Abstract
Background
Adolescents experience elevated depressive symptoms which health promotion interventions may reduce.
Purpose
This study investigated whether HIV prevention trial participation decreased depressive symptoms among African-American female adolescents.
Methods
Adolescents (N=701; M age = 17.6) first received a group-delivered HIV prevention intervention and then either 12 sexual health (intervention condition) or 12 general health (comparison condition) phone counseling contacts over 24 months. ACASI assessments were conducted at baseline, and at 6-, 12-, 18-, and 24-months post-baseline. Linear generalized estimating equations were used to detect percent relative change in depressive symptoms.
Results
Participants reported a 2.7% decrease in depressive symptoms (p = 0.001) at each assessment. Intervention participants endorsed an additional 3.6% decrease in depressive symptoms (p = 0.058).
Conclusions
Trial participation was associated with reduced depressive symptomatology, particularly among those receiving personalized sexual health counseling. HIV prevention interventions may benefit from incorporating additional content to address adolescents’ mental health needs.
Keywords: Depression, Depressive symptoms, HIV prevention intervention, African-American adolescents, adolescent women
The Substance Abuse and Mental Health Services Administration (SAMSHA) estimates that two million U.S. adolescents experience a major depressive episode each year, with an annual prevalence estimate of 8.1% among 12 to 17 year olds (SAMSHA, 2009). Young women are two times as likely to experience a major depressive episode relative to young men (SAMSHA, 2009). Furthermore, depressive symptoms have an earlier onset for young women, with symptom severity increasing during later adolescence (Collins et al., 2010; Rudolph, 2008). African-American adolescents may experience higher rates of depression relative to their non-minority peers (Khan et al., 2009). For example, 19.3% of African-American adolescent women in the National Longitudinal Study of Adolescent Health endorsed recent and chronic depressive symptoms relative to 13.0% of Caucasian adolescent women (Khan et al., 2009). Despite the prevalence of depression among adolescents, only a minority receive mental health treatment, with further health disparities for mental health care access among African-American adolescents (SAMSHA, 2009; Elster et al., 2003). A majority of adolescents receive mental health services through primary care or other general medical settings (Bower et al., 2001; Cheung & Dewa, 2007; Walker & Townsend, 1998) rather than through specialized mental health facilities, highlighting the opportunity to integrate mental health screening and treatment into general medical settings.
The sexual health of African-American adolescents is also a pressing public health concern. African-American young women experience elevated rates of sexually transmitted infections (STIs; Datta et al., 2007). Recent national estimates indicate that African-American female adolescents between the ages of 15 and 24 experience the highest rates of chlamydia and gonorrhea (CDC, 2012a). This is particularly alarming given that STIs pose a number of negative health consequences and increase susceptibility to HIV (Fleming & Wasserheit, 1999; Hook & Handsfield, 1999; Stamm, 1999). African-American adolescents are disproportionately affected by the HIV epidemic (Rangel et al., 2006) accounting for 73% of adolescent HIV infections, with a diagnosis rate nearly 23 times the rate for White adolescents (CDC, 2012b). Among African-American adolescent women, the majority of incident STI/HIV infections are acquired via heterosexual transmission (CDC, 2012b).
Reproductive health service providers and HIV risk reduction interventions offer the unique opportunity to address both the sexual and mental health of at-risk young women, especially since adolescents seeking sexual health services may experience elevated rates of depression. For instance, in one study 19% of African-American women seeking sexual health services had elevated depressive symptoms, with 40% endorsing mild to moderate symptoms and 60% endorsing symptom levels suggestive of a clinical diagnosis of depression (Collins et al., 2010). In a second study examining the prevalence of depressive symptoms, adolescents in sexual health clinics had higher levels of self-reported depressive symptoms relative to a cohort of adolescents in schools (Fernandez et al., 2009).
A growing literature suggests that elevated depressive symptoms are associated with increased engagement in sexual risk behaviors among adolescents. Cross-sectional studies indicate that elevated depressive symptoms are associated with sexual risk behaviors including inconsistent condom use (Elkington, Bauermeister, & Zimmerman, 2010; Holden et al., 2008; Paxton & Robinson, 2008; Seth et al., 2009; Smith, Buzi, & Weinman, 2010; Tolou-Shams et al., 2008), lower frequency of condom use during the last sexual encounter (Milhausen, Yarber, & Crosby, 2003; Seth et al., 2009), substance use prior to sexual activity (Milhausen et al., 2003; Seth et al., 2009; Shrier et al., 2009; Tolou-Shams et al., 2008; Wilson et al., 2010), earlier sexual debut (Milhausen et al., 2003), and decreased contraception use (Kosunen et al., 2003; Wilson et al., 2010). There is also an association between increased depressive symptoms and self-reports of a greater number of sexual partners (Elkington et al., 2010; Holden et al., 2008; Kosunen et al., 2003; Milhausen et al., 2003; Rubin, Gold, & Primack, 2009; Shrier et al., 2009), including concurrent sexual partners (Seth et al., 2009). Adolescent depression has also been associated with increased biologically-confirmed STI incidence relative to those without depressive symptoms (Lee, O'Riordan, & Lazebnik, 2009; Mazzaferro et al., 2006; Seth et al., 2009). Furthermore, longitudinal studies suggest that depressive symptom levels predict subsequent sexual risk behaviors (Brown et al., 2006; Lehrer et al., 2006). For example, a nationally-representative study found that higher baseline levels of depressive symptoms predicted substance use during the last sexual encounter one year later (Lehrer et al., 2006).
Various pathways have been proposed to explain the association between depressive symptoms and engagement in sexual risk behaviors. Adolescents may engage in sexual behavior as a coping strategy to manage negative emotional states (Brown et al., 2006; Elkington et al., 2010). Substance use may also mediate the association between psychological distress and sexual risk behaviors (Elkington et al., 2010; Khan et al., 2009). An alternative hypothesis suggests that increased depressive symptoms may be a consequence of engaging in sexual risk behaviors (Brown et al., 2006; Khan et al., 2009). Depression may also decrease an individual’s motivation to engage in protective sexual behaviors (Brown et al., 2006; Khan et al., 2009) or result in impaired information processing; thus, depressed individuals may have diminished capacity to appraise potential health risks (Holden et al., 2008; Khan et al., 2009).
Despite the literature suggesting an association between depressive symptoms and sexual risk behaviors among adolescents, few studies have investigated the efficacy of sexual risk reduction interventions to reduce depressive symptoms. Among a subset of participants endorsing elevated baseline depressive symptom levels, Sales and colleagues found that participation in an efficacious HIV prevention intervention resulted in decreased depressive symptoms among African-American adolescent females at both the 6- and 12-month follow-up assessments (Sales et al., 2010). While this study suggests that HIV prevention interventions may reduce depressive symptoms among adolescents with elevated depressive symptomatology, lacking is research examining the broader impact of HIV prevention interventions on adolescents’ depressive symptoms over more extended periods of time. A recent meta-analysis summarized the efficacy of HIV risk reduction interventions to decrease sexual risk behaviors and examined depressive symptomatology as a potential moderator of intervention efficacy (Lennon et al., 2012). Sexual risk behavior significantly decreased when participants had elevated depressive symptoms at baseline and when depressive symptom levels were reduced over the follow-up period (Lennon et al., 2012). Thus, decreasing depressive symptoms may also decrease sexual risk behaviors (Lennon et al., 2012). Additionally, studies with female participants, interventions targeting specific risk groups, and intervention content including self-management and coping skills training were more likely to reduce depressive symptoms (Lennon et al., 2012). Thus, there is some evidence to suggest that sexual health interventions may decrease depressive symptoms, particularly for interventions enrolling women and those that incorporate intervention content to improve coping skills.
The aim of the current study was to investigate the efficacy of an HIV prevention intervention to reduce depressive symptoms among a large cohort of African-American adolescent women. We hypothesized that adolescents randomized to the more intensive HIV prevention intervention with repeated personalized sexual health counseling sessions would report decreased depressive symptoms over a two-year follow-up period.
Methods
Participants
From June 2005 to June 2007 African-American adolescent females, ages 14–20 years, were recruited from three clinics providing sexual health services to predominantly inner-city adolescents in Atlanta, Georgia. An African-American female recruiter approached adolescents in clinic waiting areas, described the study, solicited participation, and assessed eligibility. Eligibility criteria included self-identifying as African-American, 14–20 years of age at time of enrollment, and reporting at least one episode of unprotected vaginal sex (vaginal sex without condom use) in the past 6 months. Adolescents were excluded from participation if they were married, pregnant, or attempting to become pregnant. Adolescents meeting inclusion criteria and interested in participating were scheduled to return to the clinic to complete informed consent procedures, baseline assessments, and be randomized to trial conditions. Written informed consent was obtained from all adolescents. Parental consent was waived for those younger than 18 due to the confidential nature of sexual health clinic services. Of the eligible adolescents, 94% (N=701) enrolled in the study, completed baseline assessments and were randomized to study conditions. Participants were compensated for travel and childcare to attend the intervention and complete assessments. The Institutional Review Board at the participating institution approved all study protocols.
Procedures
Study design
The study was a two-arm randomized controlled supplemental treatment trial (Piantadosi, 1997). In supplemental treatment trials, participants receive a “primary” treatment and, subsequently, receive a different (supplemental) treatment to enhance the effects of the primary treatment. In this study, the aim of the supplemental treatment was to extend the primary treatment’s efficacy over an extended 24-month period.
Power analysis
A power analysis was calculated to detect reductions in the primary outcome of the trial, incident chlamydial infections. We anticipated a treatment effect of 20% reduction in incident chlamydial infections over the follow-up period. Using methods outlined in Rochon, 1998 for repeated measurements, and assuming a 20% correlation for within-person measurements, 80% retention across the follow-up assessments, and setting the Type I error-rate at 0.05 for a two-tailed test, 700 participants were needed to detect the hypothesized reduction with 80% power.
Random assignment to study conditions
Random assignment to conditions was implemented subsequent to the baseline assessment using concealed allocation procedures. Prior to enrollment investigators used a computer algorithm to generate a random allocation sequence and opaque envelopes to execute the treatment assignments. Participants were randomized to either the intervention or comparison condition.
Intervention condition
Participants randomized to the intervention condition (n = 342) received as their primary treatment a CDC evidence-based, culturally- and gender-appropriate HIV prevention intervention for African-American female adolescents known as HORIZONS (DiClemente et al., 2009). HORIZONS is a group-based HIV intervention for African-American adolescent females designed to enhance HIV-preventive attitudes and beliefs, negotiation and refusal skills, safer sex norms, and preventive behaviors. The efficacy of HORIZONS has been evaluated in a randomized controlled trial with findings demonstrating significant reductions in incident chlamydial infections and high-risk sexual behaviors over a 12-month follow-up (DiClemente et al., 2009). In the current study, HORIZONS was implemented by two trained African-American female health educators with, on average, 7–8 participants attending the group session.
The supplemental treatment was phone-delivered HIV prevention maintenance counseling (PMC) that began following participation in HORIZONS. The PMC supplemental intervention consisted of a health educator administering 12 brief, tailored phone contacts to participants approximately every 8 weeks for 24 months. PMC was theory-driven, based on a directive, cognitive-behavioral problem-solving and goal-setting approach designed to identify sexual behavior change goals and strategies and reinforce prevention concepts discussed in HORIZONS (Beck, 1995; Locke & Latham, 2002; Strecher et al., 1995). Each phone contact began with adolescents completing a brief risk appraisal. Participants prioritized current factors that were contributing to HIV risk. Subsequently, a health educator delivered scripted, brief, tailored PMC based on participants’ prioritized risk appraisal. Thus, PMC was an individually tailored brief intervention designed to reinforce content from the primary treatment HORIZONS over an extended period of 24 months.
Comparison condition
Participants randomized to the comparison condition (n = 359) also received HORIZONS as their primary treatment and a time- and dose-equivalent phone-delivered General Health Promotion (GHP) supplemental treatment. The GHP condition was administered on the same schedule, 12 brief, phone contacts every 8 weeks for 24 months, by a health educator. These calls focused on discussing general health topics (e.g., diet and nutrition), rather than focusing on sexual health. Use of a placebo-attention phone condition reduces the likelihood that effects of the PMC could be attributed to differences in frequency or duration of contact with staff or in the sequencing of phone contacts over the 24-month follow-up period.
Data collection
Data collection occurred at baseline, prior to randomization, and at 6-, 12-, 18- and 24-months following participation in the primary treatment, HORIZONS. To reduce attrition participants received a reminder post-card and a retention phone call prior to each follow-up assessment. Data collection consisted of completing an audio computer-assisted self-administered interview (ACASI). ACASI enhances accurate reporting, reduces social desirability bias, and minimizes reporting errors associated with low literacy (Brown & Vanable, 2009; Turner et al., 1998).
Summary of trial findings on sexual health outcomes
Findings from the main trial have been previously reported (DiClemente, 2010; Swartzendruber et al., 2012). Briefly, there were no differences between study conditions on sociodemographic characteristics, STI prevalence or sexual behavior at the time of randomization, and retention between study arms was similar. There were statistically significant differences in incident chlamydial infections and condom use between the two arms through 18 months. Relative to participants assigned to the comparison condition, participants in the intervention condition had fewer incident chlamydial infections and a greater number of condom-protected sex acts.
Measures
Intervention condition
Intervention condition assignment was coded as 0 for the comparison condition and 1 for the intervention condition.
Assessment wave
The baseline assessment and each of the four, six-month follow-up assessment waves were coded from 1 (baseline) to 5 (24-month follow-up assessment wave).
Phone contacts
For each of the 12 phone-delivered contacts (PMC contacts in the intervention condition, GHP contacts in the comparison condition) completed phone-contacts were coded as 1 with uncompleted phone contacts coded as 0.
Sociodemographics
For descriptive purposes, participants reported their age and highest level of educational attainment.
Depressive symptoms
Respondents’ depressive symptoms at each of the assessment points were measured with the 8-item Center for Epidemiological Studies-Depression (CES-D) scale (Santor & Coyne, 1997). The CES-D assesses the presence of depressive symptoms in the past 7 days. A sample item is, “I felt sad.” For each item, participants indicated the frequency they had experienced each symptom during the past week from 1 “Less than one day” to 4 “Five to seven days.” A total score was calculated with higher scores indicative of higher depressive symptom levels; the range of possible scores is 8–32 (α = .91). Scores above 15 suggest elevated depressive symptom levels.
Data Analysis Plan
All analyses were performed using STATA version 11.2 (version 11.2; StataCorp, College Station, Texas). Descriptive statistics summarized participant characteristics, frequency of completed phone contacts, and level of depressive symptoms. Bivariate analyses examined differences for sociodemographic characteristics and depressive symptoms between conditions using one-way ANOVA for continuous variables and chi-square for categorical variables (Fleiss, Levin, & Paik, 2003). The control variable (age) and depressive symptom levels were log-transformed to facilitate ease of interpretation.
To assess intervention effects on depressive symptoms for the entire 24-month follow-up period, analyses were performed on pre-specified hypotheses using an intention-to-treat protocol with participants analyzed in their originally assigned experimental conditions irrespective of number of phone contacts completed (Lachin, 2000; Piantadosi, 1997; Pocock, 1993). We used a generalized estimating equation (GEE) model to control for repeated within-subject heterogeneity using Gaussian distributed effects with an unstructured correlation matrix between assessments (Hardin & Hilbe, 2003; Liang & Zeger, 1986). This model allows for a differential number of repeated observations of participants over the course of the study. The GEE model included condition assignment and assessment wave adjusting for baseline depressive symptomatology and age to estimate adjusted mean differences. Regression coefficients were interpreted as the percent mean difference in depressive symptoms over the 24-month period for a 1-unit change in each predictor allowing for unobserved subject-specific heterogeneity. The 95% confidence interval (CI) around the adjusted mean differences, and corresponding p value, were also computed.
Results
Participant Characteristics
African-American adolescent female participants (N = 701) were recruited from either a county health department STD clinic (n =373), a hospital-based adolescent sexual health clinic (n = 81), or a Planned Parenthood clinic (n = 247). Participants were between 14 and 20 years old with mean (SD) age of 17.6 (1.7). With regards to highest level of educational attainment, 8% completed the eighth grade or below, 53% were in high school (grades 9–12), 19% had graduated from high school or earned a GED, 16% had completed one or more years of college, and 4% described their level of education as “other.”
Baseline Differences by Experimental Condition
There were no baseline differences between conditions for the sociodemographic factors of age or educational attainment. Baseline depressive symptom levels did not differ between the intervention (M = 14.6, SD = 6.3) and comparison (M = 15.2, SD = 6.7) conditions, F (1, 699) = 1.24, p = .27.
Descriptive Statistics
Frequency of completed phone contacts
Table 1 presents descriptive statistics for the frequency of completed phone contacts by condition at each time-point. As shown in Table 1, there were no differences in the number of completed phone contacts at each time-point by condition.
Table 1.
Frequency of completed phone contacts at each assessment point by experimental condition
| Intervention Condition (n = 342) | Comparison Condition (n = 359) | Difference between Conditions∞ |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M (SD)a | 0 calls† | 1 call† | 2 calls† | 3 calls† | M (SD)a | 0 calls† | 1 call† | 2 calls† | 3 calls† | ||
| Time-point | |||||||||||
| 6-Months | 2.4 (.95) | 28 (8.2) | 30 (8.8) | 63 (18.4) | 221 (64.6) | 2.3 (.98) | 29 (8.1) | 45 (12.5) | 59 (16.4) | 226 (62.3) | F = .51, p = .48 |
| 12-Months | 2.1 (1.2) | 60 (17.5) | 41 (11.9) | 56 (16.4) | 185 (54.1) | 1.9 (1.2) | 77 (21.5) | 42 (11.7) | 64 (17.8) | 176 (49.0) | F = 1.9, p = .16 |
| 18-Months | 1.9 (1.2) | 79 (23.1) | 37 (10.8) | 63 (18.4) | 163 (47.7) | 1.8 (1.3) | 106 (29.5) | 34 (9.5) | 52 (14.5) | 167 (46.5) | F = 1.8, p = .19 |
| 24-Months | 1.6 (1.4) | 139 (40.6) | 19 (5.6) | 40 (11.7) | 144 (42.1) | 1.4 (1.4) | 160 (44.6) | 25 (6.9) | 49 (13.7) | 125 (34.8) | F = 2.6, p = .11 |
Notes.
Mean (Standard Deviation);
Frequency (Corresponding percent) for number of completed phone contacts;
One-way ANOVA examining differences in number of completed phone contacts by experimental condition
Level of depressive symptoms
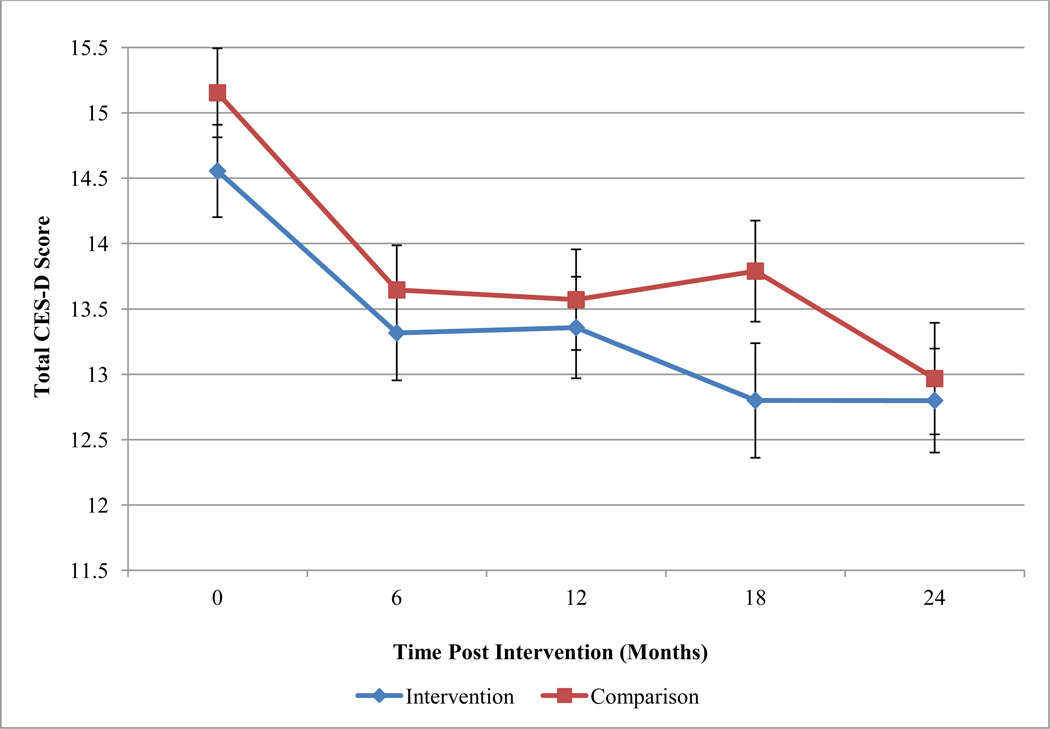
Descriptive statistics for unadjusted levels of depressive symptoms by condition at each time-point are displayed in Table 2. Table 2 also presents the frequency of participants endorsing elevated depressive symptoms (i.e., scores above the cut-off level). At baseline, 41.2% of participants endorsed elevated depressive symptoms. There were no significant bivariate differences between conditions at each assessment point for total depressive symptom levels or for the frequency of participants above the cut-off score (see Table 2). Figure 1 depicts the unadjusted mean level of depressive symptoms and corresponding standard errors by condition at each assessment point.
Table 2.
Depressive symptom levels at each assessment point by experimental condition and for the full sample
| Comparison Condition | ||||||||
|---|---|---|---|---|---|---|---|---|
| n | M (SD) | Freq. Elevatedb |
n | M (SD) | Freq. Elevatedb |
Total Score† | Frequency Elevated∞ |
|
| Time-point | ||||||||
| Baseline | 342 | 14.6 (6.3) | 38.9 (133) | 359 | 15.2 (6.7) | 43.5 (156) | F = 1.24, p = .27 | χ2 = 1.51, p = .13 |
| 6-Months | 278 | 13.3 (5.7) | 33.1 (92) | 268 | 13.6 (5.9) | 33.6 (90) | F = 0.51, p = .48 | χ2 = 0.02, p = .49 |
| 12-Months | 246 | 13.4 (6.0) | 30.1 (74) | 254 | 13.6 (6.2) | 31.5 (80) | F = 1.96, p = .16 | χ2 = 0.16, p = .38 |
| 18-Months | 230 | 12.8 (5.9) | 29.1 (67.9) | 233 | 13.8 (6.5) | 34.1 (79) | F = 1.75, p = .19 | χ2 = 1.28, p = .15 |
| 24-Months | 214 | 12.8 (6.2) | 27.1 (58) | 214 | 12.9 (5.8) | 30.8 (66) | F = 2.57, p = .11 | χ2 = 0.73, p = .23 |
Notes. Descriptive statistics reflect unadjusted depressive symptom levels.
Mean (Standard Deviation);
Freq. Elevated: % (corresponding n) of participants endorsing elevated depressive symptoms above the cut-off score of 15.; Depressive symptom levels as measured by the CES-D reflect unadjusted values;
One-way ANOVA examining differences in log-transformed depressive symptom level total score by experimental condition;
Chi-square analyses examining differences in frequency of elevated depressive scores by experimental condition.
Figure 1.
Depressive symptom levels by experimental condition.
Notes. Mean and standard error bars are depicted by experimental condition at each assessment point.
Change in Depressive Symptom Levels over Time by Condition
Table 3 presents results from GEE model. Participants in both conditions reported a 2.7% decrease in depressive symptoms at each 6-month interval (p = 0.001). Intervention condition participants also endorsed an additional 3.6% decrease in depressive symptoms relative to the comparison group (p = 0.058). Depressive symptoms levels were below the cut-off score at each follow-up assessment suggesting clinically meaningful change in depressive symptomatology levels over the 24-month follow-up period to below sub-threshold levels.
Table 3.
Intervention effects on depressive symptoms across the 24-month follow-up period
| Coefficient | Std. Error | p | 95% CI | ||
|---|---|---|---|---|---|
| Control Variable | |||||
| Age† | .015 | .126 | .906 | −.231 | .261 |
| Intervention Effects | |||||
| Assessment Wave | −.027 | .005 | .001 | −.037 | −.017 |
| Experimental Condition | −.036 | .019 | .058 | −.073 | .001 |
Notes. GEE model using Gaussian distributed individual random effects with an unstructured error structure between assessments. The fitted model was adjusted for the baseline measure of depressive symptoms.
Baseline age was log-transformed.
Discussion
Results highlight elevated levels of depressive symptoms among African-American female adolescents participating in an HIV prevention intervention. Indeed, over 40% of young women endorsed moderate to severe levels of depressive symptoms upon enrollment in a sexual health intervention. This finding coincides with previous studies noting elevated depressive symptoms among young African-American women in general (Khan et al., 2009), and specifically among young women seeking sexual health services (Collins et al., 2010; Fernandez et al., 2009). Thus, sexual health services and intervention programs have the opportunity to improve both the sexual and mental health of African-American young women.
The aim of the primary, group-delivered HIV prevention intervention (HORIZONS) received by both conditions was reducing sexual risk behaviors and improving psychosocial mediators of protective behaviors (e.g., sexual refusal self-efficacy). The primary treatment, HORIZONS, has demonstrated efficacy in the reduction of sexual risk behaviors and STIs (DiClemente et al., 2009). While the primary intervention does not specifically address mental health challenges or provide strategies to cope with depressive symptoms, participants in both study conditions reported decreased depressive symptoms over the two year follow-up period to sub-clinical levels. In contrast, the trajectory of depressive symptoms among adolescents is either curvilinear over time, with initial decreases in depressive symptoms followed by a return to baseline levels (Ge et al., 1994) or maintained at elevated levels among adolescents with moderate to severe depressive symptoms (Rushton, Forcier, & Schectman, 2002). This finding suggests that factors common between the two conditions (e.g., group intervention session followed by repeated phone contacts, provision of social support from peers and health educators, discussion of health-related information) may reduce depressive symptoms over time. Findings mirror Sales and colleagues’ finding that participation in an HIV risk reduction intervention for adolescent women with elevated baseline depressive symptomatology was associated with decreased depressive symptoms over a one-year follow-up period (Sales et al., 2010). Furthermore, the level of depressive symptom reduction seen in this sexual health intervention is on par with some depression-focused behavioral interventions (Ekers, Richards, & Gilbody, 2008). Additionally, interventions targeting depressive symptoms often reduce depressive symptoms initially, but then see depressive symptoms increase towards baseline levels over time (Horowitz & Garber, 2006). As shown in Figure 1, we did not observe a regression of depression symptoms towards baseline levels over time.
Overall improvement in depressive symptoms may be in part attributed to the social support provided through participation in the group HIV risk reduction intervention. The group-delivered format may have provided a forum to enhance support from other adolescent women. Support may have also been enhanced by participants’ relationship with the health educators leading the group and from the repeated phone contacts to discuss health-related information. For those in the intervention condition, this relationship with health educators was likely strengthened by repeated phone counseling sessions to address sexual health challenges. Thus, the therapeutic relationship established with the health educator may have served as a model for effective emotion regulation strategies (Brown et al., 2006). Alternatively, it has been suggested that increased depressive symptoms may be a consequence of engaging in sexual risk behaviors (Brown et al., 2006; Khan et al., 2009). Thus, participating in an effective HIV prevention program may have resulted in decreased sexual risk behaviors which thereby lessened depressive symptoms resulting as a consequence of engaging in risky behaviors. However, further research is needed to examine the mechanisms and mediating factors by which sexual health interventions impact depressive symptoms. For example, future investigations could examine the extent to which intervention content or intervention process variables (e.g., intervention format) affect depressive symptoms.
The supplemental intervention also focused exclusively on sexual health through tailored, individualized phone counseling. Despite a restricted focus on reducing HIV risk behaviors, participants who received more personalized sexual health counseling via the phone were marginally more likely to experience decreased depressive symptoms relative to participants who received calls that only focused upon general health promotion. The personalized phone counseling may have improved participants’ ability to cope effectively with sexual health challenges and subsequently also decrease overall level of depressive symptomatology (Lennon et al., 2012). The sexual health focused sessions may have also increased participants’ ability to correctly appraise sexual health risks and enhanced their self-efficacy to engage in protective sexual behaviors. Thus, modifying potential mechanisms underlying the association between depression and sexual risk behaviors may partially explain the additional decrease in depressive symptoms among those receiving sexual health phone counseling contacts. Future research should further explore whether differences in the content of repeated, personalized phone counseling sessions result in differential changes in mental health outcomes.
Strengths and Limitations
This study is one of the first to investigate the extent to which HIV prevention interventions affect depressive symptomatology among adolescents. This study enrolled a large sample of African- American adolescent women who are at increased risk for both adverse sexual and mental health outcomes. A third strength was the high retention across the two-year study period. Depressive symptoms were assessed using a standardized, validated measure at every assessment point. Additionally, the study employed a time- and dose-equivalent supplemental treatment providing further support that differences between study conditions were not attributable to differences in intervention dosage.
There are also limitations that should be acknowledged. Results were limited by use of single self-reported measure of depressive symptoms. While the CES-D has been correlated with depressive disorder diagnoses, a clinical diagnosis was not established by a mental health professional (Santor & Coyne, 1997). In addition, this sample consisted of urban African-American female adolescents recruited from sexual health clinics. Therefore, results may not generalize to other non-clinic recruited adolescent populations.
Conclusions
African-American young women are at increased risk for experiencing depressive symptoms and adverse sexual health outcomes. This HIV prevention program included no specific mental health content and was delivered by non-mental health practitioners, yet the intervention produced an additional benefit of decreasing depressive symptoms over a period of two years. Results suggest that young women seeking sexual health services would benefit from additional resources and skills training to address mental health challenges and depressive symptoms. Sexual health services and sexual risk reduction interventions may provide an optimal forum for addressing mental health issues. Such programming or services may benefit from additional screening of mental health symptoms. Sexual health interventions should incorporate content to specifically address co-occurring mental health problems. For example, interventions may benefit from inclusion of content to improve coping and self-management strategies (Lennon et al., 2012). Ultimately, provision of holistic intervention approaches that address multi-faceted health issues offer the potential of improving adolescents’ health.
Acknowledgements
This research was supported by a grant from the National Institute of Mental Health (5R01 MH070537) to the fifth author. Additional support was provided by the Emory Center for AIDS Research (P30 AI050409) and the Center for Contextual Genetics & Prevention Science (P30 DA027827). Jennifer L. Brown was supported by K12 GM000680 from the National Institute of General Medical Sciences. Jessica M. Sales was supported by K01 MH085506 from the National Institute of Mental Health. Andrea Swartzendruber was supported by F32 AA022058 from the National Institute on Alcohol Abuse and Alcoholism.
References
- Beck JS. Cognitive therapy: Basics and beyond. New York, NY: Guilford Press; 1995. [Google Scholar]
- Bower P, Garralda E, Kramer T, Harrington R, Sibbald B. The treatment of child and adolescent health problems in primary care: A systematic review. Family Practice. 2001;18(4):373–382. doi: 10.1093/fampra/18.4.373. [DOI] [PubMed] [Google Scholar]
- Brown JL, Vanable PA. The effects of assessment mode and privacy level on self-reports of risky sexual behaviors and substance use among young women. Journal of Applied Social Psychology. 2009;39(11):2756–2778. [Google Scholar]
- Brown LK, Tolou-Shams M, Lescano C, Houck C, Zeidman J, Pugatch D, Lourie KJ. Depressive symptoms as a predictor of sexual risk among African American adolescents and young adults. The Journal of Adolescent Health. 2006;39(3):444, e441–e448. doi: 10.1016/j.jadohealth.2006.01.015. [DOI] [PubMed] [Google Scholar]
- CDC. Chlamydia screening among sexually active young female enrollees of health plans--—United States, 2000–2007. Morbidity and Mortality Weekly Report. 2009;58(14):362–365. [PubMed] [Google Scholar]
- CDC. Sexually Transmitted Disease Surveillance. Vol. 2011. Atlanta: U.S. Department of Health and Human Services; 2012a. [Google Scholar]
- CDC. Diagnoses of HIV infection and AIDS among adolescents and young adults in the United States and 5 U.S. dependent areas, 2006–2009. HIV Surveillance Supplemental Report. 2012b;17(2) [Google Scholar]
- Cheung AH, Dewa CS. Mental health service use among adolescents and young adults with major depressive disorder and suicidality. The Canadian Journal of Psychiatry. 2007;52(4):228–232. doi: 10.1177/070674370705200404. [DOI] [PubMed] [Google Scholar]
- Collins MH, Kelch-Oliver K, Johnson K, Welkom J, Kottke M, Smith CO. Clinically Significant Depressive Symptoms in African American Adolescent Females in an Urban Reproductive Health Clinic. Journal of Clinical Psychology in Medical Settings. 2010;17(3):175–182. doi: 10.1007/s10880-010-9200-9. [DOI] [PubMed] [Google Scholar]
- Datta SD, Sternberg M, Johnson RE, Berman S, Papp JR, McQuillan G, Weinstock H. Gonorrhea and chlamydia in the United States among persons 14 to 39 years of age, 1999 to 2002. Annals of Internal Medicine. 2007;147(2):89–96. doi: 10.7326/0003-4819-147-2-200707170-00007. [DOI] [PubMed] [Google Scholar]
- DiClemente RJ. Brief cell-phone delivered counseling as a novel strategy to enhance maintenance of HIV behavioral intervention efficacy: results from a supplemental treatment effectiveness trial; Paper presented at the International AIDS Conference; Vienna, Austria. 2010. [Google Scholar]
- DiClemente RJ, Wingood GM, Rose ES, Sales JM, Lang DL, Caliendo AM, Crosby RA. Efficacy of sexually transmitted disease/human immunodeficiency virus sexual risk-reduction intervention for african american adolescent females seeking sexual health services: a randomized controlled trial. Archives Of Pediatrics & Adolescent Medicine. 2009;163(12):1112–1121. doi: 10.1001/archpediatrics.2009.205. [DOI] [PubMed] [Google Scholar]
- Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychological Medicine. 2008;38(5):611–623. doi: 10.1017/S0033291707001614. [DOI] [PubMed] [Google Scholar]
- Elkington KS, Bauermeister JA, Zimmerman MA. Psychological distress, substance use, and HIV/STI risk behaviors among youth. Journal of Youth and Adolescence. 2010;39(5):514–527. doi: 10.1007/s10964-010-9524-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elster A, Jarosik J, VanGeest J, Fleming M. Racial and ethnic disparities in health care for adolescents: a systematic review of the literature. Archives of Pediatrics & Adolescent Medicine. 2003;157(9):867–874. doi: 10.1001/archpedi.157.9.867. [DOI] [PubMed] [Google Scholar]
- Fernandez V, Kramer T, Fong G, Doig A, Garralda ME. Depressive symptoms and behavioural health risks in young women attending an urban sexual health clinic. Child Care, Health & Development. 2009;35(6):799–806. doi: 10.1111/j.1365-2214.2009.00982.x. [DOI] [PubMed] [Google Scholar]
- Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. New York, NY: John Wiley & Sons; 2003. [Google Scholar]
- Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sexually Transmitted Infections. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, Simons RL. Trajectories of stressful life events and depressive symptoms during adolescence. Developmental Psychology. 1994;30(4):467–483. [Google Scholar]
- Hardin JW, Hilbe JM. Generalized estimating equations. New York, NY: Chapman & Hall/CRC; 2003. [Google Scholar]
- Holden AEC, Shain RN, Miller WB, Piper JM, Perdue ST, Thurman AR, Korte JE. The influence of depression on sexual risk reduction and STD infection in a controlled, randomized intervention trial. Sexually Transmitted Diseases. 2008;35(10):898–904. doi: 10.1097/OLQ.0b013e31817d7a33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook EW, Handsfield HH. Gonococcal infections in the adult. In: Holmes KK, Sparling P, Markh P, editors. Sexually Transmitted Diseases. 3rd ed. New York: McGraw-Hill; 1999. pp. 451–466. [Google Scholar]
- Horowitz JL, Garber J. The prevention of depressive symptoms in children and adolescents: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2006;74(3):401–415. doi: 10.1037/0022-006X.74.3.401. [DOI] [PubMed] [Google Scholar]
- Khan MR, Kaufman JS, Pence BW, Gaynes BN, Adimora AA, Weir SS, Miller WC. Depression, sexually transmitted infection, and sexual risk behavior among young adults in the United States. Archives of Pediatrics & Adolescent Medicine. 2009;163(7):644–652. doi: 10.1001/archpediatrics.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosunen E, Kaltiala-Heino R, Rimpelä M, Laippala P. Risk-taking sexual behaviour and selfreported depression in middle adolescence - a school-based survey. Child: Care, Health and Development. 2003;29(5):337–344. doi: 10.1046/j.1365-2214.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- Lachin JM. Statistical considerations in the intent-to-treat principle. Controlled Clinical Trials. 2000;21(3):167–189. doi: 10.1016/s0197-2456(00)00046-5. [DOI] [PubMed] [Google Scholar]
- Lee SH, O'Riordan MA, Lazebnik R. Relationships among depressive symptoms, sexually transmitted infections, and pregnancy in African-American adolescent girls. Journal of Pediatric and Adolescent Gynecology. 2009;22(1):19–23. doi: 10.1016/j.jpag.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Lehrer JA, Shrier LA, Gortmaker S, Buka S. Depressive symptoms as a longitudinal predictor of sexual risk behaviors among US middle and high school students. Pediatrics. 2006;118(1):189–200. doi: 10.1542/peds.2005-1320. [DOI] [PubMed] [Google Scholar]
- Lennon CA, Huedo-Medina TB, Gerwien DP, Johnson BT. A role for depression in sexual risk reduction for women? A meta-analysis of HIV prevention trials with depression outcomes. Social Science & Medicine. 2012;75(4):688–698. doi: 10.1016/j.socscimed.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- Locke EA, Latham GP. Building a practically useful theory of goal setting and task motivation: A 35-year odyssey. American Psychologist. 2002;57(9):705–717. doi: 10.1037//0003-066x.57.9.705. [DOI] [PubMed] [Google Scholar]
- Mazzaferro KE, Murray PJ, Ness RB, Bass DC, Tyus N, Cook RL. Depression, Stress, and Social Support as Predictors of High-Risk Sexual Behaviors and STIs in Young Women. Journal of Adolescent Health. 2006;39(4):601–603. doi: 10.1016/j.jadohealth.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Milhausen RR, Yarber WL, Crosby R. Self-reported depression and sexual risk behaviors among a national sample of rural high school students. The Health Education Monograph Series. 2003;20(2):33–39. [Google Scholar]
- Paxton KC, Robinson WL. Depressive symptoms, gender, and sexual risk behavior among African-American adolescents: implications for prevention and intervention. Journal of Prevention & Intervention In The Community. 2008;35(2):49–62. doi: 10.1300/j005v35n02_05. [DOI] [PubMed] [Google Scholar]
- Piantadosi S. Clinical trials: A methodologic perspective. New York, NY: John Wiley & Sons, Inc; 1997. [Google Scholar]
- Pocock SJ. Clinical trials. New York, NY: John Wiley & Sons; 1993. [Google Scholar]
- Rangel MC, Gavin L, Reed C, Fowler MG, Lee LM. Epidemiology of HIV and AIDS among adolescents and young adults in the United States. The Journal of Adolescent Health. 2006;39(2):156–163. doi: 10.1016/j.jadohealth.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Statistics in Medicine. 1998;17(14):1643–1658. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Rubin AG, Gold MA, Primack BA. Associations between depressive symptoms and sexual risk behavior in a diverse sample of female adolescents. Journal of Pediatric And Adolescent Gynecology. 2009;22(5):306–312. doi: 10.1016/j.jpag.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD. Adolescent depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd ed. New York, NY: The Guilford Press; 2008. pp. 444–446. [Google Scholar]
- Rushton JL, Forcier M, Schectman RM. Epidemiology of depressive symptoms in the National Longitudinal Study of Adolescent Health. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(2):199–206. doi: 10.1097/00004583-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Sales JM, Lang DL, Hardin JW, DiClemente RJ, Wingood GM. Efficacy of an HIV prevention program among African American female adolescents reporting high depressive symptomatology. Journal of Women's Health. 2010;19(2):219–227. doi: 10.1089/jwh.2008.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santor DA, Coyne JC. Shortening the CES-D to improve its ability to detect cases of depression. Psychological Assessment. 1997;9(3):223–243. [Google Scholar]
- Seth P, Raiji PT, DiClemente RJ, Wingood GM, Rose E. Psychological distress as a correlate of a biologically confirmed STI, risky sexual practices, self-efficacy and communication with male sex partners in African-American female adolescents. Psychology, Health & Medicine. 2009;14(3):291–300. doi: 10.1080/13548500902730119. [DOI] [PubMed] [Google Scholar]
- Shrier LA, Schillinger JA, Aneja P, Rice PA, Batteiger BE, Braslins PG, Fortenberry JD. Depressive symptoms and sexual risk behavior in young, chlamydia-infected, heterosexual dyads. The Journal of Adolescent Health. 2009;45(1):63–69. doi: 10.1016/j.jadohealth.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Smith PB, Buzi RS, Weinman ML. Mental health screening in family-planning clinics: a sexual risk-reduction opportunity. Journal of Sex & Marital Therapy. 2010;36(3):181–192. doi: 10.1080/00926231003719475. [DOI] [PubMed] [Google Scholar]
- Stamm WE. Chlamydia trachomatis infections of the adult. In: Holmes KK, Sparling P, Mardh P, editors. Sexually Transmitted Diseases. 3rd ed. New York: McGraw-Hill; 1999. pp. 407–422. [Google Scholar]
- Strecher VJ, Seijts GH, Kok GJ, Latham GP. Goal setting as a strategy for health behavior change. Health Education Quarterly. 1995;22(2):190–200. doi: 10.1177/109019819502200207. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The NSDUH Report: Major Depressive Episode and Treatment among Adolescents. Rockville, MD: 2009. [Google Scholar]
- Swartzendruber A, Sales JM, Brown JL, Davis TL, Diclemente RJ, Rose E. Predictors of repeat Chlamydia trachomatis and/or Neisseria gonorrhoeae infections among African-American adolescent women. Sexually Transmitted Infections. 2012 doi: 10.1136/sextrans-2012-050530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolou-Shams M, Brown LK, Houck C, Lescano CM. The Association Between Depressive Symptoms, Substance Use, and HIV Risk Among Youth With an Arrest History. Journal of Studies on Alcohol & Drugs. 2008;69(1):58–64. doi: 10.15288/jsad.2008.69.58. [DOI] [PubMed] [Google Scholar]
- Turner C, Ku L, Rogers S, Lindberg L, Pleck J, Sonenstein F. Adolescent sexual behavior, drug use, and violence: Increased reporting with computer survey technology. Science. 1998;280:867–871. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- Walker Z, Townsend J. Premoting adolescent mental health in primary care: A review of the literature. Journal of Adolescence. 1998;21:621–634. doi: 10.1006/jado.1998.0183. [DOI] [PubMed] [Google Scholar]
- Wilson K, Asbridge M, Kisely S, Langille D. Associations of risk of depression with sexual risk taking among adolescents in Nova Scotia high schools. The Canadian Journal of Psychiatry / La Revue canadienne de psychiatrie. 2010;55(9):577–585. doi: 10.1177/070674371005500906. [DOI] [PubMed] [Google Scholar]