Abstract
Background
There is limited information about the clinical and prognostic significance of patient-reported recovery time.
Study Design
Prospective cohort study.
Setting & Participants
6,040 patients in the DOPPS.
Predictor
Answer to question, “How long does it take you to recover from a dialysis session?” categorized as follows: <2, 2–6, 7–12, or >12 hours.
Outcomes & Measurements
Cross-sectional and longitudinal associations between recovery time and patient characteristics, hemodialysis treatment variables, health-related quality of life (HRQoL) and hospitalization and mortality.
Results
32% reported recovery time <2 hours; 41%, 2–6 hours; 17%, 7–12 hours; and 10%, >12 hours. Using proportional odds (ordinal) logistic regression, shorter recovery time was associated with male sex, full-time employment, and higher serum albumin. Longer recovery time was associated with older age, dialysis vintage, body mass index, diabetes, and psychiatric disorder. Greater intradialytic weight loss, longer dialysis session length, and lower dialysate sodium concentration were associated with longer recovery time. In facilities that used uniform dialysate sodium concentration for ≥90% of patients, the adjusted OR of longer recovery time, comparing dialysate sodium concentration <140 vs 140 mEq/L, was 1.72 (95% CI, 1.37–2.16). Recovery time was positively correlated with symptoms of kidney failure and kidney disease burden score, and inversely correlated with HRQoL mental and physical component summary scores. Using Cox regression, adjusting for potential confounders not influenced by recovery time, it was positively associated with first hospitalization and mortality (adjusted HRs for recovery time >12 vs. 2–6 hours of 1.22 [95% CI, 1.09–1.37] and 1.47 [95% CI, 1.19–1.83], respectively).
Limitations
Answers are subjective and not supported by physiological measurements.
Conclusions
Recovery time can be used to identify patients with poorer HRQoL and higher risks of hospitalization and mortality. Interventions to reduce recovery time and possibly to improve clinical outcomes, such as increasing dialysate sodium concentration, need to be tested in randomized trials.
Keywords: hemodialysis, DOPPS, quality of life, patient reported, outcomes
End-stage renal disease (ESRD) and dialysis have major impacts on health-related quality of life (HRQOL) 1 which, in turn, predicts hospitalization and mortality 2. There is little evidence about how recovery after a dialysis session affects HRQOL or how hemodialysis can be modified to shorten it.
The question, “How long does it take you to recover from a dialysis session?” has been validated in 46 Canadian patients 3. Reported recovery time (RT) was correlated with HRQOL (more strongly with the physical than the mental component), was stable over three months, and had high test-retest correlation. Patients changing to daily or nocturnal dialysis reported a reduction in RT.
We have studied the recovery time question in a large, representative sample of hemodialysis patients from 12 countries who received treatments thrice weekly. We examined: 1) how reported RT correlates with other measures of health status; and 2) whether reported RT predicts hospitalization and mortality. We also examined the associations of reported RT with patient characteristics and treatment variables.
METHODS
Study Population
Data were from Phase 4 (2009–2011) of the Dialysis Outcomes and Practice Patterns Study (DOPPS), a prospective cohort study of a random sample of hemodialysis patients from stratified random samples of hemodialysis facilities in 12 countries: Australia, Belgium, Canada, France, Germany, Japan, Italy, New Zealand, Spain, Sweden, the United Kingdom, and the United States. The DOPPS study design and sampling scheme have been previously published4, 5.
Measurement of Recovery Time and Other Patient Characteristics
Patient demographics, comorbid conditions, dialysis prescription, medications, laboratory measurements, hospitalizations, and deaths (including cause, if known) were collected by chart abstraction or electronic medical record download at study entry and every four months thereafter 4, 6. Time of day of dialysis was not collected. Patient-reported data were collected at study entry and annually thereafter. For this analysis, patient demographics, vascular access, and comorbidities reflect information at the patient’s start of the study. Other patient covariates such as laboratory measurements and hemodialysis treatment variables reflect information from the 4-month interval closest to when the patient questionnaire was completed. Informed patient consent was obtained.
The previously validated question, “How long does it take you to recover from a dialysis session?” was included in a patient questionnaire in 2010. As in the original description 3, the question was not linked to a phase of the dialysis routine. Four answer categories were offered: <2, 2–6, 7–12, and >12 hours. Other patient-reported data were collected on the same questionnaire or at study entry.
The HRQOL was measured using the Kidney Disease Quality of Life 36 (KDQOL-36) short form. Scores for the physical component summary (PCS) and mental component summary (MCS) were calculated for patients answering all subscale questions (from the 36-Item Short Form Healthy Survey [SF-36], which is the core of the KDQOL-36). Scores for the effects of kidney disease and kidney disease burden subscales in the KDQOL-36 may vary from 0 to 100; higher scores indicate lower burden, i.e., better HRQoL. Because of the weighting used, the PCS scores ranged from 10 to 66 and MCS scores from 18 to 74 points 7. Physical activity levels were measured with the Rapid Assessment of Physical Activity (RAPA) 8; higher scores indicate greater activity.
Statistical Methods
Descriptive statistics were used to examine the means and proportions for demographics, health status and dialysis treatment variables, and patient-reported outcomes by categories of reported RT. Patients not receiving three-times-weekly dialysis or missing reported RTs were excluded from this analysis (n = 820 [12%]).
Proportional odds logistic regression models based on generalized estimating equations (GEEs) were used to identify characteristics associated cross-sectionally with reported RT treated as a 4-category ordinal outcome variable. To account for clustering within facilities, an independent working correlation was assumed. The proportional odds model estimates an adjusted common odds ratio (OR) for each predictor across all cutpoints of the ordered RT categories (i.e., <2 hours vs. ≥2 hours; ≤6 hours vs. ≥7 hours; and ≤12 hours vs. >12 hours). The proportional odds assumption was verified using a score test. Models included the following covariates: country, race (US black versus US other races), age, male, 14 summary comorbidities (coronary artery disease, congestive heart failure, other cardiovascular disease, cerebrovascular disease, cancer other than skin, diabetes, gastrointestinal bleed in the past 12 months, HIV, hypertension, lung disease, neurologic disorder, peripheral vascular disease, psychiatric disorder, and recurrent cellulitis/gangrene), full-time employment, dialysis vintage in years, body mass index (BMI), hemodialysis catheter use, albumin level, hemoglobin level, hemodiafiltration, intradialytic weight loss (IDWL), dialysate sodium concentration (DNa) prescription, change in systolic blood pressure (pre – post dialysis), dialysis session length, single-pool Kt/V, ultrafiltration rate, and prescribed blood flow rate.
For the analysis of DNa as a predictor of reported RT, patients with DNa = 125–155 mEq/L were included unless dialyzing against variable DNa concentrations (sodium modeling/profiling, 10%).
Spearman correlations were calculated to examine how reported RT varied with other measures (construct validity). To facilitate comparisons to the previous study 3, Pearson correlations were calculated as a sensitivity analysis. Mixed-effects linear regressions were used to confirm the monotonic association between MCS and PCS scores and reported RT. These models adjusted for country, race (US black only), sex, age, BMI, dialysis vintage in years, and 14 summary comorbidities.
Cox regression was used to estimate the crude and adjusted effects of reported RT on all-cause mortality and first hospitalization, stratified by country and using the robust “sandwich” variance estimator to account for facility clustering. Time at risk was defined as the time from the patient’s completion of the RT questionnaire to the outcome event (death or first hospitalization) or censoring (withdrawal from the study or end of follow-up). Model estimates were calculated for two levels of progressive adjustment: model 1) potential confounders (i.e., can predict RT—country, race [US-black only], dialysis vintage, age, BMI, sex, catheter use, and 14 summary comorbidities); and model 2) model 1 plus covariates that could be either potential confounders or potential mediators (i.e., could be predicted by RT—activities of daily living count, RAPA score, full-time employment, pruritus severity, depression severity, and sleep problems). The 2- to 6-hours category of reported RT included the largest number of patients and was used as the reference. A linear trend test was done by recoding the categories as a single interval variable (coded 2, 4, 9, and 12 hours) in the Cox model. The proportional hazards assumption was verified for all models.
To control for ascertainment differences among US comorbidity data sources, comorbidity-by–data source product terms were included in models using comorbidity data.
Data manipulations and statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC)9.
RESULTS
Descriptive Analysis
There were 6,860 patients who returned the questionnaire; 6,513 (95%) answered the RT question and 5,427 (79%) answered all relevant SF-36 subscale questions in the KDQOL-36 (allowing MCS and PCS scores to be calculated).
Table 1 provides the distribution of reported RTs; Figure S1 (provided as online supplementary material) shows the distributions by country. Sixty-eight percent of patients reported a RT of at least 2 hours; 10% reported RT longer than 12 hours. Italy had the highest percentage of patients reporting RT >6 hours (39%); Japan, the lowest (18%).
Table 1.
Distribution of demographic variables and hemodialysis treatment variables across patient-reported recovery time categories
| Characteristic | Overall | < 2 h | 2–6 h | 7–12 h | > 12 h |
|---|---|---|---|---|---|
| No. patients | 6040 | 1940 (32) | 2485 (41) | 1015 (17) | 600 (10) |
| Demographics | |||||
| Age (y) | 64.8 ± 14.0 | 63.5 ± 14.2 | 65.1 ± 13.7 | 65.4 ± 14.2 | 67.0 ± 13.6 |
| Dialysis vintage (y) | 3.3 (1.3–6.9) | 3.1 (1.2–6.7) | 3.4 (1.4–7.1) | 3.4 (1.4–6.9) | 3.2 (1.3–6.9) |
| Employed Full Time | 10 | 15 | 8 | 7 | 5 |
| BMI (kg/m2) | 24.7 (21.4–29.0) | 24.0 (21.1–28.2) | 24.8 (21.3–29.0) | 25.4 (21.8–30.0) | 25.7 (21.9–30.0) |
| Male sex | 60 | 64 | 58 | 57 | 57 |
| Black race | 7 | 9 | 6 | 7 | 7 |
| Comorbidities | |||||
| Coronary Artery Disease | 36 | 33 | 37 | 39 | 39 |
| Congestive Heart Failure | 22 | 22 | 21 | 22 | 26 |
| Diabetes | 41 | 39 | 41 | 44 | 44 |
| Hypertension | 84 | 83 | 84 | 84 | 84 |
| Cerebrovascular Disease | 14 | 14 | 14 | 14 | 17 |
| Cancer | 13 | 13 | 13 | 15 | 14 |
| Other Cardiovascular Disease | 29 | 27 | 29 | 31 | 33 |
| Gastrointestinal Bleed | 5 | 4 | 5 | 5 | 4 |
| Peripheral Vascular Disease | 26 | 24 | 25 | 29 | 31 |
| Psychiatric Disorder | 16 | 12 | 16 | 21 | 21 |
| Recurrent Cellulitis | 9 | 8 | 9 | 10 | 9 |
| Neurologic Disorder | 9 | 8 | 9 | 9 | 13 |
| Lung Disease | 12 | 9 | 12 | 15 | 14 |
| HIV | 1 | 1 | 0 | 1 | 1 |
| Laboratory and Dialysis Variables | |||||
| Albumin (g/dL) | 3.70 ± 0.44 | 3.75 ± 0.43 | 3.69 ± 0.43 | 3.68 ± 0.45 | 3.64 ± 0.46 |
| Hemoglobin (g/dL) | 11.2 ± 1.4 | 11.2 ± 1.4 | 11.2 ± 1.3 | 11.3 ± 1.4 | 11.3 ± 1.4 |
| ΔSBP (mmHg)* | 7.0 (−7.0–22.0) | 7.0 (−7.0–21.0) | 8.0 (−6.0–23.0) | 7.0 (−7.0–22.0) | 6.0 (−8.0–21.0) |
| Dialysis session length (min) | 236.4 ± 37.1 | 235.8 ± 35.5 | 236.9 ± 37.7 | 237.7 ± 40.0 | 234.5 ± 34.3 |
| Single-pool Kt/V | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.5 ± 0.3 |
| Hemodiafiltration (%) | 12 | 10 | 12 | 17 | 13 |
| Intradialytic weight loss (% of body weight) | 3.12 ± 1.50 | 3.10 ± 1.51 | 3.15 ± 1.52 | 3.08 ± 1.46 | 3.12 ± 1.45 |
| Dialysate sodiuma | |||||
| < 140 mEq/L | 32 | 28 | 32 | 39 | 36 |
| 140 mEq/L | 55 | 60 | 56 | 48 | 51 |
| > 140 mEq/L | 12 | 12 | 12 | 12 | 13 |
| Catheter use | 21 | 19 | 20 | 25 | 29 |
| Prescribed blood flow rate (ml/min) | 321.1 ± 95.0 | 314.1 ± 98.5 | 320.8 ± 95.2 | 331.7 ± 91.1 | 326.6 ± 87.1 |
| Ultrafiltration rate | |||||
| < 5 mL/min | 18 | 19 | 19 | 17 | 17 |
| 5–15 mL/min | 72 | 69 | 72 | 73 | 76 |
| ≥ 15 mL/min | 10 | 12 | 10 | 10 | 7 |
Note: Values for categorical variables are given as number (percentage) or percentage; values for continuous variables are given as mean ± standard deviation or median [interquartile range].
BMI, body mass index; SBP, systolicblood pressure;
Excludes patients with sodium modeling and dialysate sodium < 125 mEq/L or dialysate sodium > 155 mEq/L
Pre – post SBP.
Associations With Patient Demographic and Health Status Variables
The following were associated with a patient reporting a longer RT (i.e., being in a higher RT category): age (adjusted OR [AOR], 1.03 per 5 years; 95% confidence interval [CI], 1.01–1.06), dialysis vintage (AOR, 1.02 per year; 95% CI, 1.01–1.03), BMI (AOR, 1.07 per 5 units BMI higher; 95% CI, 1.02–1.12), diabetes (AOR, 1.14; 95% CI, 1.02–1.27), and psychiatric disorders (AOR, 1.39; 95% CI, 1.20–1.62). The following were associated with a patient reporting a shorter RT: male sex (AOR, 0.86; 95% CI, 0.77–0.97), full-time employment (AOR, 0.73; 95% CI, 0.59–0.91), and serum albumin level (AOR, 0.89 per 0.5 g/dl; 95% CI, 0.83–0.95) (Table 2). Note that proportional ORs can be interpreted at any cutpoint of ordered RT categories; i.e., <2 hours vs. ≥2 hours, ≤6 hours vs. ≥7 hours, or ≤12 hours vs. >12 hours.
Table 2.
Estimated ORs for associations of patient-level variables and HD treatment variables with patient-reported recovery time
| Characteristic | Model 1* | Model 2** |
|---|---|---|
| Age, per 5-y older | 1.05 (1.03–1.07) | 1.03 (1.01–1.06) |
| Male sex, vs female sex | 0.83 (0.75–0.92) | 0.86 (0.77–0.97) |
| Dialysis vintage, per 1-y longer | 1.02 (1.01–1.03) | 1.02 (1.01–1.03) |
| BMI, per 5-kg/m2 higher | 1.08 (1.03–1.13) | 1.07 (1.02–1.12) |
| Diabetes, vs no | 1.19 (1.08–1.31) | 1.14 (1.02–1.27) |
| Psychiatric disorder, vs no | 1.47 (1.28–1.70) | 1.39 (1.20–1.62) |
| Full-time employment, vs no | 0.66 (0.53–0.81) | 0.73 (0.59–0.91) |
| Catheter, vs fistula/graft | 1.16 (1.02–1.32) | 1.13 (0.99–1.29) |
| Albumin, per 1-g/dL higher | 0.87 (0.81–0.93) | 0.89 (0.83–0.95) |
| Hemoglobin, per 1-g/dL higher | 0.99 (0.95–1.04) | 1.01 (0.97–1.06) |
| Hemodiafiltration, vs standard HD | 1.08 (0.87–1.35) | 1.09 (0.88–1.35) |
| Intradialytic weight loss, per 1% higher | 1.05 (1.02–1.09) | 1.04 (1.00–1.08) |
| Dialysate sodium, vs 140 mEq/L | ||
| <140 mEq/L | 1.28 (1.07–1.53) | 1.34 (1.11–1.61) |
| >140 mEq/L | 1.08 (0.90–1.30) | 1.06 (0.89–1.27) |
| HD session length, per 30-min longer | 1.06 (1.01–1.11) | 1.05 (1.00–1.10) |
| ΔSBP, per 10–mm Hg greater | 1.00 (0.98–1.03) | 1.00 (0.98–1.03) |
| Single-pool Kt/V, per 0.1 higher | 1.01 (0.99–1.03) | 1.00 (0.98–1.02) |
| Blood flow rate, per 20-mL/min higher | 0.98 (0.96–1.01) | 0.99 (0.97–1.01) |
| Ultrafiltration rate, vs 5–15 mL/mina | ||
| <5 mL/min | 0.85 (0.74–0.97) | 0.86 (0.75–0.99) |
| ≥15 mL/min | 0.75 (0.63–0.90) | 0.73 (0.61–0.87) |
Note: Values are given as OR (95% confidence interval). Proportional ORs above (below) 1.0 indicate an increased propensity to report longer (shorter) recovery time category; i.e. <2 h vs. ≥2 h, ≤6 h vs. ≥7 h, or ≤12 h vs. >12 h.
BMI, body mass index; SBP, systolic blood pressure; OR, odds ratio; spKt/V, single-pool Kt/V; HD, hemodialysis
Model 1: adjusted for country and race (US black only), age, male sex, dialysis vintage, and BMI and accounting for facility clustering.
Model 2: Model 1 adjustments plus 14 summary comorbidities, full-time employment, catheter use, albumin, hemoglobin, hemodiafiltration use, intradialytic weight loss, dialysate sodium, session length, ΔSBP, blood flow rate, and sKt/V.
Estimated OR from a separate model excluding adjustments for session length and intradialytic weight loss due to collinearity.
Associations With Dialysis-Related Treatment Variables
The following treatment variables were associated with a patient reporting a longer RT: greater IDWL (AOR, 1.04 per percent higher; 95% CI, 1.00–1.08) and longer dialysis session duration (AOR, 1.05 per 30 minutes longer; 95% CI, 1.00–1.10). Both slow and fast ultrafiltration (UF) rate (<5 and >15 mL/min, respectively) were associated with a patient reporting a shorter RT compared with an UF rate of 5–15 mL/min (AORs of 0.86 [95% CI, 0.75–0.99] and 0.73 [95% CI, 0.61–0.87], respectively, in models excluding session length and IDWL). Separate analyses of UF rate expressed as mL/h/kg and among patients without residual kidney function and ESRD >1 year yielded similar U-shaped associations.
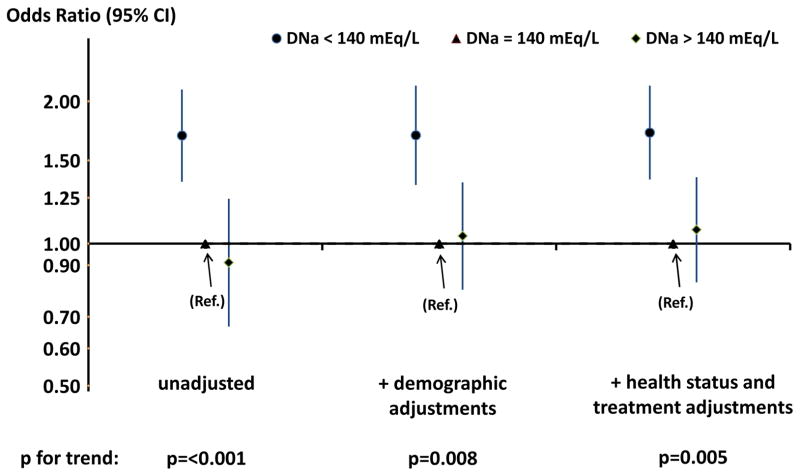
Prescription of a dialysate sodium concentration of <140 versus equal to 140 mEq/L was associated with a longer reported RT (AOR, 1.34; 95% CI, 1.11–1.61; Table 2). Additional adjustment for serum sodium did not significantly affect this association (AOR, 1.33; 95% CI, 1.11–1.59). Fifty-nine percent of DNa values <140mEq/L were exactly 138 mEq/L. A consistent association was seen specifically for DNa of 138 versus 140 mEq/L (AOR, 1.37; 95% CI, 1.11–1.70). The association of a longer reported RT with DNa of <140 vs. 140 mEq/L was stronger in facilities that used a single DNa for ≥90% of patients, i.e., ‘non-individualized’ DNa facilities (AOR, 1.72; 95% CI, 1.37–2.16; p for interaction between DNa and type of facility [individualized or non-individualized] = 0.01; n = 3,181) (Figure 1).
Figure 1.
Odds Ratios (with 95% CI) of longer reported recovery time in three categories of dialysate sodium concentration (DNa) among facilities in which ≥ 90% of patients used the same DNa (‘non-individualized’ facilities).
N=3,181 patients. “Demographic adjustments” includes country, race (US-black only), ears on dialysis, age, BMI, sex, and 14 summary comorbidities. “Health status and treatment adjustments” includes demographic adjustments plus full-time employment, dialysis session length, intradialytic weight loss, catheter use, serum albumin, hemoglobin, use of hemodiafiltration, blood flow rate, single pool K/tV, and Δ(pre-post) systolic blood pressure.
In ‘non-individualized’ facilities, patients treated with DNa<140 mEq/L had MCS and PCS scores that were respectively 1.2 (95% CI, −3.0 to 0.7) and 1.1 (95% CI, −2.5 to 0.3) points lower than patients treated with DNa equal to 140 mEq/L. Patients with DNa<140 mEq/L had a kidney disease burden score 1.4 (95% CI, −5.2 to 2.5) points lower than patients treated with DNa equal to 140 mEq/L, indicating a higher perceived burden of disease.
Dialysate potassium and bicarbonate concentrations were not associated with reported RT (dialysate potassium of 1–1.5 versus > 3 mEq/L: AOR, 1.05 [95% CI, 0.82–1.35]; dialysate potassium of 2 versus > 3 mEq/L: AOR, 1.06 [95% CI, 0.90–1.25]; dialysate bicarbonate−: AOR per 3-mEq/L increase, 1.01 [95% CI, 0.94–1.08]).
Associations With Patient-Reported Variables
Patients in longer reported RT categories tended to report symptoms related to kidney failure, including pruritus (Spearman correlation [r] = 0.12), cramping (r = 0.15), trouble falling asleep (r = 0.16), and feeling depressed (r = 0.22) (Table 3). Patients reporting fewer activities of daily living (r = −0.27) and scoring lower on the RAPA scale (aerobic) (r = −0.18) tended to report a longer RT. Reported RT was inversely correlated with KDQOL-36 measures: kidney disease burden score (r = −0.26), effects of kidney disease (r = −0.31), MCS score (r = −0.33), PCS score (r = −0.37), and subscales (r = −0.29 to −0.38). Nearly identical results were obtained when using Pearson correlations.
Table 3.
Distribution of patient symptoms and quality of life measures, by recovery time category
| Characteristic | Overall | <2 h | 2–6 h | 7–12 h | >12 h | r |
|---|---|---|---|---|---|---|
| Over the past 4 weeks, to what extent were you bothered by itchy skin? | 0.12 | |||||
| Not at all bothered | 39 | 46 | 37 | 34 | 34 | |
| Somewhat bothered | 28 | 27 | 30 | 27 | 24 | |
| Moderately bothered | 16 | 13 | 17 | 17 | 19 | |
| Very much bothered | 11 | 9 | 11 | 14 | 13 | |
| Extremely bothered | 6 | 4 | 5 | 8 | 11 | |
| Over the past 4 weeks, to what extent were you bothered by cramps? | 0.15 | |||||
| Not at all bothered | 68 | 72 | 67 | 64 | 61 | |
| Somewhat bothered | 16 | 16 | 16 | 16 | 14 | |
| Moderately bothered | 9 | 8 | 10 | 9 | 12 | |
| Very much bothered | 5 | 3 | 5 | 6 | 8 | |
| Extremely bothered | 3 | 1 | 2 | 4 | 6 | |
| How often do you have trouble falling asleep? | 0.16 | |||||
| None of the time | 21 | 28 | 20 | 15 | 16 | |
| A little of the time | 21 | 24 | 20 | 18 | 17 | |
| Some of the time | 34 | 29 | 36 | 37 | 32 | |
| All the time | 25 | 19 | 24 | 30 | 35 | |
| How often do you awaken and have trouble falling asleep again? | 0.16 | |||||
| None of the time | 18 | 25 | 16 | 12 | 13 | |
| A little of the time | 24 | 27 | 23 | 22 | 21 | |
| Some of the time | 35 | 30 | 38 | 37 | 34 | |
| All the time | 23 | 18 | 23 | 29 | 32 | |
| How often do you get enough sleep to feel rested upon waking in the morning? | 0.10 | |||||
| All the time | 30 | 37 | 27 | 26 | 29 | |
| Some of the time | 37 | 35 | 39 | 38 | 34 | |
| A little of the time | 22 | 19 | 24 | 24 | 20 | |
| None of the time | 10 | 9 | 10 | 12 | 17 | |
| During the past week, I felt depressed: | 0.22 | |||||
| <1 d | 54 | 67 | 50 | 44 | 41 | |
| 1–2 d | 26 | 22 | 29 | 27 | 26 | |
| 3–4 d | 13 | 8 | 15 | 18 | 17 | |
| 5–7 d | 7 | 3 | 6 | 10 | 17 | |
| Lawton ADL* | 5.5 | 6.3 | 5.4 | 4.8 | 4.2 | −0.27 |
| RAPA** | ||||||
| Aerobic | 3.3 | 3.7 | 3.3 | 2.9 | 2.4 | −0.18 |
| Strength and flexibility | 0.5 | 0.6 | 0.5 | 0.5 | 0.3 | −0.08 |
| KDQOL-36# | ||||||
| Kidney Disease Burden | 36 | 45 | 35 | 30 | 24 | −0.26 |
| Effects of Kidney Disease | 66 | 74 | 65 | 59 | 53 | −0.31 |
| Mental Component Summary | 45 | 49 | 44 | 42 | 38 | −0.33 |
| Vitality | 42 | 55 | 40 | 32 | 23 | −0.39 |
| Social functioning | 55 | 66 | 54 | 47 | 37 | −0.29 |
| Role-emotional | 60 | 73 | 59 | 50 | 40 | −0.34 |
| Mental health | 63 | 73 | 63 | 56 | 49 | −0.32 |
| Physical Component Summary | 37 | 43 | 37 | 33 | 30 | −0.37 |
| Physical functioning | 44 | 60 | 41 | 30 | 25 | −0.33 |
| Role-physical | 46 | 60 | 45 | 33 | 26 | −0.38 |
| Bodily pain | 60 | 74 | 58 | 49 | 41 | −0.35 |
| General health | 42 | 53 | 41 | 33 | 25 | −0.34 |
Note: Values for categorical variables are given as percentages; values for continuous variables are given as means.
KDQOL, Kidney Disease Quality of Life; ADL, activities of daily living; r, Spearman correlation; RAPA, Rapid Assessment of Physical Activity;
Lawton ADL counts the number of activities of daily living that can be performed independently.
RAPA score is calculated such that higher scores indicate greater physical activity.
KDQOL component summaries and subscales are calculated such that higher scores indicate better health.
The association of RT with MCS and PCS scores followed a dose-dependent pattern and remained after adjustment (Figure S2). Compared to patients who reported a RT of 2–6 hours, mean MCS scores were 4.9 points higher for RTs of < 2 hours, 2.4 points lower for RTs of 7–12 hours, and 6.2 points lower for RTs of >12 hours. Mean PCS scores were 4.6 points higher, 2.9 points lower, and 4.7 points lower, respectively.
Associations With Morbidity and Mortality
Median follow-up time in the study was 16 (range, 0–31; mean, 15) months. Hospitalization events were recorded for 3,119 patients (52%), and 826 patients (14%) died.
After adjustment for demographic and comorbid factors, patient-reported RT was positively and monotonically associated with the rates of first hospitalization (adjusted hazard ratio [AHR] per additional hour of RT, 1.03; 95% CI, 1.02–1.04) and all-cause mortality (AHR, 1.05; 95% CI, 1.03–1.07) (Table 4, model 1). These associations were attenuated slightly after further adjustment for patient-reported symptoms (AHRs for first hospitalization and for mortality were 1.02 and 1.03, respectively; both p<0. 01; Table 4, model 2).
Table 4.
Hazard ratios for hospitalization and mortality, by reported recovery time categories
| Unadjusted | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| First Hospitalization | ||||||
| <2 h | 0.86 (0.79–0.93) | 0.88 (0.81–0.96) | 0.92 (0.85–0.99) | |||
| 2–6 h | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| 7–12 h | 1.07 | (0.97–1.18) | 1.05 | (0.95–1.16) | 1.02 | (0.92–1.12) |
| >12 h | 1.26 | (1.12–1.41) | 1.22 | (1.09–1.37) | 1.16 | (1.03–1.30) |
| p for trend | <0.001 | <0.001 | 0.002 | |||
| All-Cause Mortality | ||||||
| <2 h | 0.86 | (0.72–1.02) | 0.88 | (0.73–1.05) | 0.98 | (0.82–1.17) |
| 2–6 h | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| 7–12 h | 1.29 | (1.07–1.54) | 1.22 | (1.02–1.46) | 1.16 | (0.96–1.39) |
| >12 h | 1.60 | (1.30–1.97) | 1.47 | (1.19–1.83) | 1.3 | (1.04–1.63) |
| p for trend | <0.001 | <0.001 | 0.006 | |||
Note: Values are given as hazard ratio (95% confidence interval). All models are stratified by country and race (US black only) and use a robust “sandwich” variance estimator. Model 1 includes sex, age, dialysis vintage, body mass index, catheter use, and 14 summary comorbidities. Model 2 includes variables in model 1 plus full-time employment, activities of daily living count, Rapid Assessment of Physical Activity score, pruritus severity, depression severity, and sleep problems.
DISCUSSION
Following a hemodialysis treatment, many patients describe feeling tired and in need of a rest or sleep. In this international population receiving unit-based three-times-weekly hemodialysis, 68% of patients reported taking more than 2 hours to recover from a dialysis session, and 27% more than 6 hours. Reported RT was more likely to be longer if patients were older, female, or had a higher BMI, diabetes, or a psychiatric disorder. Patients who suffered symptoms of kidney failure such as itching 10, cramping, sleep disturbance 11, or depression 12, 13 were more likely to report a longer RT.
The pathophysiology of the recovery process is incompletely understood. Hemodialysis causes salt and water to flow between body fluid compartments, osmotic imbalances between extra- and intracellular fluid and across the blood–brain barrier, and the transport of electrolytes across cell membranes. These changes may be greater after a stressful dialysis session, leading to slower recovery. In addition, patients who are less able to withstand the stress may experience symptoms longer. The symptoms may be related to cumulative effects of kidney failure and/or hemodialysis treatments as the likelihood of a long reported RT increased with the duration of kidney failure and dialysis vintage.
We investigated whether recovery time may be modified by alterations to the hemodialysis regimen. Reported RT was minimally associated with the use of hemofiltration/hemodiafiltration or with small solute clearance (Kt/V). Longer reported RT was associated with higher intradialytic weight loss. However, it showed a U-shaped association with the rate of fluid removal (ultrafiltration). The associations of low IDWL and slow UF rates with shorter reported RT are as expected; recovery may be quicker after treatments in which fluid shifts are slower and of smaller volume. Although patients who have maintained urine output are more likely to have lower IDWL and slow UF rates, the U-shaped association remained after adjusting for residual kidney function.
The associations of fast UF rates and shorter dialysis session time with shorter reported RT are surprising. Patients who recover slowly after treatments with a fast UF rate may adopt a lower salt and fluid intake and increased session time, selectively leaving those who recover quickly in the faster UF rate category.
Intradialytic weight loss is modestly lower in patients prescribed a lower dialysate sodium concentration14. However, after adjusting for IDWL, lower dialysate sodium concentration (DNa <140 mEq/L) was associated with a longer reported RT. The association was stronger in those facilities that did not modify the DNa 14. Patients in these facilities are quasi-randomized to receive the DNa used by the dialysis facility. Thus, restricting the analysis to these facilities reduces confounding by indication.
In the 1970s, the conventional dialysate sodium prescription increased to reduce dialysis disequilibrium syndrome caused by osmotic shifts across the blood–brain barrier 15. Our finding that lower DNa is associated with a longer reported RT suggests that symptoms during recovery may be partly a manifestation of disequilibrium. As higher DNa is not associated with an increase in hospitalization or mortality 14, a controlled trial of the effect of higher versus lower DNa on recovery after dialysis and on quality of life can be justified.
Two other interventions may be considered for patients reporting long RTs. First, increasing the frequency of HD sessions to between 5 and 7 times per week has been shown to reduce total weekly reported RT, increase quality of life, and reduce depression 16. Second, changing to peritoneal dialysis (PD) will remove the post-dialysis recovery period. Quality of life has been shown to be higher on PD than on hemodialysis in some patient groups, such as the elderly 17.
The RT question is simple to incorporate into clinical practice and can be used as a guide to a patient’s quality of life. Nearly all patients were able to give a response to the question. In contrast, even after giving informed consent, only 79% of patients answered all the questions required to calculate a summary score for mental and physical quality of life. The strength of the correlation with the PCS score of the KDQOL-36™ was similar to that reported previously 3 (−0.37 versus −0.341, respectively); the correlation with the MCS score was stronger (−0.33 versus −0.155, respectively). This strengthens the validity of the RT question as a guide to quality of life.
The RT question can also be used as a predictor of subsequent outcomes. For example, after adjustment for potential confounders, patients reporting a RT greater than 12 hours had a 22% higher rate of first hospitalization and a 47% higher mortality rate than patients reporting a RT between 2 and 6 hours.
The associations of reported RT with hospitalization and mortality were attenuated when adjusting for covariates (Table 4). The additional covariates may be intermediate variables in the causal pathways between recovery from dialysis and the two outcomes. Those results may reflect over-adjustment rather than, or in addition to, the control of confounders. Nonetheless, reported RT seems to capture important prognostic information that is not captured in any single variable.
Recovery time has a significant impact on patients and may be affected by modifiable aspects of the treatment regimen. Hence, the RT question potentially could be used as an audit measure of the quality of hemodialysis treatment. Further intervention studies are needed to provide evidence of how to reduce the length of recovery time and hence improve the quality of treatment in a dialysis unit.
The following weaknesses and limitations to this study should be considered. As in any observational study, unmeasured confounding or other sources may bias the observed associations. We cannot assign causal interpretations to estimates from cross-sectional analyses, such as the association between reported RT and patient-reported depression. Although temporal directionality might be assumed for longitudinal associations between reported RT and subsequent hospitalization or mortality, the use of time-independent predictors may not fully eliminate bias due to time-dependent confounding.
Patients were given no prompts on how to interpret the RT question, reflecting how it was originally validated 3. This may lead to variation in interpretation of the question between patients and countries. The question was asked once and responses may have varied over time, affecting the strength of associations.
A categorical choice of answers was offered to make the question easier to answer. The relative benefits of requesting an answer to the question as a time in minutes or as one of four categories require further investigation.
Patients who sleep soon after finishing dialysis may include time spent asleep in their reported RT. We did not capture the time of day of dialysis to study this possibility.
Recovery may be slower after periods of hemodynamic instability. While blood pressure before and after dialysis was recorded, hypotension during dialysis and use of fluid boluses were not. Data on the number of treatments missed by patients were not collected at the time of the RT questionnaire.
This is not a study of the pathophysiology of recovery after dialysis. Answers to the RT question are subjective and not supported by objective physiological measurements. Intervention studies measuring the effect of the dialysis prescription on recovery after dialysis are warranted.
Patients who were excluded from the study because they did not complete the patient questionnaire were more likely to be black, use a catheter for vascular access, and have greater comorbidity burdens. The impact of recovery time when generalized to other HD patients may be different than reported in this analysis.
For patients making the decision of whether to start hemodialysis, it would be useful to offer a prediction of their length of recovery time. As the majority of patients in this study were already established on hemodialysis, a further study of recovery time in incident hemodialysis patients is needed to provide a reliable prediction.
In conclusion, there is wide variation in the time it takes for patients to recover following a dialysis session. The question, “How long does it take you to recover from a dialysis session?” is a simple and meaningful self-reported measure that can be included in the clinical assessment of hemodialysis patients and possibly used as an audit measure of the quality of dialysis treatment. It helps identify patients with poorer HRQoL and higher risk of hospitalization and mortality. Interventions to reduce recovery time and possibly to improve clinical outcomes, such as increasing dialysate sodium concentration, need to be tested in randomized studies.
Supplementary Material
Acknowledgments
Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial assistance.
Support: The DOPPS is supported by research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Sanofi Renal (since 2009), Abbvie (since 2009), Baxter (since 2011), Vifor Fresenius Renal Pharma (since 2011), and Fresenius Medical Care (since 2012) without restrictions on publications. Dr Tentori is supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grant 1K01DK087762-01A1.
Footnotes
Financial Disclosure: Dr Culleton is an employee of Baxter Healthcare. Dr Tentori has received honoraria from Dialysis Clinic Inc, Amgen, and the Renal Research Institute. The other authors declare that they have no other relevant financial interests.
Figure S1: Distribution of reported recovery time categories, by DOPPS country.
Figure S2: Distribution of KDQOL-36 MCS and PCS scores, by reported recovery time category.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Descriptive Text for Online Delivery of Supplementary Material
Distribution of reported recovery time categories, by DOPPS country.
Distribution of KDQOL-36 MCS and PCS scores, by reported recovery time category.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fukuhara S, Lopes AA, Bragg-Gresham JL, et al. Worldwide Dialysis Outcomes and Practice Patterns Study. Health-related quality of life among dialysis patients on three continents: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003 Nov;64(5):1903–10. doi: 10.1046/j.1523-1755.2003.00289.x. [DOI] [PubMed] [Google Scholar]
- 2.Mapes DL, Lopes AA, Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64:339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 3.Lindsay RM, Heidenheim PA, Nesrallah G, Garg AX, Suri R. Minutes to Recovery after a Hemodialysis Session: A Simple Health-Related Quality of Life Question That Is Reliable, Valid, and Sensitive to change. Clin J Am Soc Nephrol. 2006;1(5):952–959. doi: 10.2215/CJN.00040106. [DOI] [PubMed] [Google Scholar]
- 4.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44:7–15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int. 2000;57:S74–S81. doi: 10.1046/j.1523-1755.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 6.Robinson BM, Port FK. Caring for dialysis patients: international insights from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Identifying best practices and outcomes in the DOPPS. Semin Dial. 2010;23:4–6. doi: 10.1111/j.1525-139X.2009.00674.x. [DOI] [PubMed] [Google Scholar]
- 7.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: New England Medical Center- The Health Institute; 1994. [Google Scholar]
- 8.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 9.SAS/STAT User’s Guide Version 8. Cary, North Carolina: SAS Institute; 2000. [Google Scholar]
- 10.Pisoni RL, Wikstrom B, Elder SJ, et al. Pruritus in hemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2006;21:3495–505. doi: 10.1093/ndt/gfl461. [DOI] [PubMed] [Google Scholar]
- 11.Elder SJ, Pisoni RL, Akizawa T, et al. Sleep Quality Predicts Quality of Life and Mortality Risk in Hemodialysis Patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2008;23:998–1004. doi: 10.1093/ndt/gfm630. [DOI] [PubMed] [Google Scholar]
- 12.Lopes AA, Albert JM, Young EW, et al. Screening for depression in hemodialysis patients: Associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int. 2004;66:2047–2053. doi: 10.1111/j.1523-1755.2004.00977.x. [DOI] [PubMed] [Google Scholar]
- 13.Lopes AA, Bragg J, Young E, et al. Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int. 2002;62:199–207. doi: 10.1046/j.1523-1755.2002.00411.x. [DOI] [PubMed] [Google Scholar]
- 14.Hecking M, Karaboyas A, Saran R, et al. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin J Am Soc Nephrol. 2012;7:92–100. doi: 10.2215/CJN.05440611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Port FK, Johnson WJ, Klass DW. Prevention of dialysis disequilibrium syndrome by use of high sodium concentration in the dialysate. Kidney Int. 1973;3:327–333. doi: 10.1038/ki.1973.51. [DOI] [PubMed] [Google Scholar]
- 16.Jaber BL, Lee Y, Collins AJ, et al. Effect of Daily Hemodialysis on Depressive Symptoms and Postdialysis Recovery Time: Interim Report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. Am J Kidney Dis. 2010;56:531–539. doi: 10.1053/j.ajkd.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Brown EA, Johansson L, Farrington K, et al. Broadening Options for Long-term Dialysis for the Elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients. Nephrol Dial Transplant. 2010;25:3755–3763. doi: 10.1093/ndt/gfq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.