the terminus of the O-antigen was sufficient to enable significant colonization of the Peyer’s patches, liver, and spleen of orally and intranasally immunized mice. It was also conclusively demonstrated that strains which successfully colonized deep tissues induced excellent serum antibody responses against S. Typhimurium LPS [14].
Attenuation achieved by addition as well as subtraction
Up to this point, we have considered only the construction of attenuated vaccine strains by deletion of native endogenous functions, whether they be virulence determinants or metabolic factors. However, novel approaches have recently been reported in which fully functional factors from foreign bacteria have been engineered into a pathogenic strain with the intention of disrupting pathogenicity and improving innate and adaptive immunity. This intriguing strategy has been applied to the remodeling of the lipid A moiety of LPS which is responsible for the endotoxic activity of enteric pathogens such as Salmonella. The lipid A moiety of LPS stimulates a strong innate immune response via stimulation of the Toll-like receptor 4 (TLR4)-MD2 complex, and triggers a vigorous inflammatory response that contributes to the reactogenicity of Salmonella. The endotoxicity of lipid A is dependent on the number and length of hydrophobic acyl side chains anchoring lipid A into the outer membrane [17], as well as the phosphorylation state of the disaccharide backbone [18]. Lipid A is typically biphosphorylated in Salmonella, which enables full induction of innate immunity through stimulation of TLR4-MD2. Kong et al. [19] reported that insertion of foreign genes encoding non-homologous phosphatases into S. Typhimurium resulted in attenuated strains with increased sensitivity to the bile salt deoxycholate and reduced ability to colonize the deep tissues of mice after oral immunization. The LD50 for engineered strains producing monophosphorylated lipid A increased 3–4 logs while strains with totally non-phosphorylated lipid A were completely avirulent in mice. Despite the fact that monophosphorylated strains still retained some virulence at high doses, this strategy may still prove effective in reducing reactogenicity at high doses when combined with other attenuating deletions in metabolic pathways.
LPS-remodeling strategies have also proven to be useful in the engineering of attenuated strains of Yersinia pestis, the causative agent of plague. The lipid A membrane anchor of Y. pestis LPS carries six hydrophobic side chains (hexa-acylated) when residing outside its human host, but down-regulates acylation to a tetra-acylated form after infection in response to the increase in temperature from ambient 26°C to 37°C. Hexa-acylated LPS is a potent TLR4 agonist while tetra-acylated LPS binds poorly to TLR4-MD2, resulting in a reduction in inflammatory responses. When Y. pestis was engineered for chromosomal expression of a non-homologous acyl-transferase, forcing expression of hexa-acylated LPS at 37°C, the resulting strains were highly attenuated yet immunogenic when this mutation was coupled with a metabolic deletion in a sugar utilization pathway. Mice immunized subcutaneously or intranasally with this construct were protected against both subcutaneous and intranasal challenge with fully virulent Y. pestis [20]. Interestingly, when attempts were made to further attenuate engineered plague vaccines by reducing the phosphorylation state of lipid A using a non-homologous phosphatase (an approach that proved successful when applied to Salmonella), this strategy was unsuccessful with Y. pestis [21].
Attenuation through replication
Until recently, all attenuating strategies developed to date had begun with a fully virulent pathogen and engineered insertion or deletion mutations into a wildtype strain with the goal of creating a vaccine candidate that was sufficiently attenuated to ensure safety but sufficiently robust to confer immunogenicity. A novel shift in this established paradigm was first reported by Curtiss et al. in 2009 [22], in which candidate vaccines were engineered to be fully virulent at the time of immunization, and to become attenuated as they replicated within the host. This attenuation strategy effectively avoids the over-attenuation of vaccines prior to reaching appropriate immune induction sites since the intricate balance between virulence factors and metabolic pathways of the engineered pathogen remain unchanged until limited replication within the host gradually manifests the attenuated state. This biological transition from a fully virulent organism to an attenuated vaccine is referred to as a regulated delayed attenuation phenotype (RDAP), and is engineered to be dependent on the intracellular concentration of the sugar arabinose at the time of immunization. To accomplish this requires the genetic targeting of several key metabolic chromosomal loci involved in the intracellular replication and survival of Salmonella, whose transcription levels are re-engineered to be controlled by the arabinose activator/repressor AraC [23]. In the presence of arabinose, AraC binds arabinose and subsequently activates transcription of any genes transcriptionally controlled by the arabinose promoter PBAD; in the absence of arabinose, AraC is incapable of activating PBAD and transcription ceases. Given that arabinose concentrations available to vaccine organisms after immunization will be insufficient to ensure synthesis of these critical regulatory proteins in vivo, loss of function will increase with every round of replication of vaccine organisms until maximum attenuation occurs. Using this transcriptional control system, gene cassettes encoding AraC linked to PBAD were engineered by Curtiss et al. to replace the natural promoters controlling genes involved in iron regulation (fur), catabolite repression (crp), magnesium regulation of virulence factors (phoPQ), and stationary-phase protein expression (rpoS). All engineered strains were confirmed in vitro not to grow in the absence of arabinose on otherwise rich bacteriologic media. While strains carrying individually targeted regulatory genes proved to be insufficiently attenuated in the mouse model at elevated oral doses [22], later combinations of these mutations proved to yield highly immunogenic live vaccines in orally immunized mice [24]. This attenuating strategy was recently introduced into S. Typhi strains in anticipation of Phase 1 clinical trials [25] which have now been completed; candidate RDAP vaccine strains were proven to be safe and immunogenic in orally vaccinated volunteers, demonstrating that properly attenuated live S. Typhi vaccines such as RDAP vaccines can retain the ability to undergo limited replication within humans without eliciting clinical symptoms [26].
FURTHER ENGINEERING of ATTENUATED STRAINS AS CARRIER VACCINES FOR DELIVERY of FOREIGN ANTIGENS
Foreign gene expression and metabolic stress
It is clear from efforts to construct attenuated live vaccines that disruption of the metabolism of a pathogen results in attenuation and over-attenuation results in loss of immunogenicity. It therefore follows that attempts to further engineer additional expression technologies into a candidate vaccine, which may further impact already attenuated metabolic pathways, may destroy the efficacy of the resulting live vaccine without sufficient attention being paid to maintaining the fitness of the vaccine strain. This becomes a serious consideration in the development of live multivalent vaccines (hereafter referred to as carrier vaccines) designed for immunization against several unrelated pathogens by delivering additional protective antigens to the immune system. The efficacy of any live carrier vaccine rests with its ability to present sufficient foreign antigen to the human immune system to elicit the desired protective immune responses. However, unregulated expression of foreign antigens diverts precious energy and metabolic resources away from the metabolism of the vaccine and into synthesis of proteins from which the vaccine derives no selective advantage either in growth or replication. As has been observed in clinical trials with RDAP vaccines, after immunization, live vaccines undergo a limited number of replications which allow limited colonization of lymphoid inductive sites to induce immune responses; further compromise of the metabolism of the live vaccine by unrestricted synthesis of foreign antigens will inevitably over-attenuate the vaccine and destroy immunogenicity.
The inevitable effect of foreign antigen synthesis on the metabolism of a live carrier vaccine could in principle be exploited to reduce the reactogenicity and improve the safety of a candidate vaccine with some residual reactogenicity (Figure 1D). While it is clear that over-expression of a foreign antigen will over-attenuate the resulting carrier vaccine (Figure 1E), careful attention to appropriate induction of foreign antigen synthesis, either by regulating the timing, level of synthesis, or processing of the antigen could in principle restore the balance between attenuation and immunogenicity to create a carrier vaccine with both excellent safety and immunogenicity (Figure 1F).
The concept of over-expression of foreign antigens leading to attenuation of otherwise metabolically fit organisms has been intentionally exploited recently to create carrier vaccines directly from wildtype pathogens, a strategy referred to as Attenuating Gene Expression (AGE) [27]. Support for this concept comes from the observation that over-expression of endogenous native proteins such as flagella severely attenuates wildtype S. Typhimurium by disrupting the bacterial outer membrane, resulting in elevated susceptibility to bile and an inability to replicate within murine macrophages. Although attenuated, these engineered strains remained immunogenic and conferred excellent protection against homologous challenge with fully virulent S. Typhimurium [28]. Over-expression of native proteins was also confirmed to attenuate Y. pestis, wherein over-expression the caf operon, encoding the essential virulence capsule F1, was observed to dramatically reduce both intra-macrophage survival rates and the infectivity of otherwise fully virulent Y. pestis [29]. Over-expression of the caf operon was subsequently engineered as a foreign antigen gene cassette into wildtype S. Typhimurium, resulting in a carrier strain with severely reduced survival in murine macrophages and complete loss of virulence in mice. As expected, if expression of the caf operon was tightly regulated using the native Y. pestis temperature regulated promoter, the resulting carrier strain displayed excellent expression of the F1 capsule but also retained full virulence in mice [29]. Since such over-expression of foreign proteins clearly exerts metabolic pressure on the carrier strain, a selective advantage will arise for spontaneous deletion mutations arising that destroy foreign antigen synthesis. Therefore, reliance solely on the AGE strategy for engineering carrier vaccines will not be adequate and will require additional independently attenuating deletions to ensure safety.
Metabolic stress and instability of expression plasmids
Expression and delivery of foreign antigens by attenuated carrier vaccines can be accomplished either by plasmid-mediated expression or by integration of foreign genes into the vaccine chromosome. When using multicopy expression plasmids, induction of antigen expression can introduce sufficient metabolic stress upon the carrier vaccine to result in a selective advantage for plasmid loss, which eliminates this metabolic stress and allows a restoration of fitness. If rapid plasmid loss occurs in vivo following immunization, antigen-specific immunity will be lost as well. Since the use of antibiotics for plasmid maintenance (a practice commonly used under laboratory conditions) is of little use in vivo and is currently discouraged by the Food and Drug Administration for use with human oral vaccines, non-antibiotic strategies are needed for ensuring plasmid maintenance in vivo and enhancing antigen-specific immunity. An effective yet simple solution to this dilemma was devised in which a gene encoding an essential function within the carrier vaccine was deleted from the chromosome and placed instead on a multicopy expression plasmid. Loss of the plasmid would then result in a non-viable carrier vaccine, thereby ensuring plasmid maintenance in vivo during limited replication and colonization of inductive lymphoid tissues. When chromosomal targets for such deletions involve enzymatic functions whose metabolic products can be added to the growth medium during in vitro cultivation, construction of multivalent carrier vaccines becomes reasonably straightforward. This strategy was exploited by Galán et al. [30] more than two decades ago by targeting a metabolic pathway involved in the synthesis of the S. Typhimurium cell wall. The enzyme aspartate β-semialdehyde dehydrogenase (Asd) is essential for the proper synthesis of the bacterial cell wall and several amino acids [31], and loss of Asd activity results in lysis of the bacterium resulting from an inability to correctly assemble the peptidoglycan layer of the cell wall. Attenuated strains in which Asd-encoding plasmids have not yet been introduced can be efficiently propagated by adding the metabolite diaminopimelic acid (DAP) to the growth medium until Asd-stabilized plasmids have been introduced. Using this plasmid stabilization strategy, attenuated strains of S. Typhimurium were constructed in which asd was encoded by high copy number plasmids, and the resulting carrier strains were evaluated for plasmid retention in orally immunized mice. For carrier strains recovered from deep tissues of immunized mice, 99% of recovered vaccine organisms retained Asd-stabilized expression plasmids in vivo (despite the very high copy number of these plasmids), compared to only 10% of vaccine organisms retaining unstabilized lower copy number conventional plasmids after recovery from mice [32].
This remarkably versatile strategy for plasmid maintenance was later expanded to include non-catalytic proteins such as the single-stranded binding protein (SSB) which is essential for DNA replication, recombination, and repair [33;34]. Since SSB produces no metabolic products that can be added to cultures in vitro, chromosomal deletion of ssb required the sequential use of temperature-sensitive suicide plasmids to establish this maintenance system [35]. Use of SSB-stabilized plasmids in attenuated S. Typhi carrier vaccines ultimately showed that antibody responses elicited in mice against foreign antigens delivered by S. Typhi carrier vaccines were inversely related to the metabolic burden imposed by expression of the foreign antigen, and that these responses were improved when antigens were expressed from low-copy-number SSB-stabilized plasmids carried by less attenuated carrier vaccines [35].
Minimizing metabolic stress by synchronizing antigen expression with metabolism
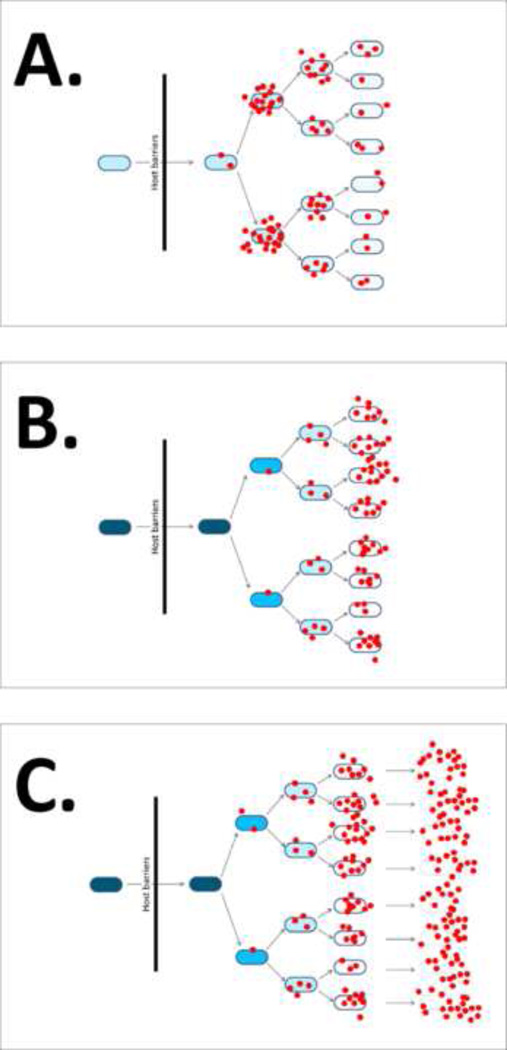
Success with plasmid maintenance systems that essentially guarantee plasmid stability in vivo can quickly lead to additional problems with over-attenuation frequently associated with plasmid-based delivery of foreign proteins. Due to the multicopy aspect of plasmids, when foreign genes are induced there is a rapid rise in antigen synthesis (Figure 2A) [36] which tends to slow growth rate and reduce the colonization capacity of the carrier vaccine due to the severe diversion of metabolic resources [37]. Over the years, vaccine developers have created a number of tools for reducing the effect of this antigen burst through tightly regulating control of the transcription, translation, and export of foreign antigens. These techniques have been reviewed elsewhere in considerable detail by ourselves [38–40] and other groups [41;42], and will not be recapitulated here. Slightly more unconventional efforts to minimize the effects of antigen burst have recently been reported in which an attempt is made to link the timing of antigen synthesis directly to the physiology of the carrier vaccine.
Figure 2. Strategies for developing safe and immunogenic carrier vaccines.
Candidate live vaccines are depicted as elongated circles, and the degree of attenuation is represented by shades of blue in which deep shades represent non-attenuated strains with shades becoming paler with increasing attenuation. Production of foreign antigen is depicted as red dots. Immunization is represented as a black arrow extending across a vertical black line labeled “host barriers”. Panel A depicts conventional strategies in which an attenuated strain is engineered for tightly regulated expression of genes encoding foreign antigens after reaching inductive sites within the host, resulting in a burst of antigen synthesis and an increase in attenuation of the carrier vaccine. Panel B depicts the combined strategies of Regulated Delayed Attenuation Phenotype (RDAP) and Regulated Delayed Antigen Synthesis (RDAS). Fully invasive carrier vaccines are used for immunization, which undergo limited replication and become increasingly attenuated with each round of replication (RDAP). As replication proceeds and attenuation increases, synthesis of foreign antigen also begins to increase as vaccine organisms reach lymphoid inductive sites (RDAS). Progression of both attenuation and antigen synthesis is inextricably linked in vivo to depletion of intracellular levels of arabinose as limited replication proceeds (see text for further details). Panel C depicts the combined strategies of RDAP and regulated delayed lysis to improve delivery of foreign antigens (or DNA vaccines) to immune inductive sites. As replication proceeds and attenuation increases (RDAP), regulated induction of foreign antigen synthesis begins, accompanied by complete lysis and release of cytoplasmic contents into antigen presenting cells as vaccine organisms reach lymphoid inductive sites (see text for more details).
One way to link the synthesis of foreign antigens to vaccine physiology is an extension of the technique of regulated delayed attenuation, in which the timing of antigen synthesis is intimately linked to replication of the vaccine in vivo. This strategy is referred to as regulated delayed antigen synthesis (RDAS, Figure 2B) [43], and was created by engineering modifications to the well characterized lactose repressor (LacI) transcriptional control system frequently employed to control foreign antigen synthesis [44]. In the absence of lactose or other synthetic sugar analogs, LacI binds to its cognate promoter and prevents transcription of the downstream open reading frame; the binding of LacI to lactose or synthetic analogs causes an allosteric shift in LacI conformation which derepresses transcription, with subsequent commencement of foreign antigen synthesis. By replacing the natural lacI promoter with an arabinose-controlled araC promoter, antigen synthesis is then linked to intracellular concentrations of arabinose, in a strategy similar to the previously discussed delayed attenuation system. Antigen synthesis is then ultimately linked to the replication and growth rate of the bacterial strain, with diminishing intracellular concentrations of arabinose leading to a shift in protein synthesis away from LacI expression and towards an increase in foreign antigen synthesis (Figure 2B). Such an approach would theoretically allow remarkable diversity in the timing of antigen delivery by a carrier vaccine. Antigen production in vaccine organisms prepared in the presence of arabinose would be tightly repressed prior to immunization; after oral administration, transient exposure to lactose after immunization could enable a quick but temporary burst of foreign antigen production, with full and sustained induction occurring as the vaccine grows and colonizes lymphoid tissues and intracellular LacI concentrations drop.
A thorough characterization of plasmid-based regulated delayed antigen synthesis in attenuated S. Typhimurium carrier vaccines was described by Wang et al. [43], and this work clearly demonstrated that the over-attenuating effects of poorly regulated antigen expression from multicopy plasmids could be effectively overcome by an arabinose-controlled LacI-mediated antigen gene expression system. When mice were orally immunized with RDAS carrier vaccines delivering a protective pneumococcal surface protein (PspA) antigen from Streptococcus pneumoniae, 52% of vaccinated animals were protected against challenge with fully virulent S. pneumoniae, while only 21% of mice receiving vaccines constitutively over-expressing the foreign antigen were protected [43].
In a related approach linking the timing of antigen synthesis directly to the physiology of the carrier vaccine, initial studies have demonstrated the feasibility of controlling the timing of foreign antigen expression encoded by chromosomally engineered expression cassettes, without the need for plasmids. Although expression of foreign antigens exclusively from chromosomally integrated gene cassettes offers the substantial advantage of minimizing any metabolic burden associated either with multicopy expression plasmids or the foreign antigens they encode, the challenge with this approach has been synthesizing sufficient levels of foreign antigen capable of eliciting relevant immune responses despite the significant drop in copy number of the cassettes encoding these antigens. Using a cassette encoding the model foreign antigen green fluorescent protein (GFP), Wang et al. [36] described a novel chromosomal expression strategy designed to compensate for the inherent disadvantage of lower gene dosage (versus plasmid-based expression) by integrating a single GFP-encoding gene cassette into multiple chromosomal sites already inactivated in an attenuated S. Typhi vaccine candidate. Using GFP-encoding cassettes integrated into both guaBA (which displays growth-regulated transcriptional control of antigen synthesis [45]) and htrA (which displays transcriptional control of antigen synthesis in response to metabolic stress during growth [46]), cumulative synthesis of GFP from these two integration sites was observed to be superior to single integrations. Most importantly, it was demonstrated that GFP expression increased in a growth phase-dependent manner, suggesting that foreign antigen synthesis could be “tuned” to the physiology of the carrier vaccine [36]. This promising chromosomal expression technology is currently being combined with plasmid-based expression from stabilized plasmids for delivery of several protective antigens from Y. pestis, delivered by a single multivalent S. Typhi carrier vaccine.
Site of antigen delivery and subsequent immune response
In addition to tightly regulated expression of foreign antigens, it is now clear that the manner in which these antigens are delivered to the immune system can have a profound impact on the resulting immune responses and ultimate success of a carrier vaccine. The induction and extent of mucosal, humoral, or cellular immunity can be significantly influenced by whether foreign antigens are expressed within the carrier vaccine or exported out of the live vaccine, as well as whether antigens are expressed prior to host cell invasion or delivered by intracellular carriers. It is now reasonably well established that antigen-specific humoral immunity can increase significantly when antigens are exported either to the carrier surface or extracellularly into the surrounding milieu, rather than remaining in the cytoplasm [47–49]. It has also been reported that cellular immunity to surface antigens delivered by intracellular carriers is superior to immunity targeting cytoplasmic antigens [50].
Cellular responses can also be improved by injection of foreign proteins from intracellular carrier vaccines into the cytoplasm of antigen-presenting cells via Salmonella type III secretion systems, a technique first described over a decade ago by Russmann et al [51]. Hegazy et al. [52] have further developed this approach by conducting a methodical analysis of the translocation efficiency of a panel of SPI-2 effector proteins when used as carriers for antigenic passenger domains fused to the carboxyl terminus of the SPI-2 carrier. They observed that for S. Typhimurium carrier vaccines delivering passenger domains of listeriolysin O (a protective antigen from Listeria monocytogenes) fused to SPI-2 effectors, translocation of fusions into murine bone marrow-derived dendritic cells displayed varying efficiencies in vitro, depending on the specific effector fusion involved. However, in mice orally immunized with these carrier strains, stimulation of L. monocytogenes antigen-specific cytotoxic T-cells did not strictly correlate with in vitro translocation efficiencies. This disparity may be a reflection of artificial induction conditions used for in vitro expression, which may have little relevance to in vivo microenvironmental induction conditions. However, it was clear from these studies that choosing the right SPI-2 effector for translocation of a vaccine antigen can elicit robust levels of cytotoxic immunity against intracellular pathogens.
Antigen delivery by regulated lysis
One rather extraordinary method for delivery of intracellular antigens to lymphoid inductive sites is through outright lysis of the carrier vaccine to release cytoplasmic contents including foreign proteins (depicted schematically in Figure 2C). Success with this approach will depend on the timing of lysis, which must occur as vaccine organisms are reaching inductive sites. Since delayed attenuation technologies proved that it was possible to control the timing of induced attenuation to coincide with deep tissue colonization and elicitation of protective immunity, it was considered plausible to adapt the delayed phenotype strategy to achieve delayed lysis of a carrier vaccine and test the immunogenicity of these novel constructs. Successful testing of this delayed lysis strategy was first reported by Kong et al. [53] who genetically engineered a programmed lysis system based on arabinose-controlled production of two key enzymes involved in the synthesis and mechanical stability of the carrier vaccine cell wall. Diaminopimelic acid and muramic acid are explicitly required to ensure the integrity of the peptidoglycan layer of the carrier vaccine cell wall, and synthesis of these two components requires enzymes encoded by chromosomal asd and murA genes respectively. The requirement for synthesis of aspartate β-semialdehyde dehydrogenase (Asd) to ensure the integrity of the cell wall was first exploited for maintenance of plasmids delivering foreign antigens (described above), with lysis of the vaccine resulting from plasmid loss and cessation of Asd synthesis. To ensure complete lysis of vaccine organisms and full release of cytoplasmic contents, arabinose-controlled synthesis of Asd was coupled with additional arabinose-controlled synthesis of MurA. To establish exquisitely stringent regulation of Asd and MurA synthesis, an arabinose-regulated anti-sense RNA system was also engineered onto the antigen-expressing multicopy plasmid. In the absence of arabinose, chromosomal transcription of both asd and murA ceased, as did transcription of an additional chromosomally encoded repressor of the anti-sense-RNA system. Therefore, cessation of both asd and murA transcription was simultaneously accompanied by induction of high levels of antisense RNA synthesized from the expression plasmid, effectively blocking residual translation from any lingering asd or murA transcripts. These concerted activities lead to cell lysis and release of vaccine antigens [53]. In mice orally immunized with carrier vaccines engineered for constitutive periplasmic synthesis of the S. pneumoniae protective PspA antigen, coupled with delayed lysis to release PspA, excellent serum antibody responses against both the foreign antigen and carrier-specific outer membrane proteins were reported. No viable vaccine organisms were detected in host tissues after three weeks, demonstrating that these engineered strains were able to proliferate long enough to stimulate humoral immunity but were eventually completely cleared from the vaccinated host [53]. This last point may have relevance to previous clinical trials in which orally administered S. Typhimurium WT05 vaccine strains proved unacceptably attenuated and were shed for greater than three weeks from vaccinees [7].
The development of the delayed lysis strategy for carrier vaccines paves the way for significant improvements in the use of carriers for delivery of DNA vaccines. Although several early reports supported the feasibility of delivering DNA vaccines using attenuated carrier strains to elicit immune responses [54–56], attempts to expand this vaccination strategy have proven frustrating. The use of delayed lysis to improve DNA vaccine delivery and ensuing immune responses has now been reported by Kong et al. [57]. In addition to the delayed lysis strategy, additional vaccine modifications required for successful delivery of the DNA vaccine involved: 1] increased expression of the SPI-1 activator HilA protein, resulting in a hyperinvasive phenotype for the carrier strain to improve intracellular delivery of the DNA vaccine, 2] inactivation of the SPI-2 effector protein SifA allowing Salmonella to escape from intracellular vacuoles into the target cell cytoplasm, 3] inactivation of additional SPI-2 effectors which normally induce apoptosis of eukaryotic cells by intracellular Salmonella, thereby allowing sufficient time for DNA vaccines to traffic to the nucleus, and 4] insertion of multiple DNA vaccine nuclear-targeting sequences to facilitate efficient delivery of the DNA vaccine to the target cell nucleus after cytoplasmic lysis of the carrier. An optimized DNA vaccine encoding the influenza hemagglutinin (HA) protective antigen, delivered by an optimized delayed lysis S. Typhimurium carrier vaccine, induced complete protection in orally immunized mice against a lethal intranasal challenge with 100 LD50s of fully virulent influenza virus [57]. Given that DNA vaccines are virtually silent in carrier strains, plasmid-mediated metabolic attenuation of the carrier is significantly reduced, and such vaccines have great potential in applications for which conventional vaccination strategies have proven unsatisfactory.
NOVEL APPLICATIONS of ENGINEERED VACCINES
Engineered vaccines as “reagent strains”
Aside from the conventional deployment of engineered strains as live and carrier vaccines, several additional applications of these strains have arisen which bear mention, including use in conjugate vaccine development and novel approaches to cancer treatment and prevention. Conjugate vaccines represent a versatile subunit vaccine strategy in which protective immunity can be targeted against capsular and outer membrane polysaccharides of a variety of Gram-negative bacteria. Along with the burgeoning interest in conjugate vaccines against pathogenic bacteria comes the problem of economic and safe purification of the polysaccharide haptens and the carrier proteins from which the conjugate vaccines are manufactured. Tennant et al. [58] have recently reported the development of “reagent strains” which have been specifically engineered for efficient purification of conjugate components from a single attenuated strain. These reagent strains can then be used to develop homologous conjugate vaccines comprised of purified endotoxin-free core-O-polysaccharides (COPS) chemically conjugated to purified flagellin monomers. Given the simplicity of the approach, this technique was applied to the engineering of reagent strains derived from both S. Typhimurium and S. Enteritidis pathogens. The engineering of these reagent strains was accomplished with three key steps in which fully virulent pathogens were first rendered auxotrophic for guanine by deletion of the chromosomal guaBA locus, followed by a deletion of a master clpP regulatory locus which resulted in hyperflagellation of the reagent strain. The final critical engineering step involved deletion of a FliD capping protein involved in the polymerization of flagellin monomers into fully functional flagella; in the absence of FliD, flagellin monomers were exported into the surrounding medium and could be efficiently purified away from intact bacteria. When evaluated in mice, Simon et al. [59] observed that conjugate vaccines developed against S. Enteritidis generated robust flagellin-specific antibody responses and higher anti-LPS IgG responses than observed in mice immunized only with unconjugated COPS. Most importantly, in mice challenged with fully virulent S. Enteritidis, conjugate vaccines conferred 100% protection in vaccinated mice receiving fractional doses down to 0.25 µg, and 90% efficacy in mice immunized with as little as 25 ng of conjugate vaccine [60]. Genetically engineered reagent strains therefore represent a remarkably straightforward method from which highly immunogenic homologous flagellin-based conjugate vaccines can be safely and economically manufactured from a single attenuated strain.
Engineered vaccines as interventions against cancer
Another intriguing application of engineered strains targets development of therapeutic interventions against metastatic cancer. Vendrell et al. [61;62] have described in two recent studies, encouraging results using an engineered S. Typhi attenuated vaccine candidate as a therapeutic intervention to promote tumor reduction when injected directly into the tumor and surrounding draining lymph nodes. This approach was used in mouse models of breast cancer [61] and T-cell lymphoma [62], and in both cases resulted in significant infiltration of activated neutrophils not observed with untreated tumors. A reduction in invasion of tumor cells into nearby tissue, along with a reduction of potentially immunosuppressive Treg cells into the draining lymph nodes, accompanied delayed development of metastases and increased survival times in both studies as well. In the case of immunotherapy against the mammary adenocarcinoma, significant infiltration of neutrophils was observed in necrotic areas that formed micro-abscesses [61]. Remarkably, immunotherapy with the metastatic T-cell lymphoma resulted in complete tumor regression in 10% of treated animals (3 out of 30 treated), which engendered tumor-specific immunity when rechallenged with the homologous tumor (in 2 of the 3 re-challenged animals) [62]. Interestingly, although the attenuated vaccine strain was engineered to be dependent on guanine for growth, viable organisms were recovered from tumor tissues for up to 7 days after injection in both studies; it was hypothesized that a nutrient-rich environment required by a rapidly growing tumor might provide just enough benefit to vaccine organisms to enhance persistence, which could theoretically enhance antitumor inflammatory responses and promote tumor-specific cytotoxicity either directly or indirectly.
In addition to therapeutic interventions, work has also progressed in the development of attenuated Salmonella carrier strains as therapeutic vaccines that target adaptive immunity in addition to innate immunity for resolution of tumors. Xiong et al. [63] have reported the construction of attenuated S. Typhimurium carrier vaccines for intracellular delivery of an important tumor associated antigen, survivin, which is involved in tumor persistence, proliferation, and invasion [64]. In these carrier vaccines, delivery of survivin is accomplished by fusion to the T3SS SPI-2 effector protein SseF, which is translocated into host antigen presenting cells to elicit antitumor activity. In this study, comparison of several strains combining various deletion mutations to achieve attenuation demonstrated that properly attenuated carrier vaccines were able to inhibit tumor growth in orally immunized mice subcutaneously challenged with colon carcinoma cells or challenged by intracranial injection of glioblastoma cells. The antitumor efficacy of these carrier vaccines was further improved in this work by enhancing innate immunity through intraperitoneal co-administration of a ligand adjuvant to stimulate natural killer T-cells [63].
Live attenuated cancer vaccines have also shown promise in a Phase 1 clinical trial in which an attenuated strain of Listeria monocytogenes was evaluated in patients with late stage metastatic cervical cancer. In this study [65], a streptomycin-resistant strain of L. monocytogenes was attenuated by chromosomal deletion of an essential gene encoding a master virulence regulator (PrfA), which was then placed on a multicopy plasmid to enhance plasmid retention in vivo. Incomplete plasmid-based complementation of the chromosomal prfA deletion mutation was reported to attenuate pathogenicity by 4–5 logs [66]. This expression plasmid further encoded export of the human papillomavirus oncoprotein E7 (HPV-16 E7) fused to listeriolysin O [67]. The carrier vaccine proved somewhat reactogenic in all patients receiving intravenous infusions of two doses ranging from 1 × 109 to 1 × 1010 CFU and spaced three weeks apart. However, 30% of patients experienced a reduction in tumor size, and overall median survival was 347 days, versus a median survival time of 6–7 months for patients treated by more conventional methods.
CONCLUSIONS
In this review, we have not attempted to provide an exhaustive recapitulation of engineering techniques and strategies currently available for constructing attenuated live strains and carrier vaccines. Rather, we have been intentionally selective in citing examples that effectively illustrate the fundamental and central theme of this review in which we stress the importance of metabolic fitness and its impact on the immunogenicity and protective efficacy of live vaccines. We have highlighted a wide array of engineering techniques and strategies which when properly and carefully applied can achieve the critical balance between safety and immunogenicity that ultimately determines the success or failure of live vaccines in clinical trials. We believe that with the availability of sufficient genomic data and armed with relevant data on mechanisms of pathogenicity, today’s vaccine developers may be constrained only by imagination and persistence in creating highly immunogenic live attenuated vaccines against an ever-increasing variety of emerging diseases of importance to public health.
Highlights.
Describes recent advances in the rational engineering of live bacterial vaccines
Explores the challenge of achieving the proper balance between attenuation and immunogenicity
The availability of powerful genetic strategies suggests a bright future in vaccine development
ACKNOWLEDGEMENTS
We are grateful to Sharon Tennant, Marcela Pasetti, and Josephine Clark-Curtiss for critically reviewing the manuscript. This work was funded by grant U01 AI077911 (J.E.G.) and R01 AI060557 (R.C).
ABBREVIATIONS
- GFP
green fluorescent protein
- LD50
50% lethal dose
- RDAP
regulated delayed attenuation phenotype
- RDAS
regulated delayed antigen synthesis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT of INTEREST STATEMENT. Neither author has any financial or personal relationships with any other people or organizations that could inappropriately influence the integrity of this work.
REFERENCES
- 1.Tacket CO, Cohen MB, Wasserman SS, et al. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El tor inaba three months after vaccination. Infect Immun. 1999 Dec;67(12):6341–6345. doi: 10.1128/iai.67.12.6341-6345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabbagh SC, Forest CG, Lepage C, Leclerc JM, Daigle F. So similar, yet so different: uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol Lett. 2010 Apr;305(1):1–13. doi: 10.1111/j.1574-6968.2010.01904.x. [DOI] [PubMed] [Google Scholar]
- 3.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008 Jan;6(1):53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 4.Srikanth CV, Mercado-Lubo R, Hallstrom K, McCormick BA. Salmonella effector proteins and host-cell responses. Cell Mol Life Sci. 2011 Nov;68(22):3687–3697. doi: 10.1007/s00018-011-0841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figueira R, Holden DW. Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology. 2012 May;158(Pt 5):1147–1161. doi: 10.1099/mic.0.058115-0. [DOI] [PubMed] [Google Scholar]
- 6.Hansen-Wester I, Hensel M. Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect. 2001 Jun;3(7):549–559. doi: 10.1016/s1286-4579(01)01411-3. [DOI] [PubMed] [Google Scholar]
- 7.Hindle Z, Chatfield SN, Phillimore J, et al. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun. 2002 Jul;70(7):3457–3467. doi: 10.1128/IAI.70.7.3457-3467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyon CE, Sadigh KS, Carmolli MP, et al. In a randomized, double-blinded, placebo-controlled trial, the single oral dose typhoid vaccine, M01ZH09, is safe and immunogenic at doses up to 1.7 × 10(10) colony-forming units. Vaccine. 2010 Apr30;28(20):3602–3608. doi: 10.1016/j.vaccine.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Levine MM, Galen JE, Pasetti MF, Sztein MB. Attenuated strains of Salmonella enterica serovars Typhi and Paratyphi as live oral vaccines against enteric fever. In: Dougan G, Good MF, Liu MA, Nabel G, Nataro JP, Rappuoli R, et al., editors. New Generation Vaccines. 4 ed. New York: Informa Healthcare USA; 2010. pp. 497–505. [Google Scholar]
- 10.Dilts DA, Riesenfeld-Orn I, Fulginiti JP, et al. Phase I clinical trials of aroA aroD and aroA aroD htrA attenuated S. typhi vaccines; effect of formulation on safety and immunogenicity. Vaccine. 2000 Feb 14;18(15):1473–1484. doi: 10.1016/s0264-410x(99)00424-7. [DOI] [PubMed] [Google Scholar]
- 11.Levine MM, Herrington D, Murphy JR, et al. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J Clin Invest. 1987 Mar;79(3):888–902. doi: 10.1172/JCI112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohmann EL, Oletta CA, Miller SI. Evaluation of a phoP/phoQ-deleted, aroA-deleted live oralSalmonella typhi vaccine strain in human volunteers. Vaccine. 1996;14(1):19–24. doi: 10.1016/0264-410x(95)00173-x. [DOI] [PubMed] [Google Scholar]
- 13.Hohmann EL, Oletta CA, Killeen KP, Miller SI. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J Infect Dis. 1996;173(6):1408–1414. doi: 10.1093/infdis/173.6.1408. [DOI] [PubMed] [Google Scholar]
- 14.Kong Q, Yang J, Liu Q, Alamuri P, Roland KL, Curtiss R., III Effect of deletion of genes involved in lipopolysaccharide core and O-antigen synthesis on virulence and immunogenicity of Salmonella enterica serovar Typhimurium. Infect Immun. 2011 Oct;79(10):4227–4239. doi: 10.1128/IAI.05398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray GL, Attridge SR, Morona R. Altering the length of the lipopolysaccharide O antigen has an impact on the interaction of Salmonella enterica serovar Typhimurium with macrophages and complement. J Bacteriol. 2006 Apr;188(7):2735–2739. doi: 10.1128/JB.188.7.2735-2739.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu B, Knirel YA, Feng L, et al. Structural diversity in Salmonella O antigens and its genetic basis. FEMS Microbiol Rev. 2013 Jul;13:10–6976. doi: 10.1111/1574-6976.12034. [DOI] [PubMed] [Google Scholar]
- 17.Resman N, Vasl J, Oblak A, et al. Essential roles of hydrophobic residues in both MD-2 and toll-like receptor 4 in activation by endotoxin. J Biol Chem. 2009 May 29;284(22):15052–15060. doi: 10.1074/jbc.M901429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng J, Lien E, Golenbock DT. MD-2-mediated ionic interactions between lipid A and TLR4 are essential for receptor activation. J Biol Chem. 2010 Mar;285(12):8695–8702. doi: 10.1074/jbc.M109.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong Q, Six DA, Liu Q, et al. Phosphate groups of lipid A are essential for Salmonella enterica serovar Typhimurium virulence and affect innate and adaptive immunity. Infect Immun. 2012 Sep;80(9):3215–3224. doi: 10.1128/IAI.00123-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun W, Six D, Kuang X, Roland KL, Raetz CR, Curtiss R., III A live attenuated strain of Yersinia pestis KIM as a vaccine against plague. Vaccine. 2011 Apr 5;29(16):2986–2998. doi: 10.1016/j.vaccine.2011.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun W, Six DA, Reynolds CM, Chung HS, Raetz CR, Curtiss R., III Pathogenicity of Yersinia pestis synthesis of 1-dephosphorylated lipid A. Infect Immun. 2013 Apr;81(4):1172–1185. doi: 10.1128/IAI.01403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtiss R, Wanda SY, Gunn BM, III, et al. Salmonella enterica serovar typhimurium strains with regulated delayed attenuation in vivo. Infect Immun. 2009 Mar;77(3):1071–1082. doi: 10.1128/IAI.00693-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schleif R. AraC protein, regulation of the l-arabinose operon in Escherichia coli, and the light switch mechanism of AraC action. FEMS Microbiol Rev. 2010 Sep;34(5):779–896. doi: 10.1111/j.1574-6976.2010.00226.x. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Wang S, Scarpellini G, et al. Evaluation of new generation Salmonella enterica serovar Typhimurium vaccines with regulated delayed attenuation to induce immune responses against PspA. Proc Natl Acad Sci U S A. 2009 Jan 13;106(2):593–598. doi: 10.1073/pnas.0811697106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi H, Santander J, Brenneman KE, et al. Live recombinant Salmonella Typhi vaccines constructed to investigate the role of rpoS in eliciting immunity to a heterologous antigen. PLoS One. 2010 Jun 18;5(6):e11142. doi: 10.1371/journal.pone.0011142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey SE, Lottenbach KR, Hill H, et al. A Phase 1 dose-escalation trial in adults of three recombinant attenuated Salmonella Typhi vaccine vectors producing Streptococcus pneumoniae surface protein antigen PspA. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.07.049. In press. [DOI] [PubMed] [Google Scholar]
- 27.Pascual DW, Suo Z, Cao L, Avci R, Yang X. Attenuating gene expression (AGE) for vaccine development. Virulence. 2013 Jul 1;4(5):384–390. doi: 10.4161/viru.24886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Thornburg T, Suo Z, et al. Flagella overexpression attenuates Salmonella pathogenesis. PLoS One. 2012;7(i10):e46828. doi: 10.1371/journal.pone.0046828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao L, Lim T, Jun S, Thornburg T, Avci R, Yang X. Vulnerabilities in Yersinia pestis caf operon are unveiled by a Salmonella vector. PLoS One. 2012;7(4):e36283. doi: 10.1371/journal.pone.0036283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galán JE, Nakayama K, Curtiss IIIR. Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene. 1990;94:29–35. doi: 10.1016/0378-1119(90)90464-3. [DOI] [PubMed] [Google Scholar]
- 31.Pittard AJ, et al. Biosynthesis of the aromatic amino acids. In: Neidhardt FC, Curtiss R, Ingraham JL, Lin ECC, Low KB, Magasanik B III, editors. Escherichia coli Salmonella: cellular,biology molecular. 2 ed. Washington, D.C.: ASM Press; 1996. pp. 458–484. [Google Scholar]
- 32.Curtiss R, Galan JE, Nakayama K, Kelly SM., III Stabilization of recombinant avirulent vaccine strains in vivo . Res Microbiol. 1990 Sep;141(7–8):797–805. doi: 10.1016/0923-2508(90)90113-5. [DOI] [PubMed] [Google Scholar]
- 33.Chase JW, Williams KR. Single-stranded DNA binding proteins required for DNA replication. Annu Rev Biochem. 1986;55:103–136. doi: 10.1146/annurev.bi.55.070186.000535. [DOI] [PubMed] [Google Scholar]
- 34.Lohman TM, Ferrari ME. Escherichia coli single-stranded DNA-binding protein: multiple DNA-binding modes and cooperativities. Annu Rev Biochem. 1994;63:527–570. doi: 10.1146/annurev.bi.63.070194.002523. [DOI] [PubMed] [Google Scholar]
- 35.Galen JE, Wang JY, Chinchilla M, et al. A new generation of stable, nonantibiotic, low-copy-number plasmids improves immune responses to foreign antigens in Salmonella enterica serovar Typhi live vectors. Infect Immun. 2010 Jan;78(1):337–347. doi: 10.1128/IAI.00916-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang JY, Harley RH, Galen JE. Novel methods for expression of foreign antigens in live vector vaccines. Hum Vaccin Immunother. 2013 Jul 1;9(7):1558–1564. doi: 10.4161/hv.23248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bumann D. Regulated antigen expression in live recombinant Salmonella enterica serovar Typhimurium strongly affects colonization capabilities and specific CD4+-T-cell responses. Infect Immun. 2001;69(12):7493–7500. doi: 10.1128/IAI.69.12.7493-7500.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galen JE, Levine MM. Can a 'flawless' live vector vaccine strain be engineered. Trends in Microbiology. 2001;9(8):372–376. doi: 10.1016/s0966-842x(01)02096-0. [DOI] [PubMed] [Google Scholar]
- 39.Galen JE, Pasetti MF, Tennant SM, Olvera-Ruiz P, Sztein MB, Levine MM. Salmonella enterica serovar Typhi Live Vector Vaccines Finally Come of Age. Immunol Cell Biol. 2009 Jul;87(5):400–412. doi: 10.1038/icb.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curtiss R, Xin W, Li Y, III, et al. New technologies in using recombinant attenuated Salmonella vaccine vectors. Crit Rev Immunol. 2010;30(3):255–270. doi: 10.1615/critrevimmunol.v30.i3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loessner H, Endmann A, Leschner S, et al. Improving live attenuated bacterial carriers for vaccination and therapy. Int J Med Microbiol. 2008 Jan;298(1–2):21–26. doi: 10.1016/j.ijmm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Hegazy WA, Hensel M. Salmonella enterica as a vaccine carrier. Future Microbiol. 2012 Jan;7(1):111–127. doi: 10.2217/fmb.11.144. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Li Y, Scarpellini G, et al. Salmonella vaccine vectors displaying delayed antigen synthesis in vivo to enhance immunogenicity. Infect Immun. 2010 Sep;78(9):3969–3980. doi: 10.1128/IAI.00444-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson CJ, Zhan H, Swint-Kruse L, Matthews KS. The lactose repressor system: paradigms for regulation, allosteric behavior and protein folding. Cell Mol Life Sci. 2007 Jan;64(1):3–16. doi: 10.1007/s00018-006-6296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Husnain SI, Thomas MS. The UP element is necessary but not sufficient for growth rate-dependent control of the Escherichia coli guaB promoter. J Bacteriol. 2008 Apr;190(7):2450–2457. doi: 10.1128/JB.01732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis C, Skovierova H, Rowley G. et al. Salmonella enterica serovar Typhimurium HtrA: regulation of expression and role of the chaperone and protease activities during infection. Microbiology. 2009 Mar;155(Pt 3):873–881. doi: 10.1099/mic.0.023754-0. [DOI] [PubMed] [Google Scholar]
- 47.Kang HY, Curtiss R., III Immune responses dependent on antigen location in recombinant attenuated Salmonella typhimurium vaccines following oral immunization. FEMS Immunol Med Microbiol. 2003 Jul 15;37(2–3):99–104. doi: 10.1016/S0928-8244(03)00063-4. [DOI] [PubMed] [Google Scholar]
- 48.Galen JE, Zhao L, Chinchilla M, et al. Adaptation of the endogenous Salmonella enterica serovar Typhi clyA-encoded hemolysin for antigen export enhances the immunogenicity of anthrax protective antigen domain 4 expressed by the attenuated live-vector vaccine strain CVD 908-htrA. Infect Immun. 2004 Dec;72(12):7096–7106. doi: 10.1128/IAI.72.12.7096-7106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galen JE, Chinchilla M, Pasetti MF, et al. Mucosal immunization with attenuated Salmonella enterica serovar Typhi expressing protective antigen of anthrax toxin (PA83) primes monkeys for accelerated serum antibody responses to parenteral PA83 vaccine. J Infect Dis. 2009 Feb 1;199(3):326–335. doi: 10.1086/596066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barat S, Willer Y, Rizos K, et al. Immunity to intracellular Salmonella depends on surface-associated antigens. PLoS Pathog. 2012;8(10):e1002966. doi: 10.1371/journal.ppat.1002966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russmann H, Shams H, Poblete F, Fu Y, Galan JE, Donis RO. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science. 1998 Jul 24;281(5376):565–568. doi: 10.1126/science.281.5376.565. [DOI] [PubMed] [Google Scholar]
- 52.Hegazy WA, Xu X, Metelitsa L, Hensel M. Evaluation of Salmonella enterica type III secretion system effector proteins as carriers for heterologous vaccine antigens. Infect Immun. 2012 Mar;80(3):1193–1202. doi: 10.1128/IAI.06056-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong W, Wanda SY, Zhang X, et al. Regulated programmed lysis of recombinant Salmonella in host tissues to release protective antigens and confer biological containment. Proc Natl Acad Sci U S A. 2008 Jul 8;105(27):9361–9366. doi: 10.1073/pnas.0803801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sizemore DR, Branstrom AA, Sadoff JC. Attenuated Shigella as a DNA delivery vehicle for DNA-mediated immunization. Science. 1995 Oct 13;270(5234):299–302. doi: 10.1126/science.270.5234.299. [DOI] [PubMed] [Google Scholar]
- 55.Sizemore DR, Branstrom AA, Sadoff JC. Attenuated bacteria as a DNA delivery vehicle for DNA-mediated immunization. Vaccine. 1997 Jun;15(8):804–807. doi: 10.1016/s0264-410x(96)00252-6. [DOI] [PubMed] [Google Scholar]
- 56.Pasetti MF, Barry EM, Losonsky G, et al. Attenuated Salmonella enterica serovar Typhi and Shigella flexneri 2a strains mucosally deliver DNA vaccines encoding measles virus hemagglutinin, inducing specific immune responses and protection in cotton rats. J Virol. 2003 May;77(9):5209–5217. doi: 10.1128/JVI.77.9.5209-5217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kong W, Brovold M, Koeneman BA, Clark-Curtiss J, Curtiss R., III Turning self-destructing Salmonella into a universal DNA vaccine delivery platform. Proc Natl Acad Sci U S A. 2012 Nov 20;109(47):19414–19419. doi: 10.1073/pnas.1217554109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tennant SM, Wang JY, Galen JE, et al. Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect Immun. 2011 Oct;79(10):4175–4185. doi: 10.1128/IAI.05278-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon R, Tennant SM, Wang JY, et al. Salmonella enterica serovar Enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. enteritidis . Infect Immun. 2011 Oct;79(10):4240–4249. doi: 10.1128/IAI.05484-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simon R, Wang JY, Boyd MA, et al. Sustained protection in mice immunized with fractional doses of Salmonella enteritidis core and o polysaccharide-flagellin glycoconjugates. PLoS One. 2013 May 31;8(5):e64680. doi: 10.1371/journal.pone.0064680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vendrell A, Gravisaco MJ, Pasetti MF, et al. A novel Salmonella Typhi-based immunotherapy promotes tumor killing via an antitumor Th1-type cellular immune response and neutrophil activation in a mouse model of breast cancer. Vaccine. 2011 Jan 17;29(4):728–736. doi: 10.1016/j.vaccine.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 62.Vendrell A, Gravisaco MJ, Goin JC, et al. Therapeutic effects of Salmonella Typhi in a mouse model of T-cell lymphoma. J Immunother. 2013 Apr;36(3):171–180. doi: 10.1097/CJI.0b013e3182886d95. [DOI] [PubMed] [Google Scholar]
- 63.Xiong G, Husseiny MI, Song L, et al. Novel cancer vaccine based on genes of Salmonella pathogenicity island 2. Int J Cancer. 2010 Jun 1;126(11):2622–2634. doi: 10.1002/ijc.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Church DN, Talbot DC. Survivin in solid tumors: rationale for development of inhibitors. Curr Oncol Rep. 2012 Apr;14(2):120–128. doi: 10.1007/s11912-012-0215-2. [DOI] [PubMed] [Google Scholar]
- 65.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009 Jun;27(30):3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 66.Wallecha A, French C, Petit R, Singh R, Amin A, Rothman J. Lm-LLO-Based Immunotherapies and HPV-Associated Disease. J Oncol. 2012;2012:542851. doi: 10.1155/2012/542851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001 Dec 1;167(11):6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]