Abstract
Background
Long-acting beta-agonists and inhaled corticosteroids have both been recommended in guidelines for the treatment of chronic obstructive pulmonary disease. Their co-administration in a combined inhaler is intended to facilitate adherence to medication regimens, and to improve efficacy. Two preparations are currently available, fluticasone/salmeterol (FPS) and budesonide/formoterol (BDF).
Objectives
To assess the efficacy of combined inhaled corticosteroid and long-acting beta-agonist preparations, compared to inhaled corticosteroids, in the treatment of adults with chronic obstructive pulmonary disease.
Search methods
We searched the Cochrane Airways Group Specialised Register of trials. The date of the most recent search is April 2007.
Selection criteria
Studies were included if they were randomised and double-blind. Studies compared combined inhaled corticosteroids and long-acting beta-agonist preparations with the inhaled corticosteroid component.
Data collection and analysis
Two reviewers independently assessed trial quality and extracted data. The primary outcome were exacerbations, mortality and pneumonia. Health-related quality of life (measured by validated scales), lung function and side-effects were secondary outcomes. Dichotomous data were analysed as fixed effect odds ratios or rate ratios with 95% confidence intervals, and continuous data as mean differences and 95% confidence intervals.
Main results
Seven studies of good methodological quality met the inclusion criteria randomising 5708 participants with predominantly poorly reversible, severe COPD. Exacerbation rates were significantly reduced with combination therapies (Rate ratio 0.91; 95% confidence interval 0.85 to 0.97, P = 0.0008). Data from two FPS studies indicated that exacerbations requiring oral steroids were reduced with combination therapy. Data from one large study suggest that there is no significant difference in the rate of hospitalisations. Mortality was also lower with combined treatment (odds ratio 0.77; 95% confidence interval 0.63 to 0.94). Quality of life, lung function and withdrawals due to lack of efficacy favoured combination treatment. Adverse event profiles were similar between the two treatments. No significant differences were found between FPS and BDP in the primary outcomes, but the confidence intervals for the BDP results were wide as smaller numbers of patients have been studied.
Authors’ conclusions
Combination ICS and LABA significantly reduces morbidity and mortality in COPD when compared with monocomponent steroid. Adverse events were not significantly different between treatments, although evidence from other sources indicates that inhaled corticosteroids are associated with increased risk of pneumonia. Assessment of BDF in larger, long-term trials is required. Dose response data would provide valuable evidence on whether efficacy and safety outcomes are affected by different steroid loads.
Medical Subject Headings (MeSH): Adrenal Cortex Hormones [administration & dosage; adverse effects], Adrenergic beta-Agonists [administration & dosage; adverse effects], Bronchodilator Agents [administration & dosage; adverse effects], Drug Combinations, Drug Therapy, Combination, Nebulizers and Vaporizers, Pneumonia [chemically induced], Pulmonary Disease, Chronic Obstructive [drug therapy; mortality], Randomized Controlled Trials as Topic, Steroids [administration & dosage; adverse effects]
MeSH check words: Humans
BACKGROUND
The use of inhaled long acting beta-agonists (LABAs) and inhaled corticosteroids (ICS) is widely recommended in COPD, on the basis of considerable trial evidence showing favourable effects in comparison with placebo (Nannini 2007a). In daily practice, at grades III and IV in GOLD 2006, patients typically seek medical attention because of dyspnoea or an exacerbation of their disease that has an impact on their quality of life.
Both the component inhaled steroid and long-acting beta-agonist are effective means of preventing COPD exacerbations and improving health-related quality of life. Inhaled steroids reduce the frequency and severity of exacerbations (Yang 2007), although there is some uncertainty as to whether the anti-inflammatory action of ICS modifies the long-term decline in forced expiratory volume in one second (FEV1) associated with COPD progression (Yang 2007; Sutherland 2003). Long-acting beta-agonists have been shown to improve FEV1 in short-term studies and also improve quality of life (Appleton 2006).
Whilst both component treatments are considered to confer some benefit in COPD, the convenience and complementary effect of combined anti-inflammatory and bronchodilator agents has brought into question whether either therapy could be supplanted as a front-line agent by their co-administration. The aim of this review is to update a previous analysis comparing combination inhaled corticosteroids (ICS) and long-acting beta-agonist (LABA) with component ICS in chronic obstructive pulmonary disease (COPD) (Nannini 2004). In view of several new studies, including a large long-term study assessing the effects of combination therapy on mortality (TORCH), we have decided to separate comparison of combination ICS/LABA with ICS from its effects against placebo (Nannini 2007a), and its effects against LABA (Nannini 2007b).
OBJECTIVES
To assess the efficacy and safety of combined inhaled corticosteroid and long-acting beta-agonists for stable COPD, as measured by clinical endpoints and pulmonary function testing against its component inhaled steroid.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised, clinical trials comparing combined inhaled corticosteroid and long acting beta-agonists with its component inhaled corticosteroid.
Types of participants
Adult patients (age > 45 years) with known, stable COPD fulfilling American Thoracic Society (ATS), European Respiratory Society (ERS) and Global Initiative for Chronic Obstructive Lung Disease (GOLD) diagnostic criteria. Patients were to be clinically stable, without evidence of an exacerbation for one month prior to study entry. Patients with significant diseases other than COPD, a diagnosis of asthma, cystic fibrosis, bronchiectasis, or other lung diseases, were to be excluded, however patients with partial reversibility on pulmonary function testing were included.
Types of interventions
Fluticasone/salmeterol (FPS) versus fluticasone (FP).
Budesonide/formoterol (BDF) versus budesonide (BD).
Study duration was for a minimum of two weeks. Concomitant therapy was permitted.
Types of outcome measures
Primary outcomes
Exacerbations, urgent visits and hospitalisations
Mortality
Pneumonia has been added as a primary outcome for this update
Secondary outcomes
Change in forced expiratory volume in 1 second (FEV1) and change in forced ventilatory capacity (FVC): trough, peak and average; and other measures of pulmonary function
Exercise performance - six minute walk and other measures
Quality of life scales - St George’s Respiratory Questionnaire (SGRQ), Chronic Respiratory Disease Questionnaire (CRDQ)
Self-rated symptom score/symptoms of breathlessness
Inhaled rescue medication used during the treatment period and other concomitant medication usage including antibiotics and steroids
“Bad days”
Area under the curve as the beta-agonist response following the first and the last morning dose of LABA/ICS (inhaled corticosteroids)
Per cent of response to salbutamol from baseline FEV1, looking for tachyphylaxis
Pharmacoeconomic advantages
Adverse events - palpitations, tremor, hoarseness/dysphonia, oral candidiasis, cataracts, skin bruising, bone fracture, bone density, plasma cortisol level
Search methods for identification of studies
The most recent search on the Register was run in April 2007. In addition, we performed a search of LILACS (all years to March 2005) and CENTRAL (The Cochrane Library Issue 1, 2006).
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO and hand searching of respiratory journals and meeting abstracts. All records in the Specialised Register coded as ‘COPD’ were searched using the following terms:
(((beta* and agonist*) and long*) or ((beta* and adrenergic*) and long*) and (*steroid or steroid* OR corticosteroid*)) or (fluticasone and salmeterol) or Seretide or Advair or (formoterol and budesonide) or Symbicort
Searching other resources
We reviewed reference lists of all primary studies and review articles for additional references. We also contacted authors of identified randomised trials about other published and unpublished studies. In addition, we contacted Allen and Hanburys for GlaxoSmithKline (GSK), the manufacturer of fluticasone/salmeterol (Advair/Seretide/Viani), and AstraZeneca who manufacture budesonide/formoterol (Symbicort), and consulted their online registers of trials.
Data collection and analysis
Selection of studies
Step I. Two authors independently identified abstracts of trials which appeared potentially relevant.
Step II. Using the full text of each study, two reviewers independently selected trials for inclusion in the review. Agreement was by simple agreement; third party adjudication was used to resolve differences.
Step III. After a preliminary review of all studies to confirm the basic requirements, two reviewers assessed the methodological quality of the included trials with particular emphasis on the concealment of allocation, ranked using Cochrane criteria (grade A: adequate concealment; grade B: uncertain; grade C: clearly inadequate concealment).
In addition, each study was assessed using the domains described by Jadad 1996:
(1) Was the study as randomised? (2) Was the study as double-blind? (3) Were withdrawals and dropouts described? (4) Was the method of randomisation well described and appropriate? (5) Was the double blinding well described and appropriate?
Data extraction and management
Two authors independently extracted data from included trials and entered results into the Cochrane Collaboration software program (Review Manager 4.2). In some cases, we estimated information regarding outcomes from graphs. This was performed independently by the two reviewers. Data extraction included the following items:
Population
Age, gender, smoking status, study setting (country, practice setting), inclusion and exclusion criteria.
Intervention
Dose, delivery device, duration
Control
Concurrent treatments (ipratropium, beta-2 agonist, inhaled and systemic corticosteroids)
Outcomes
Pulmonary function measures (baseline and follow-up FEV1 and FVC), timing of pulmonary function measures, 6-minute walk, urgent visits, admissions, self-rated symptom score/symptoms, quality-of-life instruments, adverse events (palpitations, dry mouth, blurred vision, urinary obstruction and constipation), assessors, adjudicator of clinical endpoints. Mortality outcome data were collected from studies of greater than one year’s duration where these were available.
Design
method of randomisation, presence and type of run-in period, study design (parallel, cross-over)
Measures of treatment effect
For continuous variables, a fixed effects mean difference (MD) was used for outcomes measured on the same metric. Standardised mean difference (SMD) and 95% confidence interval (CI) was calculated for outcomes where data were combined from studies using different metrics. All similar studies were to be pooled using fixed effect MD/SMD and 95% CIs.
For dichotomous variables, a fixed effect odds ratio (OR) with 95% confidence intervals (95% CI) were calculated for individual studies. All similar studies were pooled using fixed effect OR and 95% CIs. Where mean treatment differences were reported, data were entered as generic inverse variance (GIV), provided a standard error for the difference could be extracted or imputed. Where this method was used the effect size was reported from the original papers, for example as Rate Ratio. This method (GIV) was not available when the protocol was written for the review so was not pre-specified.
The reported confidence interval or P value were used to calculate standard deviations, or standard errors, for results when these were not reported and could not be obtained from the authors of the papers.
Assessment of heterogeneity
For pooled effects, heterogeneity was tested using I2 measurement of the degree of variation between the studies, not attributable by the play of chance. If heterogeneity was found, (I2 statistic more than 20%), a random effects model was used to determine the impact of heterogeneity on the overall pooled effect. In addition, the robustness of the results was tested using a sensitivity analysis based on the quality of the trials where possible
Subgroup analysis and investigation of heterogeneity
Whilst we separated the type of steroid and long-acting beta-agonist, we pooled studies with differing dosages of the same drug. We planned a priori subgroups as:
Disease severity (related to baseline FEV1 and placebo group exacerbation rate) according to the GOLD staging = IIA, IIB (moderate COPD, characterised by deteriorating lung function (A = FEV1 </=80% predicted; B = </=50% predicted) and progression of symptoms) and III (severe COPD, characterised by severe airflow limitation (FEV1 <30% predicted) and presence of respiratory failure or clinical signs of right heart failure. (GOLD 2006).
Prior inhaled corticosteroid plus long acting beta agonists use (dichotomised as yes/no).
Concurrent therapy with routine beta-agonist use (short or long-acting), corticosteroid (systemic or inhaled) or theophylline use (dichotomised as yes/no).
Reversibility of airflow obstruction with beta-2-agonist therapy (dichotomised as partial/none).
Definition: >12% and > 200 ml from baseline FEV1 or > 12% as a per cent of the predicted normal value following MDI salbutamol 200 to 400.
Dose, duration and delivery method of therapy.
Sensitivity analysis
In addition, sensitivity analyses were performed using the following domains:
Methodological quality: using a quality-weighted analysis to allow for the use of all trials.
Random effects versus fixed effect modelling.
RESULTS
Description of studies
See: Characteristics of included studies.
For details of search history, see Table 1. For an illustration of how the combined therapies have been split for the update see Figure 1.
Figure 1. Flow chart to illustrate separation of review between three comparisons. Six RCTs met the original entry criteria of the review. All of these had a placebo and long-acting beta-agonist arm, and five assessed combination against steroids. Seven new studies with one or more control comparisons were identified: five had a placebo arm, four had a long-acting beta-agonist arm, and two had an inhaled steroid treatment arm.
Seven studies met the review entry criteria. For a full description of baseline characteristics, methods used and inclusion and exclusion entry criteria of individual studies, see Characteristics of included studies.
Design
All trials had a randomised, double-blind parallel group design. Methods of randomisation were described in one study (Mahler 2002). Method of blinding was not fully described in all studies. Following correspondence from GSK, trial methodology was confirmed for TRISTAN, and AstraZeneca confirmed methodology for Szafranski 2003. Study characteristics were sufficiently described in one study without full text journal publication to justify inclusion in the review (SFCT01).
Participants
Participants suffered from COPD, with variable definition of COPD and reversibility. COPD was defined by national or international criteria: ATS (Hanania 2003; Mahler 2002) ERS (TORCH; TRISTAN) and GOLD (Calverley 2003; Szafranski 2003). In one study, definition were based on lung function tests and smoking history (SFCT01). Patient populations in the studies suffered from moderate and severe COPD. Hanania 2003 and Mahler 2002 enrolled participants with reversible and non-reversible COPD.
Interventions
In one study all participants had a two-week run-in treatment with oral corticosteroids, inhaled formoterol and prn short-acting beta-agonists (Calverley 2003).
In one study the combination of ICS/LABA was fluticasone/salmeterol (FPS) was 250 mcg/50 mcg twice daily (Hanania 2003). In the remainder of the FPS studies the dose was 500 mcg/50 mcg twice daily. In Calverley 2003 and Szafranski 2003 the combination inhaled corticosteroid/long-acting beta agonist was budesonide/formoterol (BDF) (320 mcg/9 mcg twice daily). This was compared with budesonide (BD - 400 mcg twice daily). The dosage of the combined preparation and the separate medications remained stable throughout the studies.
Concomitant therapy was as-needed short-acting beta agonist, or oral steroids and/or antibiotics in the case of exacerbations. However in two studies, theophylline was also used. Eleven percent of participants in Hanania 2003, in addition to the study drugs. The exact proportion of patients in TRISTAN who were taking theophylline was not reported.
Duration
24 weeks: Hanania 2003; Mahler 2002
52 weeks: Calverley 2003; Szafranski 2003; SFCT01; TRISTAN.
156 weeks: TORCH.
Outcomes
Exacerbations were stratified by medication given (oral steroid and/or antibiotic treatment in Calverley 2003; SFCT01; Szafranski 2003; TORCH; TRISTAN) or hospitalisation (TORCH; TRISTAN). Hanania 2003 and Mahler 2002 withdrew participants who exacerbated. Lung function was measured as FEV1 or PEF in all the studies. Quality of life assessment by the SGRQ or CRDQ were available for Calverley 2003; Hanania 2003; Mahler 2002; SFCT01; Szafranski 2003; TORCH; TRISTAN. All cause mortality was reported by TORCH.
Risk of bias in included studies
Intention-to treat (ITT) analyses were reported in all studies for their primary outcomes. TORCH reported incomplete data for FEV1 and SGRQ scores. Concealment of allocation was reported in Calverley 2003; Szafranski 2003; TORCH; TRISTAN. Blinding of treatment was reported for all studies. Identical delivery device for treatment groups was reported in Calverley 2003; Szafranski 2003; TORCH; TRISTAN.
An overview of the judgements we have made regarding the risk of bias for each study is given in Figure 2
Figure 2. Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Effects of interventions
Primary outcomes
1. Requirement for additional treatment, urgent visits & hospitalisations
Ratio of exacerbations
Pooled results for FPS and BDF versus ICS alone
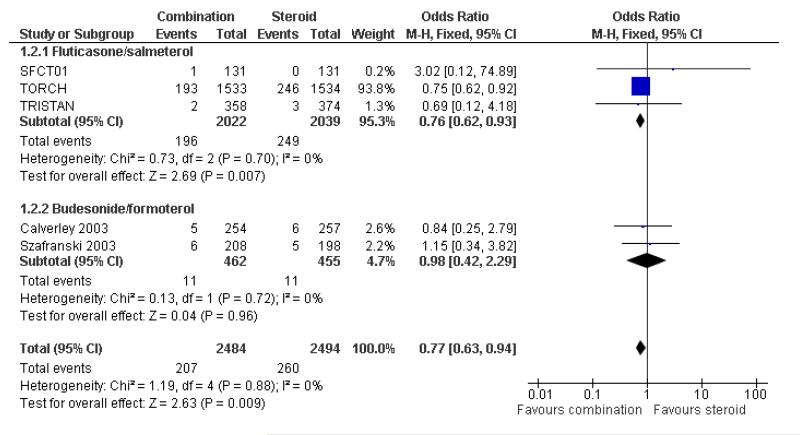
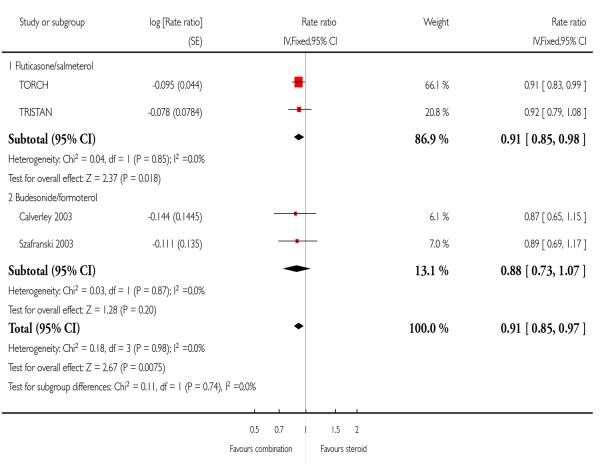
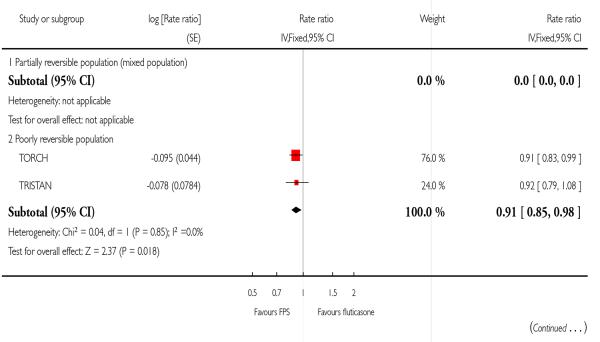
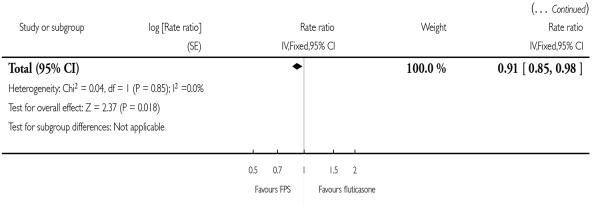
There was a significant reduction in the rate of exacerbations with combination therapy when compared with inhaled corticosteroid (0.91; 95% CI 0.85 to 0.97, four trials, N = 4706, Figure 3).
Figure 3. Forest plot of comparison: 1 All Combined Inhalers - Primary Outcomes, outcome: 1.1 Exacerbations.
FPS versus FP
There was a significant reduction in the rate of exacerbations with combination therapy when compared with FP (0.91; 95% CI 0.85 to 0.98, two trials, N = 3789).
BDF versus BD
There was no significant effect on pooled exacerbation rates between treatments (0.88; 95% CI 0.73 to 1.07). There is no significant difference between the results for FPS and BDP, as the confidence intervals for the BDF comparison are wide.
Exacerbation by type
FPS versus FP
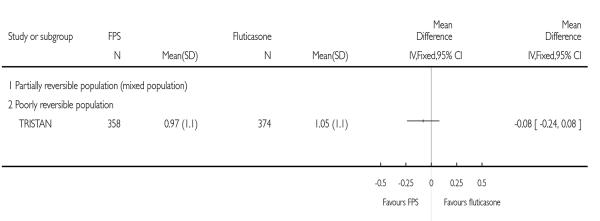
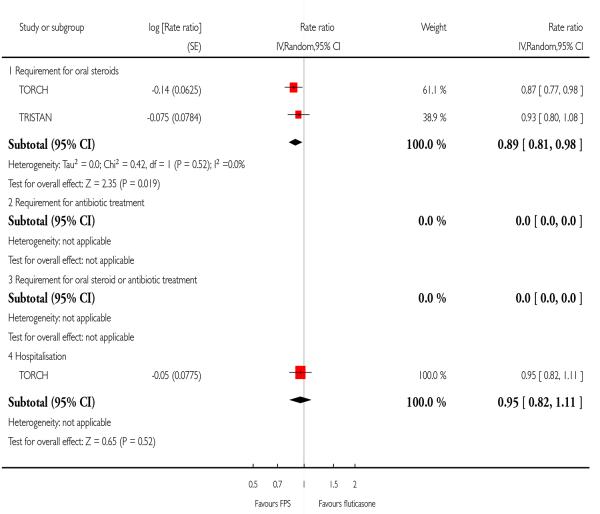
FPS led to fewer exacerbations which required oral steroids (0.89; 95% CI 0.81 to 0.98, Analysis 2.4). One large study did not find a significant difference in the rate of hospitalisations between treatments (0.95; 95% CI 0.82 to 1.11).
Dichotomous data (number of people experiencing an exacerbation)
FPS versus FP
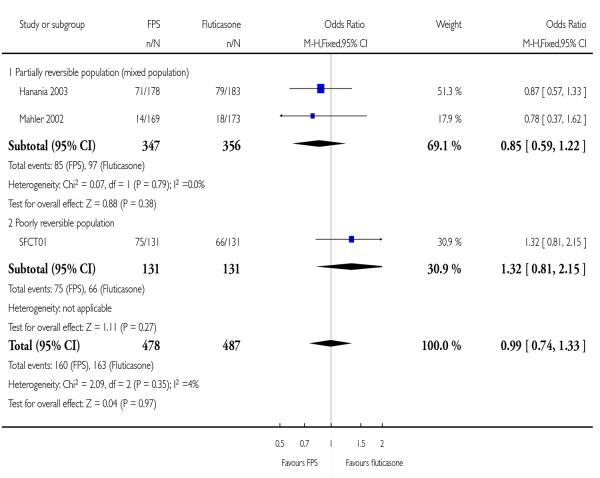
There was no significant difference between FPS and FP (OR 0.99; 95% CI 0.74 to 1.33, Analysis 2.1).
2. Mortality
Pooled results for FPS and BDF versus ICS alone
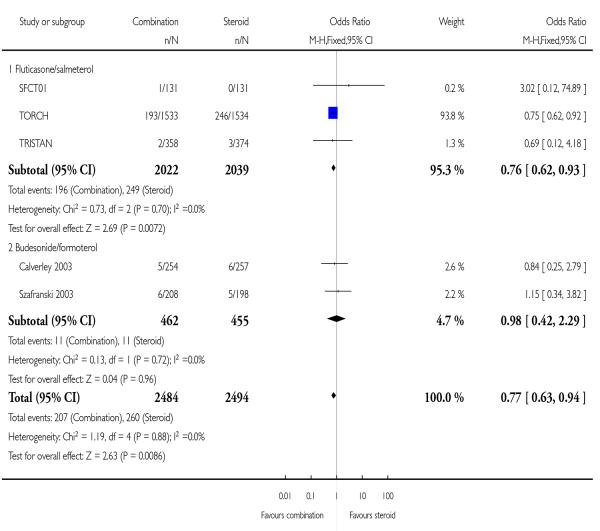
When data were combined with both treatments and their respective comparators, the odds of death were significantly lower following combination treatment compared mono component steroid (0.77; 95% CI 0.63 to 0.94, Figure 4). Since differing lengths of follow-up across the studies hinders the calculation of a pooled NNT, we have tabulated this for each study individually (see Table 2). The three year NNT (using the baseline risk of 16% in the ICS arm of TORCH) to prevent one extra death is 32 (95% CI 19 to 123). In contrast, in lower risk patients (using the baseline risk of 0.8% in the ICS arm of TRISTAN) the one year NNT is much higher at 547 to prevent one extra death (95% CI 340 to 2100).
Figure 4. Forest plot of comparison: 1 All Combined Inhalers - Primary Outcomes, outcome: 1.2 Mortality.
FPS versus FP
Data were separated according to study duration. Compared with FP there was a significant reduction in the odds of death at the end of treatment (OR 0.76; 95% CI 0.62 to 0.93, three studies, N = 4061).
BDF versus BD
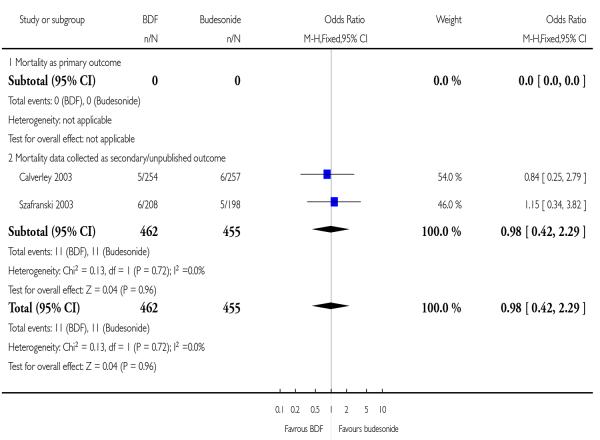
The two studies of one year duration did not identify a significant difference between treatments.
3. Pneumonia
Pooled results for FPS and BDF versus ICS alone
When data were combined with both treatments and their respective comparators, the odds of pneumonia were not significantly different following combination treatment compared mono component steroid (OR 1.13; 95% CI 0.92 to 1.38, Figure 5).
Figure 5. Forest plot of comparison: 1 All Combined Inhalers - Primary Outcomes, outcome: 1.3 Pneumonia.
FPS versus FP
Data were separated according to study duration. Compared with FP there was a significant difference in the odds of pneumonia at the end of treatment (OR 1.12; 95% CI 0.91 to 1.37, three studies, N = 4061).
BDF versus BD
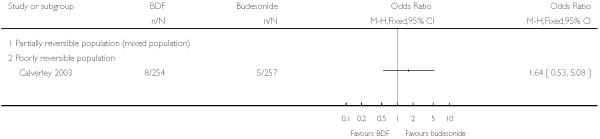
The single study that reported pneumonia Calverley 2003 did not identify a significant difference between treatments.
Secondary outcomes
Quality of life
FPS versus FP
There was a significant improvement in favour of FPS over FP of −1.30 units on the SGRQ; 95% CI −2.04 to −0.57, three studies, N = 3001. Due to the high rate of attrition in TORCH, the data were presented for only a subset of those who were randomised (2007/3091). Removing this study from the analysis resulted in a similar effect estimate (−1.56; 95% CI −2.66 to −0.46).
Data from two studies reporting quality of life as mean change in CRDQ suggested high levels of statistical variation (I square 76%).
Neither fixed effect nor random effects modelling gave significant differences (2.12 units; 95% CI −0.50 to 4.75 and 2.34 units; 95% CI −3.15 to 7.82 respectively).
BDF versus BD
There was a significant effect in favour of BDF compared with BD of −3.26 (95% CI −5.1 to −1.42).
Symptom score
FPS versus FP
Pooled data from Mahler 2002 and Hanania 2003 gave no significant difference between treatments in TDI scores (mean difference 0.31; 95% confidence interval −0.45 to 1.08).
TRISTAN reported improvements in symptoms after treatment in favour of FPS versus FP on breathlessness scores (FPS mean: 1.47; FP mean: 1.59. Improvement in night time awakenings compared with FP was reported, but there was no statistically significant difference (P = 0.591). Cough scores were not significantly different compared with FP (P = 0.34 respectively).
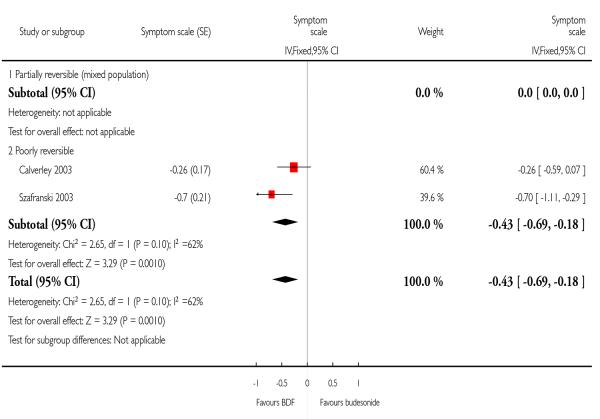
BDF versus BD
When data were pooled for the comparison with BD there was a high level of heterogeneity (I2 = 62.3%). A Random Effects model generated a marginally significant result (−0.46; 95%CI −0.89 to −0.03). The possible cause for the difference in response may be study design, with the effects of pre-dosing treatment being maintained by BD treatment. The addition of further studies to this analysis would help to elucidate whether the variation between the studies represents an important difference in treatment protocols.
Lung function
Predose & post dose FEV1 - Change from baseline
FPS versus FP
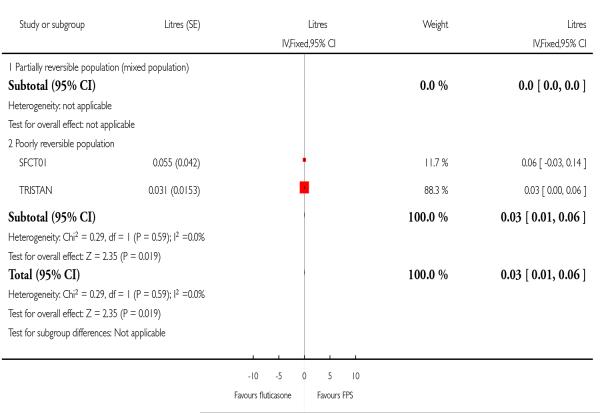
Data pooled from Mahler 2002 and Hanania 2003 gave a MD of 0.05 L; 95%CI 0.02 to 0.09. Post dose FEV1 from TORCH significantly favoured FPS by 0.04 Litres.
BDF versus BD
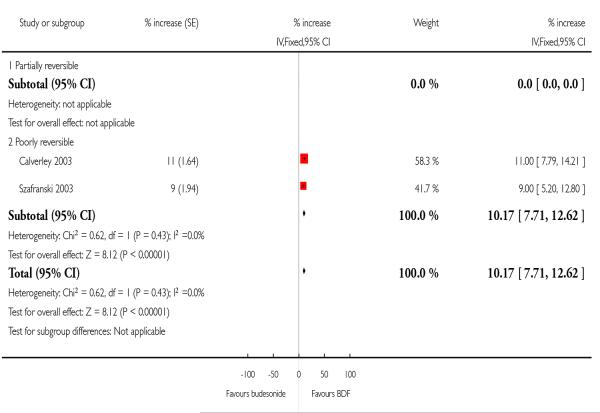
There was a significant difference in favour of BDF versus BD (MD 10.17%; 95%CI 7.71 to 12.62).
Predose FEV1 absolute values
FPS versus FP
TRISTAN presented mean differences between treatment regimens. There was an increase in predose FEV1 in those treated with FPS versus FP: 95 ml; 95% CI 67 to 122.
Rescue medication
FPS versus FP
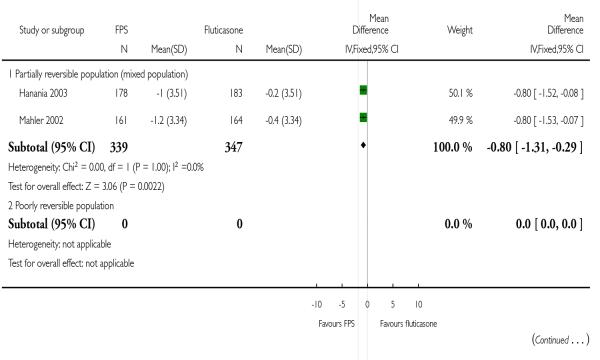
Pooled data from Mahler 2002 and Hanania 2003 indicated a significant reduction in mean puffs per day of short-acting beta-agonist usage in favour of FPS over FP (−0.8 puffs/day; 95% CI −1.31 to −0.29).
Mahler 2002 reported no significant increases in the percentage of nights between FPS and FP.
TRISTAN reported a significant difference in median % of days without use of relief medication in favour of FPS over FP (P < 0.001).
BDF versus BD
BDF treatment reduced the requirement for reliever medication when compared with BD (−0.80 puffs per day; 95%CI −1.06 to −0.54).
Safety & tolerability
FPS versus FP
There was no significant difference between FPS and FP in the odds of any adverse event, headache, URTI, and candidiasis.
BDF versus BD
No data were reported on specific events such as candidiasis or headaches.
Withdrawals
FPS versus FP
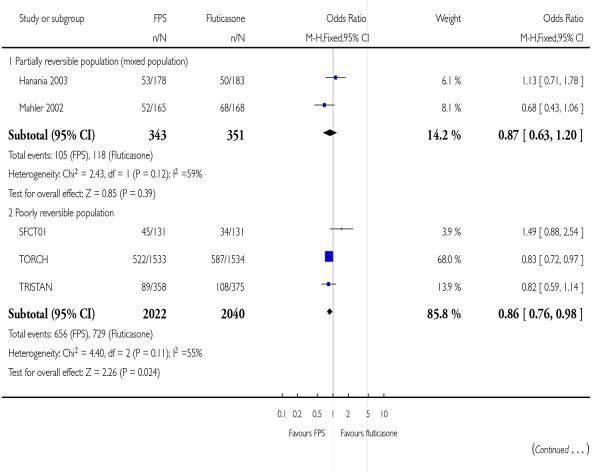
Study withdrawal occurred significantly less frequently on FPS than FP (OR 0.86; 95% CI 0.76 to 0.97, five studies, N = 4786). When expressed as withdrawal due to lack of efficacy there was no significant difference between treatments (OR 0.73; 95% CI 0.49 to 1.08, four studies, N = 4395). However there were fewer withdrawals due to adverse events in FPS treated participants than among those treated with FP (OR 0.75; 95% CI 0.64 to 0.88, four studies, N = 4424).
BDF versus BD
Data were pooled from Calverley 2003 and Szafranski 2003 for withdrawals due to worsening COPD symptoms and adverse events.
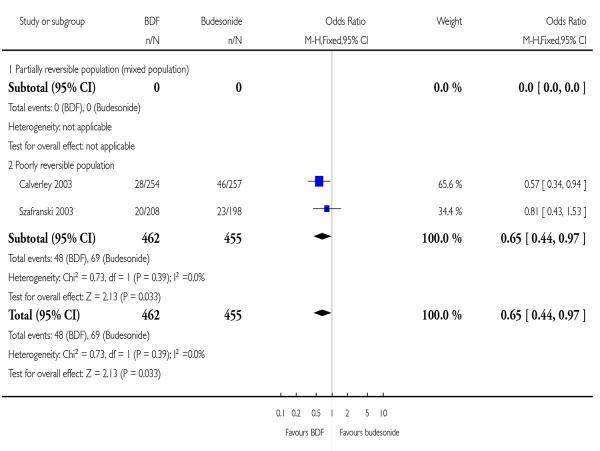
There was no significant difference in withdrawals due to worsening of COPD symptoms when BDF was compared with BD: 1.05; 95%CI 0.64 to 1.71.
There was no significant difference between BDF and its comparators on the likelihood withdrawal due to adverse events other than COPD deterioration (OR 1.05; 95%CI 0.64 to 1.71).
DISCUSSION
We have reviewed data from seven randomised controlled trials (5708 participants) assessing the effectiveness of combined inhaled corticosteroid and long-acting beta-agonist in the treatment of chronic obstructive pulmonary disease (COPD). Whilst the consensus regarding the definitions of COPD and COPD exacerbations evolve, the trial evidence to date indicates that in severe COPD where this is defined by low FEV1, significant smoking history, and a recent history of reduced disease control, combination therapy is more effective than its monocomponent inhaled steroid in reducing exacerbations which lead to unscheduled additional treatment. Although the BDF studies did not exhibit significant differences in rates of exacerbations, when pooled with FPS the effect estimate significantly favoured combination treatment, and there was no significant difference between FPS and BDP. The pooled rate ratio of 0.91 translates to a 9% reduction in the mean rate of exacerbations. The clinical relevance of this effect would depend on how frequently exacerbations occur in individual patients.
The issue of rate ratios and their analysis is discussed elsewhere (Nannini 2007a). Inhaled steroids have been shown to reduce exacerbations and improve quality of life compared with placebo (Yang 2007). The statistically significant effects favouring combination therapy over inhaled steroids in this review on these same endpoints should therefore be regarded as being of potentially great importance. When considered by type of exacerbation, there was a significant reduction in requirement for oral steroid treatment associated with FPS over FP, but there was no significant difference in hospitalisation in TORCH. Data for this outcome is of particular interest in planning and allocation of resources in COPD services, and the lack of an effect on this outcome indicates that FPS is superior to FP in the prevention of exacerbations of moderate intensity, but that there is currently little difference shown between these therapies in the deterioration of symptoms leading to hospitalisation. Lung function, quality of life and study withdrawal favoured combination therapy. This review demonstrates that adding LABA to ICS is of significant benefit in reducing morbidity of COPD. The estimate of the long-term change in quality of life is at risk of bias since only two-thirds of randomised participants contributed data to this endpoint. Given the rate of attrition observed in studies of prolonged duration such as NETT and TORCH, the question of how loss to follow-up and mortality affect reliability of outcome measurements is unlikely to be resolved simply.
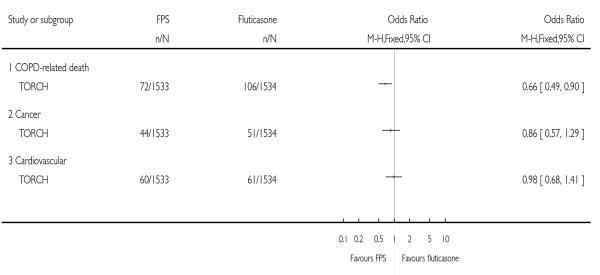
The question of whether mortality in COPD can be modified with maintenance pharmacological intervention has been addressed in one long-term study of FPS and FP. Given the lack of a significant difference between FPS and FP on pneumonia, and the significant increased risk of this event when FPS was compared with placebo and salmeterol (Nannini 2007a; Nannini 2007b), it is feasible that this particular event is one of many risk factors in mortality. The choice of all cause mortality (rather than cause-specific mortality) as an outcome in this review reflects the availability of data in the original studies, and the challenging nature of cause ascertainment processes (McGarvey 2007). Since mortality in people with COPD is likely to be affected by co morbidities such as cardiovascular disease and lung cancer, establishing a primary cause will demand careful consideration.
Other secondary outcomes such as quality of life and lung function measurements indicated that combination therapy was more effective than its mono component inhaled steroid. There was no significant difference in adverse event profiles. Five of the seven studies to date have compared FPS with FP. The two efficacy endpoints which combined both treatment-control comparisons did not indicate that there was excessive statistical heterogeneity across the studies. A direct comparison between FPS and BDF treatments would provide valuable information. Further work looking at different doses of ICS affect harms and benefits in this population, and would help to clarify the validity of combining estimates from different treatment comparisons.
AUTHORS’ CONCLUSIONS
Implications for practice
In participants with moderate and severe COPD, there is clinical benefit when long acting beta-agonist and inhaled corticosteroid are co-administered compared to treatment with mono component steroid. Exacerbation rates are significantly reduced by combination inhalers, although the confidence intervals for this outcome are wide for the budesonide/formoterol studies as numbers of participants was smaller than for fluticasone/salmeterol. The impact of the estimated difference in the mean rate of exacerbations of 9% will depend on the frequency of these events in individual patients. Given the uncertainty as to the dose of steroids known to be efficacious in COPD, the optimum dose of inhaled steroids remains unclear. There was no significant difference between fluticasone/salmeterol and fluticasone when adverse events such as pneumonia and candidiasis were considered. This suggests that these may be related primarily to the steroid component.
Implications for research
Additional work is required assessing budesonide/formoterol with budesonide on exacerbations and mortality outcomes. The frequency of pneumonia requires assessment, with adequate diagnostic procedures in place to confirm these events. Data on the nature and severity of exacerbations would enhance this review, with attention to hospitalisation and the recording of short courses of steroids or antibiotics of particular interest. The optimum dose of ICS in COPD has not yet been explored adequately and further work in this area would assist in clarifying uncertainties surrounding the importance of ICS dosing.
PLAIN LANGUAGE SUMMARY.
Combination therapy of inhaled steroids and long-acting beta-agonists versus inhaled steroids alone
Combinations of two classes of medication (long-acting beta-agonists and inhaled corticosteroids) in one inhaler have been developed to treat people with COPD as it may make it easier to take the medication. Two brands of combined inhaler exist currently: budesonide/formoterol (BDF - ‘Symbicort’), and fluticasone/salmeterol (FPS - ‘Advair’ or ‘Seretide’). The results of the studies showed that BDF and FPS were effective and reduced the frequency of flare ups compared with inhaled corticosteroid alone. The studies showed that on average there was a relative reduction of 9% in the mean rates of exacerbations. The impact of this difference on individuals will vary depending on how frequently they experience exacerbations. Quality of life and lung function showed improvements with combination treatment compared with steroids. Future research should assess the benefits and harms of BDF since the majority of evidence to date has been drawn from FPS studies.
ACKNOWLEDGEMENTS
The authors are indebted to the Hamamellis Trust who very generously funded the return travel for Dr Nannini to London inorder to spend a week working on the development of the review. Thanks to Liz Arnold, Susan Hansen and Veronica Stewart for technical and clerical support. We would also like to acknowledge the efforts of Inge Vestbo, Diane Grimley and Karen Richardson of GSK who helped us in our attempts to obtain unpublished information on TRISTAN, and those of Goran Tornling, Moira Coughlan and Roger Metcalf of AstraZeneca in helping us obtain data for Szafranski 2003. We thank Dr Nick Hanania and Prof Donald Mahler for corresponding with us in our attempts to obtain unpublished data from their studies.
SOURCES OF SUPPORT
Internal sources
NHS Cochrane Grant scheme, UK.
External sources
No sources of support supplied
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Parallel group study. Randomisation: unclear Blinding: double-blind (identical inhaler devices) Trial duration: 52 weeks with two week run-in of treatment optimisation. Allocation concealment: unclear Withdrawals: stated Intention to treat analysis: stated Jadad score: 4 |
|
| Participants |
|
|
| Interventions | Run-in phase: All participants received 30mg oral prednisolone BiD and 2×4.5mg formoterol BiD (2 weeks). 1) BDF: 320/9mcg bid. 2) Placebo (lactose monohydrate). Additional treatmentgroups not covered in this review: 3) BUD: 400mcg bid. 3) F: 9mcg bid. Inhaler device: Turbuhaler | |
| Outcomes | Time to first exacerbation; change in post-medication FEV1; number of exacerbations; time to and number of OCS-treated episodes; am and pm PEF, slow VC, HRQL, symptoms, use of reliever medication, AEs | |
| Notes | Classified as ‘poorly reversible population’. P values used to calculate pooled SEMs for the following outcomes: Health related quality of life; FEV1; rescue medication | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Described as randomised; no other information reported |
| Allocation concealment? | Unclear | Information not available |
| Blinding? All outcomes |
Yes | Identical inhaler devices |
| Methods | Parallel group study. Randomisation: method unclear. Blinding: double blind. Allocation concealment: unclear Excluded: described. Withdrawals: stated. Trial duration: 24 weeks with 2-week run-in period. Intention to treat analysis: not stated. Jadad score: 4 |
|
| Participants |
|
|
| Interventions | Run-in: 2 weeks treatment with placebo inhaler and prn SABA. 1) FPS 50/250 mcgbid. 2) Placebo Additional treatment groups not covered in this review 3) SAL 50 mcgbid. 4) FP 250 mcg bid. Inhaler device: Diskus | |
| Outcomes | Lung function: Change in FEV1 from baseline to end of study (M). PEF data not stratified by reversibility. Quality of life: CRDQ, CBSQ not stratified by reversibility. Dyspnoea and symptoms: Transitional dyspnoea index, Baseline dyspnoea index not stratified by reversibility. Exacerbations. Rescue salbutamol use | |
| Notes | FEV1 reversibility < 12% or 200ml (of baseline FEV1) Reversibility stratified data. Mean % increase non-reversible patients = 8.8 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Described as randomised; information not available |
| Allocation concealment? | Unclear | Information not available |
| Blinding? All outcomes |
Yes | Identical inhaler devices |
| Methods | Parallel group study. Randomisation: stratified by reversibility and investigative site. Blinding: Double blind. Allocation concealment: unclear Trial duration: 24 weeks. Withdrawals: stated Intention to treat analysis: stated Jadad score: 3 |
|
| Participants |
|
|
| Interventions | Run-in: 2 weeks treatment with placebo inhaler and prn SABA.
|
|
| Outcomes | Lung function: Change in FEV1 from baseline to end of study (M). Quality of life: CRDQ, CBSQ not stratified by reversibility. Dyspnoea and symptoms: End of study dyspnoea (TDI). Exacerbations. Rescue salbutamol use | |
| Notes | COPD subjects reversible and non-reversible, < 15% (baseline) improvement in FEV1 to salbutamol. Reversibility stratified data. Mean FEV1 reversibility 11.0% | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Described as randomised; information not available |
| Allocation concealment? | Unclear | Information not available |
| Blinding? All outcomes |
Yes | Identical inhaler devices |
| Methods | Parallel group design. Randomisation: not clear. Blinding: double blind. Allocation concealment: unclear. Excluded: not described. Withdrawals: described. Trial duration: 52 weeks. Withdrawals: Stated Intention to treat analysis stated. Jadad Score: 3 |
|
| Participants |
|
|
| Interventions | Run-in: 2 weeks. All maintenance LABA and ICS treatment ceased
|
|
| Outcomes | Withdrawals; exacerbations; FEV1; adverse events | |
| Notes | Unpublished study downloaded from ctr.gsk.co.uk | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Described as randomised; information not available |
| Allocation concealment? | Unclear | Information not available |
| Blinding? All outcomes |
Yes | Identical inhaler devices |
| Methods | Parallel group study. Randomisation: Randomised, double-blind, placebo-controlled parallel group trial. Duration: 52 weeks. Methods of randomisation: Computer-generated scheme at AstraZeneca, Lund, Sweden. At each centre, eligible patients received an enrolment code and then after run-in, participants were allocated the next consecutive patient number. Allocation concealment: adequate. Blinding: All the Turbuhaler inhalers were identical to ensure that the patient, pharmacist and the investigator were blinded to the allocated treatment Withdrawals: Stated Intention to treat analysis: Stated Jadad sore: 5 |
|
| Participants |
|
|
| Interventions | Run-in: 2 weeks. Treatment with prn SABA only.
|
|
| Outcomes | Symptoms, adverse events, exacerbations, lung function. | |
| Notes | Classified as ‘poorly reversible’ subgroup. Jadad score: 5. Exacerbation defined as requirement of oral steroids and/or antibiotics and/or hospitalisation for respiratory symptoms. Mild exacerbation defined as requirement of >/= 4 inhalations per day. P values used to calculate pooled SEMs for following outcomes: Symptoms; rescue medication usage | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | As described above |
| Allocation concealment? | Yes | As described above |
| Blinding? All outcomes |
Yes | Identical inhaler devices |
| Methods | Parallel group design. Randomisation: Permuted block randomisation with stratification for smoking status and country Blinding: double blind (identical inhaler devices) Allocation concealment: Adequate Excluded: described Withdrawals: described Trial duration: 156 weeks Withdrawals: Stated Intention to treat analysis: stated Jadad Score: 5 |
|
| Participants |
|
|
| Interventions | Run-in: 2 weeks. All maintenance treatment with ICS and LABA ceased. 1) FP/SAL combination 500/50mcg BID 2) Placebo Additional treatment groups not covered in this review: 3) FP 500mcg BID 4) SAL 50mcg BID Inhaler device: DPI | |
| Outcomes | All cause mortality; change in SGRQ; exacerbations (requiring antibiotics, steroids, hospitalisation or combination of these); lung function; withdrawals; adverse events | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | As described above |
| Allocation concealment? | Yes | As described above |
| Blinding? All outcomes |
Yes | Idenitical inhaler devices |
| Methods | Parallel group design. Randomisation: computer generated. Numbers were generated off-site. Once a treatment number had been assigned to a participant it could not be assigned to any other participant. Blinding: Double blind. Participants received identically packaged and presented placebos. Withdrawals: Described. Trial duration: 2 week run-in period, 52 weeks treatment, 2-week follow-up Intention to treat analysis: stated Jadad Score: 5 |
|
| Participants |
|
|
| Interventions | Run-in: 2 weeks. All maintenance treatment with ICS and LABA ceased. 1) FPS 50 mcg/500 mcg bid. 2) Placebo Additional treatment groups not covered in this review: 3) SAL 50 mcg bid. 4) FP 500 mcg bid. Inhaler device: DPI | |
| Outcomes | FEV1; PEF; exercise tolerance; quality of life: SGRQ; dyspnoea and symptoms (symptom score for shortness of breath, cough and sputum production); exacerbations (defined as requirement for antibiotics, oral steroids or both); rescue salbutamol use | |
| Notes | FEV1 reversibility (% predicted normal) Mean Reversibility (% predicted) = 3.8 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | As described above |
| Allocation concealment? | Yes | As described above |
| Blinding? All outcomes |
Yes | Idenitical inhaler devices |
DATA AND ANALYSES
Comparison 1. All Combined Inhalers - Primary Outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations | 4 | Rate ratio (Fixed, 95% CI) | 0.91 [0.85, 0.97] | |
| 1.1 Flut icasone/salmeterol | 2 | Rate ratio (Fixed, 95% CI) | 0.91 [0.85, 0.98] | |
| 1.2 Budesonide/formoterol | 2 | Rate ratio (Fixed, 95% CI) | 0.88 [0.73, 1.07] | |
| 2 Mortality | 5 | 4978 | Odds Ratio (M-H, Fixed, 95% CI) | 0.77 [0.63, 0.94] |
| 2.1 Fluticasone/salmeterol | 3 | 4061 | Odds Ratio (M-H, Fixed, 95% CI) | 0.76 [0.62, 0.93] |
| 2.2 Budesonide/formoterol | 2 | 917 | Odds Ratio (M-H, Fixed, 95% CI) | 0.98 [0.42, 2.29] |
| 3 Pneumonia | 5 | 5033 | Odds Ratio (M-H, Fixed, 95% CI) | 1.13 [0.92, 1.38] |
| 3.1 Fluticasone/salmeterol | 4 | 4522 | Odds Ratio (M-H, Fixed, 95% CI) | 1.12 [0.91, 1.37] |
| 3.2 Budesonide/formoterol | 1 | 511 | Odds Ratio (M-H, Fixed, 95% CI) | 1.64 [0.53, 5.08] |
Comparison 2. Fluticasone/salmeterol (FPS) versus fluticasone (FP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with one or more exacerbation | 3 | 965 | Odds Ratio (M-H, Fixed, 95% CI) | 0.99 [0.74, 1.33] |
| 1.1 Partially reversible population (mixed population) | 2 | 703 | Odds Ratio (M-H, Fixed, 95% CI) | 0.85 [0.59, 1.22] |
| 1.2 Poorly reversible population | 1 | 262 | Odds Ratio (M-H, Fixed, 95% CI) | 1.32 [0.81, 2.15] |
| 2 End of treatment mean number of exacerbations per participant | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Partially reversible population (mixed population) | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 2.2 Poorly reversible population | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 3 Exacerbations | 2 | Rate ratio (Fixed, 95% CI) | 0.91 [0.85, 0.98] | |
| 3.1 Partially reversible population (mixed population) | 0 | Rate ratio (Fixed, 95% CI) | Not estimable | |
| 3.2 Poorly reversible population | 2 | Rate ratio (Fixed, 95% CI) | 0.91 [0.85, 0.98] | |
| 4 Exacerbations by type | 2 | Rate ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Requirement for oral steroids | 2 | Rate ratio (Random, 95% CI) | 0.89 [0.81, 0.98] | |
| 4.2 Requirement for antibiotic treatment | 0 | Rate ratio (Random, 95% CI) | Not estimable | |
| 4.3 Requirement for oral steroid or antibiotic treatment | 0 | Rate ratio (Random, 95% CI) | Not estimable | |
| 4.4 Hospitalisation | 1 | Rate ratio (Random, 95% CI) | 0.95 [0.82, 1.11] | |
| 5 Mortality | 4 | 4394 | Odds Ratio (M-H, Fixed, 95% CI) | 0.76 [0.62, 0.93] |
| 5.1 Mortality: three year data | 1 | 3067 | Odds Ratio (M-H, Fixed, 95% CI) | 0.75 [0.62, 0.92] |
| 5.2 Mortality: >one and <three year data | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Mortality: one year data | 2 | 994 | Odds Ratio (M-H, Fixed, 95% CI) | 1.03 [0.23, 4.57] |
| 5.4 Mortality: 6 month data | 1 | 333 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Change from baseline in St George’s Respiratory Questionnaire (total score) | 3 | SGRQ units (Fixed, 95% CI) | −1.30 [−2.04, −0.57] | |
| 6.1 Partially reversible population (mixed population) | 0 | SGRQ units (Fixed, 95% CI) | Not estimable | |
| 6.2 Poorly reversible population | 3 | SGRQ units (Fixed, 95% CI) | −1.30 [−2.04, −0.57] | |
| 7 Change from baseline in St George’s Respiratory Questionnaire (domain - symptoms) | 1 | SGRQ units (Fixed, 95% CI) | Totals not selected | |
| 7.1 Partially reversible population (mixed population) | 0 | SGRQ units (Fixed, 95% CI) | Not estimable | |
| 7.2 Poorly reversible population | 1 | SGRQ units (Fixed, 95% CI) | Not estimable | |
| 8 Change from baseline in St George’s Respiratory Questionnaire (domain - activity) | 1 | SGRQ units (Fixed, 95% CI) | Totals not selected | |
| 8.1 Partially reversible population (mixed population) | 0 | SGRQ units (Fixed, 95% CI) | Not estimable | |
| 8.2 Poorly reversible population | 1 | SGRQ units (Fixed, 95% CI) | Not estimable | |
| 9 Change from baseline in St George’s Respiratory Questionnaire (domain - impact) | 1 | SGRQ units (Fixed, 95% CI) | Totals not selected | |
| 9.1 Partially reversible population (mixed population) | 0 | SGRQ units (Fixed, 95% CI) | Not estimable | |
| 9.2 Poorly reversible population | 1 | SGRQ units (Fixed, 95% CI) | Not estimable | |
| 10 End of treatment St George’s Respiratory Questionnaire scores (total score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Partially reversible population (mixed population) | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 10.2 Poorly reversible population | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 11 End of treatment St George’s Respiratory Questionnaire scores (domain - symptoms) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Partially reversible population (mixed population) | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 11.2 Poorly reversible population | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 12 Change from baseline in Canadian Respiratory Disease Questionnaire scores | 2 | 696 | Mean Difference (IV, Random, 95% CI) | 2.34 [−3.15,7.82] |
| 12.1 Partially reversible population (mixed population) | 2 | 696 | Mean Difference (IV, Random, 95% CI) | 2.34 [−3.15, 7.82] |
| 12.2 Poorly reversible population | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 13 Change from baseline in Transitional Dyspnoea Index (TDI) | 2 | 690 | Mean Difference (IV, Random, 95% CI) | 0.31 [−0.45, 1.08] |
| 13.1 Partially reversible population (mixed population) | 2 | 690 | Mean Difference (IV, Random, 95% CI) | 0.31 [−0.45, 1.08] |
| 13.2 Poorly reversible population | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 14 Change from baseline in FEV1 (Litres) | 2 | L/min (Fixed, 95% CI) | 0.09 [0.06, 0.12] | |
| 14.1 Partially reversible population (mixed population) | 0 | L/min (Fixed, 95% CI) | Not estimable | |
| 14.2 Poorly reversible population | 2 | L/min (Fixed, 95% CI) | 0.09 [0.06, 0.12] | |
| 15 Change from baseline in predose FEV1 (Litres) | 2 | 690 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.02, 0.09] |
| 15.1 Reversible population | 2 | 380 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [0.01, 0.12] |
| 15.2 Partially reversible population (mixed population) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Poorly reversible population | 2 | 310 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [−0.01, 0.09] |
| 16 End of treatment FEV1 (Litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 Partially reversible population (mixed population) | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 16.2 Poorly reversible population | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 17 End of treatment postdose FEV1 | 2 | Litres (Fixed, 95% CI) | 0.03 [0.01, 0.06] | |
| 17.1 Partially reversible population (mixed population) | 0 | Litres (Fixed, 95% CI) | Not estimable | |
| 17.2 Poorly reversible population | 2 | Litres (Fixed, 95% CI) | 0.03 [0.01, 0.06] | |
| 18 Change from baseline in postdose FEV1 | 1 | Litres (Fixed, 95% CI) | Totals not selected | |
| 18.1 Reversible population | 0 | Litres (Fixed, 95% CI) | Not estimable | |
| 18.2 Partially reversible population (mixed population) | 0 | Litres (Fixed, 95% CI) | Not estimable | |
| 18.3 Poorly reversible population | 1 | Litres (Fixed, 95% CI) | Not estimable | |
| 18.4 Unclear reversibility | 0 | Litres (Fixed, 95% CI) | Not estimable | |
| 19 End of treatment am PEF (L/min) | 1 | L/min (Fixed, 95% CI) | Totals not selected | |
| 19.1 Partially reversible population (mixed population) | 0 | L/min (Fixed, 95% CI) | Not estimable | |
| 19.2 Poorly reversible population | 1 | L/min (Fixed, 95% CI) | Not estimable | |
| 20 Absolute shuttle walk test | 1 | Metres (Fixed, 95% CI) | Totals not selected | |
| 20.1 Partially reversible population (mixed population) | 0 | Metres (Fixed, 95% CI) | Not estimable | |
| 20.2 Poorly reversible population | 1 | Metres (Fixed, 95% CI) | Not estimable | |
| 21 Change from baseline in rescue medication usage (puffs/day) | 2 | 686 | Mean Difference (IV, Fixed, 95% CI) | −0.80 [−1.31, −0.29] |
| 21.1 Partially reversible population (mixed population) | 2 | 686 | Mean Difference (IV, Fixed, 95% CI) | −0.80 [−1.31, −0.29] |
| 21.2 Poorly reversible population | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 22 Withdrawals | 5 | 4756 | Odds Ratio (M-H, Fixed, 95% CI) | 0.86 [0.76, 0.97] |
| 22.1 Partially reversible population (mixed population) | 2 | 694 | Odds Ratio (M-H, Fixed, 95% CI) | 0.87 [0.63, 1.20] |
| 22.2 Poorly reversible population | 3 | 4062 | Odds Ratio (M-H, Fixed, 95% CI) | 0.86 [0.76, 0.98] |
| 23 Withdrawal due to lack of efficacy/exacerbation | 4 | 4395 | Odds Ratio (M-H, Fixed, 95% CI) | 0.73 [0.49, 1.08] |
| 23.1 Partially reversible population (mixed population) | 1 | 333 | Odds Ratio (M-H, Fixed, 95% CI) | 1.02 [0.20, 5.12] |
| 23.2 Poorly reversible population | 3 | 4062 | Odds Ratio (M-H, Fixed, 95% CI) | 0.72 [0.48, 1.08] |
| 24 Withdrawals due to adverse events | 4 | 4424 | Odds Ratio (M-H, Fixed, 95% CI) | 0.75 [0.64, 0.88] |
| 24.1 Partially reversible population (mixed population) | 1 | 342 | Odds Ratio (M-H, Fixed, 95% CI) | 0.48 [0.22, 1.02] |
| 24.2 Poorly reversible population | 3 | 4082 | Odds Ratio (M-H, Fixed, 95% CI) | 0.77 [0.65, 0.90] |
| 25 Adverse events - any event | 5 | 4795 | Odds Ratio (M-H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| 25.1 Partially reversible population (mixed population) | 2 | 703 | Odds Ratio (M-H, Fixed, 95% CI) | 0.92 [0.66, 1.30] |
| 25.2 Poorly reversible population | 3 | 4092 | Odds Ratio (M-H, Fixed, 95% CI) | 0.94 [0.78, 1.13] |
| 26 Adverse events - candidiasis | 4 | 1697 | Odds Ratio (M-H, Fixed, 95% CI) | 1.08 [0.74, 1.58] |
| 26.1 Partially reversible population (mixed population) | 2 | 703 | Odds Ratio (M-H, Fixed, 95% CI) | 1.07 [0.62, 1.83] |
| 26.2 Poorly reversible population | 2 | 994 | Odds Ratio (M-H, Fixed, 95% CI) | 1.09 [0.63, 1.86] |
| 27 Adverse events - pneumonia | 4 | 4522 | Odds Ratio (M-H, Fixed, 95% CI) | 1.12 [0.91, 1.37] |
| 27.1 Partially reversible population (mixed population) | 2 | 694 | Odds Ratio (M-H, Fixed, 95% CI) | 0.73 [0.14, 3.72] |
| 27.2 Poorly reversible population | 2 | 3828 | Odds Ratio (M-H, Fixed, 95% CI) | 1.12 [0.91, 1.38] |
| 27.3 Unclear reversibility | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 28 Adverse events - headache | 4 | 4538 | Odds Ratio (M-H, Fixed, 95% CI) | 0.97 [0.79, 1.20] |
| 28.1 Partially reversible population (mixed population) | 2 | 694 | Odds Ratio (M-H, Fixed, 95% CI) | 1.07 [0.72, 1.60] |
| 28.2 Poorly reversible population | 2 | 3844 | Odds Ratio (M-H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
| 28.3 Unclear reversibility | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 29 Adverse events - upper respiratory tract infection | 4 | 4538 | Odds Ratio (M-H, Random, 95% CI) | 1.03 [0.67, 1.58] |
| 29.1 Partially reversible population (mixed population) | 2 | 694 | Odds Ratio (M-H, Random, 95% CI) | 1.22 [0.79, 1.90] |
| 29.2 Poorly reversible population | 2 | 3844 | Odds Ratio (M-H, Random, 95% CI) | 0.89 [0.39, 2.01] |
| 29.3 Unclear reversibility | 0 | 0 | Odds Ratio (M-H, Random, 95% CI) | Not estimable |
| 30 Mortality - cause specific | 1 | Odds Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 30.1 COPD-related death | 1 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable | |
| 30.2 Cancer | 1 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable | |
| 30.3 Cardiovascular | 1 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 3. Budesonide/formoterol (BDF) versus budesonide (BD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe Exacerbations | 2 | Rate ratio (Fixed, 95% CI) | 0.88 [0.73, 1.07] | |
| 1.1 Partially reversible | 0 | Rate ratio (Fixed, 95% CI) | Not estimable | |
| 1.2 Poorly reversible | 2 | Rate ratio (Fixed, 95% CI) | 0.88 [0.73, 1.07] | |
| 2 Mean exacerbation rates per patient per year | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Partially reversible population (mixed population) | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 2.2 Poorly reversible population | 2 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 3 Quality of life - change scores | 2 | SGRQ (Fixed, 95% CI) | −3.26 [−5.10, −1.42] | |
| 3.1 Partially reversible (mixed population) | 0 | SGRQ (Fixed, 95% CI) | Not estimable | |
| 3.2 Poorly reversible | 2 | SGRQ (Fixed, 95% CI) | −3.26 [−5.10, −1.42] | |
| 4 Rescue medication use | 2 | Puffs per day (Fixed, 95% CI) | −0.8 [−1.06, −0.54] | |
| 4.1 Partially reversible | 0 | Puffs per day (Fixed, 95% CI) | Not estimable | |
| 4.2 Poorly reversible | 2 | Puffs per day (Fixed, 95% CI) | −0.8 [−1.06, −0.54] | |
| 5 Symptoms (change scores) | 2 | Symptom scale (Fixed, 95% CI) | −0.43 [−0.69, −0.18] | |
| 5.1 Partially reversible (mixed population) | 0 | Symptom scale (Fixed, 95% CI) | Not estimable | |
| 5.2 Poorly reversible | 2 | Symptom scale (Fixed, 95% CI) | −0.43 [−0.69, −0.18] | |
| 6 Mortality | 2 | 917 | Odds Ratio (M-H, Fixed, 95% CI) | 0.98 [0.42, 2.29] |
| 6.1 Mortality as primary outcome | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Mortality data collected as secondary/unpublished outcome | 2 | 917 | Odds Ratio (M-H, Fixed, 95% CI) | 0.98 [0.42, 2.29] |
| 7 Mean FEV1 (% increase from baseline) | 2 | % increase (Fixed, 95% CI) | 10.17 [7.71, 12.62] | |
| 7.1 Partially reversible | 0 | % increase (Fixed, 95% CI) | Not estimable | |
| 7.2 Poorly reversible | 2 | % increase (Fixed, 95% CI) | 10.17 [7.71, 12.62] | |
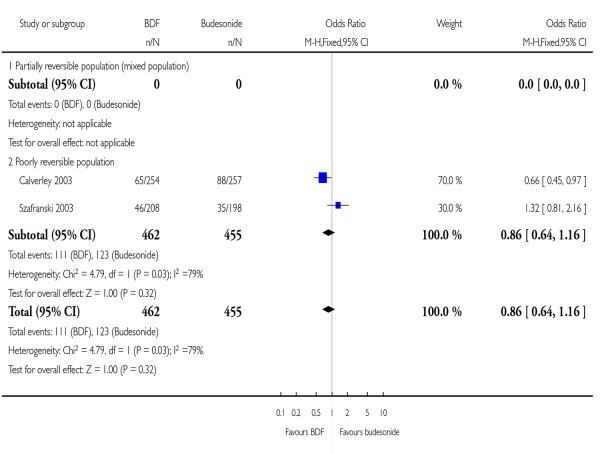
| 8 Adverse events - ‘serious’ events | 2 | 917 | Odds Ratio (M-H, Fixed, 95% CI) | 0.86 [0.64, 1.16] |
| 8.1 Partially reversible population (mixed population) | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Poorly reversible population | 2 | 917 | Odds Ratio (M-H, Fixed, 95% CI) | 0.86 [0.64, 1.16] |
| 9 Adverse events - candidiasis | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Partially reversible population (mixed population) | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Poorly reversible population | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Withdrawals due to worsening COPD symptoms | 2 | 917 | Odds Ratio (M-H, Fixed, 95% CI) | 0.65 [0.44, 0.97] |
| 10.1 Partially reversible population (mixed population) | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Poorly reversible population | 2 | 917 | Odds Ratio (M-H, Fixed, 95% CI) | 0.65 [0.44, 0.97] |
| 11 Withdrawals due to adverse events | 2 | 917 | Odds Ratio (M-H, Fixed, 95% CI) | 1.05 [0.64, 1.71] |
| 11.1 Partially reversible population (mixed population) | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Poorly reversible population | 2 | 917 | Odds Ratio (M-H, Fixed, 95% CI) | 1.05 [0.64, 1.71] |
| 12 Adverse events - pneumonia | 1 | Odds Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 12.1 Partially reversible population (mixed population) | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable | |
| 12.2 Poorly reversible population | 1 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
Analysis 1.1. Comparison 1 All Combined Inhalers - Primary Outcomes, Outcome 1 Exacerbations.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 1 All Combined Inhalers - Primary Outcomes
Outcome: 1 Exacerbations
|
Analysis 1.2. Comparison 1 All Combined Inhalers - Primary Outcomes, Outcome 2 Mortality.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 1 All Combined Inhalers - Primary Outcomes
Outcome: 2 Mortality
|
Analysis 1.3. Comparison 1 All Combined Inhalers - Primary Outcomes, Outcome 3 Pneumonia.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 1 All Combined Inhalers - Primary Outcomes
Outcome: 3 Pneumonia

|
Analysis 2.1. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 1 Number of participants with one or more exacerbation.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 1 Number of participants with one or more exacerbation
|
Analysis 2.2. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 2 End of treatment mean number of exacerbations per participant.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 2 End of treatment mean number of exacerbations per participant
|
Analysis 2.3. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 3 Exacerbations.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 3 Exacerbations
|
|
Analysis 2.4. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 4 Exacerbations by type.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 4 Exacerbations by type
|
Analysis 2.5. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 5 Mortality.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 5 Mortality

|
Analysis 2.6. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 6 Change from baseline in St George’s Respiratory Questionnaire (total score).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 6 Change from baseline in St George’s Respiratory Questionnaire (total score)
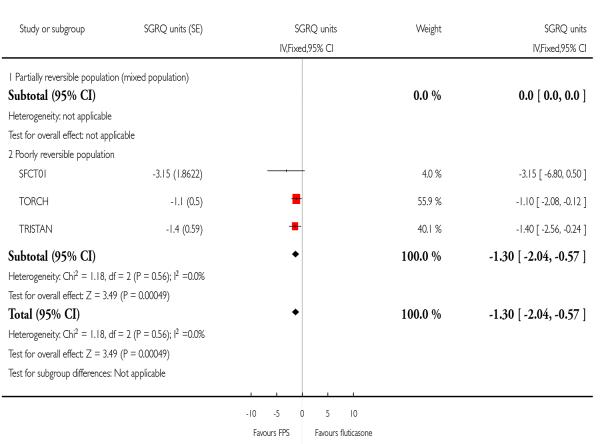
|
Analysis 2.7. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 7 Change from baseline in St George’s Respiratory Questionnaire (domain - symptoms).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 7 Change from baseline in St George’s Respiratory Questionnaire (domain - symptoms)
|
Analysis 2.8. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 8 Change from baseline in St George’s Respiratory Questionnaire (domain - activity).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 8 Change from baseline in St George’s Respiratory Questionnaire (domain - activity)

|
Analysis 2.9. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 9 Change from baseline in St George’s Respiratory Questionnaire (domain - impact).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 9 Change from baseline in St George’s Respiratory Questionnaire (domain - impact)
|
Analysis 2.10. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 10 End of treatment St George’s Respiratory Questionnaire scores (total score).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 10 End of treatment St George’s Respiratory Questionnaire scores (total score)
|
Analysis 2.11. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 11 End of treatment St George’s Respiratory Questionnaire scores (domain - symptoms).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 11 End of treatment St George’s Respiratory Questionnaire scores (domain - symptoms)
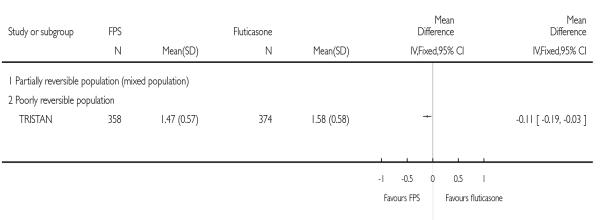
|
Analysis 2.12. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 12 Change from baseline in Canadian Respiratory Disease Questionnaire scores.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 12 Change from baseline in Canadian Respiratory Disease Questionnaire scores

|
Analysis 2.13. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 13 Change from baseline in Transitional Dyspnoea Index (TDI).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 13 Change from baseline in Transitional Dyspnoea Index (TDI)

|
Analysis 2.14. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 14 Change from baseline in FEV1 (Litres).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 14 Change from baseline in FEV1 (Litres)
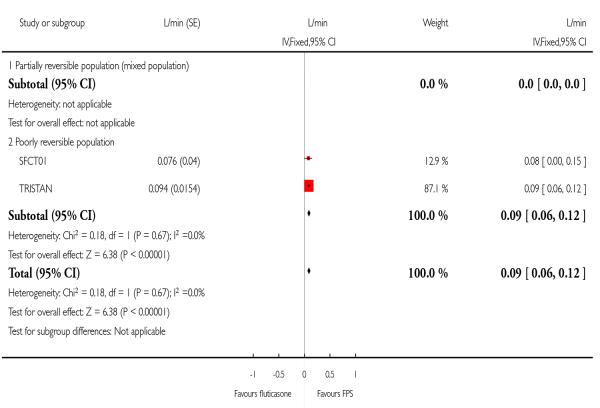
|
Analysis 2.15. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 15 Change from baseline in predose FEV1 (Litres).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 15 Change from baseline in predose FEV1 (Litres)
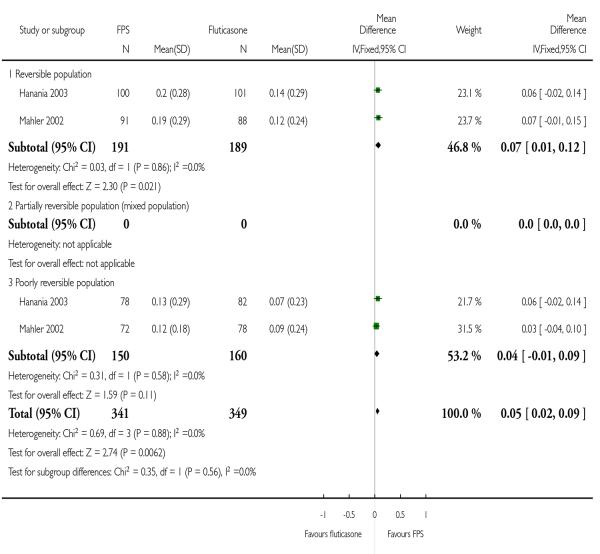
|
Analysis 2.16. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 16 End of treatment FEV1 (Litres).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 16 End of treatment FEV1 (Litres)
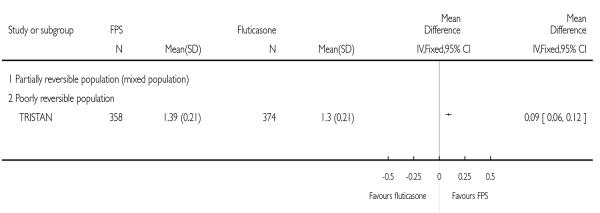
|
Analysis 2.17. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 17 End of treatment postdose FEV1.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 17 End of treatment postdose FEV1
|
Analysis 2.18. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 18 Change from baseline in postdose FEV1.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 18 Change from baseline in postdose FEV1

|
Analysis 2.19. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 19 End of treatment am PEF (L/min).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 19 End of treatment am PEF (L/min)

|
Analysis 2.20. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 20 Absolute shuttle walk test.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 20 Absolute shuttle walk test
|
Analysis 2.21. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 21 Change from baseline in rescue medication usage (puffs/day).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 21 Change from baseline in rescue medication usage (puffs/day)
|

|
Analysis 2.22. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 22 Withdrawals.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 22 Withdrawals
|

|
Analysis 2.23. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 23 Withdrawal due to lack of efficacy/exacerbation.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 23 Withdrawal due to lack of efficacy/exacerbation
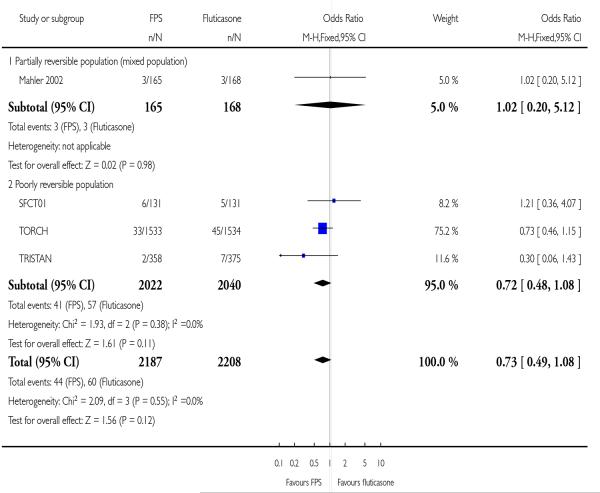
|
Analysis 2.24. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 24 Withdrawals due to adverse events.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 24 Withdrawals due to adverse events

|
Analysis 2.25. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 25 Adverse events - any event.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 25 Adverse events - any event

|
Analysis 2.26. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 26 Adverse events - candidiasis.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 26 Adverse events - candidiasis
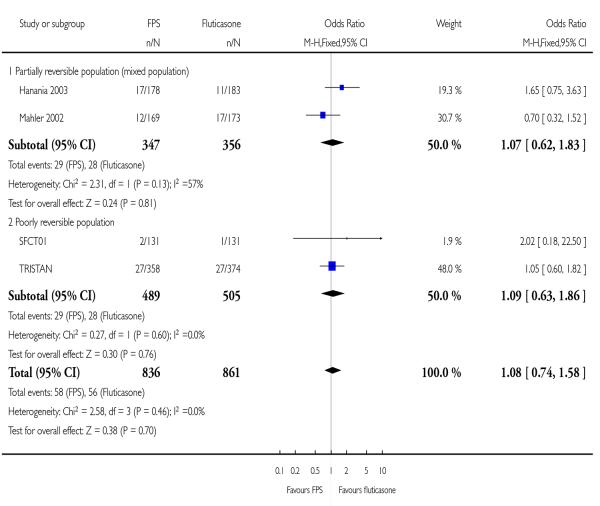
|
Analysis 2.27. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 27 Adverse events - pneumonia.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 27 Adverse events - pneumonia

|
Analysis 2.28. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 28 Adverse events - headache.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 28 Adverse events - headache
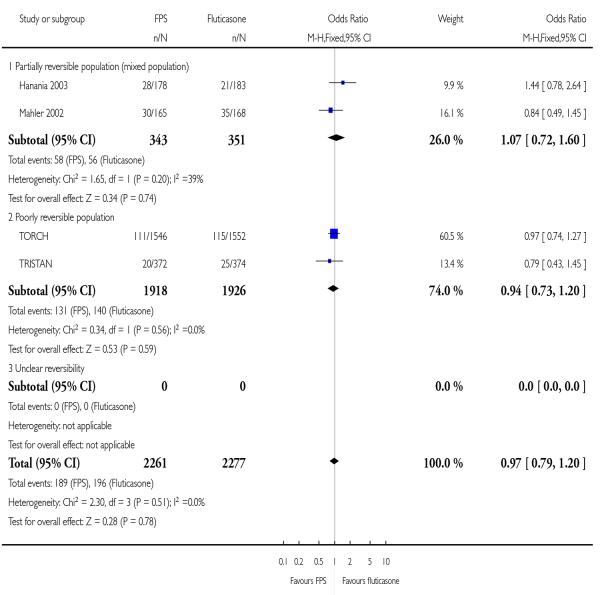
|
Analysis 2.29. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 29 Adverse events - upper respiratory tract infection.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 29 Adverse events - upper respiratory tract infection
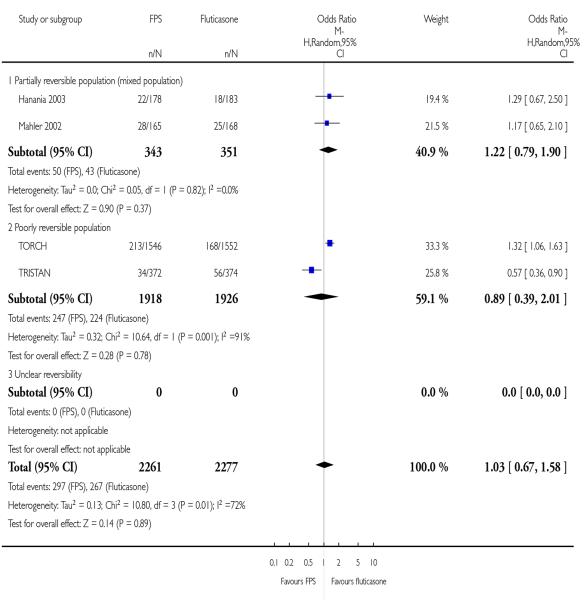
|
Analysis 2.30. Comparison 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP), Outcome 30 Mortality - cause specific.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 2 Fluticasone/salmeterol (FPS) versus fluticasone (FP)
Outcome: 30 Mortality - cause specific
|
Analysis 3.1. Comparison 3 Budesonide/formoterol (BDF) versus budesonide (BD), Outcome 1 Severe Exacerbations.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 3 Budesonide/formoterol (BDF) versus budesonide (BD)
Outcome: 1 Severe Exacerbations

|
Analysis 3.2. Comparison 3 Budesonide/formoterol (BDF) versus budesonide (BD), Outcome 2 Mean exacerbation rates per patient per year.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 3 Budesonide/formoterol (BDF) versus budesonide (BD)
Outcome: 2 Mean exacerbation rates per patient per year
|
Analysis 3.3. Comparison 3 Budesonide/formoterol (BDF) versus budesonide (BD), Outcome 3 Quality of life - change scores.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 3 Budesonide/formoterol (BDF) versus budesonide (BD)
Outcome: 3 Quality of life - change scores

|
Analysis 3.4. Comparison 3 Budesonide/formoterol (BDF) versus budesonide (BD), Outcome 4 Rescue medication use.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 3 Budesonide/formoterol (BDF) versus budesonide (BD)
Outcome: 4 Rescue medication use

|
Analysis 3.5. Comparison 3 Budesonide/formoterol (BDF) versus budesonide (BD), Outcome 5 Symptoms (change scores).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 3 Budesonide/formoterol (BDF) versus budesonide (BD)
Outcome: 5 Symptoms (change scores)
|
Analysis 3.6. Comparison 3 Budesonide/formoterol (BDF) versus budesonide (BD), Outcome 6 Mortality.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 3 Budesonide/formoterol (BDF) versus budesonide (BD)
Outcome: 6 Mortality
|
Analysis 3.7. Comparison 3 Budesonide/formoterol (BDF) versus budesonide (BD), Outcome 7 Mean FEV1 (% increase from baseline).
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 3 Budesonide/formoterol (BDF) versus budesonide (BD)
Outcome: 7 Mean FEV1 (% increase from baseline)
|
Analysis 3.8. Comparison 3 Budesonide/formoterol (BDF) versus budesonide (BD), Outcome 8 Adverse events - ‘serious’ events.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 3 Budesonide/formoterol (BDF) versus budesonide (BD)
Outcome: 8 Adverse events - ‘serious’ events
|
Analysis 3.10. Comparison 3 Budesonide/formoterol (BDF) versus budesonide (BD), Outcome 10 Withdrawals due to worsening COPD symptoms.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 3 Budesonide/formoterol (BDF) versus budesonide (BD)
Outcome: 10 Withdrawals due to worsening COPD symptoms
|
Analysis 3.11. Comparison 3 Budesonide/formoterol (BDF) versus budesonide (BD), Outcome 11 Withdrawals due to adverse events.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 3 Budesonide/formoterol (BDF) versus budesonide (BD)
Outcome: 11 Withdrawals due to adverse events

|
Analysis 3.12. Comparison 3 Budesonide/formoterol (BDF) versus budesonide (BD), Outcome 12 Adverse events - pneumonia.
Review: Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Comparison: 3 Budesonide/formoterol (BDF) versus budesonide (BD)
Outcome: 12 Adverse events - pneumonia
|
ADDITIONAL TABLES
Table 1. Search history.
| Version | Detail |
|---|---|
| 1st published version - Issue 4, 2003 (All years to April 2002) | References identified: 34 References retrieved: 7 Studies excluded 3 (Cazzola 2000; Chapman 2002; Soriano 2002) Studies identified from supplementary searching: 4 (Dal Negro 2003; Hanania 2003 - both included; Cazzola 2002a; Cazzola 2004 - both excluded). Studies included: 4 |
| 2nd published version - Issue 3, 2004 (April 2003-April 2004) | References identified: 12 References retrieved: 3 (2 papers full publication of a previously included or cited studies study (Dal Negro 2003; Hanania 2003). Hand searching identified two further references to the COSMIC 2003 study. Studies identified from supplementary searching: 1 (TRISTAN) New studies included: 2 Total studies included: 6 |
| 3rd published version - Issue 3, 2005 (April 2004-April 2005) | References identified: 52 References retrieved: 46 (references to studies already included/excluded/ongoing: 24) New unique studies identified: 10 (ongoing studies: 2) New studies included: 0 Total studies included: 6 |
| 4th published version - April 2005 - April 2007 | References identified: 66 References retrieved: 27 (references to studies already included/ excluded/ongoing: ) New unique studies identified: 8 (ongoing studies: 0) New studies included: 7 Total studies included: 13 |
Table 2. Rates and NNT of mortality.
| Study ID | Study duration | ICS rate (%) | NNT |
|---|---|---|---|
| SFCT01 | 52 | 0 | NA |
| TORCH | 156 weeks | 16 | 32 (19 to 123) |
| TRISTAN | 52 weeks | 0.8 | 547 (340 to 2100) |
| Calverley 2003 | 52 weeks | 2.3 | 193 (120 to 741) |
| Szafranski 2003 | 52 weeks | 2.5 | 178 (110 to 683) |
WHAT’S NEW
Last assessed as up-to-date: 20 August 2007.
| Date | Event | Description |
|---|---|---|
| 11 November 2009 | Amended | Spelling mistakes corrected and minor changes to wording. Changes made to formatting |
HISTORY
Protocol first published: Issue 3, 2002
Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 8 April 2008 | Amended | Converted to new review format. |
| 21 August 2007 | New citation required and conclusions have changed | This review contains evidence from 5 studies previously included in a review of combination therapy in COPD (Nannini L, Cates CJ, Lasserson TJ, Poole P Combined corticosteroid and long-acting beta-agonist in one inhaler for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2004, Issue 3), with new data from two studies (TORCH; SFCT01). New findings There is a significant reduction on mortality with combination therapy compared with ICS alone. Exacerbation rates are lower with combination therapy compared with ICS. Additional work should focus on budesonide and formoterol, and the collection of data on pneumonia |
Footnotes
DECLARATIONS OF INTEREST: The authors who have been involved in this review have done so without any known conflicts of interest. None of the authors is considered a paid consultant by any pharmaceutical company which produces agents discussed in this review.
References to studies included in this review
- Calverley 2003 {published and unpublished data} .Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]; American Thoracic Society 100th International Conference; 2004; May 21-26, p. C22. Poster 505. [Google Scholar]
- *; Calverley PM, Bonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal. 2003;22(6):912–9. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- Calverley PMA, Cseke Z, Peterson S. Budesonide/formoterol reduces the use of oral corticosteroids in the treatment of COPD [Abstract] European Respiratory Journal. 2003;22(Suppl 45):P436. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- Calverley PMA, Kuna P, Olsson H. COPD exacerbations are reduced by budesonide/formoterol in a single inhaler [Abstract] European Respiratory Journal. 2003;22(Suppl 45):P1587. [Google Scholar]
- Calverley PMA, Olsson H, Symbicort International COPD Study Group Budesonide/formoterol ina single inhaler sustains improvements in lung function over 12 months compared with monocomponents and placebo in patients with COPD [abstract]; American Thoracic Society 99th International Conference; 2003; p. B024. Poster 418. [Google Scholar]
- Calverley PMA, Peterson S. Combining budesonide/formoterol in a single inhale reduces exacerbation frequency in COPD [abstract]; American Thoracic Society 99th International Conference; 2003; p. D092. Poster 211. [Google Scholar]
- Calverley PMA, Ståhl E, Jones PW. Budesonide/formoterol improves the general health status of patients with COPD [Abstract]; American Thoracic Society 2005 International Conference; San Diego, California. 2005; May 20-25, p. B93. Poster 303. [Google Scholar]
- Calverley PMA, Szafranski W, Andersson A. Budesonide/formoterol is a well-tolerated long term maintenance therapy for COPD. European Respiratory Journal. 2005;26(Suppl 49) Poster 1917. [Google Scholar]
- Calverley PMA, Thompson NC, Olsson H. Budesonide/formoterol in a single inhaler sustains lung function improvements in COPD [Abstract] European Respiratory Journal. 2003;22(Suppl 45):P435. [Google Scholar]
- Halpin D, Ståhl E, Lundback B, Anderson F, Peterson S. Treatment costs and number needed to treat (NNT) with budesonide/formoterol to avoid one exacerbation of COPD [Abstract]; American Thoracic Society 100th International Conference; 2004; May 21-26, p. D22. Poster 525. [Google Scholar]
- Halpin DMG, Larsson T, Calverley PMA. How many patients with COPD must be treated with budesonide/formoterol compared with formoterol alone to avoid 1 day of oral steroid use? [Abstract]; American Thoracic Society 2005 International Conference; San Diego, California. 2005; May 20-25, p. B93. Poster 314. [Google Scholar]
- Jones PW, Stahl E. Budesonide/formoterol in a single inhaler improves health status in patients with COPD [abstract]; American Thoracic Society 99th International Conference; 2003; p. B024. Poster 419. [Google Scholar]
- Jones PW, Ståhl E. Budesonide/formoterol sustains clinically relevant improvements in health status in COPD [Abstract] European Respiratory Journal. 2005;26(Suppl 49) Abstract 1352. [Google Scholar]
- Jones PW, Ståhl E. Reducing exacerbations leads to a better health-related quality of life in patients with COPD; 13th ERS Annual Congress; Vienna. 27th September 2003.p. P1586. [Google Scholar]
- Lofdahl CG. Reducing the impact of COPD exacerbations: Clinical efficacy of budesonide/formoterol. European Respiratory Review. 2004;13(88):14–21. [Google Scholar]
- Lofdahl CG, Andreasson E, Svensson K, Ericsson A. Budesonide/formoterol in a single inhaler improves health status in patients with COPD without increasing healthcare costs [Abstract] European Respiratory Journal. 2003;22(Suppl 45):P433. [Google Scholar]
- Lofdahl CG, Ericsson A, Svensson K, Andreasson E. Cost effectiveness of budesonide/formoterol in a single inhaler for COPD compared with each monocomponent used alone. Pharmacoeconomics. 2005;23(4):365–75. doi: 10.2165/00019053-200523040-00006. [DOI] [PubMed] [Google Scholar]
- Hanania 2003 {published and unpublished data} .*; Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 micro g)/salmeterol (50 micro g) combined in the diskus inhaler for the treatment of COPD. Chest. 2003;124(3):834–43. doi: 10.1378/chest.124.3.834. [DOI] [PubMed] [Google Scholar]
- Hanania NA, Ramsdell J, Payne K, Davis S, Horstman D, Lee B, et al. Improvements in airflow and dyspnea in COPD patients following 24 weeks treatment with salmeterol 50mcg and fluticasone propionate 250mcg alone or in combination via the diskus. American Journal of Respiratory & Critical Care Medicine. 2001;163(5 Suppl):A279. [Google Scholar]
- Horstman D, Darken P, Davis S, Lee B. Improvements in FEV1 and symptoms in poorly reversible COPD patients following treatment with salmeterol 50mcg/fluticasone propionate 250mcg combination [Abstract] European Respiratory Journal. 2003;22(Suppl 45):P434. [Google Scholar]
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD) [Abstract]; National COPD Conference; Arlington, Virginia. 2003; Abstract 1081. [Google Scholar]
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to salmeterol/fluticasone propionate combination therapy in COPD [Abstract] European Respiratory Journal. 2003;22(Suppl 45):P429. [Google Scholar]
- SFCA3007 A randomized, double-blind, placebo-controlled, parallel-group trial evaluating the safety and efficacy of the DISKUS formulations of Salmeterol (SAL) 50mcg BID and Fluticasone Propionate (FP) 250mcg BID individually and in combination as Salmeterol 50mcg/Fluticasone Propionate 250mcg BID (SFC 50/250) compared to placebo in COPD subjects. 2005 GlaxoSmithKline Clinical Trials Register: http://ctr.gsk.co.uk.
- Spencer M, Wire P, Lee B, Chang CN, Darken P, Horstman D. Patients with COPD using salmeterol/fluticasone propionate combination therapy experience improved quality of life. European Respiratory Journal. 2003;22(Suppl 45):51s. [Google Scholar]
- Spencer MD, Karia N, Anderson J. The clinical significance of treatment benefits with the salmeterol/fluticasone propionate 50/500mcg combination in COPD. European Respiratory Journal. 2004;24(Suppl 48):290s. [Google Scholar]
- Mahler 2002 {published and unpublished data} .Mahler DA, Darken P, Brown CP, Knobil K. Predicting Lung Function Responses to Combination Therapy in Chronic Obstructive Pulmonary Disease (COPD) 2003 http://www.abstracts2view.com.
- *; Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. [Effectiveness of fluticasone propionate and salmeterol combination delivered via the diskus device in the treatment of chronic obstructive pulmonary disease] American Journal of Respiratory Critical Care Medicine. 2002;166(8):1084–91. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
- SFCA3006 A randomized, double-blind, placebo-controlled, parallel-group trial evaluating the safety and efficacy of the DISKUS formulations of Salmeterol (SAL) 50mcg BID and Fluticasone Propionate (FP) 500mcg BID individually and in combination as Salmeterol 50mcg/Fluticasone Propionate 500mcg BID (SFC 50/500) compared to placebo in COPD subjects. 2005 GlaxoSmithKline Clinical Trials Register: http://ctr.gsk.co.uk.
- Spencer M, Wire P, Lee B, Chang CN, Darken P, Horstman D. Patients with COPD using salmeterol/fluticasone propionate combination therapy experience improved quality of life. European Respiratory Journal. 2003;22(Suppl 45):51s. [Google Scholar]
- Spencer MD, Anderson JA. Salmeterol/fluticasone combination produces clinically important benefits in dyspnea and fatigue [Abstract]; American Thoracic Society 2005 International Conference; San Diego, California. 2005; May 20-25, p. B93. Poster 308. [Google Scholar]
- Spencer MD, Karia N, Anderson J. The clinical significance of treatment benefits with the salmeterol/fluticasone propionate 50/500mcg combination in COPD. European Respiratory Journal. 2004;24(Suppl 48):290s. [Google Scholar]
- SFCT01 {unpublished data only} .*; SFCT01 A Multicentre, Randomised, Double-Blind, Parallel Group, Placebo-Controlled Study to Compare the Efficacy and Safety of Inhaled Salmeterol/Fluticasone Propionate Combination Product 25/250 μg Two Puffs Bd and Fluticasone Propionate 250μg Two Puffs Bd Alone, All Administered Via Metered Dose Inhalers (MDI), in the Treatment of Subjects with Chronic Obstructive Pulmonary Disease (COPD) for 52 Weeks. 2005 GlaxoSmithKline Clinical Trials Register: http://ctr.gsk.co.uk.
- Szafranski 2003 {published and unpublished data} .Anderson P. [Budesonide/formoterol in a single inhaler (Symbicort) provides early and sustained improvement in lung function in moderate to severe COPD [Abstract]] Thorax. 2002;57(Suppl III):iii43. [Google Scholar]
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]; American Thoracic Society 100th International Conference; 2004; May 21-26, p. C22. Poster 505. [Google Scholar]
- Calverley P, Pauwels R, Lofdahl CG, Svensson K, Higenbottam T, et al. Relationship between respiratory symptoms and medical treatment in exacerbations of COPD. European Respiratory Journal. 2005;26(3):406–13. doi: 10.1183/09031936.05.00143404. [DOI] [PubMed] [Google Scholar]
- Calverley PMA. Effect of budesonide/formoterol on severe exacerbations and lung function in moderate to severe COPD. Thorax; BTS Winter meeting; 2002.p. S145. [Google Scholar]
- Calverley PMA, Szafranski W, Andersson A. Budesonide/formoterol is a well-tolerated long term maintenance therapy for COPD. European Respiratory Journal. 2005;26(Suppl 49) Poster 1917. [Google Scholar]
- Calverley PMA, Thompson NC, Olsson H. Budesonide/formoterol in a single inhaler sustains lung function improvements in COPD [Abstract] European Respiratory Journal. 2003;22(Suppl 45):P435. [Google Scholar]
- Campbell LM, Szafranski W. Budesonide/formoterol in a single inhaler (Symbicort) provides sustained relief from symptoms in moderate to severe COPD. Thorax; BTS Winter meeting; 2002.p. S143. [Google Scholar]
- Campell LW, Szafranski W. Budesonide/Formoterol in a single inhaler (Symbicort) reduces severe exacerbations in patients with moderate-severe COPD. Thorax; BTS Winter meeting; 2002.p. S141. [Google Scholar]
- Dahl R, Cukier A, Olsson H. Budesonide/formoterol in a single inhaler reduces severe and mild exacerbations in patients with moderate to severe COPD. European Respiratory Journal. 2002;20(Suppl 38):242. P1575. [Google Scholar]
- Egede F, Menga G. Budesonide/formoterol in a single inhaler provides sustained relief from symptoms and night-time awakenings in moderate-severe COPD: results from symptoms and night-time awakenings in moderate to severe COPD: results from a 1-year study. European Respiratory Journal. 2002;20(Suppl 38):242. P1574. [Google Scholar]
- Halpin D, Stahl E, Lundback B, Anderson F, Peterson S. Treatment costs and number needed to treat (NNT) with budesonide/formoterol to avoid one exacerbation of COPD [Abstract]; American Thoracic Society 100th International Conference; 2004; May 21-26, p. D22. Poster 525. [Google Scholar]
- Jones PW, Stahl E, Svensson K. Improvement in health status in patients with moderate to severe COPD after treatment with budesonide/formoterol in a single inhaler. European Respiratory Journal. 2002;20(Suppl 38):250. P1613. [Google Scholar]
- Korsgaard J, Sansores R. Budesonide/formoterol (single inhaler) provides sustained relief from shortness of breath and chest tightness in a 1-year study of patients with moderate to severe COPD. European Respiratory Journal. 2002;20(Suppl 38):242. P1577. [Google Scholar]
- Lange P, Saenz C. Budesonide/formoterol in a single inhaler is well tolerated in patients with moderate to severe COPD: results of a 1 year study. European Respiratory Journal. 2002;20(Suppl 38):242. P1573. [Google Scholar]
- Lofdahl CG. Reducing the impact of COPD exacerbations: Clinical efficacy of budesonide/formoterol. European Respiratory Review. 2004;13(88):14–21. [Google Scholar]
- Milanowski J, Nahabedian S. Budesonide/formoterol in a single inhaler acts rapidly to improve lung function and relieve symptoms in patients with moderate to severe COPD. European Respiratory Journal. 2002;20(Suppl 38):242. P1576. [Google Scholar]
- *; Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. European Respiratory Journal. 2003;21(1):74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- TORCH {published and unpublished data} .*; Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine. 2007;356(8):775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- Calverley PMA, Celli B, Ferguson G, Jenkins C, Jones PW, Pride NB, et al. Baseline characteristics of the first 5,000 COPD patients enrolled in the TORCH survival study. European Respiratory Journal. 2003;22(Suppl 45):578s. [Google Scholar]
- Celli B, Calverley PMA, Anderson JA, Ferguson GT, Jenkins C, Jones PW, et al. The TORCH (TOwards a Revolution in COPD Health) study: salmeterol/fluticasone propionate (SFC) improves health status, reduces exacerbations and improves lung function over three years. European Respiratory Journal. 2006;28(Suppl 50):34s. [Google Scholar]
- Ferguson GT, Calverley PMA, Anderson JA, et al. The TORCH (TOwards a Revolution in COPD Health) study: salmeterol/fluticasone propionate (SFC) improves survival in COPD over three years. European Respiratory Journal. 2006;28(Suppl 50):34s. [Google Scholar]
- Jenkins CR, Calverley PMA, Celli B, Ferguson G, Jones PW, Pride N, et al. Seasonal Patterns of Exacerbation Rates in the TORCH Survival Study. 2007:A839. http://www.abstracts2view.com.
- Jones PW, Calverley P, Celli B, Ferguson G, Jenkins C, Pride N. Trans-Regional Validity of the SGRQ in the TORCH Survival Study. 2007:A122. http://www.abstracts2view.com.
- McGarvey LP, John M, Anderson JA, Zvarich MT, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62:411–5. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCO30003 A multicentre, randomised, double-blind, parallel group, placebo-controlled study to investigate the long-term effects of salmeterol/fluticasone propionate (SERETIDE®/VIANI®/ADVAIR®) 50/500mcg bd, salmeterol 50mcg bd and fluticasone propionate 500mcg bd, all delivered via the DISKUS®/ACCUHALER® inhaler, on the survival of subjects with chronic obstructive pulmonary disease (COPD) over 3 years of treatment. 2006 www.ctr.gsk.co.uk.
- Vestbo J, Calverley P, Celli B, Ferguson G, Jenkins C, Jones P, et al. The TORCH (TOwards a Revolution in COPD Health) survival study protocol. European Respiratory Journal. 2004;24(2):206–10. doi: 10.1183/09031936.04.00120603. [DOI] [PubMed] [Google Scholar]
- Wise RA, McGarvey LP, John M, Anderson JA, Zvarich MT. Reliability of cause-specific mortality adjudication in a COPD clinical trial. 2007:A120. http://www.abstracts2view.com.
- TRISTAN {published and unpublished data} .*; Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Clinical improvements with salmeterol / fluticasone propionate combination in differing severities of COPD. 2003:A035. http://www.abstracts2view.com. Poster D50.
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Salmeterol/Fluticasone propionate combination for one year provides greater clinical benefit than its individual components; Proceedings of the 98th International American Thoracic Society Conference; 2002; p. A98. http://www.abstracts-on-line.com/abstracts/ATS. Poster 306. [Google Scholar]
- Calverly PMA, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Safety of salmeterol/fluticasone propionate combination in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal. 2002;20(Suppl 38):242. P1572. [Google Scholar]
- Hunjan MK, Chandler F. Numbers needed to treat (NNT) to avoid an exacerbation in patients with chronic obstructive pulmonary disease (COPD) using salmeterol/fluticasone propionate combination (SFC) and associated costs [Abstract]; American Thoracic Society 100th International Conference; 2004; May 21-26, p. D22. Poster 503. [Google Scholar]
- Hunjan MK, Williams DT. Costs of avoiding exacerbations in patients with chronic obstructive pulmonary disease (COPD) treated with salmeterol/fluticasone propionate combination (seretide) and salmeterol. European Respiratory Journal. 2004;24(Suppl 48):291s. [Google Scholar]
- Hunjan MK, Williams DT. Salmeterol/fluticasone propionate combination is clinically effective in avoiding exacerbations in patients with moderate/severe COPD. European Respiratory Journal. 2004;24(Suppl 48M):513s. [Google Scholar]
- Jones PW, Edin HM, Anderson J. Salmeterol/fluticasone propionate combination improves health status in COPD patients; Proceedings of the 98th International American Thoracic Society Conference; 2002; p. A39. http://www.abstracts-online.com/abstracts/ATS. Poster K39. [Google Scholar]
- Jones PW, Ståhl E. Budesonide /formoterol sustains clinically relevant improvements in health status in COPD [Abstract] European Respiratory Journal. 2005;26(Suppl 49) Abstract 1352. [Google Scholar]
- Jones PW, Vestbo J, Pauwels RA, Calverley PMA, Anderson JA, Spencer MD. Informative drop out in COPD studies. Investigation of health status of withdrawals in the TRISTAN study; 13th ERS Annual Congress; Vienna. 27th September 2003.p. P1593. [Google Scholar]
- Nitschmann S. Inhalational combination therapy in chronic obstructive lung disease. Tristan study. German Internist. 2004;45(6):727–8. doi: 10.1007/s00108-004-1177-8. [DOI] [PubMed] [Google Scholar]
- Pauwels R, Vestbo J, Calverley PMA, Jones PW, Pride NB, Gulsvik A. Characterization of exacerbations in the TRISTAN study of salmeterol / fluticasone propionate (SFC) combination in moderate to severe COPD. 2003 http://www.abstracts2view.com.
- Pauwels RA, Calverly PMA, Vestbo J, Jones PW, Pride N, Gulsvik A, et al. Reduction of exacerbations with salmeterol/fluticasone combination 50/500 mcg bd in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal. 2002;20(Suppl 38):240. P1569. [Google Scholar]
- SFCB3024 A multicentre, randomised, double-blind, parallel group, placebo-controlled study to compare the efficacy and safety of the salmeterol/FP combination product at a strength of 50/500mcg bd with salmeterol 50mcg bd alone and FP 500mcg bd alone, delivered via the DISKUS™/ACCUHALER™, in the treatment of subjects with chronic obstructive pulmonary disease (COPD) for 12 months. 2005 GlaxoSmithKline Clinical Trials Register: http://ctr.gsk.co.uk.
- Spencer M, Briggs AH, Grossman RF, Rance L. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics. 2005;23(6):619–37. doi: 10.2165/00019053-200523060-00008. [DOI] [PubMed] [Google Scholar]
- Spencer MD, Karia N, Anderson J. The clinical significance of treatment benefits with the salmeterol/fluticasone propionate 50/500mcg combination in COPD. European Respiratory Journal. 2004;24(Suppl 48):290s. [Google Scholar]
- Vestbo J, Calverley PMA, Pauwels R, Jones P, Pride N, Gulsvik A, et al. Absence of gender susceptibility to the combination of salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal. 2002;20(Suppl 38):240. P1570. [Google Scholar]
- Vestbo J, Pauwels R, Anderson JA, Jones P, Calverley P. Early onset of effect of salmeterol and fluticasone propionate in chronic obstructive pulmonary disease. Thorax. 2005;60(4):301–4. doi: 10.1136/thx.2004.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestbo J, Pauwels RS, Calverley PMA, Jones PW, Pride NB, Gulsvik A. Salmeterol / fluticasone propionate combination produces improvement in lung function detectable within 24 Hours in moderate to severe COPD. 2003 http://www.abstracts2view.com.
- Vestbo J, Soriano JB, Anderson JA, Calverley P, Pauwels R, Jones P. Gender does not influence the response to the combination of salmeterol and fluticasone propionate in COPD. Respiratory Medicine. 2004;98(11):1045–50. doi: 10.1016/j.rmed.2004.03.017. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Aaron 2007 {published data only} .Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine. 2007;146(8):545–55. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- Kaplan A. Effects of tiotropium combined with either salmeterol or salmeterol/fluticasone in moderate to severe COPD. Primary Care Respiratory Journal. 2007;16(4):258–60. doi: 10.3132/pcrj.2007.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbeau 2007 {published data only} .Bourbeau J, Christodoulopoulos P, Maltais F, Yamauchi Y, Olivenstein R, Hamid Q. Effect of salmeterol/fluticasone propionate on airway inflammation in COPD: A randomised controlled trial. Thorax. 2007;62(11):938–43. doi: 10.1136/thx.2006.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukier 2007 {published data only} .Cukier A, Ferreira CAS, Stelmach R, Ribeiro M, Cortopassi F, Calverley PMA. The effect of bronchodilators and oxygen alone and in combination on self-paced exercise performance in stable COPD. Respiratory Medicine. 2007;101(4):743–53. doi: 10.1016/j.rmed.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Golabi 2006 {published data only} .Golabi P, Topaloglu N, Karakurt S, Celikel T. Effects of tiotropium and salmeterol/fluticasone combination on lung hyperinflation dyspnea and exercise tolerance in COPD [Abstract] European Respiratory Journal. 2006;28(Suppl 50):33s. [Google Scholar]
- Haque 2006 {published data only} .Haque RA, Torrego A, Essilfie-Quaye S, Kharitonov SA, Johnson M, Adcock IM, et al. Effect of salmeterol and fluticasone on glucocorticoid receptor translocation in sputum macrophages and peripheral blood mononuclear cells from patients with chronic obstructive pulmonary disease; Proceedings of the American Thoracic Society; 2006.p. A848. [Google Scholar]
- INSPIRE {published data only} .GlaxoSmithKline (SCO40036) [accessed 8th April 2008];Multicentre, randomised, double-blind, double-dummy, parallel group, 104-week study to compare the effect of the salmeterol/fluticasone propionate combination product (SERETIDE*) 50/500mcg delivered twice daily via the DISKUS*/ACCUHALER* inhaler with tiotropium bromide 18 mcg delivered once daily via the HandiHaler inhalation device on the rate of health care utilisation exacerbations in subjects with severe chronic obstructive pulmonary disease (COPD) http://ctr.gsk.co.uk.
- Seemungal T, Stockley R, Calverley P, Hagan G, Wedzicha JA. Investigating new standards for prophylaxis in reduction of exacerbations - The INSPIRE study methodology. Journal of Chronic Obstructive Pulmonary Disease. 2007;4(3):177–83. doi: 10.1080/15412550701407862. [DOI] [PubMed] [Google Scholar]
- Wedzicha J, Stockley R, Seemungal T, Hagan G, Calverley P. The INSPIRE study: effect of salmeterol/fluticasone propionate versus tiotropium bromide on COPD exacerbations. Respirology. 2007;12(Suppl 4):A112. [Google Scholar]
- Lindberg 2007 {published data only} .Lindberg A, Szalai Z, Pullerits T, Radeczky E. Fast onset of effect of budesonide/formoterol versus salmeterol/fluticasone and salbutamol in patients with chronic obstructive pulmonary disease and reversible airway obstruction. Respirology. 2007;12(5):732–9. doi: 10.1111/j.1440-1843.2007.01132.x. [DOI] [PubMed] [Google Scholar]
- Lindberg A, Szalai Z, Pullertis T, Radeczky El. Budesonide/formoterol (B/F) has an onset of action that is similar to salbutamol and faster than salmeterol/fluticasone in patients with COPD. European Respiratory Journal. 2006;28(Suppl 50):214s. [Google Scholar]
- Schermer 2007 {published data only} .Schermer TR, Albers JM, Verblackt HW, Costongs RJ, Westers P. Lower inhaled steroid requirement with a fluticasone/salmeterol combination in family practice patients with asthma or COPD. Family Practice. 2007;24(2):181–8. doi: 10.1093/fampra/cml076. [DOI] [PubMed] [Google Scholar]
- SCO100250 {unpublished data only} .GlaxoSmithKline (SCO100250) [accessed 8th April 2008];A randomized, double-blind, parallel-group, 52-week study to compare the effect of fluticasone propionate/salmeterol DISKUS 250/50mcg bid with salmeterol DISKUS 50mcg bid on the annual rate of moderate/severe exacerbations in subjects with chronic obstructive pulmonary disease (COPD) http://ctr.gsk.co.uk.
- SCO40043 {unpublished data only} .SCO40043 [accessed 8th April 2008];A randomized, double-blind, parallel-group, 52-week study to compare the effect of fluticasone propionate/salmeterol DISKUS® 250/50mcg bid with salmeterol DISKUS® 50mcg bid on the annual rate of moderate/severe exacerbations in subjects with chronic obstructive pulmonary disease (COPD) http://ctr.gsk.co.uk.
- Sethi 2006 {published data only} .Sethi S, Grove L, Wrona C, Maloney J. Prevalence of bacterial colonization in COPD is not altered by fluticasone/salmeterol; Proceedings of the American Thoracic Society; 2006.p. A115. [Google Scholar]
- SHINE {unpublished data only} .Astrazeneca (D5899C00002) [Accessed 8th April, 2008];A 6-Month double-blind, double-dummy, randomized, parallel group, multicenter efficacy & safety study of Symbicort® pMDI 2 × 160/4.5mg & 80/4.5mg bid compared to Formoterol TBH, Budesonide pMDI (& the combination) & placebo in COPD patients. 2008 www.astrazenecaclinicaltrials.com.
- Sutherland 2006 {published data only} .Sutherland ER, Moss TA, Stevens AD, Pak J, Martin RJ. Modulation of sputum gene expression in COPD by fluticasone /salmeterol. European Respiratory Journal. 2006;28(Suppl 50):662s. [Google Scholar]
- Trofimenko 2006 {published data only} .Trofimenko IN, Chernyak BA. The efficacy of salmeterol/fluticasone (SF) for 6 month’s therapy at severe COPD patients. European Respiratory Journal. 2006;28(Suppl 50):30s. [Google Scholar]
- Zheng 2006 {published data only} .Zheng J, Zhong N, Yang L, Wu Y, Chen P, Wen Z, et al. The efficacy and safety of fluticasone propionate 500 mg/salmeterol 50 mg combined via diskus/accuhaler in Chinese patients with chronic obstructive pulmonary disease (COPD) Chest. 2006;130(4):182s. [Google Scholar]
- Zhong N, Zheng J, Yang L, Wu Y, Chen P, Wen Z, et al. The efficacy and safety of salmeterol 50μg/fluticasone propionate 500μg combined via accuhaler in Chinese patients with chronic obstructive pulmonary disease [Abstract] Respirology. 2006;11(Suppl 5):A150. [Google Scholar]
Additional references
- Appleton 2006 .Appleton S, Poole P, Smith B, Veale A, Lasserson TJ, Chan MM, Cates CJ. Long-acting beta2-agonists for poorly reversible chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD001104.pub2. Art. No.: CD001104. DOI: 10.1002/14651858.CD001104.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATS 1995 .American Thoracic Society Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(5 Pt 2):S77–121. [PubMed] [Google Scholar]
- GOLD 2006 .Global Strategy for Diagnosis, Management, Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2006. http://www.goldcopd.org. [Google Scholar]
- Jones 2002 .Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. European Respiratory Journal. 2002;19:398–404. doi: 10.1183/09031936.02.00063702. [DOI] [PubMed] [Google Scholar]
- McGarvey 2007 .McGarvey LP, John M, Anderson JA, Zvarich MT, Wise RA. Ascertainment of Cause-Specific Mortality in COPD - Operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62:411–5. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannini 2007a .Nannini L, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and longacting beta-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2007 doi: 10.1002/14651858.CD003794.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannini 2007b .Nannini L, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and longacting beta-agonist in one inhaler versus long-actng beta-agonist for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2007 [Google Scholar]
- NETT .National Emphysema Treatment Trial Research Group A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. The New England Journal of Medicine. 2003;348(21):2059–73. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- NICE 2004 .National Collaborating Centre for Chronic Conditions Managament of chronic obstructive pulmonary disease in primary and secondary care. 2004 http://www.nice.org.uk.
- Roisin 2000 .Rodriguez-Roisin R. Toward a consensus definition of for COPD exacerbations. Chest. 2000;117(5):398s–401s. doi: 10.1378/chest.117.5_suppl_2.398s. [DOI] [PubMed] [Google Scholar]
- Suissa 2006 .Suissa S. Statistical treatment of exacerbations in therapeutic trials of chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine. 2006;173(8):842–6. doi: 10.1164/rccm.200508-1338PP. [DOI] [PubMed] [Google Scholar]
- Sutherland 2003 .Sutherland ER, Allmers H, Ayas NT, Venn AJ, Martin RJ. Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta-analysis. Thorax. 2003;58:937–41. doi: 10.1136/thorax.58.11.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang 2007 .Yang IA, Fong KM, Sim EHA, Black PN, Lasserson TJ. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2007;(2) doi: 10.1002/14651858.CD002991.pub2. Art. No.: CD002991. DOI: 10.1002/14651858.CD002991.pub2. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Nannini 2003 .Nannini L, Lasserson TJ, Poole P. Combined corticosteroid and longacting beta-agonist in one inhaler for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2003;(4) doi: 10.1002/14651858.CD003794. CD003794. [DOI] [PubMed] [Google Scholar]
- Nannini 2004 .Nannini L, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and longacting beta-agonist in one inhaler for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2004;(3):CD003794. doi: 10.1002/14651858.CD003794. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study