Abstract
Background
Sepsis is characterized by metabolic disturbances, and previous data suggest a relative carnitine deficiency may contribute to metabolic dysfunction. Studies regarding safety and patient-centered efficacy of carnitine during septic shock are lacking.
Methods
This was a double-blind randomized control trial of levocarnitine (L-carnitine) infusion vs normal saline for the treatment of vasopressor-dependent septic shock. Patients meeting consensus definition for septic shock with a cumulative vasopressor index ≥3 and sequential organ failure assessment (SOFA) score ≥5 enrolled within 16 hours of the recognition of septic shock were eligible. The primary safety outcome was difference in serious adverse events (SAEs) per patient between groups. Efficacy outcomes included proportion of patients demonstrating a decrease in SOFA score of 2 or more points at 24 hours and short- and long-term survival.
Results
Of the 31 patients enrolled, 16 were in the L-carnitine and 15 were in the placebo arm. There was no difference in SAEs between placebo and intervention (2.1 vs 1.8 SAEs per patient, P = .44). There was no difference in the proportion of patients achieving a decrease in SOFA score of 2 or more points at 24 hours between placebo and treatment (53% vs 44%, P = .59). Mortality was significantly lower at 28 days in the L-carnitine group (4/16 vs 9/15, P = .048), with a nonsignificant improved survival at 1 year (P = .06).
Conclusion
L-carnitine infusion appears safe in vasopressor-dependent septic shock. Preliminary efficacy data suggest potential benefit of L-carnitine treatment, and further testing is indicated.
Keywords: L-carnitine, carnitine, sepsis, shock, randomized control trial
Introduction
Severe sepsis remains a significant public health concern, with more than 750,000 patients affected annually in the United States.1 Septic shock portends a particularly poor prognosis, and despite several therapeutic advances in the past several decades, the estimated mortality rate remains 30%–65%.2,3 These data suggest the need to investigate additional novel therapeutic strategies for the treatment of septic shock.
Emerging evidence suggests the presence of mitochondrial dysfunction during sepsis and that this dysfunction is associated with adverse clinical outcomes.4 Among the mitochon-drial alterations reported in sepsis are inhibition of pyruvate dehydrogenase (PDH) and carnitine palmitoyl transferase 1 (CPT-1).5,6 Specifically, with regard to CPT-1, a systemic car-nitine deficiency results from increased urinary secretion of carnitine.7 Animal models of sepsis demonstrate favorable effects of carnitine on mortality, and the 1 published clinical trial of L-carnitine in the setting of septic shock demonstrated improvements in hemodynamic parameters, including right atrial pressure, mean arterial pressure, and arterial oxygenation.8 However, this trial was completed more than 20 years ago, and treatment for septic shock has evolved considerably over this time frame. In addition, no safety- or patient-centered outcome data were reported in this study. Therefore, we sought to establish preliminary safety and efficacy data for use of intravenous (IV) L-carnitine administration in septic shock to support a larger dose-finding phase II trial.
Methods
This was a prospective double-blind randomized control trial of patients with septic shock. The study took place from October 2010 through December 2011 at a single, large urban tertiary care center in the United States (Carolinas Medical Center, Charlotte, NC). The research protocol was approved by the institutional review board and performed in accordance with good clinical practice guidelines. Each patient or the patient’s legally authorized surrogate provided written informed consent prior to enrollment in the trial and collection of data. If the patient was enrolled by a surrogate, the patient was approached if he or she survived and regained full decision-making capacity and was reconsented for inclusion in the study. The trial was conducted under the authority of the Food and Drug Administration (initial new drug [IND] 107,086) and was registered on clinicaltrials.gov (NCT01193777).
Patient Selection
All patients who presented with septic shock during the study period were screened for inclusion. To be enrolled, patients were required to meet consensus criteria for septic shock, including suspected or confirmed infection, 2 or more systemic inflammatory response criteria,9 and hypotension requiring vasopressor infusion despite at least a 20-mL/kg IV crystalloid bolus. Furthermore, patients were required to have a Sequential Organ Failure Assessment (SOFA) score10 of 5 or more at enrollment and demonstrate persistent vasopressor requirement, defined as a cumulative vasopressor index (CVI)11 of 3 or more for at least 4 continuous hours. Patients were excluded if they were <18 years old, could not be enrolled within 16 hours of recognition of septic shock, were known to be pregnant or breastfeeding, had a primary diagnosis other than sepsis, had an established do not resuscitate (DNR) order, had any known history of seizures or seizure disorder, had any known inborn error of metabolism, had an anticipated requirement for surgery that would interfere with the infusion time, were actively enrolled in another interventional study, were unable to provide informed consent, had received chest compression or cardioversion prior to enrollment during the current hospitalization, or had a known systemic allergy to L-carnitine.
Screening and Consent
Using a 24-hour day, 7-day week method previously established for the routine clinical care of patients with sepsis, an alert was sent to inform clinical care resources when patients began early protocolized emergency department resuscitation.12 This alert was also received by study staff who responded and screened the patients for study enrollment. To screen patients who were transferred to the intensive care unit (ICU) after initial hospital admission, an automated query of the electronic medical record of all patients in the ICU who had both antibiotics and vasopressors ordered was generated, and the list was evaluated for eligible patients within the enrollment window. Given the 16-hour enrollment window, all patients were enrolled on hospital day and ICU day 0.
Randomization and Blinding
Patients were randomized by a sealed, blinded envelope method to receive either L-carnitine or normal saline placebo in a predefined 1:1 random sequence using a permutated block randomization in blocks of 4. The randomization scheme was generated by an independent statistician prior to trial initiation, and the codex to permit study unblinding was held in a sealed manila envelope by the statistician. Upon enrollment patient enrollment, the principal investigator (PI) signed an order for the study drug that was faxed to the clinical pharmacy that mixed the drug. To ensure blinding, the pharmacist prepared either L-carnitine or placebo in identical polypropylene infusion bags and labeled the bags with identical labels that included the patient’s study ID number, patient name, medical record number, and infusion rate. The bags were not otherwise identifiable. Study drug or placebo was administered by members of the clinical care team who were blinded to group assignment. Unblinding did not occur until all patient data had been collected, adverse events had been assessed, and patient follow-ups had been completed and the database was locked.
Study Drug Administration Protocol
L-carnitine solution or normal saline placebo was infused over 12 hours in the emergency department and/or ICU. L-carnitine was administered in an unmarked syringe as a 4-g bolus injection (20 mL) over 2–3 minutes followed by 8-g infusion (8 g in 1000 mL of 0.9% normal saline or approximately 8 mg/mL) over the following 12 hours (83 mL/h). Placebo patients received an equivalent-volume (20 mL) bolus in an unmarked syringe followed by an equivalent volume of 0.9% NaCl at the same infusion rate in identical bags.
Clinical Management
Other than the study intervention, all clinical management was provided via standard care at the discretion of the emergency department and ICU attending physicians. The institution has standing protocols for the management of severe sepsis—namely, a well-defined and previously described early quantitative resuscitation protocol,12 a standardized protocol for the management of hyperglycemia using either continuous IV or intermittent subcutaneous insulin management to target blood glucose levels <180 mg/dL based on clinical trial data,13 and guidance regarding the use of preferential early enteral feeding unless contraindicated.
Safety Outcomes
The primary safety outcome was difference in number of serious adverse events (SAEs) per patient between L-carnitine and placebo groups. Secondary outcomes included difference in adverse events (AEs), excluding SAEs, per patient between groups. SAEs and AEs were determined by study coordinators blinded to treatment group assignment and confirmed by the blinded PI. SAEs were defined as any clinical change or laboratory abnormality falling outside the normal range as reported by the central laboratory that (1) resulted in a change in clinical management, (2) was not present prior to initiation of L-carnitine or placebo infusion, and (3) resulted in either prolonged hospital length of stay or death. This definition of AEs was used based on the Food and Drug Administration (FDA) guidance regarding the IND, and the definitions were developed in coordination with the FDA. Of note, relatedness to study drug was not considered in the simple determination of the presence of either SAEs or AEs. The entire medical record of the patient’s hospitalization was evaluated for the existence of SAEs, which were subsequently recorded. Death at any time point up to 28 days following enrollment was considered an SAE. AEs were defined as any clinical change or laboratory abnormality falling outside the normal range as reported by the central laboratory that (1) resulted in a change in clinical management, (2) was not present prior to initiation of L-carnitine or placebo infusion, and (3) occurred within the 12 hours following L-carnitine or placebo infusion but did not extend hospital stay or lead to death. It is important to note that all AEs meeting this definition were recorded, irrespective of their suspected relationship to the study drug.
Efficacy Outcomes
During the study treatment period, the patient’s physiological parameters were measured per standard care. All data needed to calculate the SOFA score were collected at enrollment and 24 and 48 hours. Patients were followed until hospital discharge or death. If the patient was discharged, follow-up was performed at 3, 6, and 12 months after discharge using a predefined protocol that included telephone, electronic medical record, and Social Security death certificate search as previously described.14 The primary efficacy outcome was the proportion of patients demonstrating a decrease in SOFA score of 2 or more points at 24 hours. Secondary efficacy outcomes included change in SOFA score at 48 hours and 28-day mortality. As an exploratory analysis, prospectively collected time to vasopressor withdrawal and 3-month, 6-month, and 1-year survival data were analyzed.
Statistical Analysis
We compared baseline demographics and clinical characteristics between the groups using the Student unpaired t test, Mann-Whitney U test, χ2 test, or Fisher exact test as appropriate. The difference in the proportion of patients who achieved the primary end point of a decrease in SOFA of 2 or more points over the first 24 hours between the L-carnitine and placebo groups was evaluated with the χ2 test and proportions with 95% confidence intervals. Kaplan-Meier survival curves were constructed to determine difference in long-term mortality between groups and compared using the log-rank test. Data were analyzed using an intent-to-treat analysis. All statistical tests were 2-sided with P < .05 considered significant, and only unadjusted data analyses were performed. The sample size was set to determine a difference of 0.15 SAEs per patient assuming a standard deviation of 0.12, requiring 12 patients in each treatment arm. A previously approved drug for sepsis reported that 12% of patients experienced an SAE in a phase III clinical trial with a similar severity of illness, providing the basis for this estimation.15 Data were analyzed using commercially available statistical software (StatsDirect 2.7.7 [StatsDirect Ltd, Cheshire, England] and STATA 10.0 [StataCorp, College Station, TX]).
Results
Of the 585 patients screened, 554 were excluded for the reasons shown in Figure 1, primarily insufficient severity of illness to meet inclusion criteria as assessed by vasopressor dose or SOFA score. Thirty-one patients were enrolled, all of whom underwent randomization, with 16 patients assigned to L-carnitine and 15 to placebo (Figure 1). Baseline characteristics between groups were well matched and are shown in Table 1. Of note, patients assigned to the L-carnitine arm had significantly more organ dysfunction, measured by the SOFA score, as compared with placebo patients. Similarly, there were no significant differences in supportive treatments administered to the 2 groups (Table 2). Telephone follow-up was achieved with all patients or their surrogates at 12 months.
Figure 1.
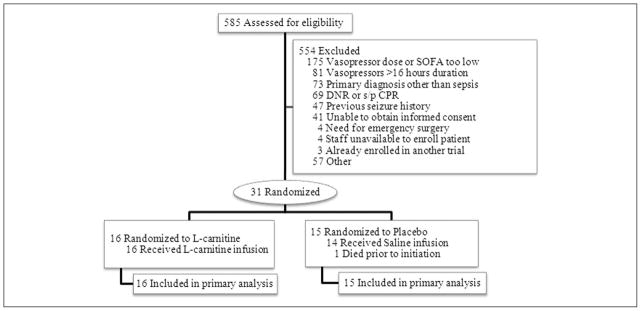
Study flow diagram of study participants (CONSORT). CPR, cardiopulmonary resuscitation; DNR, do not resuscitate; SOFA, Sequential Organ Failure Assessment; s/p, status post.
Table 1.
Patient Demographics and Clinical Characteristics at Enrollment.
| Variable | L-carnitine (n = 16) | Placebo (n = 15) | P Value |
|---|---|---|---|
| Age, y | 63 (55, 72) | 65 (56, 71) | .66 |
| Race | |||
| White | 13 (81) | 12 (80) | .93 |
| African American | 3 (19) | 3 (20) | |
| Male sex | 11 (69) | 10 (67) | .91 |
| Height, cm | 177 (166, 178) | 175 (170, 180) | .48 |
| Weight, kg | 85 (71, 99) | 80 (68, 96) | .89 |
| BMI, kg/m2 | 29.1 (24.3, 33.6) | 24.8 (23.4, 29.4) | .29 |
| Comorbidities | |||
| Coronary artery disease | 4 (25) | 3 (20) | .76 |
| COPD | 5 (31) | 3 (20) | .51 |
| Hypertension | 11 (69) | 11 (73) | .80 |
| Diabetes mellitus | 4 (25) | 7 (47) | .24 |
| Cirrhosis | 2 (13) | 2 (13) | .95 |
| Chronic renal insufficiency | 5 (31) | 2 (13) | .27 |
| Baseline laboratory values | |||
| Platelets, cells/mm3 | 147 (79, 196) | 254 (174, 335) | .03 |
| Creatinine, mg/dL | 2.0 (1.5, 2.7) | 1.6 (1.1, 1.9) | .16 |
| Total bilirubin, mg/dL | 1.4 (0.8, 3.9) | 1.2 (0.9, 2.4) | .61 |
| Lactate, mmol/L | 2.7 (2.0, 3.6) | 2.6 (1.8, 6.6) | .71 |
| Cumulative vasopressor index | 4 (4, 7) | 4 (4, 8) | .96 |
| SOFA score | 14 (11.5, 14) | 10 (7, 10) | .01 |
| Respiratory | 3 (2.5, 3) | 2 (0, 2) | .03 |
| Coagulation | 0.5 (0, 2) | 0 (0, 1) | .27 |
| Liver | 1 (0, 2) | 0 (0, 2) | .47 |
| Cardiovascular | 4 (4, 4) | 4 (4, 4) | .53 |
| Central nervous system | 3 (2,4) | 1 (0, 3) | .14 |
| Renal | 1.5 (1, 2) | 1 (0, 1) | .08 |
Continuous data presented as medians and interquartile range and discrete data as absolute numbers with percentages (%). BMI, body mass index; COPD, chronic obstructive pulmonary disease; SOFA, Sequential Organ Failure Assessment.
Table 2.
Treatments Administered.
| Variable | L-carnitine (n = 16) | Placebo (n = 15) | P Value |
|---|---|---|---|
| Intravenous fluids, La | 4.0 (2.1, 5.3) | 4.1 (2.4, 5.2) | .75 |
| Vasopressors administeredb | 16 (100) | 15 (100) | |
| Norepinephrine | 13 (81) | 10 (71) | .39 |
| Dopamine | 1 (6) | 1 (7) | .97 |
| Vasopressin | 5 (31) | 4 (29) | .80 |
| Phenylephrine | 3 (19) | 4 (29) | .63 |
| Epinephrine | 1 (6) | 1 (7) | .97 |
| Packed red blood cells | 7 (44) | 3 (20) | .19 |
| Mechanical ventilation | 14 (88) | 8 (53) | .049 |
| Intravenous steroids | 8 (50) | 5 (33) | .38 |
Continuous data presented as medians and interquartile range and categorical data as absolute numbers with percentages (%).
In the first 6 hours of resuscitation.
Vasopressors administered at enrollment.
There was no significant difference in the safety outcome of difference in SAEs or AEs between the L-carnitine and placebo groups. Excluding patients who died, there were 1.7 SAEs per patient in the placebo group vs 1.6 in L-carnitine–treated patients (P = .85). Including deaths at 28 days, placebo patients had 2.1 compared with 1.8 SAEs per patient in the L-carnitine group (P = .44). Excluding SAEs, placebo patients had an additional 0.2 AEs per patient vs 0.3 in the intervention group (P = .71). All observed SAEs and AEs are summarized by organ system and treatment group in Table 3. Importantly, there was no difference in the incidence of seizures between L-carnitine–treated patients and patients receiving placebo in the infusion period or the 12 hours following infusion, which is relevant given the presumed association between L-carnitine administration and seizure activity. There was a nonsignificant trend toward a higher incidence of recurrent infection in the placebo-treated group. The trial was stopped following enrollment of an adequate number of patients to establish safety, so that further efficacy testing in a larger phase II study could begin.
Table 3.
Summary of Serious Adverse Events and Adverse Events by Treatment Group.
| Serious Adverse Event | L-carnitine (n = 16) | Placebo (n = 15) |
|---|---|---|
| Respiratory, thoracic, and mediastinal disorders | ||
| Exacerbation of COPD | 1 | 1 |
| Pneumothorax | 1 | 0 |
| Acute respiratory failure | 1 | 2 |
| Pneumoniaa | 1 | 1 |
| Pulmonary embolus | 1 | 0 |
| Cardiac disorders | ||
| Atrial fibrillation with RVR | 1 | 0 |
| Cardiac arrest with ROSC | 0 | 1 |
| Patent foramen ovale diagnosed | 0 | 1 |
| Endocrine disorders | ||
| Hypoglycemia | 1 | 0 |
| Renal/urinary disorders | ||
| Acute on chronic renal failure | 1 | 0 |
| Acute renal failure | 2 | 1 |
| End-stage renal disease (new dialysis) | 2 | 0 |
| Hematological disorders | ||
| Heparin-induced thrombocytopenia | 1 | 0 |
| Worsening anemia | 1 | 1 |
| Worsening thrombocytopenia | 2 | 0 |
| Disseminated intravascular coagulation | 1 | 1 |
| Deep venous thrombosis | 0 | 1 |
| Gastrointestinal disorders | ||
| Gastrointestinal bleeding | 2 | 0 |
| Diarrhea | 0 | 1 |
| Nausea/vomiting | 0 | 1 |
| Hepatobiliary disorders | ||
| Worsening liver function | 0 | 1 |
| Obstructive jaundice | 0 | 1 |
| Immune system disorders | ||
| Infections and infestationsb | 1 | 5 |
| Neoplasms benign, malignant and unspecified | ||
| Newly diagnosed neoplasm during hospitalization | 0 | 2 |
| Nervous system disorders | ||
| Seizure | 1 | 2 |
| CVA | 1 | 0 |
| Central cord compression | 1 | 0 |
| Critical illness myopathy and/or encephalopathy | 2 | 0 |
| Skin and subcutaneous tissue disorders—abscess | 0 | 1 |
| Electrolyte imbalances | ||
| Hypokalemia | 1 | 1 |
| Hypomagnesemia | 0 | 1 |
| Death | ||
| 28 days | 4 | 9 |
| 3 months | 9 | 10 |
| 6 months | 9 | 12 |
| 12 months | 9 | 12 |
Data presented as absolute numbers. COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; ROSC, return of spontaneous circulation; RVR, rapid ventricular response.
Pneumonia only counted under respiratory disorders but not recounted under new infections.
New infections were as follows: in the L-carnitine–treated group, there was 1 case of sepsis not otherwise specified, of unclear source. In the placebo group, 2 patients had all 5 new infections. One patient had an episode of sepsis from a biliary source. The second patient had a prolonged intensive care unit course complicated by an intra-abdominal abscess, candidemia, multidrug-resistant bacteremia from a urinary source, and later recurrent sepsis from a urinary source. This patient also had H1N1 influenza, which was counted under pneumonia.
No difference in the primary efficacy outcome of a decrease in SOFA score of 2 points at 24 hours (7/16 patients in the L-carnitine–treated group vs 8/15 patients in the placebo group; P = .59) or at 48 hours (10/16 patients in the L-carnitine–treated group vs 7/15 patients in the placebo group; P = .37) was observed in the study. Median time to vasopressor withdrawal was not significantly different between L-carnitine–treated and placebo-treated patients: 2775 (interquartile range [IQR], 1954, 4380) minutes vs 1979 (IQR, 980, 3371) minutes (P = .15), respectively.
Mortality was significantly lower at 28 days in the L-carnitine group compared with the placebo group (4/16 vs 9/15, P = .048), despite a significantly higher severity of illness at enrollment. As a post hoc exploratory analysis, we wished to further analyze long-term effects of L-carnitine on clinical outcome. At 28 days, 2 of 6 (33%) survivors in the placebo group and 3 of 12 (25%) survivors in the intervention group were still hospitalized. Survival analysis at 1 year demonstrated a non-significant improved survival in the L-carnitine group, as shown on the Kaplan-Meier curve in Figure 2, although this result did not reach statistical significance (P = .06).
Figure 2.
Kaplan-Meier survival curve comparing long-term survival between placebo group (black) and L-carnitine group (red).
Discussion
In this double-blind randomized control trial of L-carnitine administration for vasopressor-dependent septic shock, we found no significant difference in SAEs observed in L-carnitine–treated patients vs patients treated with placebo. Furthermore, we gathered preliminary efficacy data, which showed no statistical difference in the primary outcome of change in SOFA score at 24 hours but did show a significant reduction in mortality at 28 days and a nonsignificant reduction at 1 year in the L-carnitine arm. Given the small sample and the lack of power to detect an efficacy outcome difference, we cautiously interpret these data to suggest the possibility of a beneficial effect and the absence of harm.
The known AEs of L-carnitine treatment are mostly minor and include nausea, vomiting, diarrhea, headache, and a change in body odor. The most serious AE of L-carnitine is the potential to decrease the seizure threshold. In this study, we observed no difference in the incidence of seizures between the treatment arms. Furthermore, we observed none of the other known AEs of L-carnitine treatment, perhaps because these critically ill patients were unlikely to notice or relay these relatively minor AEs.
We chose to conduct a blinded randomized control trial to distinguish SAEs that can be expected with critical illness from those caused by L-carnitine. To reduce bias, we employed explicit and standardized definitions of SAEs in this study (not preexisting and resulting in a change in clinical management, death, or prolongation of hospitalization), and assessors were blinded to group assignment.
Previously, Gasparetto et al8 randomized 115 patients with circulatory shock to receive 12 g of acetyl-L-carnitine over 12 hours or placebo. Of these 115 patients, 72 had sepsis as their etiology of shock. Among the 72 patients with septic shock, those who received acetyl-L-carnitine had significantly higher systolic and mean arterial pressures, lower right atrial pressure, and higher arterial partial pressure of oxygen and hemoglobin oxygen saturation at the end of the acetyl-L-carnitine infusion compared with placebo-treated patients. These results suggested the potential for acetyl-L-carnitine to improve dysfunction of multiple organ systems in septic shock and provided a rationale for using change in organ failure as the primary outcome measure in the present study. However, this study was conducted more than 20 years ago, and the clinical care of sepsis has changed considerably in the interim. Furthermore, no patient-centered outcomes were reported in this study.
In our study, we evaluated several patient-centered efficacy outcomes between L-carnitine and placebo for preliminary data regarding the clinical effectiveness of L-carnitine administration in septic shock. We found no difference in the decrease in SOFA score of 2 points at 24 hours. We chose this dichotomous outcome a priori rather than total change in SOFA score due to difficulty in defining change in SOFA score among patients who die prior to calculation of the delayed score. Although this decision eliminated our need to impute severity of illness data, it did decrease our power to detect a significant difference between groups.
We did note significantly lower mortality in the L-carnitine–treated patients at 28 days and a nonsignificant improved survival at 1 year, despite a higher SOFA score at enrollment in the L-carnitine group prior to the initiation of infusion. Of note, previous research has suggested that the mortality of patients with an initial SOFA score of >11 is >90%.16 Among our placebo group, the mortality was 80% (4/5 patients with an initial SOFA score of >11), consistent with prior data. In contrast, in our interventional arm, the mortality of this group of patients was 62% (8/13 patients). These data suggest that within the limitations of wide confidence intervals resulting from our sample size, the mortality reported in this study is consistent with prior reports.
In a post hoc aim, we investigated the possibility that L-carnitine treatment was simply keeping patients alive longer without improving their long-term outcome. Two observations suggest that this is not the case. First, the fact that most patients had been discharged from the hospital at the time of mortality determination suggests that patients treated with L-carnitine were not simply being kept alive longer in the hospital but were rather being discharged home at a rate similar to patients treated with placebo. These data suggest that the improvement in survival at 28 days is not attributable to more prolonged hospitalizations and increased morbidity. Second, inspection of the Kaplan-Meier curve (Figure 2) suggests that the major difference between study groups appears early after enrollment and may suggest clinical improvement that we did not capture through our use of the surrogate end point of change in SOFA score. We found no significant difference in time to vasopres-sor withdrawal, with a trend toward more rapid withdrawal of vasopressors in the control group. Taken together with cardiovascular SOFA score data, and assuming the mortality benefit was not due to random chance, this would suggest a mechanism of action independent of effects on the macrovascular cardiovascular system, such as beneficial effects on mitochon-drial and/or microcirculatory dysfunction. However, evaluation of mechanisms of action was beyond the scope of this study, therefore remaining purely speculative. It is important to note that this study was underpowered to detect significant differences in either change in SOFA score or mortality. Rather, the suggestion of safety and preliminary efficacy documented in this report is of interest and supports a large phase II dose-finding study. Such a study has been funded; it is registered at clinicaltrials.gov (NCT01665092) and has begun enrollment.
This study has several strengths. The use of blinded randomized methodology minimized bias in both the assessment of AEs and treatment efficacy. Primary and secondary outcomes of the study were predefined, and the number of analyses was not excessive, decreasing the chance of false-positive results. The trial also has several important limitations. It was a single-center study limited to a severely ill cohort of patients with vasopressor-dependent septic shock, potentially limiting generalizability. Second, we did not gather detailed baseline or interventional nutrition assessments and cannot account for the potential confounding between groups. Most important, however, this study lacked adequate sample size to reliably determine a valid efficacy and thus only indicates potential benefit in the absence of evidence of harm, supporting further study of L-carnitine administration in early sepsis.
Conclusion
Data from this double-blind randomized control trial suggest that L-carnitine administration appears safe for the treatment of vasopressor-dependent septic shock. Preliminary efficacy data revealed no difference in early SOFA score reduction but did show a significant reduction in mortality at 28 days. Given the small sample and the lack of power to detect an efficacy outcome difference, we cautiously interpret these data to suggest the possibility of a beneficial effect and the absence of harm. Thus, we conclude that further efficacy evaluation in the form of a phase II dose-finding study is indicated.
Clinical Relevancy Statement.
Septic shock is a condition characterized by numerous metabolic disturbances, and patients with sepsis have been reported to have a relative carnitine deficiency. Even with optimal medical management, mortality from septic shock remains strikingly high. To our knowledge, this study is the first to demonstrate safety and preliminary efficacy of L-carnitine infusion in this patient population.
Footnotes
Financial disclosure: This project was supported by a grant from the American Heart Association to Dr Puskarich (AHA 10POST356001), as well as a foundation grant from the Cannon Research Center (SRG10–004). The sponsors had no role in conduct, analysis or decision to publish. Dr Jones has received salary support from National Institute of General Medical Sciences (R01 GM103799).
References
- 1.Angus D, Linde-Zwirble W, Lidicker J, Clermont G, Carcillo J, Pinsky M. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 3.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 4.Fink M. Bench-to-bedside review: cytopathic hypoxia. Crit Care. 2002;6:491–499. doi: 10.1186/cc1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eaton S, Fukumoto K, Stefanutti G, Spitz L, Zammit VA, Pierro A. Myocardial carnitine palmitoyltransferase 1 as a target for oxidative modification in inflammation and sepsis. Biochem Soc Trans. 2003;31:1133–1136. doi: 10.1042/bst0311133. [DOI] [PubMed] [Google Scholar]
- 6.Vary TC. Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: effects on plasma lactate [abstract] Shock. 1996;6:89–94. doi: 10.1097/00024382-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Nani G, Pittiruti M, Giovannini I, Boldrini G, Ronconi P, Castagneto M. Plasma carnitine levels and urinary carnitine excretion during sepsis. JPEN J Parenter Enteral Nutr. 1985;9:483–490. doi: 10.1177/0148607185009004483. [DOI] [PubMed] [Google Scholar]
- 8.Gasparetto A, Corbucci GG, de Blasi RA, et al. Influence of acetyl-L-car-nitine infusion on haemodynamic parameters and survival of circulatory-shock patients. Int J Clin Pharm Res. 1991;11:83–92. [PubMed] [Google Scholar]
- 9.Bone R, Balk R, Cerra F, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/ Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 10.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqui FS, Kumar A, Woodward B, Wang Y. Cumulative vasopressor index (CVI) as an assessment of cardiovascular organ dysfunction and indicator of outcome in patients with septic shock [abstract] Crit Care Med. 2007;35:A7. [Google Scholar]
- 12.Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department–based early goal directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132:425–432. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 14.Jones A, Stiell I, Nesbitt L, et al. Nontraumatic out-of-hospital hypotension predicts in-hospital mortality. Ann Emerg Med. 2004;43:106–113. doi: 10.1016/j.annemergmed.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 15.FDA clinical review drotrecogin alfa (activated) [recombinant human activated protein C (rhAPC)] Xigris. 2012 http://www.fda.gov/downloads/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/ucm113438.pdf.
- 16.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]



