Abstract
Testosterone (T) impacts LH secretion through negative feedback via the androgen receptor (AR) in the hypothalamo-pituitary system. An untested postulate is that increasing body mass index (BMI), abdominal visceral fat (AVF) or total abdominal fat (TAF) with aging decreases LH secretion by heightening T negative feedback via AR. This hypothesis was tested in a prospective, randomized double-blind cross-over study of 19 healthy men comparing the effects of flutamide, a selective nonsteroidal AR antagonist, and placebo administration on basal and pulsatile LH secretion as a function of age and obesity measures. To this end, serum levels of 2-hydroxyflutamide (2-OHF), a major active flutamide metabolite, were measured by mass spectrometry, and AVF/TAF quantified by abdominal computerized tomography. Statistical analysis showed that antiandrogen administration elevated 6-hr mean LH concentrations to 5.4 ± 1.3 IU/L compared with 3.3 ± 1.2 IU/L for placebo (P<10−3), and total T by 35% (P<10−4). The LH-T concentration product doubled (P<10−8). According to deconvolution analysis, flutamide exposure increased total LH secretion (P<10−3) and pulsatile LH secretion (P=0.0077), along with LH pulse frequency (P=0.019). Despite feedback inhibition, the LH-T product declined as a linear function of AVF (P=0.021) and TAF (P=0.017). This was explained by the fact that higher BMI was associated with lower 2-OHF concentrations (R=-0.562, P=0.012). In contrast, age was associated with less pulsatile LH secretion (R=-0.567, P=0.011) even when LH responses were normalized to antiantrogen levels. In conclusion, increased AVF, TAF and BMI predict decreased LH and flutamide blood levels, whereas older age is marked by impaired stimulation of pulsatile LH secretion even when normalized for antiandrogen levels, suggesting different mechanisms of regulation by adiposity and age.
Keywords: aging, human, testosterone, gonadotropin, inhibition, flutamide
Introduction
Testosterone (T) is the primary androgen responsible for anabolism in men ‘Kelly & Jones (2013)’. Investigators have recognized that T concentrations decline with age, along with changes in LH and T pulse frequency, amplitude and regularity ‘Giagulli et al. (1994)’. Other potential factors also decrease T production, including medications, acute illness, chronic disease and possibly obesity ‘Isidori & Lenzi (2005)’, ‘Yeap (2009)’. The exact mechanisms mediating hypoandrogenemia in aging and obesity are currently unknown ‘Lapauw et al. (2008)’, ‘Veldhuis et al. (1992)’, ‘Vermeulen (1996)’. T secretion is dependent upon intact GnRH neurons, gonadotropes and Leydig cells, and also an intact feedback system, whereby elevated T concentrations reduce LH secretion, and vice versa ‘Urban et al. (1988b)’. Thus, one potential mechanism for hypoandrogenemia in aging and/or obesity would be heightened T feedback via androgen receptors (AR) within the hypothalamo-pituitary system. In earlier tests of the aging hypothesis, T feedback was muted by inhibiting T secretion or T action ‘Liu et al. (2006)’, or T feedback was augmented by exogenous T or DHT administration ‘Sahlin et al. (1994)’, ‘Winters & Wang (2010)’. However, none of these studies of T feedback in aging individuals evaluated possible confounding by adiposity, and none normalized feedback to blood drug levels.
Flutamide is a brain-permeant selective antiandrogen, whose 2-hydroxy metabolite (2-OHF) binds to the androgen receptor (AR) with high affinity ‘Goldspiel & Kohler (1990)’. This feedback probe stimulates pulsatile LH secretion primarily by accelerating hypothalamic GnRH/LH pulse frequency ‘Urban et al. (1988a)’. The present study utilizes flutamide to unmask AR-mediated feedback inhibition, mass spectrometry to measure 2-hyproxyflutamide blood levels, and men of various BMI’s, abdominal visceral fat estimates and ages to test the hypothesis that the effect of age on feedback disinhibition of pulsatile LH secretion is explained by BMI and abdominal adiposity.
Methods
Study design
The design was prospective, double-blind and randomized with intrasubject cross-over, as described in an initial study ‘Veldhuis et al. (2010b)’. However, the present study recruited a separate group of 19 healthy aging men so as to incorporate a wide range of BMI and obtain AVF and TAF estimates by computerized tomography (CT). Each participant gave written informed consent approved by the Mayo Institutional Review Board. The FDA also reviewed the protocol for off-label use of flutamide. A data and safety monitoring board at Mayo provided oversight.
Setting
The study was conducted at the Mayo Clinic Center for Translational Science Activities (CTSA) in Rochester, MN.
Inclusion and exclusion criteria
All 19 subjects were community dwelling, ambulatory and healthy 20 to 74 yr-old men with normal 0800 hr serum prolactin < 20 μg/L, LH < 20 IU/L, FSH < 30 IU/L, and total testosterone > 300 ng/dL at baseline. None had received psychiatric or neuroactive medications, anabolic steroids or glucocorticoids for at least 3 mo. Other exclusions comprised acute or chronic systemic illness, including diabetes mellitus; inflammatory disease; drug or alcohol abuse; hemoglobin < 12.0 g/dL; untreated hypothyroidism; cardiopulmonary, hepatic, renal or hematological disease; cancer; and/or unwillingness to provide informed consent. Screening physical examination and biochemical screening were also normal, including endocrine (cortisol, IGF-I, TSH), metabolic (electrolytes, calcium), hepatic, renal and hematological testing.
Clinical protocol
Eligible consenting volunteers underwent 2 separate prospectively randomly ordered studies in the CRU. Each participant received 4 days of oral placebo or flutamide (250 mg) 3 times daily, with CRU sampling on the fourth morning. A forearm i.v. catheter was placed at 0645 hr to allow blood sampling every 10 min from 0800 until 1600 (8 hr) to monitor LH and T. After 6 hr of baseline sampling, participants received a single submaximal i.v. dose of GnRH (100 ng/kg) at 1400 hr, followed by 2 more hr of blood sampling. Breakfast and lunch were provided. There was a minimum washout period of 1 mo (maximum 3.5 mo) between study visits.
Drug assay
The main bioactive metabolite of flutamide, 2-OHF was assayed by liquid chromatography-tandem mass spectrometry, exactly as described earlier ‘Veldhuis et al. (2010b)’. Sensitivity was 0.1 μg/mL (0.342 μmol/L) and the interassay variability was 10.3%. The drug assay was performed on a single pool of serum for each visit, comprising 0.05 mL aliquots of each of the 49 samples over the 8 hr, thereby providing a mean estimate.
Hormone assays
LH concentrations were measured in duplicate using the DxI automated immunoenzymatic assay system (Beckman Instruments, Chaska, MN). Intraassay coefficients of variation (CV) were 4.3 and 4.0% at 1.2 and 38.5 IU/L, respectively. Interassay CVs were 9.3, 6.0, and 4.2% at 1.4, 15.6, and 48.8 IU/L, respectively. The procedural sensitivity was 0.2 IU/L and the upper analytic limit 250 IU/L, based upon World Health Organization Second International Reference Preparation 30/552. T concentrations were also measured on the DxI automated immunoenzymatic assay system. Intraassay CVs were 6.5% at 69 ng/dL and 3.3% at 862 ng/dL (convert ng/dL to nmol/L by multiplying by 0.0347). Interassay CVs were 8.6% at 407 ng/dl, 4.0% at 761 ng/dL and 7.4% at 1,116 ng/dl. The analytic range was 54-1650 ng/dL. The coefficient of determination was R2=0.98 between the immunoassay and liquid chromatography-tandem mass spectrometer (LC-MS/MS: ThermoFisher Scientific, Franklin MA, and Applied Biosystems-MDS Sciex, Foster City, CA). LC-MS/MS was used to verify T estimates on the 0800 hr samples ‘Singh (2008)’. The LC-MS/MS intraassay CVs were 3.3, 2.8, 2.2 and 2.0% at 16, 64, 184 and 927 ng/dL, respectively, and interassay CVs 5.1, 3.8, 3.7, and 2.8% at 17, 65, 177 and 919 ng/dL respectively (analytic range 7-2,000 ng/dL). Estradiol (E2) was also assayed at 0800 hr by LC-MS/MS with sensitivity of 3.5 pg/mL and interassay CV 4.5% (to convert E2 concentrations to pmol/L from pg/mL multiply by 3.69) ‘Nelson et al. (2004)’.
Sex-hormone-binding globulin (SHBG) was measured in each subject’s pooled serum on an Immulite 2000 Siemans (Diagnostic Products, Los Angeles, CA). Interassay and intraassay CVs were 4.5-6.0% at SHBG concentrations ranging from 5.4 to 96 nmol/L. Albumin was quantified on the Roche/Hitachi 912 (Roche Diagnostics, Basal, Switzerland). Inter- and intraassay CVs were 0.8-2.0% at albumin concentrations from 2.5 and 4.6 g/dL. Free and bioavailable T concentrations were calculated from SHBG, albumin and total T concentrations, as described in the appendix of ‘Takahashi et al. (2007)’.
Abdominal visceral (AVF) and total abdominal fat (TAF)
AVF and TAF were estimated by computerized tomography (CT) at the L3-L4 interspace, as reported ‘Veldhuis et al. (2005)’.
Deconvolution analysis
The 8-hr LH time series were analyzed using a validated deconvolution algorithm with sensitivity and specificity both ≥ 93%. The Matlab (The MathWorks, Natick, MA) program detrends the data and normalizes concentrations to the unit interval, while compiling all possible pulse-time sets via a boundary-detection algorithm ‘Liu et al. (2009)’. This is a maximum-likelihood estimation method. Half-lives of LH were represented as 18 min for rapid decay and 90 min for slow decay ‘Veldhuis et al. (1986)’. Half-lives of T were 3.5 min for rapid decay and 28 min for slow decay ‘Veldhuis et al. (2010a)’. The parameters of LH and T secretion were basal (non-pulsatile), pulsatile and total (sum of basal and pulsatile) secretion (concentration units/session), mass secreted per burst (concentration units) and waveform mode (time delay to maximal secretion).
LH-T concentration product
Since flutamide elevates both LH and T, the product of mean LH and T concentrations provides a simple (model-free) outcome.
Approximate entropy (ApEn)
Approximate entropy (ApEn) was used as a model-independent measure of the regularity of hormone release, as previously described. Sensitivity and specificity both exceed 90% for quantifying greater pattern irregularity due to lesser feedback on hormone secretion ‘Pincus et al. (1999)’.
Statistical comparisons
The primary statistic for placebo-flutamide comparisons was a paired two-tailed Student’s t-test. Linear regressions was applied to evaluate possible correlations between LH and T changes and subject characteristics. Data are given as mean ± SD or median and range.
Results
Subjects
All 19 men completed the study without adverse drug reactions. The median age was 51 yr (range 20-74 yr) and BMI 27 kg/m2 (range 20-38 kg/m2). Other characteristics are noted in Table 1.
Table 1. Epidemiological Characteristics of Subjects.
| Parameter |
Mean ± SD |
Median |
Range |
|---|---|---|---|
| Age (yr) | 52 ± 14 | 51 | 20-74 |
| Height (cm) | 176 ± 5.0 | 177 | 168-185 |
| Weight (kg) | 87 ± 13 | 91 | 62-113 |
| BMI (kg/m2) | 28 ± 4.7 | 27 | 20-38 |
| AVF (cm2) | 168 ± 103 | 165 | 10-440 |
| TAF (cm2) | 356 ± 205 | 356 | 42-671 |
| Total T (ng/dL) | 475 ± 128 | 462 | 278-821 |
| Free T (ng/dL) | 12 ± 1.1 | 11 | 5.5-23 |
| E2 (pg/mL) | 23 ± 7.7 | 21 | 12-37 |
| Prolactin (μg/L) | 7.6 ± 3.5 | 6.0 | 4-16 |
| TSH (mU/L) | 2.1 ± 0.84 | 1.9 | 1.1-4.3 |
| SHBG (nM) | 29 ± 14 | 26 | 15-63 |
| Albumin (g/dL) | 4.4 ± 0.16 | 4.5 | 4.1-4.7 |
| FSH (IU/L) | 5.7 ± 1.6 | 5.5 | 1.9-8.0 |
| LH (IU/L) | 3.5 ± 1.4 | 3.2 | 1.6-7.1 |
Baseline characteristics of 19 healthy men prior to initiation of study visits. Abbreviations; bmi, body mass index; AVF, abdominal visceral fat; TAF, total abdominal fat; T, testosterone; E2, estradiol; TSH, thyroid stimulating hormone; SHBG, sex-hormone binding globulin; FSH, follicle stimulating hormone; LH luteinizing hormone. Multiply T by 0.347 and E2 by 3.69 to convert to matching international units (nmol/L and pmol/L, respectively). Data are mean ± SD, median and range.
Mean LH, T and drug concentrations
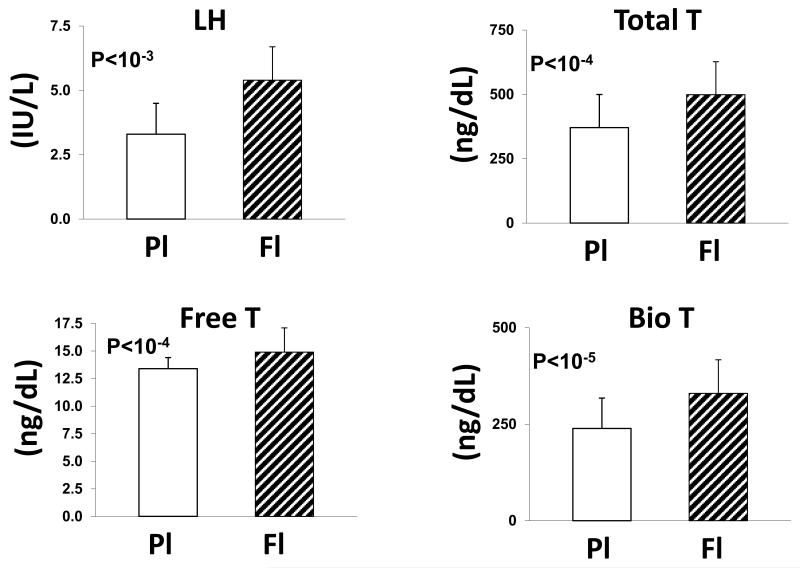
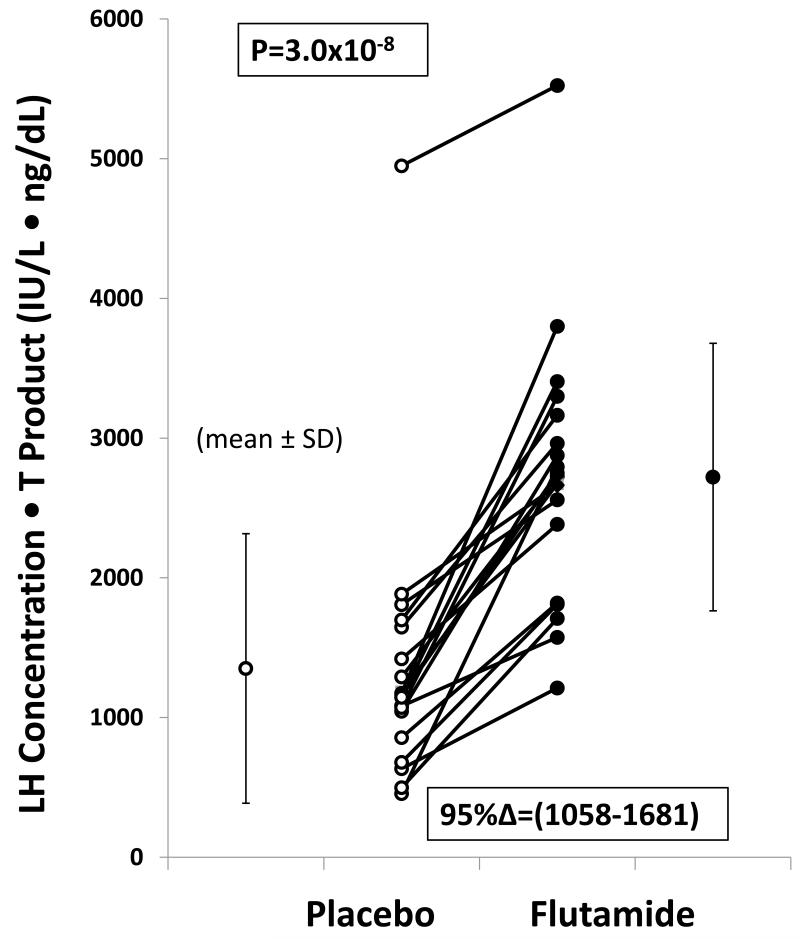
Mean concentrations of LH, total T, bioavailable T and free T were significantly higher after flutamide than placebo administration (Fig. 1). LH concentrations were 5.4±1.3 IU/L after flutamide vs 3.3±1.2 IU/L after placebo (P<10−3). Total T concentrations were 499±128 ng/dL (flutamide) compared with 371±129 ng/dL (placebo) [P<10−4]. Free and bioavailable T concentrations also rose, viz: to 14.9±2.2 from 13.4±1.1 ng/dL for free T (P<10−4); and to 330±128 from 239±79 ng/dL for bioavailable T, P<10−5). E2 rose to 26 ± 4.3 from 18 ± 3.1 pg/mL (P<0.01) (Fig. 2). In relation to the LH and T concentration product, flutamide exposure induced a marked incremental change, viz: (95% C.I. range) 1058-1681 IU/L*ng/dL, (P=3.0x10−8) (Fig. 3). Two-hydroxyflutamide levels averaged 0.99 ± 0.52 (SD) μg/mL (median 0.99, absolute range 0.44-2.23 μg/mL). (To convert to μmol/L multiply by 3.42).
Figure 1. LH and Testosterone Responses to Flutamide in Men.
Comparisons of gonadal-axis responses to placebo and flutamide administration in 19 healthy men. Data are the mean (± SD) LH, total T, free T and bioavailable T concentrations. Abbreviations: PL, placebo; Fl, flutamide; LH luteinizing hormone; T, testosterone.
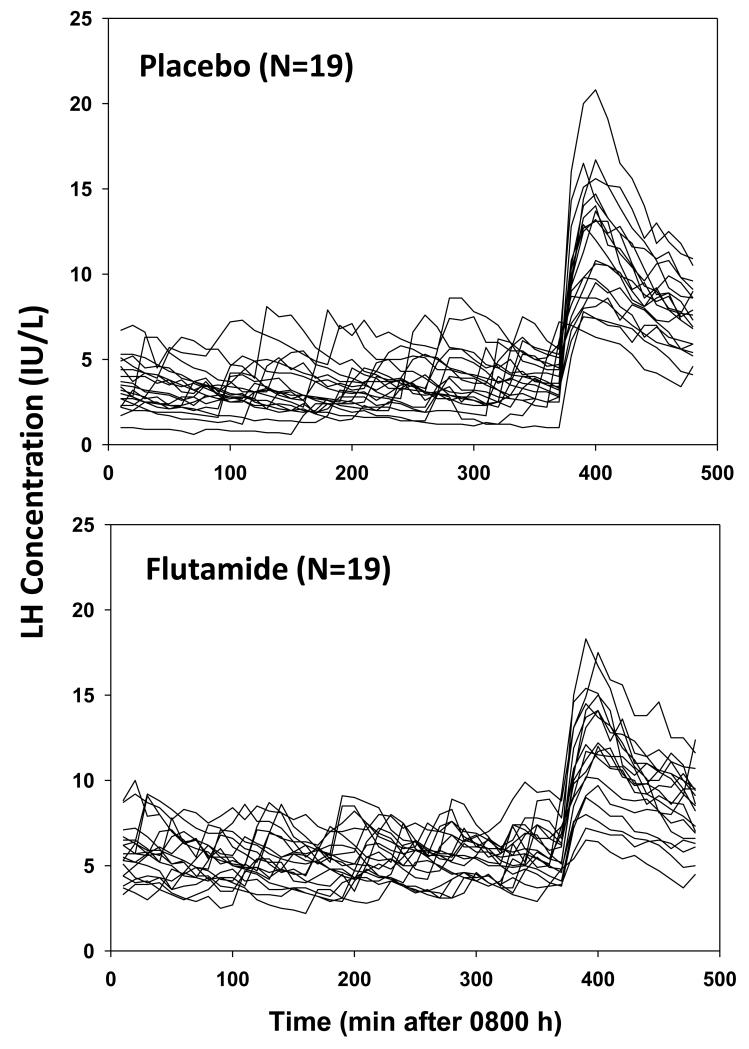
Figure 2. LH Concentration vs Time Course.
LH concentration time series sampled every 10 min for 8 hr in 19 men given placebo (top) or flutamide (bottom) for 4 days. The first 6 hr (360 min) of 10-min sampling was performed before, and the last 2 after, bolus i.v. GnRH (100 ng/kg) injection.
Figure 3. Feedback Disinhibition by Flutamide.
Individual LH-T concentration product after placebo (left) and flutamide (right) exposure in 19 healthy men. Ninety-five percent confidence intervals (C.I.) for this incremental treatment effect are noted. P value is estimated by 2-tailed paired Student’s t-test.
Deconvolution analysis
Deconvolution analysis revealed that flutamide exposure increased total LH secretion (P<10−3) by elevating both basal LH secretion (P<10−3) and pulsatile LH secretion (P=0.0077). The number of pulses increased to 3.4±1.2 with flutamide from 2.4±1.1 with placebo (P=0.019). Burst mode and mass/burst did not change: Table 2.
Table 2. Deconvolution Analysis of LH Time Series.
| Placebo | Flutamide | P value | |
|---|---|---|---|
|
|
|
|
|
| No. pulses (per 6 hr) | 2.4 ± 1.1 [2.0] | 3.4 ± 1.2 [4.0] | 0.019 |
| Burst mode (min) | 15 ± 6.0 [14] | 14 ± 5.6 [13] | 0.43 |
| Basal secretion* | 7.2 ± 3.1 [7.0] | 13 ± 5.7 [13] | <10−3 |
| Pulsatile secretion* | 7.3 ± 3.1 [6.1] | 10 ± 5.0 [9.3] | 0.0077 |
| Total secretion* | 15 ± 5.2 [14] | 24 ± 6.1 [23] | <10−3 |
| Mass/burst (IU/L) | 3.4 ± 1.4 [3.8] | 3.4 ± 1.6 [3.1] | 0.98 |
N=19 subjects.
Data are the mean±SD [median]. P values are paired two-tailed Student’s t-test results.
units are IU/L/6 hr.
Boldface are p<0.05.
ApEn
LH ApEn, a sensitive measure of feedback change, increased significantly with flutamide administration (P<10−4), thus quantifying greater irregularity, viz., less pattern orderliness: Table 3. T ApEn did not differ between flutamide and placebo.
Table 3. Orderliness (ApEn) of LH and T Secretion.
| Placebo |
Flutamide |
*P value |
|
|---|---|---|---|
| ApEn LH | 0.6859 ± 0.16 (0.6477) | 0.9482 ± 0.11 (0.9646) | P<10−4 |
| ApEn T | 1.0952 ± 0.096 (1.0927) | 1.0900 ± 0.082 (1.0927) | 0.83 |
paired Student’s t-test (N=19).
Data are mean ± SD (median).
GnRH-stimulated LH release
Intravenous injection of GnRH elevated LH concentrations after both placebo and flutamide, with no difference in mean (P=0.17) or peak (P=0.63) LH concentrations: Table 4. There were also no differences in GnRH-stimulated LH mass/burst, burst mode, or basal LH secretion between treatments.
Table 4. GnRH-Stimulated LH Release.
| Placebo |
Flutamide |
P value* |
|
|---|---|---|---|
| Mean** | 8.4 ± 2.2 [8.8] | 8.8 ± 2.3 [9.5] | 0.17 |
| Peak** | 11.7 ± 3.7 [13] | 11.5 ± 3.4 [12] | 0.63 |
| Mass/burst** | 16 ± 5.0 [16] | 16 ± 6.6 [14] | 0.84 |
| Mode (min) | 9.8 ± 5.2 [9.0] | 8.7 ± 3.4 [8.1] | 0.42 |
| Basal sec*** | 13.8 ± 9.9 [12] | 14.1 ± 9.7 [13] | 0.91 |
Data are mean ± SD [median] (N=19).
paired t test
IU/L
IU/L/2 hr
Linear regression
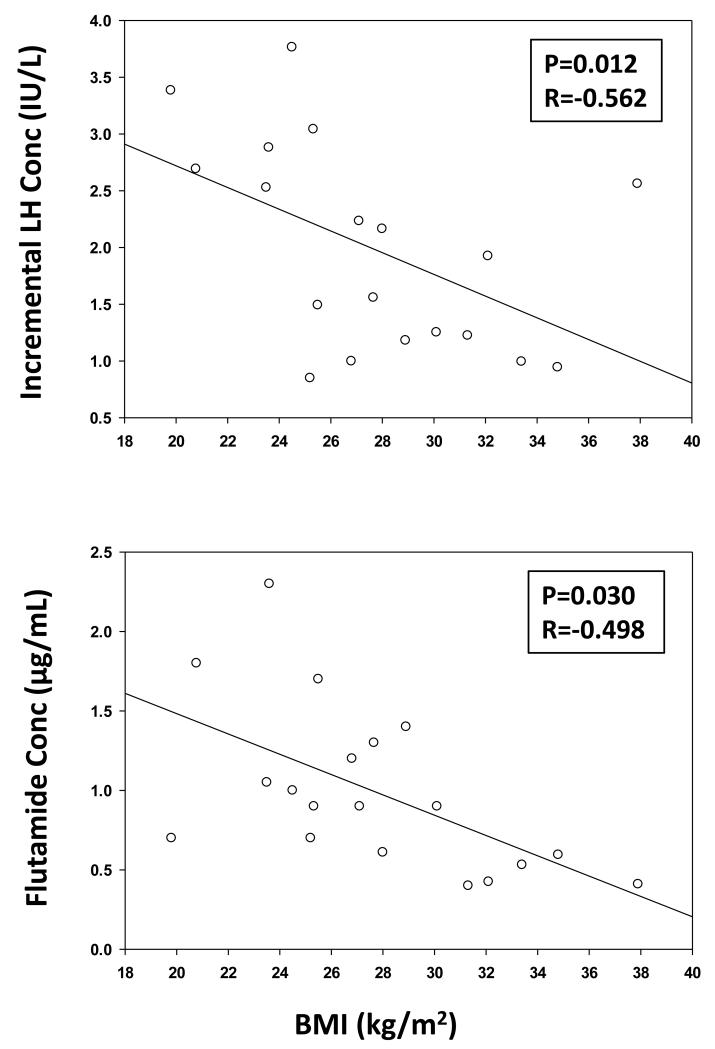
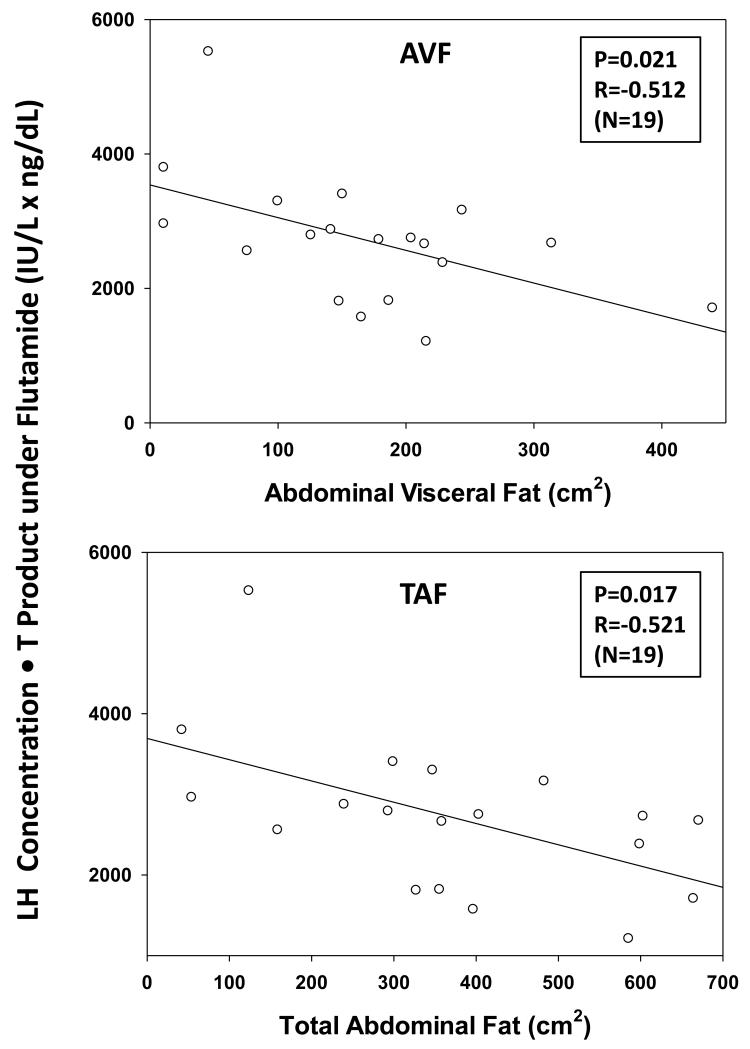
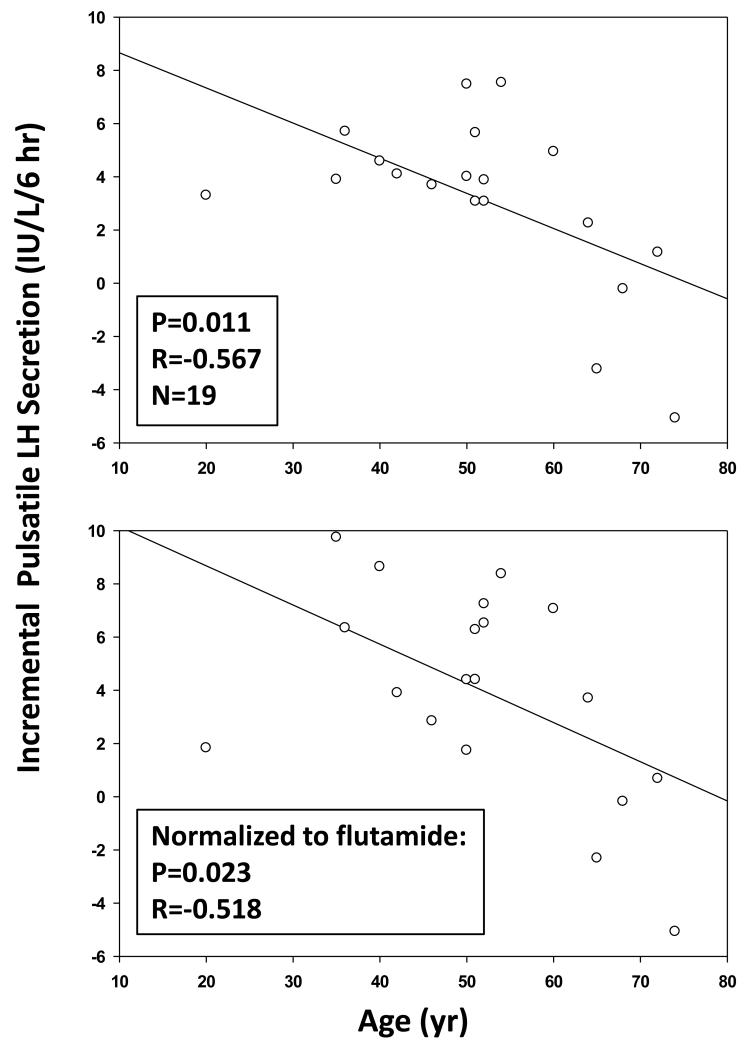
There was a negative linear correlation between the LH-concentration increment induced by flutamide administration and BMI, viz., −0.096 slope, R=−0.498 and P=0.030 (Fig. 4 (top)). Flutamide concentration was also negatively correlated with BMI viz: slope −0.064, R=−0.562, and P=0.012 (Fig. 4 (bottom)). Weight (kg) behaved similarly to BMI (P=0.0124). Basal LH secretion decreased with increasing BMI (P=0.026), whereas pulsatile LH secretion decreased with increasing age (P=0.034). Moreover, the LH-T concentration product during flutamide exposure varied inversely with AVF and TAF (Fig. 5). There was a similar directional trend (P=0.073) in the case of BMI. However, age did not influence serum flutamide levels (P=0.91). When incremental pulsatile LH secretion was normalized against (divided by) the 2-OHF concentration, the resulting correlation coefficients were not significant for BMI (P=0.779), AVF (P=0.581) or TAF (P=0.735), but remained significant for age (R=−0.518, P=0.023) (Fig. 6).
Figure 4. BMI Negatively Determines LH Response to Flutamide and Reduces Flutamide Drug Levels.
Top: Negative impact of BMI on incremental LH concentration (the differences between 6-hr mean LH values in the flutamide vs placebo visit). Bottom: Increasing body mass index reduces 2-hydroxyflutamide concentrations. Multiply the latter by 3.42 to obtain μmol/L. Pearson’s P and R values are noted.
Figure 5. Flutamide Disinhibition is Reduced by AVF and TAF.
Arithmetic mean (± SD) LH-T concentration product calculated after flutamide administration in 19 men. The product of LH and T concentrations decreased with increasing abdominal visceral fat (AVF, top) and total abdominal fat (TAF, bottom).
Figure 6. Negative Effect of Age on Incremental Pulsatile LH Secretion in Men.
Incremental stimulation of pulsatile LH secretion varies negatively with age, whether LH responses are (bottom panel) or are not (top panel) normalized to blood flutamide concentrations. Pearson’s Ph and R values are noted.
Discussion
In this prospective double-blind study of 19 healthy men selected for wide ranges of both BMI (20-38 kg/m2) and age (20-74 yr), flutamide compared with placebo administration elevated mean LH concentrations by 64%, total T concentrations by 35%, bioavailable T by 38%, free T by 44%, E2 by 44% and the LH-T product by 201%. As predicted by the main hypothesis, AVF (P=0.021) and TAF (P=0.017) were negative correlates of the incremental LH-T concentration product, a surrogate of the gonadal-axis response to AR-feedback disinhibition. Regression analysis revealed that serum 2-hydroxyflutamide (2-OHF) concentrations (the primary active flutamide metabolite) decreased with increasing weight (P=0.0124) and BMI (P=0.012), but not with age. The mechanisms behind these findings are unclear; however, we speculate that there is an increase in the volume of distribution and/or more rapid clearance. Importantly, after adjusting for 2-OHF drug levels, there was no correlation between the stimulated LH-T concentration product and any of BMI, AVF or TAF. Accordingly, the reduction in flutamide blood levels with increasing BMI could explain lower AR-mediated feedback disinhibition of LH-T concentrations with BMI. In contrast, age with or without adjustment for antiandrogen drug levels was associated with attenuated (incremental) pulsatile LH secretion. An early study with flutamide and bicalutamide found similar effects of age on incremental LH secretion ‘Veldhuis et al. (2010b)’. However, body composition data were not available. Altogether, age but not obesity is a viable marker of altered (decreased) AR-mediated feedback responses in men.
Flutamide is a highly selective nonsteroidal AR antagonist, approved by the US FDA for use in patients with prostate cancer ‘Mahler et al. (1998)’, ‘Schulz et al. (1988)’. Flutamide does not have significant intrinsic androgenicity. In the rat, 2-OHF’s affinity for the AR is ~ 55 nmol/L ‘Belanger et al. (1988)’, ‘Kolvenbag et al. (1998)’, ‘Simard et al. (1986)’. In the human prostate, one-half maximally inhibitory concentrations of 2-OHF are 0.6 to 2.7 nmol/L ‘Simard et al. (1986)’. Thus, the 1000-fold higher serum concentrations of 2-OHF attained here (mean 3400 nmol/L=3.4 μmol/L) would predict at least 90% inhibition of AR.
The mechanistic effects of flutamide upon LH secretion corroborate previous inferences of enhanced basal (nonpulsatile) LH secretion and LH secretory-burst frequency (and thereby pulsatile LH secretion) ‘Veldhuis et al. (2010b)’. As before, LH concentrations after administration of GnRH were unaffected by flutamide treatment. The collective outcomes support the notion that AR (or unconfirmed unrelated effects of flutamide) mediate suppression of LH pulse frequency and interpulse basal LH release. Concomitantly, age and BMI limited the flutamide-induced increase in LH secretion. Notably, the negative effect of age, but not that of BMI, remained significant when stimulated LH secretion was normalized for 2-OHF concentrations (P=0.023, R=−0.518). Thus, the present investigation introduces two mechanisms for decreased LH stimulation under low effective T (androgen) feedback. One mechanism is 2-OHF drug-level dependent (AVF, TAF, BMI) and the other is 2-OHF drug-level independent (age).
Prolonged androgen deprivation in the treatment of prostate cancer results in increased insulin and triglyceride concentration, BMI, AVF and possibly blood pressure ‘Basaria et al. (2006)’, ‘Braga-Basaria et al. (2006)’. A similar pathophysiology occurs in AR-knockdown mice ‘Yanase et al. (2008)’. Since increased BMI was associated with decreased 2-OHF concentrations in the present study, the long-term changes in body composition during extended flutamide therapy might also diminish flutamide bioavailability. This hypothesis remains to be tested longitudinally. However, our findings have clinical implications for treatment of obese patients with prostate cancer or polycystic ovarian disease, for whom flutamide dosing is not currently targeted to drug levels.
The inference of decreased AR-mediated negative feedback in older men could in principle reflect reduced androgen delivery to hypothalamo-pituitary unit, lower AR expression in androgen feedback sites, and/or more rapid T clearance. The issue is important, in that in experimental models T mediates neuronal survival ‘Hammond et al. (2001)’. Decreased sex-steroid receptors, including AR, are described in aging brain, pituitary and other T targets ‘Haji et al. (1980)’, ‘Ishunina et al. (2002)’, ‘Rajfer et al. (1980)’, ‘Shain & Axelrod (1973)’, ‘Tohgi et al. (1995)’. In contrast, more rapid systemic T clearance does not seem to be the case in older men, in whom higher SHBG levels delay T’s elimination from blood ‘Vermeulen et al. (1982)’. Thus, if our outcome of reduced feedback escape (lesser disinhibition) of LH secretion in aging men is verified, the most plausible explanation may be net neuronal loss of AR at central nervous-system feedback sites. This consideration seems more likely than AR changes in the pituitary gland, since exogenous GnRH stimulation of LH release was unrelated to age or flutamide exposure in the cohort of men studied here. The GnRH data also suggest that E2 feedback at the pituitary level was not greatly altered by flutamide, inasmuch as E2 feedback would be expected to blunt GnRH action ‘Urban et al. (1988b)’. Moreover, E2 feedback itself does not seem to change with age ‘Ten Kulve et al. (2010)’.
This study has potential weaknesses. The sample size of 19 individuals is relatively small, requiring confirmation in larger cohorts. Even so, each subject was studied by frequent blood sampling, CT estimates of abdominal fat, and mass spectrometry quantification of AR-antagonist levels. With respect to volunteer adherence to three times daily oral administration of flutamide, serum 2-OHF concentrations were consistently within the expected therapeutic range ‘Anon. (2011b)’, ‘Simard et al. (1986)’. Since the study population of Olmsted County, Minnesota is primarily Caucasian ‘Anon. (2011a)’, further investigations would be needed to ascertain whether there are ethnic or racial differences in androgen negative feedback.
In summary, increased BMI, AVF and TAF are associated with decreased flutamide-stimulated LH and T secretion in proportion to their reduction in blood 2-OHF levels. In contrast, the association of age with attenuated AR-mediated feedback escape is independent of 2-OHF concentrations, indicating distinct mechanisms of obesity- and age-related alterations in T-LH feedback regulation.
Acknowledgments
We thank Jill Smith for support of manuscript preparation; Sandra Cabral for assisting in data analysis; the Mayo Immunochemical Laboratory for performing assays; and the Mayo research nursing staff for implementing the protocol. Supported in part via R01 AG23133 and P30 DK050456 (Metabolic Studies Core of the Minnesota Obesity Center) from the National Institutes of Health (Bethesda, MD). Matlab versions of ApEn and deconvolution methodology are available from Veldhuis.johannes@mayo.edu. Contents are solely the responsibility of the authors and do not necessarily represent the official views of any federal institution.
Funding
Supported in part via R01 AG23133 and P30 DK050456 (Metabolic Studies Core of the Minnesota Obesity Center) from the National Institutes of Health (Bethesda, MD).
Footnotes
Disclosures
The authors have nothing to disclose.
Author Contributions
Paul Y. Takahashi, M.D. performed the experiments, drafted the manuscript, edited and revised the manuscript, and approved the final copy of the manuscript.
Peter Y. Liu, M.D., Ph.D. performed the experiments, edited and revised the manuscript, and approved the final copy of the manuscript.
Johannes D. Veldhuis, M.D. contributed to the conception and design of the research, analyzed the data, prepared the figures, edited and revised the manuscript, and approved the final copy of the manuscript.
Reference List
- 1.Census Information. Olmsted County, MN: 2011a. 2010. http://quickfacts.census.gov/qfd/states/27/27109.html. [Google Scholar]
- 2.Product Monograph Euflex (flutamide 250 mg tablets) Merck (Schering-plough); Quebec: 2011b. [Google Scholar]
- 3.Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581–588. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 4.Belanger A, Giasson M, Couture J, Dupont A, Cusan L, Labrie F. Plasma levels of hydroxy-flutamide in patients with prostatic cancer receiving the combined hormonal therapy: an LHRH agonist and flutamide. Prostate. 1988;12:79–84. doi: 10.1002/pros.2990120110. [DOI] [PubMed] [Google Scholar]
- 5.Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, Basaria S. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 6.Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab. 1994;79:997–1000. doi: 10.1210/jcem.79.4.7962311. [DOI] [PubMed] [Google Scholar]
- 7.Goldspiel BR, Kohler DR. Flutamide: an antiandrogen for advanced prostate cancer. Drug Interaction and Clinical Pharmacy. 1990;24:616–623. doi: 10.1177/106002809002400612. [DOI] [PubMed] [Google Scholar]
- 8.Haji M, Kato KI, Nawata H, Ibayashi H. Age-related changes in the concentrations of cytosol receptors for sex steroid hormones in the hypothalamus and pituitary gland of the rat. Brain Res. 1980;204:373–386. doi: 10.1016/0006-8993(81)90596-5. [DOI] [PubMed] [Google Scholar]
- 9.Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 10.Ishunina TA, Fisser B, Swaab DF. Sex differences in androgen receptor immunoreactivity in basal forebrain nuclei of elderly and Alzheimer patients. Exp Neurol. 2002;176:122–132. doi: 10.1006/exnr.2002.7907. [DOI] [PubMed] [Google Scholar]
- 11.Isidori AM, Lenzi A. Risk factors for androgen decline in older males: lifestyle, chronic diseases and drugs. J Endocrinol Invest. 2005;28:14–22. [PubMed] [Google Scholar]
- 12.Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217:R25–R45. doi: 10.1530/JOE-12-0455. [DOI] [PubMed] [Google Scholar]
- 13.Kolvenbag GJ, Furr BJ, Blackledge GR. Receptor affinity and potency of non-steroidal antiandrogens: translation of preclinical findings into clinical activity. Prostate Cancer Prostatic Dis. 1998;1:307–314. doi: 10.1038/sj.pcan.4500262. [DOI] [PubMed] [Google Scholar]
- 14.Lapauw B, Goemaere S, Zmierczak HG, Van Pottelbergh I, Mahmoud A, Taes Y, De Bacquer D, Vansteelandt S, Kaufman JM. The decline of serum testosterone levels in community-dwelling men over 70 years of age: descriptive data and predictors of longitudinal changes. Eur J Endocrinol. 2008;159:459–468. doi: 10.1530/EJE-07-0873. [DOI] [PubMed] [Google Scholar]
- 15.Liu PY, Keenan DM, Kok P, Padmanabhan V, O’Byrne KT, Veldhuis JD. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endo Metab. 2009;297:E538–E544. doi: 10.1152/ajpendo.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu PY, Takahashi PY, Roebuck PD, Veldhuis JD. Age or factors associated with aging attenuate testosterone’s concentration-dependent enhancement of the regularity of luteinizing hormone secretion in healthy men. J Clin Endocrinol Metab. 2006;91:4077–4084. doi: 10.1210/jc.2005-2811. [DOI] [PubMed] [Google Scholar]
- 17.Mahler C, Verhelst J, Denis L. Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer. Clin Pharmacokinet. 1998;34:405–417. doi: 10.2165/00003088-199834050-00005. [DOI] [PubMed] [Google Scholar]
- 18.Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 19.Pincus SM, Hartman ML, Roelfsema F, Thorner MO, Veldhuis JD. Hormone pulsatility discrimination via coarse and short time sampling. Am J Physiol. 1999;277:E948–E957. doi: 10.1152/ajpendo.1999.277.5.E948. [DOI] [PubMed] [Google Scholar]
- 20.Rajfer J, Namkung PC, Petra PH. Identification, partial characterization and age-related changes of a cytoplasmic androgen receptor in the rat penis. J Steroid Biochem. 1980;13:1489–1492. doi: 10.1016/0022-4731(80)90064-3. [DOI] [PubMed] [Google Scholar]
- 21.Sahlin L, Norstedt G, Eriksson H. Androgen regulation of the insulin-like growth factor-I and the estrogen receptor in rat uterus and liver. J Steroid Biochem Mol Biol. 1994;51:57–66. doi: 10.1016/0960-0760(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 22.Schulz M, Schmoldt A, Donn F, Becker H. The pharmacokinetics of flutamide and its major metabolites after a single oral dose and during chronic treatment. Eur J Clin Pharmacol. 1988;34:633–636. doi: 10.1007/BF00615229. [DOI] [PubMed] [Google Scholar]
- 23.Shain SA, Axelrod LR. Reduced high affinity 5 alpha-dihydrotestosterone receptor capacity in the ventral prostate of the aging rat. Steroids. 1973;21:801–812. doi: 10.1016/0039-128x(73)90122-0. [DOI] [PubMed] [Google Scholar]
- 24.Simard J, Luthy I, Guay J, Belanger A, Labrie F. Characteristics of interaction of the antiandrogen flutamide with the androgen receptor in various target tissues. Mol Cell Endocrinol. 1986;44:261–270. doi: 10.1016/0303-7207(86)90132-2. [DOI] [PubMed] [Google Scholar]
- 25.Singh RJ. Validation of a high throughput method for serum/plasma testosterone using liquid chromatography tandem mass spectrometry (LC-MS/MS) Steroids. 2008;73:1339–1344. doi: 10.1016/j.steroids.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi PY, Votruba P, Abu-Rub M, Mielke K, Veldhuis JD. Age attenuates testosterone secretion driven by amplitude-varying pulses of recombinant human luteinizing hormone during acute gonadotrope inhibition in healthy men. J Clin Endocrinol Metab. 2007;92:3626–3632. doi: 10.1210/jc.2006-2704. [DOI] [PubMed] [Google Scholar]
- 27.Ten Kulve JS, De Jong FH, de RW. The effect of circulating estradiol concentrations on gonadotropin secretion in young and old castrated male-to-female transsexuals. Aging Male. 2010;13:155–161. doi: 10.3109/13685538.2010.511328. [DOI] [PubMed] [Google Scholar]
- 28.Tohgi H, Utsugisawa K, Yamagata M, Yoshimura M. Effects of age on messenger RNA expression of glucocorticoid, thyroid hormone, androgen, and estrogen receptors in postmortem human hippocampus. Brain Res. 1995;700:245–253. doi: 10.1016/0006-8993(95)00971-r. [DOI] [PubMed] [Google Scholar]
- 29.Urban RJ, Davis MR, Rogol AD, Johnson ML, Veldhuis JD. Acute androgen receptor blockade increases luteinizing-hormone secretory activity in men. J Clin Endocrinol Metab. 1988a;67:1149–1155. doi: 10.1210/jcem-67-6-1149. [DOI] [PubMed] [Google Scholar]
- 30.Urban RJ, Evans WS, Rogol AD, Kaiser DL, Johnson ML, Veldhuis JD. Contemporary aspects of discrete peak detection algorithms. I. The paradigm of the luteinizing hormone pulse signal in men. Endocr Rev. 1988b;9:3–37. doi: 10.1210/edrv-9-1-3. [DOI] [PubMed] [Google Scholar]
- 31.Veldhuis JD, Fraioli F, Rogol AD, Dufau ML. Metabolic clearance of biologically active luteinizing hormone in man. J Clin Invest. 1986;77:1122–1128. doi: 10.1172/JCI112411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veldhuis JD, Keenan DM, Liu PY, Takahashi PY. Kinetics of removal of intravenous testosterone pulses in normal men. Eur J Endocrinol. 2010a;162:787–794. doi: 10.1530/EJE-09-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veldhuis JD, Patrie J, Frick K, Weltman JY, Weltman AL. Administration of recombinant human GHRH-1,44-amide for three months reduces abdominal visceral fat mass and increases physical-performance measures in postmenopausal women. Eur J Endocrinol. 2005;153:669–677. doi: 10.1530/eje.1.02019. [DOI] [PubMed] [Google Scholar]
- 34.Veldhuis JD, Takahashi PY, Keenan DM, Liu PY, Mielke KL, Weist SM. Age disrupts androgen receptor-modulated negative feedback in the gonadal axis in healthy men. Am J Physiol Endocrinol Metab. 2010b;299:E675–E682. doi: 10.1152/ajpendo.00300.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veldhuis JD, Urban RJ, Lizarralde G, Johnson ML, Iranmanesh A. Attenuation of luteinizing hormone secretory burst amplitude is a proximate basis for the hypoandrogenism of healthy aging in men. J Clin Endocrinol Metab. 1992;75:707–713. doi: 10.1210/jcem.75.3.1517359. [DOI] [PubMed] [Google Scholar]
- 36.Vermeulen A. Decreased androgen levels and obesity in men. Ann Med. 1996;28:13–15. doi: 10.3109/07853899608999068. [DOI] [PubMed] [Google Scholar]
- 37.Vermeulen A, Ando S, Verdonck L. Prolactinomas, testosterone-binding globulin, and androgen metabolism. J Clin Endocrinol Metab. 1982;54:409–412. doi: 10.1210/jcem-54-2-409. [DOI] [PubMed] [Google Scholar]
- 38.Winters SJ, Wang C. LH and non-SHBG testosterone and estradiol levels during testosterone replacement of hypogonadal men: further evidence that steroid negative feedback increases as men grow older. J Androl. 2010;31:281–287. doi: 10.2164/jandrol.109.009035. [DOI] [PubMed] [Google Scholar]
- 39.Yanase T, Fan W, Kyoya K, Min L, Takayanagi R, Kato S, Nawata H. Androgens and metabolic syndrome: lessons from androgen receptor knock out (ARKO) mice. J Steroid Biochem Mol Biol. 2008;109:254–257. doi: 10.1016/j.jsbmb.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Yeap BB. Testosterone and ill-health in aging men. Nat Clin Pract Endocrinol Metab. 2009;5:113–121. doi: 10.1038/ncpendmet1050. [DOI] [PubMed] [Google Scholar]