Abstract
Photooxidation of A2E may be involved in diseases of the macula and antioxidants could serve as therapeutic agents for these diseases. Inhibitors of A2E photooxidation were prepared by Mannich reaction of the antioxidants quercetin and sesamol. These compounds contain water-solubilizing amine groups and several were more potent inhibitors of A2E photooxidation than quercetin.
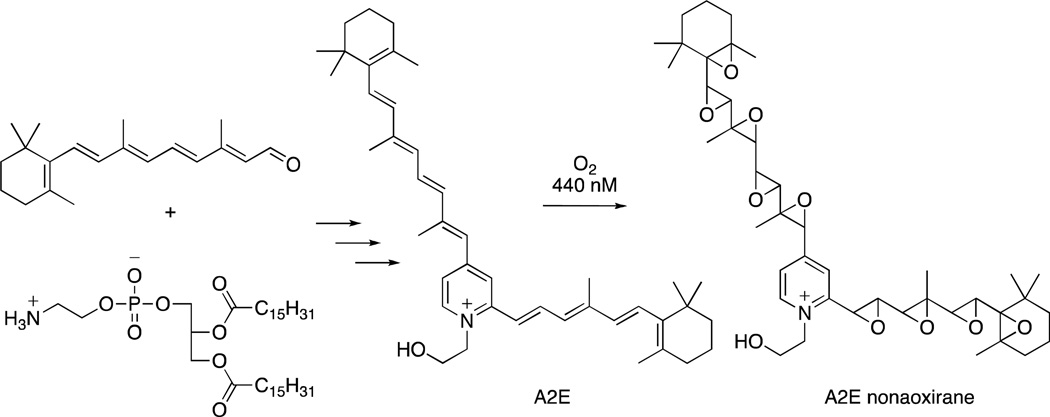
A2E, a pigment of the lipofuscin of retinal pigment cells, is thought to play a role in diseases of the retina such as macular degeneration, and methods to prevent its formation, or ameliorate its destructive effects, have been proposed as therapeutic approaches.1–3 A2E is a pyridinium bis-retinoid4,5 and is formed intracellularly from retinal and phosphatidylethanolamine6 (Figure 1). Studies have shown that A2E photosensitizes singlet oxygen production upon irradiation in the visible region leading to formation of autoxidation products, including the nonaoxirane shown in Figure 1, resulting from epoxidation of the A2E olefins.7,8 These oxidation products have been proposed to lead to cellular damage and death.2,9 Antioxidants, such as anthocyanins isolated from bilberry, vitamin E, and resveratrol, can inhibit A2E autoxidation.9 In an effort to prepare more stable and effective antioxidants than the anthocyanins, a recent study from our laboratories utilized quercetin linked to antioxidants such as curcumin and caffeic acid to inhibit A2E oxidation.10
Figure 1.
Formation of A2E from retinal and phosphatidylethanolamine and oxidation to A2E nonaoxirane.
The Mannich reaction is a versatile reaction that leads to the incorporation of amines into organic molecules. Amines have been used extensively as water-solubilizing groups in drugs to improve physicochemical properties (e.g. solubility) leading to improved bioavailability and formulation. We have used the Mannich reaction to prepare compounds that combine multiple antioxidants with water-solubilizing amine groups. These substances have been evaluated in non-cellular and intracellular assays of A2E photooxidation and shown to prevent irradiation-induced destruction of A2E.
Irradiation of A2E at its absorption maximum of 440 nm leads to singlet oxygen generation and subsequent oxidation of A2E. The epoxide oxidation products of A2E are hypothesized to act as destructive agents within cells causing cell damage and death and may be responsible for several diseases of the retina. A potential treatment for retinal damage would be to inhibit the oxidation of A2E with antioxidants and several natural products and their synthetic derivatives have been shown to inhibit photooxidation of A2E.9,10 We prepared previously analogues wherein quercetin, caffeic acid, and curcumin were linked through aliphatic groups for this purpose.10 A different approach to covalent modification is utilized in the present study, where the Mannich reaction is used to connect antioxidants through amine linkers. The chemistry in this approach is straightforward and leads to analogues containing water-solubilizing amines, which are found in many therapeutic agents and confer desirable physicochemical properties and improved bioavailability and formulation.
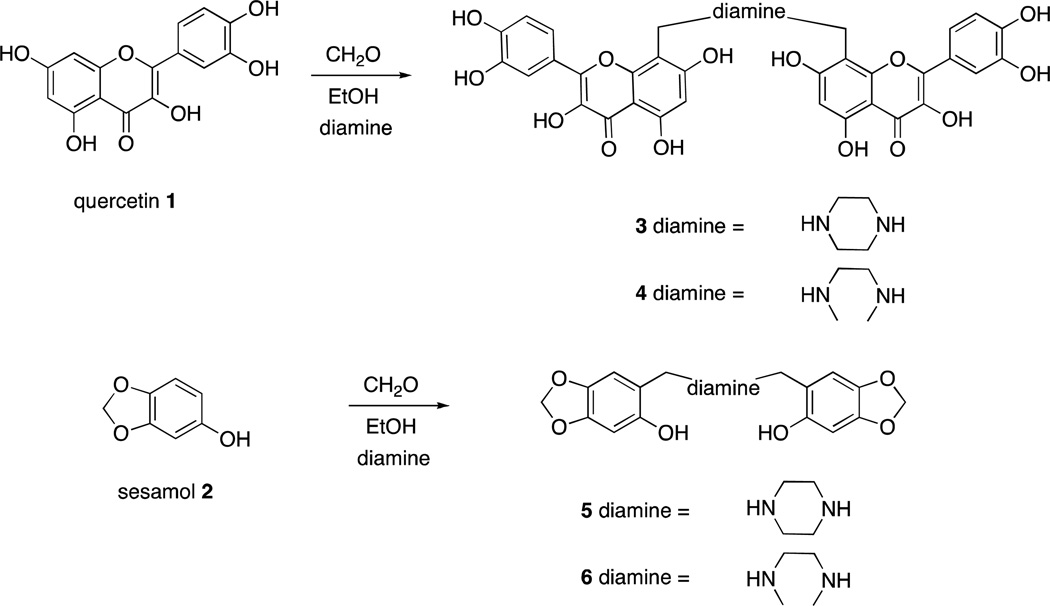
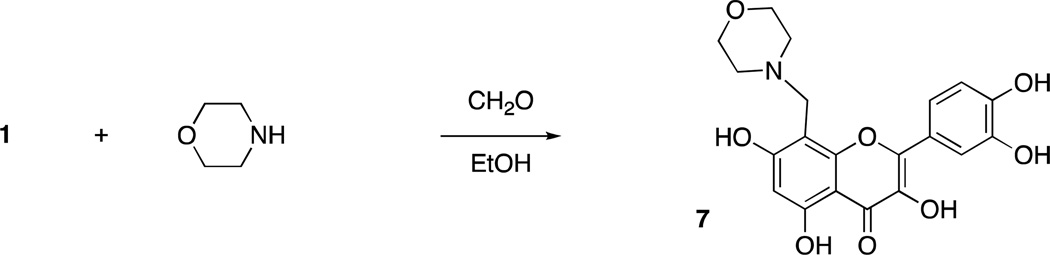
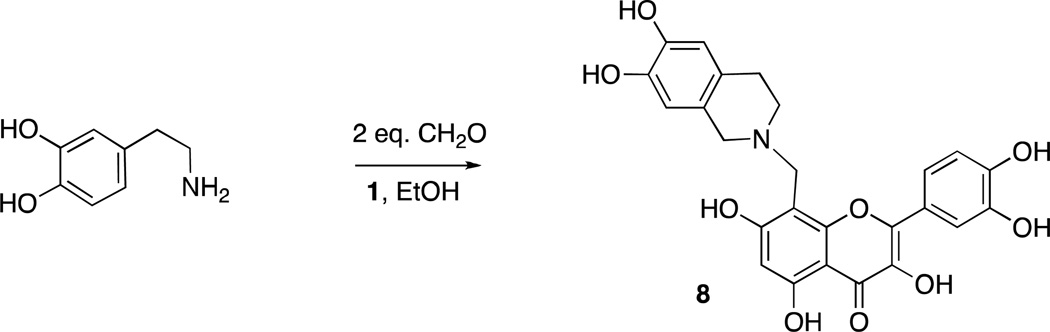
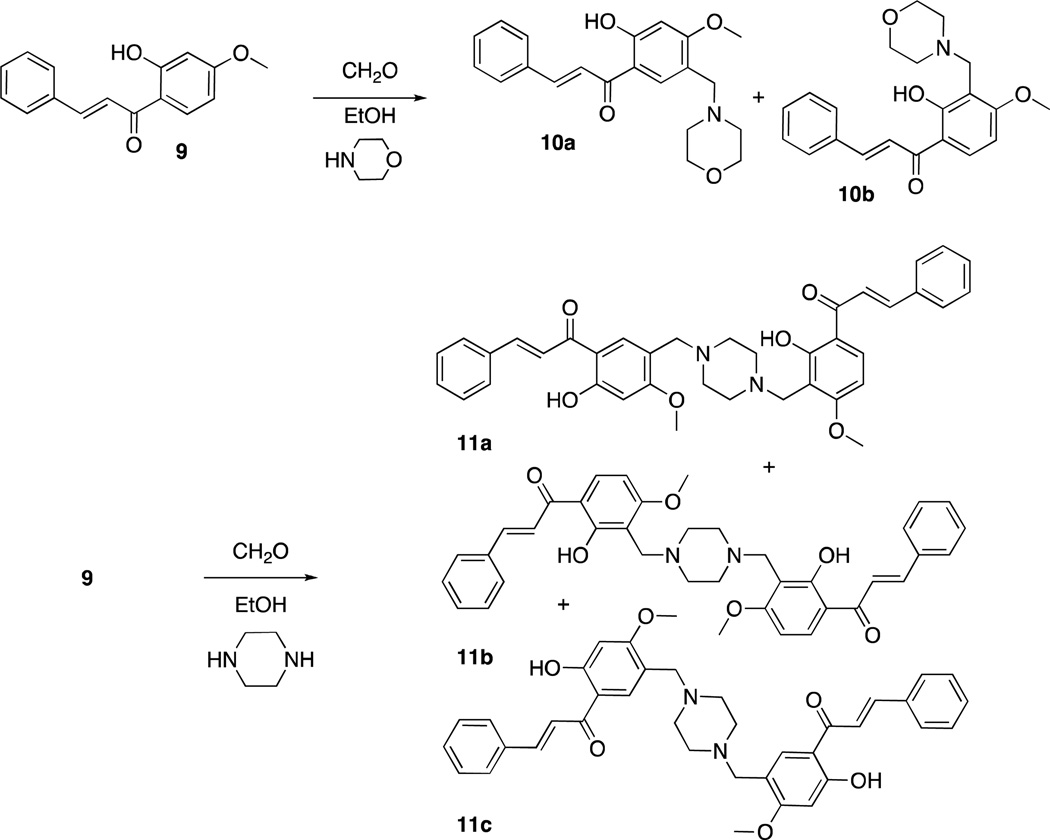
Quercetin11 (1) and sesamol12 (2) can undergo regioselective Mannich reactions under certain conditions and we were able to selectively synthesize dimers of quercetin (3 and 4) and sesamol (5 and 6), by utilizing diamines (piperazine or N,N-dimethylethylenediamine, respectively) in the Mannich reaction of quercetin and sesamol with formaldehyde (Scheme 1). Reaction of quercetin with morpholine and formaldehyde regioselectively gave the Mannich adduct 7 (Scheme 2).11 Mannich reaction of quercetin (1) with dopamine occurred with concomitant Pictet-Spengler reaction of dopamine and formaldehyde to give compound 8 (Scheme 3). Whereas sesamol (2) and quercetin (1) underwent Mannich reaction regioselectively at room temperature with stoichiometric quantities of base and formaldehyde, catechol 9 required elevated temperatures and excess reagents and gave equimolar mixtures of regioisomers 10a and 10b when morpholine was used as the amine (Scheme 4). When treated with piperazine and formaldehyde, three possible regioisomeric dimers of the catechol were obtained (11a – c), as expected.
Scheme 1.
Synthesis of homodimeric antioxidants of quercetin (1) and sesamol (2).
Scheme 2.
Regioselective formation of morpholine/quercetin adduct 7.
Scheme 3.
Synthesis of quercetin/tetrahydroisoquinoline conjugate 8.
Scheme 4.
Mannich reaction of catechol 9.
Only antioxidants containing quercetin (3, 4, 8, and 9) showed sufficient antioxidant ability to inhibit non-cellular and intracellular photooxidation of A2E (Figure 2). While sesamol and catechol are known antioxidants, they were ineffective inhibitors of A2E photooxidation at 200 µM. This finding is consistent with the lack of ortho phenolic groups in sesamol and catechol and the correlation of ortho phenolic groups with antioxidant capacity.13 The quercetin-containing analogues were tested at 50 µM, 100 µM, and 200 µM concentrations and compared with quercetin at the same concentration (Figure 2B). A 400 µM quercetin sample was also tested to allow for comparison with the quercetin dimers 3 and 4 at 200 µM. Interestingly, while 7 and 8 showed inhibition and dose response curves comparable to that of quercetin, compounds 3 and 4 gave shallow dose response curves, so that the inhibition of A2E oxidation at 50 µM was comparable to that of quercetin at 200 µM. This is twice the antioxidant capacity that would be expected based on the stoichiometry of two quercetin units per molecule for 3 and 4. Similarly, single concentration determinations at 100 µM showed that compounds 3 and 4 outperformed quercetin in inhibiting A2E photooxidation. (Figure 2A). That compounds 3 and 4 contain a diamine (piperazine or N1,N2–dimethylethane-1, 2-diamine, respectively) may explain the increase in antioxidant ability. Phenolic compounds have been shown to be better antioxidants as the corresponding phenolate ions at basic pH.14 Hence, the basicity of the diamine group in proximity to the phenolic hydroxy groups may give rise to the increased antioxidant effect seen in the present investigation.
Figure 2.
Protective effects of antioxidants on the photooxidation of A2E. (A) Comparison of the inhibition of in vitro (non-cellular) A2E photooxidation by quercetin (1) and compounds 3, 4, 7, and 8 at 100 µM. (B) Inhibition of in vitro (non-cellular) A2E photooxidation upon varying the concentrations of antioxidants 3, 4, 7, 8, and quercetin (1). (C) Comparison of the cellular (RPE cells accumulated antioxidant from a 20 µM concentration in medium) inhibition of A2E photooxidation by compounds 3, 4, 7, 8, and quercetin (1).
To evaluate the efficacy of these compounds in inhibiting intracellular photooxidation of A2E, human adult RPE cells (ARPE-19 cell line) were allowed to accumulate A2E by incubation with 10 µM A2E. Subsequently, cells were allowed to accumulate antioxidants 3, 4, 7, 8, and quercetin (1) by treatment of the cells with 20 µM solution. Irradiation of the cells at 430 nm led to inhibition of A2E photooxidation as determined by quantitation of cellular A2E levels. The results obtained (Figure 2C) are very similar to those obtained in vitro (non-cellular) (Figure 2A) indicating that the compounds are able to penetrate the cells to provide protection to intracellular A2E upon irradiation.
In conclusion, the Mannich reaction has been instrumental in the design and synthesis of novel antioxidants. Several of these compounds containing quercetin were potent inhibitors of A2E photooxidation and superior to quercetin itself. The water solubilizing amine groups in these antioxidants were designed to provide drug-like physicochemical properties and may be responsible for the improved antioxidant activity.
EXPERIMENTAL SECTION
General Experimental Procedures
A2E was prepared from retinal and ethanolamine by the method described in the literature.15 1H NMR spectra were recorded with a Bruker DRX300 or Bruker Avance III 500 instrument. 13C NMR spectra were recorded with a Bruker Avance III 500 instrument. Chemical shift values are given in δ (ppm) using the peak signals of the solvent DMSO-d6 (δH 2.50 and δC 39.50) as references, and coupling constants are reported in Hz. FAB MS (3KV Xe beam) data were measured with an HX110 JEOL Ltd (Tokyo Japan) Double Focusing Sector type Mass Spectrometer. Column chromatography was performed on silica gel (particle size 40−63 µm) (Sorbent Technologies, Atlanta, GA, USA) and TLC plates (w/UV 254) were used for fraction and compound detection. The spots were visualized using UV light at 254 nm. All final compounds were >95% pure as determined by analytical reversed-phase HPLC.
Analytical reversed-phase HPLC-measurements were carried out on an Alliance System (Waters Corp., Milford, MA) equipped with 2695 separation module, 2996 photodiode array detector, and a 2475 multi-λ fluorescence detector. For chromatographic separation, an analytical scale Atlantis dC18 (3 µm, 4.6 mm × 150 mm, Waters) column was utilized with an acetonitrile and water gradient and 0.1% trifluoroacetic acid (85–100%, 0.8 mL/min 25 min; 100% acetonitrile, 0.8 mL/min 15 min; monitoring at 430 nm; 20 µL injection volume). Peak area was determined using Empower (Waters) software. Alternatively, analytical reversed-phase HPLC-measurements were carried out on a JASCO System equipped with MD-1510 multiwavelength detector. For chromatographic separation, an analytical scale Thermo Scientific Hypersil Gold C18 (150 × 4.6 mm) column was utilized with an acetonitrile and water gradient and 0.1% trifluoroacetic acid and 80 µL injection volume monitoring at 440 nm. Peak area was determined using ChromNAV (JASCO) software.
8,8'-(Piperazine-1,4-diylbis(methylene))bis(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen- 4-one (3)
To a solution of quercetin dihydrate (200 mg, 0.59 mmol) and piperazine (26 mg, 0.30 mmol) in ethanol (5 mL) was added 37% aqueous formaldehyde (0.044 mL, 0.59 mmol). A precipitate formed slowly over 30 min. After 48 h the reaction mixture was filtered to provide the product as a yellow powder (185 mg, 85% yield). 1H NMR (DMSO-d6) δ 2.63 (8H, s, bd), 3.88 (4H, s), 6.19 (2H, s,), 6.89 (2H, d, J = 9.0 Hz), 7.58 (2H, dd, J = 2.1, 9.0 Hz), 7.71 (2H, d, J = 2.1 Hz), 12.52 (2H, s, bd); 13C NMR (DMSO-d6) δ 50.9, 52.1, 98.1, 99.9, 102.8, 115.0, 115.7, 119.9, 122.2, 135.7, 145.1, 146.5, 147.7, 153.8, 159.6, 164.0, 176.0; MS m/z MH+ = 715.
8,8'-(Ethane-1,2-diylbis(methylazanediyl))bis(methylene)bis(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one) (4)
To a solution of quercetin dihydrate (100 mg, 0.30 mmol) and N1,N2-dimethylethane-1,2-diamine (0.036 mL, 0.33 mmol) in ethanol (2 mL) was added 37% aqueous formaldehyde (0.041 mL, 0.33 mmol). After 48 h the resulting precipitate was collected to provide the product as a yellow powder. 1H NMR (DMSO-d6) δ 2.29 (6H, s), 2.86 (4H, s), 3.93 (4H, s), 6.10 (2H, s), 6.86 (2H, d, J = 8.5 Hz), 7.53 (2H, d, J = 8.5 Hz), 7.67 (2H, s), 12.53 (2H, s, bd); 13C NMR (DMSO-d6) 41.1, 50.5, 51.4, 53.9, 79.4, 98.8, 99.6, 101.8, 114.9, 115.5, 119.9, 122.3, 135.4, 145.4, 146.2, 147.7, 154.3, 160.0, 166.8, 175.6; MS m/z MH+ = 717.
6,6'-(Piperazine-1,4-diylbis(methylene))dibenzo[d][1,3]dioxol-5-ol (5)
To a solution of sesamol (200 mg, 1.44 mmol) and piperazine (62 mg, 0.72 mmol) in ethanol was added 37% aqueous formaldehyde (0.108 mL, 1.44 mmol) over 1 min. After 2 – 3 min, a precipitate formed. After 18 h, the reaction mixture was filtered and the resulting solid washed with ethanol. The product was obtained as a tan solid (213 mg, 77% yield). 1H NMR (DMSO-d6) δ 2.4 (8H, s, bd), 3.50 (4H, s), 5.87 (4H, s), 6.37 (2H, s), 6.65 (2H, s); 13C NMR (DMSO-d6) δ 51.9, 58.1, 97.7, 100.5, 108.8, 113.5, 139.5, 146.7, 151.5; MS m/z M+ = 386.
6,6'-(Ethane-1,2-diylbis(methylazanediyl))bis(methylene)dibenzo[d][1,3]dioxol-5-ol (6)
To a solution of sesamol (100 mg, 0.72 mmol) and N1,N2-dimethylethane-1,2-diamine (0.039 mL, 0.36 mmol) in ethanol was added 37% aqueous formaldehyde (0.054 mL, 0.72 mmol). The resulting precipitate was collected to provide the product as a powder (62 mg, 44% yield). 1H NMR (DMSO-d6) δ 2.14 (6H, s), 2.54 (4H, s), 3.49 (4H, s), 5.87 (4H, s), 6.37 (2H, s), 6.67 (2H, s); 13C NMR (DMSO-d6) δ 41.0, 53.3, 58.0, 97.7, 100.4, 108.8, 114.3, 139.4, 146.6, 151.7; MS m/z MH+ = 389.
8-((6,7-Dihydroxy-3,4-dihydroisoquinolin-2(1H)-yl)methyl)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one (8)
To a solution of quercetin dihydrate (100 mg, 0.30 mmol) and dopamine hydrochloride (63 mg, 0.33 mmol) in ethanol (2 mL) was added triethylamine (0.046 mL, 0.33 mmol) followed by 37% aqueous formaldehyde (0.025 mL, 0.33 mmol). After 72 hours the reaction was filtered to provide a brown powder (68 mg). Silica gel chromatography (20% MeOH in CH2Cl2) provided the product as a yellow solid (38 mg, 43%). 1H NMR (DMSO-d6, 300 MHz) δ 2.73 (2H, s, bd) 3.00 (2H, s, bd) 3.79 (2H, s, bd), 4.16 (2H, s, bd), 6.23 (1H, s), 6.43 (1H, s), 6.49 (1H, s), 6.88 (1H, d, J = 8.4 Hz), 7.55 (1H, dd, J = 1.8, 8.4 Hz), 7.74 (1H, d, J = 1.8 Hz), 12.56 (1H, s, bd); MS m/z MH+ = 480.
(E)-1-(2-Hydroxy-4-methoxy-5-(morpholinomethyl)phenyl)-3-phenylprop-2-en-1-one (10a) and (E)-1-(2-hydroxy-4-methoxy-3-(morpholinomethyl)phenyl)-3-phenylprop-2-en-1-one (10b)
To a solution of 9 (0.443 g, 1.74 mmol) in isopropanol (5.0 mL) was added paraformaldehyde (0.131 g, 4.37 mmol) and morpholine (330 µL, 3.52 mmol). The mixture was refluxed for 6 h and then stirred at room temperature overnight to give a yellow precipitate (489 mg). Silica gel chromatography (EtOAc/CH2Cl2) gave 10a as a yellow solid (151 mg, 25% yield).16 Further elution of the column with MeOH/EtOAc gave 10b as a yellow solid (139 mg, 23% yield). 1H NMR of 10a δ 2.41-2.38 (4H, m), 3.46 (1H, s), 3.57-3.54 (4H, m), 3.89 (3H, s), 6.57 (1H, s), 7.59-7.48 (3H, m), 7.82 (1H, d, J =15.5 Hz), 7.92-7.89 (2H, m), 7.97 (1H, d, J = 15.5), 8.12 (1H, s), 13.39 (1H, s); MS of 10a m/z MH+ = 354. 1H NMR of 10b (DMSO-d6) δ 2.41 (4H, s, bd), 3.51–3.53 (4H, m), 3.55 (2H, s), 3.90 (3H, s), 6.72 (1H, d, J = 9.1 Hz), 7.48-7.47 (3H, m), 7.82 (1H, d, J = 15.5 Hz), 7.91-7.90 (2H, m), 8.03 (1H, d, J = 15.5 Hz), 8.29 (1H, d, J = 9.0 Hz). 13C NMR of 10b (DMSO-d6) δ 49.27, 53.06, 56.15, 66.20, 102.86, 111.65, 114.59, 121.76, 128.95, 129.08, 130.78, 132.34, 134.59, 143.92, 163.23, 164.17, 192.06; MS of 10b m/z MH+ = 354.
(E)-1-(2-Hydroxy-3-((4-(4-hydroxy-2-methoxy-5-((E)-3-phenylprop-2-enoyl)benzyl)piperazin-1-yl)methyl)-4-methoxyphenyl)-3-phenylprop-2-en-1-one (11a), (2E,2'E)-1,1'-(3,3'-(piperazine-1,4-diylbis(methylene))bis(2-hydroxy-4-methoxy-3,1-phenylene))bis(3-phenylprop-2-en-1-one) (11b) and (2E,2'E)-1,1'-(5,5'-(piperazine-1,4-diylbis(methylene))bis(2-hydroxy-4-methoxy-5,1-phenylene))bis(3-phenylprop-2-en-1-one) (11c)
To a solution of 9 (63 mg, 0.247 mmol) in isopropanol (3.0 mL) was added paraformaldehyde (12.0 mg, 0.40 mmol) and anhydrous piperazine (15.0 mg, 0.174 mmol). The mixture was refluxed for 5 h and then stirred at room temperature overnight. The resulting precipitate was collected to give a mixture of regioisomers as a yellow powder (41 mg). MS m/z MH+ = 619.
Non-cellular Photooxidation of A2E Assay
To test the effect of antioxidants on A2E photooxidation 100 µL of A2E stock (200 µM in DMSO) was added to 200 µL DPBS as described in the literature.9 To these samples, 1.5 µL of antioxidant (20 µM in DMSO) was added to obtain a final concentration of 100 µM. These mixtures were irradiated (430 nm; light intensity of 2.8 mW/cm2) for 5 min. Samples were analyzed by reversed-phase HPLC with sample preparation and injection performed under dim red light. To test the effects of varying concentrations of antioxidants on A2E photooxidation the appropriate volume of antioxidant solution (20 µM in DMSO) was added to the solution prepared by adding 100 µL of A2E stock solution (200 µM in DMSO) to 200 µL DPBS and the samples were irradiated as described above. Samples were diluted with EtOH (0.5 mL) and analyzed by reversed-phase HPLC. A2E loss relative to unirradiated samples was interpreted as percent photooxidation.
Cellular Photooxidation of A2E Assay
A human adult RPE cell line (ARPE-19; American Type Culture Collection, Manassas, VA) that is devoid of endogenous A2E was cultured in 35 mm cell culture dishes (Corning, NY) to confluence for 5 days as previously reported.17,18 The cells were allowed to accumulate synthesized A2E15 for 18 days from a 10 µM concentration in culture media that included 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA). From day 10–18 the cells were also incubated with 20 µM antioxidant. Subsequently, the cultures were washed 10 times with DPBS (total 500 µL volume) and then in DPBS were irradiated at 430 nm (2.8 mW/cm2) for 10 min. The cells were then detached from the culture dish with trypsin, washed, pelleted and counted 3 times (Bright Light Counting Chamber, Hausser Scientific Company, Horsham, PA). The cells were dried under vacuum in a desiccator for 2 hours following which 100 µL of methanol was added to each vial for homogenization. After centrifugation, 30 µL of supernatant solution was used for HPLC analysis. A2E loss relative to unirradiated samples was interpreted as percent photooxidation.
Supplementary Material
ACKNOWLEDGMENTS
Janet R. Sparrow and Heike Schönherr were supported by National Institutes of Health grant RO1 EY12951 (JRS) and by a grant from Research to Prevent Blindness to the Department of Ophthalmology, Columbia University.
Footnotes
ASSOCIATED CONTENT
Supporting Information
1H and 13C NMR spectra of are available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Ben-Shabat S, Parish CA, Hashimoto M, Liu L, Nakanishi K, Sparrow JR. Bioorg. Med. Chem. Lett. 2001;11:1533–1540. doi: 10.1016/s0960-894x(01)00314-6. [DOI] [PubMed] [Google Scholar]
- 2.Sparrow JR, Zhou J, Ben-Shabat S, Vollmer H, Itagaki Y, Nakanishi K. Invest. Ophthal. Visual Sci. 2002;43:1222–1226. [PubMed] [Google Scholar]
- 3.Kaufman Y, Ma Y, Washington I. J. Biol. Chem. 2011;286:7958–7965. doi: 10.1074/jbc.M110.178640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai N, Decatur J, Nakanishi K, Eldred GE. J. Am. Chem. Soc. 1996;118:1559–1560. [Google Scholar]
- 5.Ren RX-F, N. Sakai N, Nakanishi K. J. Am. Chem. Soc. 1997;119:3619–3620. [Google Scholar]
- 6.Ben-Shabat S, Parish CA, Vollmer HR, Itagaki Y, Fishkin N, Nakanishi K, Sparrow JR. J. Biol. Chem. 2001;277:7183–7190. doi: 10.1074/jbc.M108981200. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Shabat JR, Itagaki Y, Jockusch S, Sparrow JR, Turro NJ, Nakanishi K. Angew. Chem. Int. Ed. 2002;41:814–817. doi: 10.1002/1521-3773(20020301)41:5<814::aid-anie814>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Washington I, Jockusch S, Itagaki Y, Turro NJ, Nakanishi K. Angew. Chem. Int. Ed. 2005;44:7097–7100. doi: 10.1002/anie.200501346. [DOI] [PubMed] [Google Scholar]
- 9.Sparrow JR, Vollmer HR, Zhou J, Jang YP, Jockusch S, Itagaki Y, Nakanishi K. J. Biol. Chem. 2003;278:18207–18213. doi: 10.1074/jbc.M300457200. [DOI] [PubMed] [Google Scholar]
- 10.Yanase E, Jang YP, Nakanishi K. Heterocycles. 2011;82:1151–1155. [Google Scholar]
- 11.Zhang S, Ma J, Bao Y, Yang P, Zou L, Li K, Sun X. Bioorg. Med. Chem. 2008;16:7127–7132. doi: 10.1016/j.bmc.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 12.Lange J, Hoogeveen S, Veerman W, Wals H. Heterocycles. 2000;53:197–204. [Google Scholar]
- 13.Wright JS, Johnson ER, DiLabi GA. J. Am. Chem. Soc. 2001;123:1173–1183. doi: 10.1021/ja002455u. [DOI] [PubMed] [Google Scholar]
- 14.Amorati R, Pedulli GF, Cabrini L, Zambonin L, Landi L. J. Agric. Food Chem. 2006;54:2932–2937. doi: 10.1021/jf053159+. [DOI] [PubMed] [Google Scholar]
- 15.Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow JR. Proc. Natl Acad. Sci. USA. 1998;95:14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy MVB, Su CR, Chiou WF, Liu YN, Chen RYH, Bastow KF, Lee KH, Wu TS. Bioorg. Med. Chem. 2008;16:7358–7370. doi: 10.1016/j.bmc.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. Invest. Ophthalmol. Visual Sci. 1999;40:2988–2995. [PubMed] [Google Scholar]
- 18.Sparrow JR, Nakanishi K, Parish CA. Invest. Ophthalmol. Visual Sci. 2000;41:1981–1989. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.