Abstract
Background
Recent studies suggest an association between higher latitude, a proxy of vitamin D (VD) status, and allergic diseases. Chile provides an ideal setting to study this association due to its latitude span and high rates of VD deficiency in southern regions. The aim of this study is to explore the associations of latitude and solar radiation with anaphylaxis admission rates.
Methods
We reviewed anaphylaxis admissions in Chile’s hospital discharge database between 2001 and 2010 and investigated associations with latitude and solar radiation.
Results
2316 anaphylaxis admissions were registered. Median age of patients was 41 years; 53% were female. National anaphylaxis admission rate was 1.41 per 100,000 persons per year. We observed a strong north-south increasing gradient of anaphylaxis admissions (β 0.04, P=0.01), with increasing rates south of latitude 34°S. A significant association was also observed between solar radiation and anaphylaxis admissions (β −0.11, P=0.009). Latitude was associated with food-induced (β 0.05, P=0.02), but not drug-induced (β −0.002, P=0.27), anaphylaxis. The association between latitude and food-induced anaphylaxis was significant in children (β 0.01, P=0.006), but not adults (β 0.003, P=0.16). Anaphylaxis admissions were not associated with regional sociodemographic factors like poverty, rurality, educational level, ethnicity, or physician density.
Conclusions
Anaphylaxis admission rates in Chile are highest at higher latitudes and lower solar radiation, used as proxies of VD status. The associations appear driven by food-induced anaphylaxis. Our data support a possible role of VD deficiency as an etiological factor in the high anaphylaxis admission rates found in southern Chile.
Keywords: allergy, anaphylaxis, food allergy, latitude, solar radiation, vitamin D
Introduction
Vitamin D (VD) is a pleiotropic hormone with multiple effects on the immune system [1]. A growing number of epidemiologic studies support an association between VD deficiency and allergic diseases. In 2007, Camargo and colleagues reported that sales of epinephrine autoinjectors in the USA had a strong latitude gradient, a proxy of sun exposure and VD status [2]; the investigators replicated this finding in Australia [3]. Further data from the USA and Australia have shown that the prevalence of different allergic diseases, including food allergy (FA), anaphylaxis, and atopic dermatitis increase at higher latitudes, used as a proxy of sun exposure and VD status [4–7].
Chile is a South American country that spans 39° of latitude without significant longitudinal variations, and includes the southernmost populated region in the world. Chile has an estimated population of 17 million with a relatively homogeneous ethnic background composed of a blend of European (mainly Spanish) and indigenous ethnicities. In Chile, only few foods are weakly enriched with VD and there is low consumption of fish [8]. The population inhabiting regions south of Santiago, the capital city, are at higher risk of VD deficiency due to lower availability of solar radiation (SR) needed for VD photoconversion [9]. These characteristics make Chile an ideal location for studying the effect of latitude and SR on allergic diseases.
The objective of our study was to explore the associations of latitude and SR with anaphylaxis admission rates in Chile. We hypothesized confirmation of the latitude-anaphylaxis association in Chile, particularly south of Santiago (latitude 33.5°S). Moreover, we hypothesized that the association would be strongest for food-induced anaphylaxis, due to a potentially stronger role of VD deficiency on the pathogenesis of food-related allergic reactions [10].
Methods
Using the national hospital discharge database from the Department of Health Statistics and Information of the Chilean Ministry of Health, we analyzed admissions due to anaphylaxis between 2001 and 2010 [11]. The database is a mandatory registry of all hospitalizations in the public and private health systems throughout Chile. During the study period, Chile was divided into 13 administrative regions from north to south. Latitude for each region was taken at its center (e.g., latitude 19°34’S was used for the Tarapacá Region located between latitudes 17°30′ and 21°38′). Chile’s health care system combines public insurance (middle–low socioeconomic level population) and private insurance (high socioeconomic level population). Forty percent of the population inhabits the capital city of Santiago in central Chile, 22% lives in the northern regions and 38% in the south.
Anaphylaxis was defined by the following ICD-10 codes: T78.0 (food-induced anaphylaxis), T78.2 (anaphylaxis, unspecified), and T88.6 (drug-induced anaphylaxis). To avoid coding bias, we also analyzed whether anaphylaxis admission rates changed when including the ICD-10 diagnostic code for angioedema (T78.3), as anaphylaxis may be miscoded as angioedema, particularly if under-recognized [12]. Admission rates were expressed as cases per 100,000 persons per year. The childhood anaphylaxis admission rate was calculated in the Chilean population younger than 18 years, while the adult rate was calculated for ages 18 years and older. Standardized incidence ratios (SIRs) were calculated to evaluate any excess in anaphylaxis-related admissions. The national average was used as the reference for SIR (i.e., SIR 1.0). Due to large variations in size and population among regions, these were grouped into five different latitude ranges of similar size from north to south in order to show changes in anaphylaxis admission rates and SIRs.
Average annual SR intensity data, expressed as MJ/m2/day, was obtained from the Chilean Solarimetric Registry [13]. The regional poverty and rurality data were obtained from the 2009 National Socioeconomic Characterization Survey from the Chilean Ministry of Social Development [14]. Average years of schooling and indigenous ethnicity for each region were extracted from the 2002 Chilean Population and Housing Census [15]. Regional physician density was obtained from the Department of Health Statistics and Information of the Chilean Ministry of Health [11].
This study was approved by the Ethics Committee of the School of Medicine of Pontificia Universidad Católica de Chile.
Statistical analyses
All rates are shown with 95% confidence intervals (95%CI). Simple linear regressions were used to evaluate the association between the main exposures (years, regional latitude, SR, poverty, years of schooling, indigenous ethnicity rates) and main outcome (regional anaphylaxis admission rate). Unstandardized β coefficients and 95% confidence intervals are reported for each regression. Tolerance statistics were calculated to examine collinearity among exposure variables. Comparison between two rates was performed by binomial exact test. A second-order polynomial regression with 95% confidence intervals was used to fit the curve of SIR variation throughout Chile. A two-sided P<0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics 21.0 (SPSS Inc, Chicago, USA) and OpenEpi software version 2.3.1 [16].
Results
Over the 10-year study period, 2316 anaphylaxis admissions were registered, with the following diagnoses: food-induced anaphylaxis in 230 cases (10%), drug-induced anaphylaxis in 208 cases (9%), and unspecified anaphylaxis in 1878 cases (81%). Demographic characteristics of the anaphylaxis admissions are shown in Table 1. The median age of cases was 41 years (range: 0 – 97 years); 17% of cases were children (age: <18 years). Females had a slightly higher admission rate than males. A higher anaphylaxis admission rate was observed during spring-summer than autumn-winter seasons (P<0.001).
Table 1.
Demographic characteristics and seasonal distribution of anaphylaxis admissions in Chile, 2001–2010.
| n (%) | Admission rate per 100,000 persons (95% Confidence Intervals) | |
|---|---|---|
|
| ||
| Age (years) | ||
| 0 to 9 | 166 (7) | 0.6 (0.5–0.7) |
| 10 to 19 | 294 (13) | 1.0 (0.9–1.1) |
| 20 to 29 | 278 (12) | 1.1 (1.0–1.2) |
| 30 to 39 | 347 (15) | 1.4 (1.3–1.6) |
| 40 to 49 | 382 (16) | 1.7 (1.5–1.8) |
| 50 to 59 | 374 (16) | 2.3 (2.1–2.6) |
| 60 to 69 | 254 (11) | 2.4 (2.2–2.8) |
| 70 to 79 | 158 (7) | 2.6 (2.2–3.0) |
| 80 to 99 | 63 (3) | 2.4 (1.9–3.0) |
|
| ||
| Sex | ||
| Male | 1093 (47) | 1.3 (1.2–1.4) |
| Female | 1223 (53) | 1.5 (1.4–1.6) |
|
| ||
| Insurance | ||
| Public | 1536 (66) | 1.3 (1.2–1.4) |
| Private | 780 (34) | 1.7 (1.6–1.8) |
|
| ||
| Season | ||
| Summer | 645 (28) | 0.39 (0.36–0.43) |
| Fall | 546 (24) | 0.33 (0.3-0-36) |
| Winter | 482 (21) | 0.29 (0.27–0.32) |
| Spring | 619 (27) | 0.38 (0.35-0.4) |
| Unknown | 24 | |
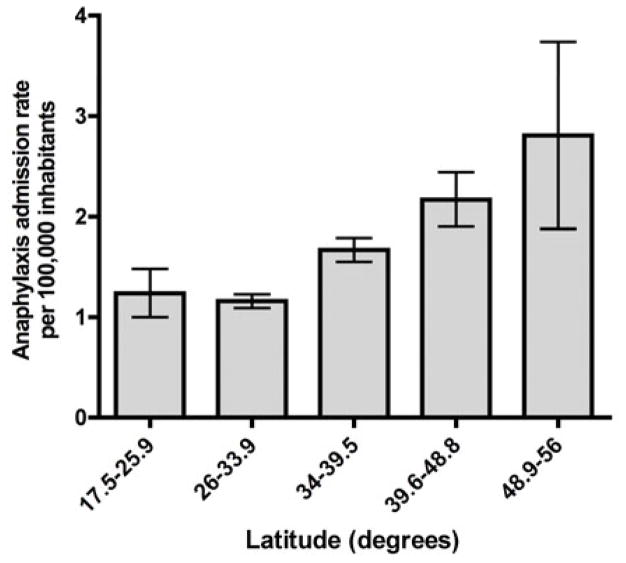
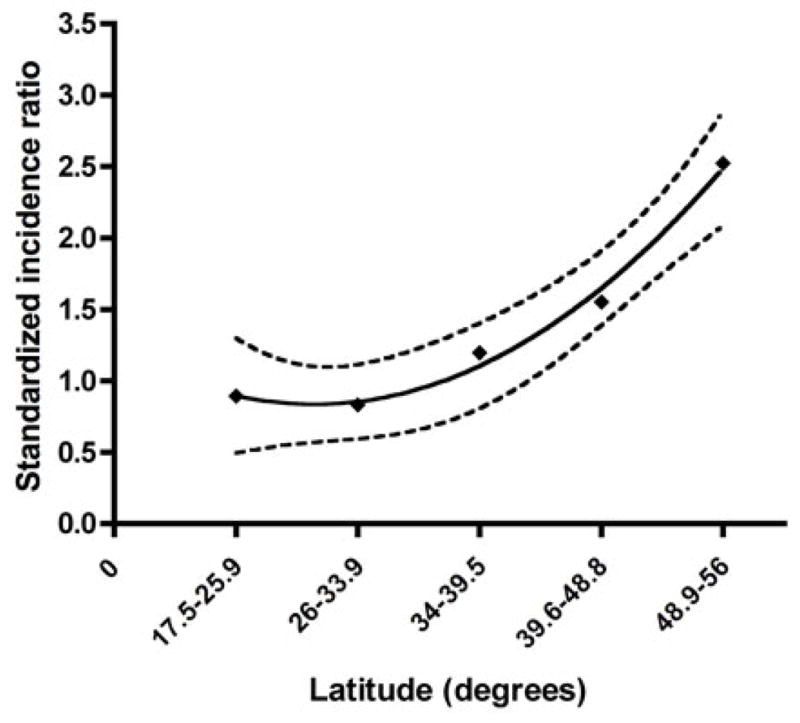
The national anaphylaxis admission rate was 1.41 per 100,000 persons per year (95%CI, 1.36 – 1.47). There was no significant variation in yearly anaphylaxis admission rate during the study period (P=0.27) (Figure 1). With regard to latitude, we found a strong north-south increasing gradient of anaphylaxis admissions (P=0.01) (Table 2). As we hypothesized, admission rates particularly increased south of Santiago (latitude 33.5°S), with the southernmost region (latitude 48.9–56°S) having the highest rate (2.82; 95%CI, 2.1 – 3.7) (Figure 2). To quantify the excess in anaphylaxis admissions throughout Chile, we calculated standardized incidence ratios (SIR) for five latitude ranges of similar size from north to south. As expected, the incidence of anaphylaxis admissions increased further from the Equator, exceeding the expected rate south of latitude 33.9°S and reaching its highest value at latitude 48.8–56°S (SIR 2.52; 95%CI, 2.00 – 3.05) (Figure 3). A second-order polynomial curve best fit the latitudinal variation in SIR (R2=0.99).
Figure 1.
Annual national anaphylaxis admission rates in Chile, 2001–2010. Error bars show 95% confidence intervals.
Table 2.
Association of latitude and solar radiation with anaphylaxis and angioedema admission rates.
| Admissions (ICD-10 code) | Latitude (degrees)
|
Solar radiation (MJ/m2/day)
|
||||
|---|---|---|---|---|---|---|
| β | 95%CI | P | β | 95%CI | P | |
| Anaphylaxis (T78.0, T88.6, T78.2) | ||||||
| Total (n=2316) | 0.04 | 0.01, 0.07 | 0.01 | −0.106 | −0.18, −0.033 | 0.009 |
| Children (n=403) | 0.035 | 0.008, 0.062 | 0.01 | −0.092 | −0.157, −0.028 | 0.009 |
| Adults (n=1913) | 0.042 | 0.005, 0.079 | 0.03 | −0.11 | −0.2, −0.021 | 0.02 |
| Food-induced anaphylaxis (T78.0) | ||||||
| Total (n=230) | 0.05 | 0.001, 0.01 | 0.02 | −0.014 | −0.025, −0.003 | 0.02 |
| Children (n=63) | 0.01 | 0.003, 0.016 | 0.006 | −0.024 | −0.04, −0.009 | 0.005 |
| Adults (n=167) | 0.003 | −0.002, 0.008 | 0.16 | −0.009 | −0.021, 0.003 | 0.14 |
| Drug-induced anaphylaxis (T88.6) | ||||||
| Total (n=208) | −0.002 | −0.006, 0.002 | 0.27 | 0.005 | −0.006, 0.016 | 0.36 |
| Children (n=35) | −0.005 | −0.008, −0.001 | 0.02 | 0.011 | 0.002, 0.02 | 0.02 |
| Adults (n=173) | −0.001 | −0.007, 0.005 | 0.7 | 0.002 | −0.014, 0.018 | 0.79 |
| Unspecified anaphylaxis (T78.2) | ||||||
| Total (n=1878) | 0.037 | 0.009, 0.066 | 0.02 | −0.097 | −0.167, −0.027 | 0.01 |
| Children (n=305) | 0.026 | 0.001, 0.052 | 0.04 | −0.071 | −0.133, −0.008 | 0.03 |
| Adults (n=1573) | 0.041 | 0.009, 0.074 | 0.02 | −0.107 | −0.188, −0.027 | 0.01 |
| Angioedema (T78.3) | ||||||
| Total (n=812) | 0.025 | 0.009, 0.041 | 0.006 | −0.066 | −0.105, −0.026 | 0.004 |
| Children (n=204) | 0.018 | 0.006, 0.030 | 0.006 | −0.044 | −0.075, −0.012 | 0.01 |
| Adults (n=608) | 0.027 | 0.006, 0.049 | 0.02 | −0.074 | −0.125, −0.022 | 0.009 |
Figure 2.

Latitudinal distribution of anaphylaxis admissions in Chile, 2001–2010. Error bars show 95% confidence intervals.
Figure 3.
Latitude-related increase in standardized incidence ratios of anaphylaxis admissions in Chile, 2001–2010. Black line: second-order polynomial curve fit, dotted lines: 95% confidence intervals.
We next analyzed whether regional levels of SR correlated with anaphylaxis admission rates. Latitude is highly correlated with SR (R2 = 0.93, P<0.001). As expected, we found a significant association between SR and anaphylaxis admission rates (P=0.009). A multivariable linear regression entering both latitude and SR was not possible due to high collinearity (tolerance 0.07, variance inflation factors 15.2). However, when both factors (latitude and SR) were included in a stepwise linear regression, only SR remained in the model, suggesting its association was slightly stronger, consistent with our hypothesis that it is a more direct proxy of VD status than latitude.
When analyzing childhood and adult anaphylaxis admission rates separately, latitude was significantly associated among both children (P=0.01) and adults (P=0.03). Similarly, regional SR was significantly associated with anaphylaxis rates among both children (P=0.009) and adults (P=0.02).
To test the hypothesis that the influence of VD deficiency is stronger on food-related allergic reactions than other forms of allergy [10], we investigated the association of latitude with anaphylaxis was related to the trigger of anaphylaxis (e.g., food versus drug). Consistent with our hypothesis, a significant association was found between latitude and SR with food-induced anaphylaxis (P=0.02 and P=0.02, respectively), but not with drug-induced anaphylaxis (P=0.27 and P=0.36, respectively). Further, we assessed if the association of latitude and SR with food-induced anaphylaxis was age-dependent. The associations of latitude and SR with food-induced anaphylaxis were found to be statistically significant among children (P=0.006 and P=0.005, respectively), but not among adults, (P=0.16 and P=0.14, respectively). Although overall drug-induced anaphylaxis was not significantly associated with latitude or SR, in children these associations were significant (P=0.02 and P=0.02, respectively). However, the small number of children with drug-induced anaphylaxis (n=35) precludes firm conclusions.
A higher admission rate was seen in the population with private insurance vs. public insurance (P<0.001), an indirect marker of socioeconomic status. However, anaphylaxis admission rates were not correlated to regional poverty rates (β −0.005; 95%CI, −0.074 to 0.064; P=0.88), rurality (β 0.005; 95%CI, −0.025 to 0.034; P=0.74), average years of schooling (β −0.131; 95%CI, −0.672 to 0.409; P=0.60), indigenous ethnicity rates (β 0.019; 95%CI, −0.036 to 0.074; P=0.46) or physician density (β −0.022; 95%CI, −0.129 to 0.085; P=0.66). In addition, the highest regional admission rates were observed in the southernmost region among those with public insurance (2.7; 95%CI, 1.8 – 3.8) and among those with private insurance (3.1; 95%CI, 1.9 – 4.8).
Miscoding may bias analysis of ICD-10 code databases. Thus, we also analyzed admissions due to angioedema (ICD-10 code T78.3) to test the associations of latitude and SR with anaphylaxis admissions. A total of 812 angioedema admissions were registered during the study period, with a median age of 37 years (range 0–96) and 54% of female gender; 25% of angioedema admissions were children. Both latitude and SR were significantly associated with angioedema admission rates (P=0.006; and P=0.004; respectively), and with childhood and adult angioedema admission rates evaluated separately (Table 2). When adding angioedema admissions to anaphylaxis admissions, the association of latitude and SR with anaphylaxis admissions actually strengthened (β 0.065 [95%CI, 0.023 to 0.107], P=0.006; and β −0.172 [95%CI, −0.272 to −0.071], P=0.003; respectively).
Discussion
Incidence rates of anaphylaxis appear to be increasing worldwide [17–19]. The identification of factors that drive this increase is essential to understand the pathogenesis of the allergy pandemic and to eventually curb this phenomenon. We found a significant association between latitude and SR (proxies of vitamin D status) and anaphylaxis admission rates in Chile, with rates highest in areas furthest from the equator and a surplus of anaphylaxis at the extreme south of Chile. A similar latitude gradient of anaphylaxis admissions was suggested by Mullins and colleagues in Australia, but was not statistically significant [3]. Because latitude and SR are co-linear in Chile (r = −0.97), it is difficult to disentangle which factor is driving the association. However, our analyses suggest the association between SR and anaphylaxis admissions is a little stronger than that of latitude.
Our data provide further evidence for a potential role of VD in the pathogenesis of allergic diseases such as food-induced anaphylaxis. The effect of latitude on VD status is mostly through sunlight exposure, with populations living furthest from the Equator being less likely to have SR exposure, and thus higher rates of VD deficiency. A previous report showed a decreasing southward trend in 25(OH)D levels in Chile, with the lowest levels being found between latitudes 49–56°S [20]. These data support a possible role of VD deficiency in the high anaphylaxis admission rates found in the southernmost regions of Chile.
The possible role of VD on the development of allergic diseases seems to be greater when only food-related allergies are considered. For example, an emergency department-based study from the US previously found a stronger latitude gradient for FA-related visits than other causes of acute allergic reactions [4]. In a Korean sample of patients with atopic dermatitis and food sensitization, lower 25(OH)D levels were associated with more severe atopic dermatitis [21]. In Australian infants VD insufficiency has been associated with higher odds of having challenge-proven FA [22]. However, a prospective study of infants in the same country, showed a significant association of cord-blood 25(OH)D with eczema, but not IgE-mediated food sensitization or allergy, at 12 months of age [23]. In the current study, we found that latitude-anaphylaxis association was statistically significant in food-induced, but not drug-induced, anaphylaxis admissions suggesting a stronger association between latitude and FA than other etiologies of acute allergic reactions. Interestingly, the association of latitude with food-induced anaphylaxis appeared stronger in children than adults. Evidence for a stronger influence of VD status on the development of allergy in children than adults was also provided by the National Health and Nutrition Examination Survey 2005–2006 that demonstrated in a nationally-representative US-based sample that 25(OH)D levels were associated with IgE sensitization in children, but not in adults [24]. Our study showed an association of latitude/SR with angioedema admissions, another possible proxy of FA, in both children and adults, suggesting a more direct role of latitude/SR in triggering anaphylaxis per se rather than anaphylaxis only acting as a proxy of FA rates.
While the data are consistent with our a priori hypothesis about latitude, SR, vitamin D status and anaphylaxis risk, it is important to examine other possible explanations. Anaphylaxis may act as a proxy for underlying FA prevalence and thus, increased rates may be presumed to reflect an underlying increase in FA. Nonetheless, higher anaphylaxis admission rates may also be related to more severe food allergies or higher rates in poorly controlled food allergic disease at high latitudes. Unfortunately, there are no studies examining the prevalence or severity of FA throughout the country. It is also possible that the association between latitude/SR and anaphylaxis could be independent of VD. For example, studies have shown that ultraviolet light may have VD-independent effects on immune function [25]. In addition, several studies suggest that higher, instead of lower, 25(OH)D levels could be associated with higher rates of allergic disease [26–29]. The increased rates of anaphylaxis admissions in the southern regions of Chile do not appear to be explained by regional differences in demographic factors, socioeconomic status, rurality, education, ethnicity or physician density. The southernmost regions of Chile until very recently did not have any medical schools and do not have any specialists in allergy/immunology so the excess is not due to greater awareness or incidental diagnosis of allergy in southern Chile.
At first glance, the higher anaphylaxis admission rates in spring-summer compared to fall-winter may appear to conflict with the VD hypothesis. We believe, however, that seasonal variations that lead to VD deficiency during a developmentally critical period may increase the susceptibility to develop allergic diseases [10], but do not necessarily affect distribution of acute allergic reactions. It is likely that seasonal differences observed were influenced by higher rates of winter-related respiratory illnesses that require hospitalization, reducing hospital bed availability for other diseases. In addition, as has been observed in the United States [30], insect sting allergy increases in spring and summer elevating anaphylaxis incidence during these seasons.
Several limitations of this work must be considered, most of which are inherent to the characteristics of the variables included in the database. Most admissions were coded as unspecified anaphylaxis providing no information on specific triggers for large part of the sample. In addition, we did not have patients’ clinical information such as other diagnoses that may act as confounders (e.g. asthma) other than the sociodemographic information reported in the database. We attempted to minimize potential bias due to ICD-10 miscoding by performing additional analyses using codes that may represent miscoded anaphylaxis. Lastly, this study only included patients that were admitted to a hospital, used as proxy of the overall incidence of anaphylaxis. A better proxy might be clinic and emergency department visits due to anaphylaxis, but unfortunately this is not registered systematically throughout Chile.
In summary, our study provides further evidence for a potential role of VD deficiency in the etiology of anaphylaxis. Additional studies are needed to confirm the role of VD deficiency on the pathogenesis of allergic diseases, including cohort studies with population from different latitudes and cross-sectional population sampling at different latitudes to directly evaluate the association of VD, acute allergic reactions, and allergic sensitization. Chile provides an outstanding locale for such research.
Acknowledgments
Funding: Dr. Borzutzky was supported by FONDECYT grant 1130615. Dr. Camargo was supported by NIH U01 AI-87881.
Footnotes
Disclosures: Dr Camargo has worked as consultant for Dey/Mylan and Sanofi-Aventis. The other authors have no potential conflicts of interest to declare.
References
- 1.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Rheum Dis Clin North Am. 2012;38:125–39. doi: 10.1016/j.rdc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Camargo CA, Jr, Clark S, Kaplan MS, Lieberman P, Wood RA. Regional differences in EpiPen prescriptions in the United States: the potential role of vitamin D. J Allergy Clin Immunol. 2007;120:131–6. doi: 10.1016/j.jaci.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 3.Mullins RJ, Clark S, Camargo CA., Jr Regional variation in epinephrine autoinjector prescriptions in Australia: more evidence for the vitamin D-anaphylaxis hypothesis. Ann Allergy Asthma Immunol. 2009;103:488–95. doi: 10.1016/S1081-1206(10)60265-7. [DOI] [PubMed] [Google Scholar]
- 4.Rudders SA, Espinola JA, Camargo CA., Jr North-south differences in US emergency department visits for acute allergic reactions. Ann Allergy Asthma Immunol. 2010;104:413–6. doi: 10.1016/j.anai.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Osborne NJ, Ukoumunne OC, Wake M, Allen KJ. Prevalence of eczema and food allergy is associated with latitude in Australia. The Journal of allergy and clinical immunology. 2012;129:865–7. doi: 10.1016/j.jaci.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 6.Mullins RJ, Clark S, Camargo CA., Jr Regional variation in infant hypoallergenic formula prescriptions in Australia. Pediatr Allergy Immunol. 2010;21:e413–20. doi: 10.1111/j.1399-3038.2009.00962.x. [DOI] [PubMed] [Google Scholar]
- 7.Sheehan WJ, Graham D, Ma L, Baxi S, Phipatanakul W. Higher incidence of pediatric anaphylaxis in northern areas of the United States. J Allergy Clin Immunol. 2009;124:850–2. e2. doi: 10.1016/j.jaci.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crovetto M, Uauy R. Changes in processed food expenditure in the population of Metropolitan Santiago in the last twenty years. Rev Med Chil. 2012;140:305–12. doi: 10.4067/S0034-98872012000300004. [DOI] [PubMed] [Google Scholar]
- 9.Diaz S, Vernet M, Paladini A, Fuenzalida H, Deferrari G, Booth CR, Cabrera S, Casiccia C, Dieguez M, Lovengreen C, Pedroni J, Rosales A, Vrsalovic J. Availability of vitamin D photoconversion weighted UV radiation in southern South America. Photochem Photobiol Sci. 2011;10:1854–67. doi: 10.1039/c1pp05162h. [DOI] [PubMed] [Google Scholar]
- 10.Vassallo MF, Camargo CA., Jr Potential mechanisms for the hypothesized link between sunshine, vitamin D, and food allergy in children. The Journal of allergy and clinical immunology. 2010;126:217–22. doi: 10.1016/j.jaci.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Departamento de Estadísticas e Información de Salud. [last accessed 03/05/2013.];Egresos Hospitalarios. http://deis.minsal.cl/BDPublica/BD_Egresos.aspx.
- 12.Hocagil H, Karakilic E, Hocagil C, Senlikci H, Buyukcam F. Underdiagnosis of anaphylaxis in the emergency department: misdiagnosed or miscoded? Hong Kong medical journal = Xianggang yi xue za zhi/Hong Kong Academy of Medicine. 2013 doi: 10.12809/hkmj133895. [DOI] [PubMed] [Google Scholar]
- 13.Comisión Nacional de Energía. Irradiancia Solar en Territorios de la República de Chile. 1. Santiago, Chile: Margen Impresores; 2008. [Google Scholar]
- 14.Ministerio de Desarrollo Social. [Last accessed 04-05-2012.];Encuesta CASEN. 2009 www.ministeriodesarrollosocial.gob.cl/observatorio/casen/
- 15.Withers BT. Horner’s syndrome from food allergy: diagnosis by provocative intradermal testing. The Annals of otology, rhinology, and laryngology. 1962;71:74–8. doi: 10.1177/000348946207100105. [DOI] [PubMed] [Google Scholar]
- 16.Dean AG, Sullivan KM, Soe MM. [accessed 2013/01/22.];OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version 2.3.1. www.OpenEpi.com, updated 2011/23/06.
- 17.Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. The Journal of allergy and clinical immunology. 2009;123:434–42. doi: 10.1016/j.jaci.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 18.Decker WW, Campbell RL, Manivannan V, Luke A, St Sauver JL, Weaver A, Bellolio MF, Bergstralh EJ, Stead LG, Li JT. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008;122:1161–5. doi: 10.1016/j.jaci.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheikh A, Hippisley-Cox J, Newton J, Fenty J. Trends in national incidence, lifetime prevalence and adrenaline prescribing for anaphylaxis in England. J R Soc Med. 2008;101:139–43. doi: 10.1258/jrsm.2008.070306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez G, Jimenez M, Rosowski J, Viviani P, Montalvo D, Besancon F, Grant C, Sapunar J, Aguayo CG. Is there a geographic variability in the detection of hypovitaminosis D during winter?: National Multicenter Study in healthy elderly women. Rev Med Chil. 2004;123:1290–91. [Google Scholar]
- 21.Lee SA, Hong S, Kim HJ, Lee SH, Yum HY. Correlation between serum vitamin d level and the severity of atopic dermatitis associated with food sensitization. Allergy Asthma Immunol Res. 2013;5:207–10. doi: 10.4168/aair.2013.5.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen KJ, Koplin JJ, Ponsonby AL, Gurrin LC, Wake M, Vuillermin P, Martin P, Matheson M, Lowe A, Robinson M, Tey D, Osborne NJ, Dang T, Tina Tan HT, Thiele L, Anderson D, Czech H, Sanjeevan J, Zurzolo G, Dwyer T, Tang ML, Hill D, Dharmage SC. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. J Allergy Clin Immunol. 2013;131:1109–16. 16 e1–6. doi: 10.1016/j.jaci.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Jones AP, Palmer D, Zhang G, Prescott SL. Cord blood 25-hydroxyvitamin d3 and allergic disease during infancy. Pediatrics. 2012;130:e1128–35. doi: 10.1542/peds.2012-1172. [DOI] [PubMed] [Google Scholar]
- 24.Sharief S, Jariwala S, Kumar J, Muntner P, Melamed ML. Vitamin D levels and food and environmental allergies in the United States: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2011;127:1195–202. doi: 10.1016/j.jaci.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milliken SV, Wassall H, Lewis BJ, Logie J, Barker RN, Macdonald H, Vickers MA, Ormerod AD. Effects of ultraviolet light on human serum 25-hydroxyvitamin D and systemic immune function. J Allergy Clin Immunol. 2012;129:1554–61. doi: 10.1016/j.jaci.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Wjst M. Introduction of oral vitamin D supplementation and the rise of the allergy pandemic. Allergy Asthma Clin Immunol. 2009;5:8. doi: 10.1186/1710-1492-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothers J, Wright AL, Stern DA, Halonen M, Camargo CA., Jr Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J Allergy Clin Immunol. 2011;128:1093–9. e1–5. doi: 10.1016/j.jaci.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norizoe C, Akiyama N, Segawa T, Tachimoto H, Mezawa H, Ida H, Urashima M. Increased Food Allergy with Vitamin D: A Randomized, Double-blind, Placebo-controlled Trial. Pediatrics international : official journal of the Japan Pediatric Society. 2013 doi: 10.1111/ped.12207. [DOI] [PubMed] [Google Scholar]
- 29.Weisse K, Winkler S, Hirche F, Herberth G, Hinz D, Bauer M, Roder S, Rolle-Kampczyk U, von Bergen M, Olek S, Sack U, Richter T, Diez U, Borte M, Stangl GI, Lehmann I. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy. 2013;68:220–8. doi: 10.1111/all.12081. [DOI] [PubMed] [Google Scholar]
- 30.Clark S, Long AA, Gaeta TJ, Camargo CA., Jr Multicenter study of emergency department visits for insect sting allergies. J Allergy Clin Immunol. 2005;116:643–9. doi: 10.1016/j.jaci.2005.06.026. [DOI] [PubMed] [Google Scholar]