Abstract
Background
Adaptive functioning is not often examined in childhood brain tumor (BT) survivors, with the few existing investigations relying on examiner interviews. Parent questionnaires may provide similar information with decreased burden. The purpose of this study was: (1) to examine adaptive behaviors in BT survivors relative to healthy peer and cancer survivor groups, and (2) to explore the validity of a parent questionnaire in relation to an examiner administered interview.
Procedure
Participants (age 13.11±2.98 years) were BT survivors treated with conformal radiation therapy (n=50), healthy siblings of BT survivors (n=39) and solid tumor (ST) survivors who did not receive CNS-directed therapy (n=40). Parents completed the Adaptive Behavior Assessment System–2nd Edition (ABAS-II). For a subset of the BT cohort (n=32), examiners interviewed the parents using the Vineland Adaptive Behavior Scales (VABS) within 12 months.
Results
Groups differed significantly on each of the ABAS-II indices and the general adaptive composite, with the BT group scoring lower than the sibling and ST groups across indices. Executive functioning, but not IQ, was associated with adaptive skills; no clear pattern of clinical and demographic predictors was established. VABS scores were correlated with ABAS-II scores on nearly all indices.
Conclusions
BT survivors showed significantly lower adaptive functioning when compared to healthy and cancer controls. The ABAS-II proved sensitive to these behavioral limitations and was consistent with scores on the VABS. The use of a parent questionnaire to assess adaptive functioning enhances survivorship investigations by increasing flexibility of assessment and decreasing examiner burden.
Keywords: cancer, conformal radiation, late effects, adaptive functioning, parent report
INTRODUCTION
Adaptive functioning is the ability to independently complete tasks of daily living at an age-appropriate level. It represents real-world application of intellectual abilities. In typically developing individuals, correlations between adaptive and intellectual functioning are small to moderate [1], suggesting these measures tap related but not identical constructs. The assessment of adaptive functioning has historically been most relevant in diagnosis of intellectual disabilities. It is now gaining application with populations that exhibit an inability to functionally perform at the level anticipated by cognitive skills, resulting in a discrepancy between intellectual abilities and adaptive functioning (e.g., children with Attention-Deficit/Hyperactivity Disorder [ADHD]). Deficits in executive functions (e.g., planning, organizing, strategizing), as seen in ADHD, can reduce adaptive functioning in otherwise cognitively intact children [2,3].
Adaptive functioning has seldom been examined in childhood brain tumor (BT) survivors, individuals with known cognitive risks. In this population, adaptive functioning declines have been identified in children undergoing surgery only [4] and in those receiving adjuvant therapy [5–7]. To our knowledge, we are the only group [8,9] to investigate adaptive functioning in children who have undergone modern irradiation - conformal or intensity modulated radiation therapy (CRT/IMRT). Using caregiver interview, we found that CRT/IMRT affords relative sparing of adaptive functioning, but functional communication remains vulnerable. There have been studies of activities of daily living following stereotactic conformal radiotherapy [10,11], but these focused on hygiene and self-care, rather than more extensive adaptive behavior (e.g., social, communication). Using sophisticated imaging and planning technology, CRT/IMRT targets the most concentrated dose of radiation to the tumor site, minimizing damage to healthy tissue and potentially protecting functional outcomes [12,13]. In addition to potential cognitive late effects impacting adaptive functioning (IQ declines, executive functioning deficits and academic difficulties [13–16]), childhood BT survivors are also at risk for endocrine dysfunction, vision deficits and motor problems [17,18] that may adversely affect these skills.
Investigation of adaptive functioning in this population has relied heavily on examiner-administered parent interviews. These interviews require a trained psychological examiner to administer, often within a clinical setting. Parent questionnaires may serve to decrease examiner burden while efficiently gathering the same information. Questionnaires can be sent home with or mailed to families and require less time to complete and less training to score. This flexibility could prove advantageous as focus turns to quality of life of an expanding survivor cohort. Within the realm of adaptive functioning, parent rating scales have proven highly correlated with parent interview measures in typically developing children [19]. Despite wide use clinically and in collaborative trials, we are unaware of published studies evaluating the utility of these measures in the context of childhood cancer.
To address these omissions in the literature, the current study examined adaptive behaviors in BT survivors treated with CRT/IMRT relative to healthy peers and cancer survivors not receiving CNS-directed therapy. Inclusion of a cancer control group allows for examination of effects of the cancer experience, something not previously accounted for in this literature. This study also explored the validity of a parent questionnaire in relation to a “gold standard” examiner-administered parent interview. We hypothesized parents of BT survivors would rate their children as having lower adaptive functioning compared to parents of healthy siblings and solid tumor participants; we planned to explore possible clinical and cognitive (IQ and executive function) predictors of these anticipated discrepancies. We also hypothesized that parent ratings would be consistent with parent interview.
METHODS
Participants
Participants (ages 8 to 18 years at evaluation) included three groups: a patient group of 50 childhood BT survivors treated with CRT/IMRT, a healthy comparison group of 40 siblings of BT survivors, and a patient comparison group of 40 solid tumor (ST) survivors not receiving CNS-directed therapy. Enrollment for all three groups was stratified by gender and age (8–12; 13–18); the BT group was stratified based on tumor location (infratentorial; supratentorial). Initial recruitment was based on order of routine medical visits with a targeted approach near the end of the study as strata were filled. For the current study, 159 individuals were approached, with an overall participation rate of 82% (50/62 BT patients [81%], 40/52 ST patients [77%], and 40/45 siblings [89%]).
BT survivors were treated for a primary CNS tumor (low-grade glioma, ependymoma or craniopharyngioma) on an institutional phase II trial of CRT (RT-1). Treatment was initiated at least two years prior to current study enrollment with patients having no evidence of recurrent disease requiring additional treatment since CRT. No patients had a history of neurofibromatosis (NF-1) or posterior fossa syndrome. Radiation treatment parameters have been described previously [12]. All participants received CRT/IMRT, using conventional fractionation (1.8 Gy/day) with a prescribed dose of 59.4 Gy. The dose was attenuated to 54.0 Gy for children younger than 18 months of age after gross-total resection. Irradiated clinical target volume included a 10-mm margin surrounding the tumor and/or tumor bed to control microscopic disease, and an additional 3- to 5-mm margin expansion in three dimensions to form the planning target volume and account for uncertainty in patient positioning and image registration.
Sibling participants were healthy siblings of BT patients treated at St. Jude Children’s Research Hospital (SJCRH; 15 of whom participated in this study). ST patients received treatment for their tumor (Ewing sarcoma, osteosarcoma, soft tissue/rhabdomyosarcoma, neuroblastoma, or Wilms tumor) without CNS-directed therapy (e.g., cranial radiation therapy, intrathecal chemotherapy, high dose IV methotrexate), were diagnosed at least two years prior to enrollment on the study and had completed all treatment at the time of enrollment.
Individuals with global intellectual impairment (IQ <70 for BT patients [obtained during previous RT-1 assessment]; history of special education services for siblings/ST survivors) were excluded from participation. Participants were also excluded for a history of CNS injury or disease (predating cancer diagnosis in BT patients), documented ADHD (predating cancer diagnosis for BT patients), treatment with psychotropic or stimulant medication within two weeks of study participation, or major sensorimotor impairment that would preclude valid testing (e.g., blindness, hemiparesis). All participants were English speakers. This study was approved by the Institutional Review Board of SJCRH; written informed consent was required. Study enrollment occurred between April 2007 and December 2009.
Procedures
Assessment of Adaptive Behavior
Parents of each participant were given the Adaptive Behavior Assessment System – 2nd Edition (ABAS-II) [19], requiring 15 to 20 minutes to complete. The ABAS-II consists of 211 items, covering nine areas, from which three Adaptive Domain scores (Conceptual, Social, Practical) and a General Adaptive Composite (GAC) are calculated. This measure was standardized on a large, representative sample. Reliability of the ABAS-II has been demonstrated (inter-rater: GAC=0.91, Adaptive Domains=0.76–0.91) [19]. All scores are age standardized (100±15).
A subset of the BT group (n=32) completed the Vineland Adaptive Behavior Scales (VABS) [1] within 12 months of the ABAS-II (median=4.25 months; range=0–11.63 months) as part of RT-1 serial cognitive assessment and scores were included retrospectively. This is a parent interview administered by an individual with advanced training and experience in psychological assessment, requiring 20 to 60 minutes to complete. Because of its interview format, the VABS is still widely viewed as a “gold standard” for assessment of adaptive function, yielding Index scores in four domains [Communication, Daily Living Skills, Socialization and Motor Skills (up to age 5)], plus an Adaptive Behavior Composite (ABC) score. All scores are age standardized (100±15). This measure was also standardized on a large, representative sample and has demonstrated high levels of reliability (inter-rater: ABC=0.74; Index scores=0.62–0.78) [1].
Assessment of General Cognitive Ability
To obtain an estimate of overall cognitive functioning, all participants were administered the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) [20]. This abbreviated IQ is age standardized (100±15).
Assessment of Executive Functioning
Parents completed the Behavior Rating Inventory of Executive Function (BRIEF) [21]. The BRIEF consists of 86-items, from which eight clinical scales, two indices (Behavioral Regulation and Metacognitive), and an overall Global Executive Composite (GEC) are derived. Scores are standardized by age and gender (50±10). Internal consistency for the BRIEF is high across clinical scales (0.80–0.98) [21].
Survey of Clinical and Demographic Characteristics
Parents completed a questionnaire to assess family demographics and child development. An estimate of socioeconomic status (SES) was calculated using the Barratt Simplified Measure of Social Status [22]. These scores (range=8–66; higher scores indicating higher SES) are derived using maternal and paternal education and occupation. Relevant clinical variables (diagnosis, tumor location, number of surgeries, extent of resection, hydrocephalus, shunt placement, visual field impairment, visual acuity impairment, chemotherapy, age at CRT) were abstracted from the RT-1 database for the BT group.
Statistical Analyses
In order to characterize the sample and evaluate group similarity, qualitative analyses of demographic and clinical variables were performed using analyses of variance (ANOVA) or independent t-tests when a variable did not apply to the sibling group (i.e., age at diagnosis). For dichotomous variables, Chi Square tests were employed. ANOVAs were also used to examine group differences in ABAS-II scores, with post-hoc comparisons as appropriate. The predictive value of clinical, cognitive and demographic variables on ABAS-II scores was examined using linear mixed models. Pearson correlation coefficients were calculated to investigate the association between IQ and ABAS-II scores, as well as the relationship between ABAS-II and VABS scores. An a priori significance level of p<.05 (one-tailed) was used for all analyses.
RESULTS
Demographic and Clinical Characteristics
Prior to approach for study participation, 40 BT survivors were excluded based on chart review (19 for stimulant/psychotropic medication, 9 previously documented IQ<70, 4 NF1, 3 blindness, 2 ADHD, 3 some combination). One hundred and twenty nine children were included in this study (one sibling control family failed to complete the ABAS-II). Due to stratification, groups were predictably balanced on gender and age at assessment. ST survivors were younger at diagnosis and, by association, farther out from treatment at the time of their participation. The three groups did not differ on estimates of SES. The BT group had lower IQ than both siblings and ST survivors, but mean IQ remained in the average range. Demographic variables are outlined in Table 1 and clinical characteristics of the BT group in Table 2.
Table 1.
Participant Demographic and Clinical Characteristics
| Brain Tumor (N=50) | Siblings (N=39) | Solid Tumor (N=40) | p | |
|---|---|---|---|---|
| Gender (% Male) | 50 | 51.3 | 50 | 0.99 |
| Age at Diagnosis (Y) | 6.38 ± 3.43 | NA | 4.50 ± 4.18 | 0.02* |
| Time from Diagnosis to CRT (Y) | 1.14 ± 1.86 | NA | NA | NA |
| Age at Assessment (Y) | 13.18 ± 2.88 | 12.98 ± 2.61 | 13.21 ± 3.46 | 0.93 |
| Time since Diagnosis (Y) | 6.80 ± 2.60 | NA | 8.71 ± 3.94 | <0.01* |
| SES (BSMSS) | 37.61 ± 12.20 | 42.71 ± 12.19 | 42.09 ± 13.32 | 0.11 |
| Abbreviated IQ (WASI std score) | 98.20 ± 13.91 | 108.59 ± 12.68 | 107.88 ± 12.44 | <0.01* |
P-value indicates whether group is equally distributed across sub-categories using One-Way ANOVA, independent t-test or Chi-square. Y – Years; CRT – conformal radiation therapy; SES – socioeconomic status; BSMSS - Barratt Simplified Measure of Social Status (Scores range from 8 to 66 with higher scores indicative of higher SES); IQ – intelligence quotient; WASI – Wechsler Abbreviated Scale of Intelligence
Table 2.
Clinical Characteristics of Brain Tumor Survivors
| N | % | p | |
|---|---|---|---|
| Tumor Diagnosis | |||
| Ependymoma | 22 | 44 | 0.22 |
| Low Grade Glioma | 12 | 24 | |
| Craniopharyngioma | 16 | 32 | |
| Tumor Location | |||
| Infratentorial | 22 | 44 | 0.40 |
| Supratentorial | 28 | 56 | |
| Pre-CRT Chemotherapy | |||
| No | 44 | 88 | <0.01* |
| Yes | 6 | 12 | |
| Extent of Surgical Resection | |||
| Biopsy/STR | 25 | 50 | 1.00 |
| NTR/GTR | 25 | 50 | |
| Pre-CRT Surgery | |||
| n = 1 | 31 | 65 | 0.04* |
| n = 2–4 | 17 | 35 | |
| Hydrocephalus | |||
| No | 21 | 42 | 0.26 |
| Yes | 29 | 58 | |
| CSF Shunting | |||
| No | 29 | 58 | 0.26 |
| Yes | 21 | 42 | |
| Visual Acuity Impairment | |||
| None | 20 | 71 | <0.01* |
| Unilateral | 7 | 25 | |
| Bilateral | 1 | 4 | |
| Visual Field Impairment | |||
| None | 18 | 64 | <0.01* |
| Unilateral | 3 | 11 | |
| Bilateral | 7 | 25 | |
p<.05
P-value indicates whether group is equally distributed across sub-categories using Chi-square or Fisher’s Exact test when necessary. CRT – conformal radiation therapy; STR – subtotal resection; NTR – near total resection; GTR – gross total resection; CSF – cerebrospinal fluid
Group Differences in Adaptive Functioning
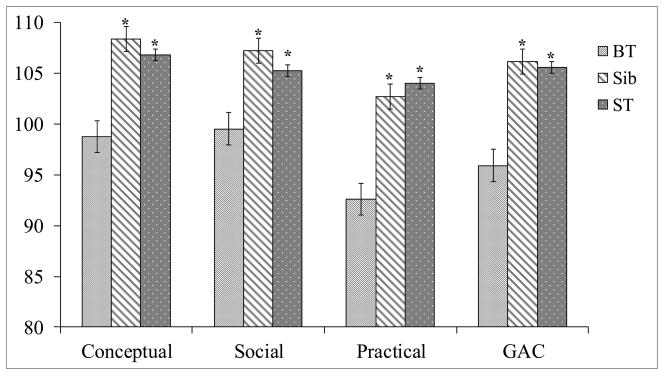
Mean scores for each group and post-hoc analyses are presented in Figure 1 for the three ABAS-II Adaptive Domain scores (Conceptual, Social and Practical) and the GAC score. Groups differed significantly on each of these domains and the GAC (p<.04). Post-hoc tests revealed BT survivors scored lower than siblings (p<.01) and ST survivors (p<.04) across all domains. (This finding was upheld if siblings from the same family [n=15] were removed from analysis.) Siblings and ST survivors did not differ.
Figure 1.
ABAS-II Index Scores by Participant Group
Group means with standard error bars; ANOVA revealed main effect for group across domains and GAC (ps<.04); ABAS-II – Adaptive Behavior Assessement System – 2nd Edition; *p<.05
Clinical and Demographic Predictors of Adaptive Functioning
Results of univariate analyses did not reveal clear patterns of predictors of adaptive functioning among BT survivors. None of the demographic variables (gender, SES, age at assessment) reached statistical significance. Among the clinical variables of interest (Table 2), a limited number of significant predictors were identified. Tumor diagnosis proved significant, with low-grade glioma survivors scoring lower than craniopharyngioma survivors in the Social (p=.004) and Practical (p=.041) domains as well as the GAC (p=.026). Longer duration between diagnosis and CRT was also associated with poorer Social domain scores (p=.024). Higher number of surgeries was related to lower Practical domain scores (p=.041). Despite a number of significant findings, there was not enough evidence to warrant running multivariate predictive models. Number of surgeries and duration between diagnosis and CRT were highly correlated (r=.560; p=<.0001). Further analyses showed low-grade glioma survivors had a significantly longer duration between diagnosis and CRT (F=.0064) than ependymoma (p=.003) or craniopharyngioma (p=.005) survivors. Additionally, craniopharyngioma survivors had fewer surgeries (F=.0727) than low-grade glioma survivors (p=.029).
No relationship was identified between IQ and any domains of adaptive functioning. Previously outlined executive functioning (BRIEF) difficulties in this sample [23] did prove to be significantly related to adaptive behavior. Univariate models show that each of the ABAS-II domain scores and the GAC were negatively associated with BRIEF composite scores (Behavior Regulation, Metacognitive and GEC; p<.033). As parents reported more problems with executive functions, they reported lower adaptive functioning.
Parent Report vs. Parent Interview
In the subset of BT survivors with VABS scores (n=32), analysis revealed a significant relationship between many ABAS-II scores and VABS indices (rs=0.37–0.57, p<.05). Pearson coefficients for ABAS-II and VABS scores are presented in Table 3.
Table 3.
ABAS-II Association with VABS (BT Cohort)
| ABAS-II Index | ||||
|---|---|---|---|---|
|
| ||||
| VABS Index | Conceptual | Social | Practical | GAC |
| Communication | 0.43* | 0.29 | 0.34 | 0.42* |
| Daily Living | 0.37* | 0.33 | 0.53** | 0.47** |
| Socialization | 0.41* | 0.34 | 0.50** | 0.48** |
| Adaptive Behavior Composite | 0.50** | 0.41* | 0.56** | 0.57** |
p<.05;
p<.01
Values are Pearson correlation coefficients; ABAS-II – Adaptive Behavior Assessment Scale – 2nd Edition; VABS – Vineland Adaptive Behavior Scale; BT – Brain Tumor; GAC – General Adaptive Composite
DISCUSSION
The current study examined adaptive behaviors in BT survivors treated with CRT/IMRT compared to healthy peers and cancer survivors who did not receive CNS-directed therapy. Consistent with a priori hypotheses, BT survivors showed significantly lower adaptive functioning across all domains. These results suggest that, even with group mean scores in the average range, BT survivors are functioning significantly lower than siblings and ST survivors in all areas of adaptive skills.
We did not find a distinct profile of clinical or demographic variables that predicted adaptive functioning difficulties in the BT group. Of note, vision variables examined were not significantly related to adaptive functioning. This had been a particular worry for the craniopharyngioma and low grade glioma groups. There was some evidence, however, that the number of surgeries and time between diagnosis and CRT were associated with lower adaptive functioning scores. Given that low-grade glioma diagnosis was correlated with higher number of surgeries and longer duration between diagnosis and radiation therapy, these survivors may be at greater risk of adaptive deficits than survivors of craniopharyngioma or ependymoma. We also observed a strong association between parent reported executive dysfunction and lower adaptive skills. While these associations have been observed in other clinical populations using traditional, comprehensive batteries [24,25], our study was able to highlight this relationship in cancer survivors using only parent report measures.
In addition to its utility in identifying adaptive difficulties, we also found the ABAS-II questionnaire to be consistent with VABS parent interview in a previously unexamined clinical population. This lends credence to the ABAS-II’s wide clinical use and inclusion in cooperative group trials.
In previous examinations of adaptive functioning in BT survivors, investigators have compared observed outcomes to published norms. When scores are in the average range, as in the current study, the data suggests that these children function adequately. Average scores provide encouraging evidence of the relative sparing afforded by CRT/IMRT; however, without a comparison group, the literature may overestimate these adaptive skills. Given that our sibling and ST control groups were matched on age, gender and SES, we believe the current study to be a more rigorous comparison than published normative means.
The BT literature has widely cited younger age at treatment as a risk factor for cognitive late effects [15,26], but we did not observe better adaptive functioning for children who were older at CRT. Craniopharyngioma survivors in our sample were significantly older at treatment, but as noted, this diagnosis was also associated with other clinical factors directly related to better adaptive outcomes (i.e., fewer surgeries, shorter wait to CRT). The lack of association between age at treatment and adaptive functioning is consistent with recent studies that fail to show protection with older age at treatment [27,28]. However, the current study may have been limited by a small sample size when examining clinical and demographic predictors of adaptive functioning.
This study is cross-sectional in design and, therefore, we cannot address the question of adaptive function as survivors age. Netson et al. [8,9] examined adaptive functioning longitudinally across these BT diagnosis groups using the VABS, noting relative sparing of skills, but did not have controls. Given the demonstrated utility of the ABAS-II and importance of comparison beyond published norms, future studies may examine adaptive functioning over time, taking advantage of the ABAS-II’s flexibility to increase sample size and incorporate control groups to the longitudinal design.
Our study is limited to parent report and would be improved by including teacher report of adaptive skills within the school setting to expand the scope of examination. Also, we have both questionnaire and parent interview data, but these are separated by as much as 12 months and we have no laboratory measure of overall adaptive functioning or individual adaptive domains. While incorporating “real-world” measures of adaptive skills may be expensive and time-consuming, there are direct measures that would serve to deepen this look into adaptive outcomes [29 (review)]. Finally, our sample of BT survivors may be more functional due to exclusions (e.g., below average IQ, visual impairments) but this results in a more conservative estimate of group differences and supports the risk to adaptive functioning.
Future studies should work towards identifying specific risk factors that lead to decreased adaptive functioning. Elucidation of vulnerable areas may lead to treatment planning and adaptive remediation to further protect functional outcomes. Adaptive skills training (e.g., direct skills based teaching, computer simulations), established in other clinical populations (mental illness [30], autism [31,32], traumatic brain injury [33,34]), may be an area of possible intervention to enhance the quality of life for BT survivors. Current findings also suggest that executive functions could be targeted for intervention with secondary improvements expected in adaptive skills.
This study has important implications for examination of adaptive functioning. Multi-site trials, long term patient follow-up and survivor investigations are areas that stand to benefit from wider use of a robust parent questionnaire. The ABAS-II can increase the flexibility of these studies as it does not require training in psychological assessment for administration and can be completed outside of a clinical setting. There is decreased examiner burden as the ABAS-II does not necessitate a 20 to 60 minute interview and can be completed independently by the informant. This will facilitate multi-site trials, since parent report forms can be easily scored and transferred across sites. The growing and increasingly dispersed survivor cohort can be followed and monitored while maintaining consistency and reliability.
With improved treatment outcomes for childhood BT, focus is being placed more often on quality of life. Adaptive functioning should remain a component of this initiative as it has proven to be vulnerable, is associated with ability to live independently and is potentially modifiable. By successfully assessing and addressing adaptive skills, we can continue to increase the quality of childhood cancer survivorship.
Acknowledgments
The authors thank the patients and their families who volunteered their time to participate in this study. This work was supported in part by the National Cancer Institute (St. Jude Cancer Center Support [CORE] Grant [P30 CA21765]; H.C., R21 CA131616); the International Neuropsychological Society (H.C., Rita G. Rudel Award); and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
Portions of this paper were presented at the annual meeting of the International Neuropsychological Society in Boston, Massachusetts, 2011.
References
- 1.Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, Inc; 1984. [Google Scholar]
- 2.Roizen NJ, Blondis TA, Irwin M, Stein MA. Adaptive functioning in children with attention-deficit hyperactivity disorder. Arch Pediatr Adolesc Med. 1994;148(11):1147–32. doi: 10.1001/archpedi.1994.02170110023004. [DOI] [PubMed] [Google Scholar]
- 3.Stein MA, Szumowski E, Blondis TA, Roizen NJ. Adaptive skills dysfunction in ADD and ADHD children. J Child Psychol Psychiatry. 1995;36(4):663–70. doi: 10.1111/j.1469-7610.1995.tb02320.x. [DOI] [PubMed] [Google Scholar]
- 4.Ris MD, Beebe DW, Armstrong FD, Fontanesi J, Holmes E, Sanford RA, Wisoff JH Children’s Oncology Group. Cognitive and adaptive outcome in extracerebellar low-grade brain tumors in children: a report from the Children’s Oncology Group. J Clin Oncol. 2008;26(29):4765–70. doi: 10.1200/JCO.2008.17.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulhern RK, Horowitz ME, Kovnar EH, Langston J, Sanford RA, Kun LE. Neurodevelopmental status of infants and young children treated for brain tumors with preirradiation chemotherapy. J Clin Oncol. 1989;7(11):1660–6. doi: 10.1200/JCO.1989.7.11.1660. [DOI] [PubMed] [Google Scholar]
- 6.Stargatt R, Rosenfeld JV, Anderson V, Hassall T, Maixner W, Ashley D. Intelligence and adaptive function in children diagnosed with brain tumour during infancy. J Neurooncol. 2006;80(3):295–303. doi: 10.1007/s11060-006-9187-0. [DOI] [PubMed] [Google Scholar]
- 7.Papazoglou A, King TZ, Morris RD, Morris MK, Krawiecki NS. Attention mediates radiation’s impact on daily living skills in children treated for brain tumors. Pediatr Blood Cancer. 2008;50(6):1253–7. doi: 10.1002/pbc.21495. [DOI] [PubMed] [Google Scholar]
- 8.Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. A 5-year investigation of children’s adaptive functioning following conformal radiation therapy for localized ependymoma. Int J Radiat Oncol Biol Phys. 2012;84(1):217–223. doi: 10.1016/j.ijrobp.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. Longitudinal investigation of adaptive functioning following conformal irradiation for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys. 2012;85(5):1301–6. doi: 10.1016/j.ijrobp.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jalali R, Dutta D, Develekar R, Sarin R. Prospective assessment of ADL using Barthel’s scoring system in children with low grade brain tumours treated with SCRT. Pediatr Blood Cancer. 2007;49(4):430. [Google Scholar]
- 11.Jalali R, Dutta D, Kamble R, Gupta T, Munshi A, Sarin R, Dinshaw K. Prospective assessment of activities of daily living using Modified Barthel’s Index in children and young adults with low-grade gliomas treated with stereotactic conformal radiotherapy. J Neurooncol. 2008;90:321–328. doi: 10.1007/s11060-008-9666-6. [DOI] [PubMed] [Google Scholar]
- 12.Merchant TE, Mulhern RK, Krasin MJ, Kun LE, Williams T, Li C, Xiong X, Khan RB, Lustig RH, Boop FA, Sanford RA. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22(15):3156–62. doi: 10.1200/JCO.2004.11.142. [DOI] [PubMed] [Google Scholar]
- 13.Conklin HM, Li C, Xiong X, Ogg RJ, Merchant TE. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol. 2008;26(24):3965–70. doi: 10.1200/JCO.2007.15.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kun LE, Mulhern RK, Crisco JJ. Quality of life in children treated for brain tumors. Intellectual, emotional, and academic function. J Neurosurg. 1983;58(1):1–6. doi: 10.3171/jns.1983.58.1.0001. [DOI] [PubMed] [Google Scholar]
- 15.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 16.Butler RW, Haser JK. Neurocognitive effects of treatment for childhood cancer. Ment Retard Dev Disabil Res Rev. 2006;12(3):184–91. doi: 10.1002/mrdd.20110. [DOI] [PubMed] [Google Scholar]
- 17.Packer RJ, Gurney JG, Punyko JA, Donaldson SS, Inskip PD, Stovall M, Yasui Y, Mertens AC, Sklar CA, Nicholson HS, Zeltzer LK, Neglia JP, Robison LL. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21(17):3255–61. doi: 10.1200/JCO.2003.01.202. [DOI] [PubMed] [Google Scholar]
- 18.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27(22):3691–7. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison PL, Oakland T. Adaptive Behavior Assessment System. 2. San Antonio, TX: The Psych Corp; 2003. [Google Scholar]
- 20.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 21.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Odessa, FL: Psychological Assessment Resources, Inc; 2000. [Google Scholar]
- 22.Barratt WR. The Barratt Simplified Measure of Social Status. Retrieved from the Indiana State University website: http://socialclassoncampus.blogspot.com/2012/06/barratt-simplified-measure-of-social.html.
- 23.Howarth RA, Ashford JM, Merchant TE, Ogg RJ, Santana V, Wu S, Xiong X, Conklin HM. The utility of parent report in the assessment of working memory among childhood brain tumor survivors. J Int Neuropsychol Soc. 2013;19(4):380–9. doi: 10.1017/S1355617712001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stavro GM, Ettenhofer ML, Nigg JT. Executive functions and adaptive functioning in young adult attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc. 2007;13(2):324–34. doi: 10.1017/S1355617707070348. [DOI] [PubMed] [Google Scholar]
- 25.Ware AL, Crocker N, O’Brien JW, Deweese BN, Roesch SC, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN CIFASD . Executive function predicts adaptive behavior in children with histories of heavy prenatal alcohol exposure and attention deficit/hyperactivity disorder. Alcohol Clin Exp Res. 2012;36(8):1431–41. doi: 10.1111/j.1530-0277.2011.01718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol. 2001;19(15):3470–6. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 27.Mabbott DJ, Penkman L, Witol A, Strother D, Bouffet E. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22(2):159–68. doi: 10.1037/0894-4105.22.2.159. [DOI] [PubMed] [Google Scholar]
- 28.Ellenberg L, Liu Q, Gioia G, Yasui Y, Packer RJ, Mertens A, Donaldson SS, Stovall M, Kadan-Lottick N, Armstrong G, Robison LL. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23(6):705–17. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore DJ, Palmer BW, Patterson TL, Jeste DV. A review of performance-based measures of functional living skills. J Psychiatr Res. 2007;41(1–2):97–118. doi: 10.1016/j.jpsychires.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Patterson TL, McKibbin C, Taylor M, Goldman S, Davila-Fraga W, Bucardo J, Jeste DV. Functional adaptation skills training (FAST): a pilot psychosocial intervention study in middle-aged and older patients with chronic psychotic disorders. Am J Geriatr Psychiatry. 2003;11(1):17–23. [PubMed] [Google Scholar]
- 31.Eapen V, Črnčec R, Walter A. Clinical outcomes of an early intervention program for preschool children with Autism Spectrum Disorder in a community group setting. BMC Pediatr. 2013;13(1):3. doi: 10.1186/1471-2431-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichow B, Barton EE, Boyd BA, Hume K. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD009260.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Whitlock JA, Hamilton BB. Functional outcome after rehabilitation for severe traumatic brain injury. Arch Phys Med Rehabil. 1995;76(12):1103–12. doi: 10.1016/s0003-9993(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 34.Yip BC, Man DW. Virtual reality (VR)-based community living skills training for people with acquired brain injury: A pilot study. Brain Inj. 2009;23(13–14):1017–26. doi: 10.3109/02699050903379412. [DOI] [PubMed] [Google Scholar]