Abstract
The aryl hydrocarbon receptor nuclear translocator (ARNT), also designated as hypoxia-inducible factor (HIF)-1β, plays a pivotal role in the adaptive responses to (micro-)environmental stresses such as dioxin exposure and oxygen deprivation (hypoxia). ARNT belongs to the group of basic helix-loop-helix (bHLH)–Per-ARNT-Sim (PAS) transcription factors, which act as heterodimers. ARNT serves as a common binding partner for the aryl hydrocarbon receptor (AhR) as well as HIF-α subunits. HIF-α proteins are regulated in an oxygen-dependent manner, whereas ARNT is generally regarded as constitutively expressed, meaning that neither the arnt mRNA nor the protein level is influenced by hypoxia (despite the name HIF-1β). However, there is emerging evidence that tumor cells derived from different entities are able to upregulate ARNT, especially under low oxygen tension in a cell-specific manner. The objective of this review is therefore to highlight and summarize current knowledge regarding the hypoxia-dependent upregulation of ARNT, which is in sharp contrast to the general point of view described in the literature. Elucidating the mechanism behind this rare cellular attribute will help us to gain new insights into HIF biology and might provide new strategies for anti-cancer therapeutics. In conclusion, putative treatment effects on ARNT should be taken into account while studying the HIF pathway. This step is of great importance when ARNT is intended to serve as a loading control or as a reference.
INTRODUCTION
The aryl hydrocarbon receptor nuclear translocator (ARNT), also designated as hypoxia-inducible factor (HIF)-1β, is a transcription factor belonging to the basic helix-loop-helix (bHLH)–Per-ARNT-Sim (PAS) family. The bHLH-PAS proteins act as heterodimers, consisting of one signal-regulated as well as one unregulated subunit. ARNT is considered to fit into the second group and serves as a binding partner for several signal-dependent bHLH-PAS members (discussed below) (1). The human arnt gene is located on chromosome 1q21, encoding a protein of 789 amino acids (2). ARNT is composed of a bHLH domain required for DNA binding, two PAS domains (PAS-A and PAS-B) essential for dimerization and one transactivation domain (3). In addition, ARNT comprises a nuclear localization signal (NLS), mediating the import into the nucleus via the classical importin α/β-dependent pathway (4). In general, ARNT expression is regarded to be ubiquitous (1), constitutive (5) and in abundance (6).
ARNT plays a key role in two distinct cellular signaling cascades responding to environmental conditions: the aryl hydrocarbon receptor (AhR) and the hypoxia-inducible factor (HIF) pathways (7,8). The AhR pathway senses ecological pollutants such as dioxins, which are considered to be among the most toxic chemicals known (9). AhR is a cytosolic bHLH-PAS transcription factor consisting of a similar domain structure as described for ARNT. Appropriate compounds bind within the PAS domain of AhR, thereby inducing conformational changes and unmasking a NLS. Subsequently, AhR is translocated into the nucleus, where it heterodimerizes with ARNT. The transcriptional active AhR/ARNT complex binds to xenobiotic-responsive elements within the regulatory region of target genes and initiates transcription (7).
The HIF pathway mediates cellular adaptive responses to reduced oxygen supply (hypoxia). It consists of three α subunits (HIF-1α, HIF-2α, HIF-3α) as well as the two β subunits ARNT and ARNT2 (3), which are two paralogues encoded by separate genes (1,10). By contrast to ARNT, ARNT2 expression exerts a tissue-restricted pattern and was detected in the central nervous system and the kidney as well as in breast cancer. However, there is evidence for an important role of ARNT2 during cellular hypoxic adaptation, but many functions remain to be elucidated (11). (For a detailed description regarding differences of ARNT versus ARNT2, see the article by Hankinson [10].)
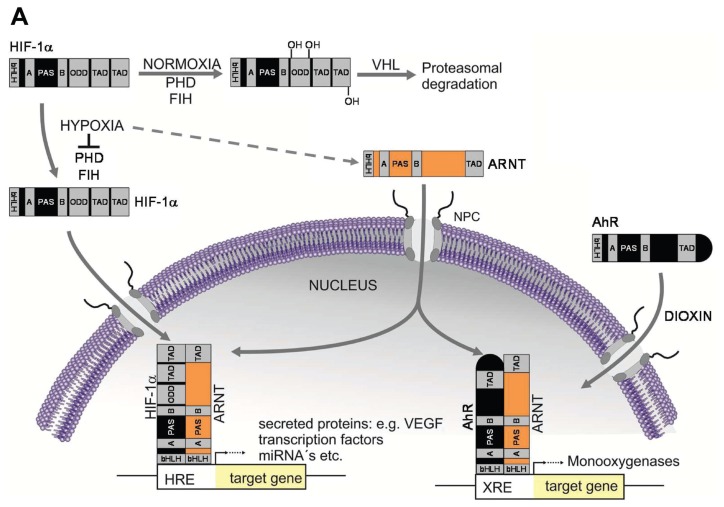
All HIF proteins belong to the bHLH-PAS family and act as heterodimers composed of one α and one β subunit (3). The major difference between both groups is the type of regulation. The α subunits are regulated in an oxygen-dependent manner, whereas the β subunits are considered as constitutively expressed (1,12). Briefly, under sufficient oxygen supply (normoxia), HIF-1α is hydroxylated at two proline residues (Pro402 and Pro564 [13]) within its oxygen-dependent degradation domain, followed by ubiquitination and proteasomal degradation (Figure 1A). In hypoxia, caused by the missing cofactor oxygen, this posttranslational modification is prevented, leading to HIF-1α accumulation, nuclear translocation and heterodimerization with ARNT (12). The HIF-1α/ARNT complex (designated as HIF-1) is the master regulator mediating adaptive responses to low oxygen tension and initiates the transcription of numerous target genes by binding to hypoxia-responsive elements in conjunction with the cofactors CBP/p300 (5,14).
Figure 1.
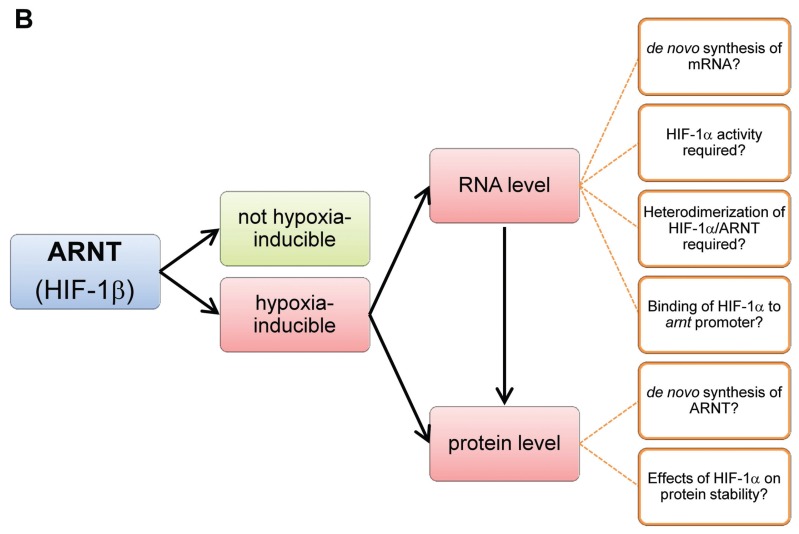
The aryl hydrocarbon receptor nuclear translocator. (A) ARNT interconnects the HIF and AhR pathway. Under sufficient oxygen supply (normoxia), HIF-1α is hydroxylated at two conserved proline residues within its oxygen-dependent degradation domain (ODD) by prolylhydroxylase domain (PHD) enzymes. This posttranslational modification is recognized by the von Hippel-Lindau (VHL) tumor suppressor protein leading to ubiquitination and proteasomal degradation of HIF-1α. Asparaginyl hydroxylation residue within the C-terminal transactivation domain (TAD) of HIF-1α catalyzed by factor inhibiting HIF (FIH) prevents the recruitment of cofactors required for target gene expression. Hypoxia inhibits both PHD and FIH, thus leading to HIF-1α accumulation and nuclear translocation. Heterodimerization of HIF-1α and ARNT is mediated by PAS domains. Subsequently, the HIF-1α/ARNT complex (HIF-1) initiates target gene expression in conjunction with cofactors (that is, CBP/p300; not shown) (3). By contrast, the AhR pathway is activated by environmental pollutants (for example, dioxin exposure), leading to nuclear translocation of AhR. Subsequently, AhR/ARNT complexes initiate the expression of target genes such as monooxygenases (7). ARNT is regarded as constitutively expressed but can be upregulated in response to hypoxia in a cell-specific manner (dotted arrow; see text for details). HRE, hypoxia responsive element; NPC, nuclear pore complex; VEGF, vascular endothelial growth factor; XRE, xenobiotic responsive element. (B) Proposed concept of hypoxia-dependent upregulation of ARNT and associated research questions.
In addition to the oxygen-dependent regulation, the HIF pathway is controlled by phosphatidylinositol 3-kinase (PI3K)/Akt signaling. This cascade can be activated by growth factors or oncogenic events (for example, loss-of-function of the negative regulator PTEN [phosphatase and tensin homolog]), leading to enhanced HIF-1α mRNA translation and thus elevated HIF activity (3,15).
HIF signaling significantly contributes to tumor progression by promoting invasion/metastasis, metabolic alterations and the induction of angiogenesis. Furthermore, it is associated with the resistance against radiation and chemotherapy, thus leading to poor patient survival (13,16,17). Inhibition of this pathway by various compounds and strategies, including the disruption of HIF-α/ARNT heterodimerization, is therefore an attractive approach in cancer therapy (16,18–21).
Following this strategy, Park et al. (22) showed by way of example that targeting the PAS domain of HIF-1α by small molecules is a feasible approach to developing novel HIF inhibitors.
This review focuses on the capability of certain cells to upregulate ARNT under hypoxia, which is in sharp contrast to the current point of view described in the literature. The aim of this article is therefore to highlight recent findings regarding the expression of ARNT and to identify future research questions to close this gap of knowledge in HIF biology.
HYPOXIA-DEPENDENT UPREGULATION OF ARNT
According to the point of view described in the vast majority of the literature, ARNT is regarded to be constitutively expressed (5,15,23). This means that arnt mRNA and protein levels are maintained at constant levels independent of oxygen availability (5).
However, there was early evidence that ARNT is a hypoxia-inducible protein similar to its counterpart HIF-1α. In 1995, Wang et al. (24) published that ARNT was elevated on both mRNA and protein levels in cells exposed to hypoxia. Another study published by Huang et al. in 1996 (25) reported that ARNT levels remained constant regardless of pO2. Herein, the authors challenged the results reported by Wang et al. (24) and argued against them to explain contradictory data (25). Interestingly, the study published by Wang et al. (24) claiming that ARNT is elevated under hypoxic conditions was cited approximately four times more often than the study of Huang et al. (25), which denies this observation (SCOPUS: 2892 versus 750; as of April 2014). Nevertheless, in the following years, it became part of the general notion that ARNT is not regulated by the oxygen tension.
Evidence for a cell line–specific hypoxic inducibility of ARNT was provided from Chilov et al. (26). Herein, the authors demonstrated that exposure of human HeLa, Hep3B and LN229 cells to hypoxia did not alter ARNT levels. By contrast, ARNT was induced in murine L929 and Hepa1 cell lines under hypoxic conditions. Therefore, the authors concluded that only certain cells are able to upregulate ARNT under oxygen deprivation (26).
The inducibility of ARNT in response to various stimuli was demonstrated by Zhong et al. (27). In this study, the authors tested the hypothesis whether HIF-1α and ARNT are regulated by similar signaling pathways in human prostate cancer cells. Exposure of PC-3 cells to hypoxia, the hypoxia-mimetic cobalt chloride (CoCl2) and growth factors increased ARNT protein expression. These effects were partly reversed by pharmacological PI3K/Akt inhibition. Interestingly, the authors mentioned that HIF-1α was more susceptible to PI3K/Akt suppression compared with ARNT, but this was not shown in more detail. Because of the observation that ARNT was elevated by the same stressors/stimuli as required for HIF-1α accumulation, it was deduced that both proteins have regulatory mechanisms in common within this cell type (27).
Vavilala et al. (28) provided additional support for the cell line specificity of hypoxia-dependent ARNT upregulation. The data imply that ARNT was upregulated on mRNA level under hypoxia in at least two out of four cell lines. However, the regulation of ARNT was not the aim of this study, and the authors did not discuss this observation (28).
Recently, Mandl et al. (29) investigated the hypoxia-dependent regulation of ARNT in human melanoma cells. Herein, time course experiments using the hypoxia-mimetic cobalt chloride revealed that ARNT was inducible on protein level in two of five cell lines. In addition, the study elucidated that ARNT was upregulated under hypoxia in an HIF-1α–dependent manner in 518A2 human melanoma cells. This report provides the first evidence that HIF-1α obviously controls the expression of its binding partner ARNT in a cell type–specific manner. Overall, it was concluded that hypoxia-dependent up-regulation of ARNT might prevent this transcription factor from becoming a limiting factor (29). The concepts published in the previous study were in line with Wolff et al. (30). The authors demonstrated the inducibility of ARNT in response to hypoxia as well as hypoxia mimetics (that is, cobalt chloride, dimethyloxalylglycine) in a number of cell lines, including MCF-7 breast cancer cells. Interestingly, this study revealed that hypoxia-dependent upregulation of ARNT is not necessarily accompanied with an elevated arnt mRNA level (30). This result implies different mechanisms acting on both mRNA and/or protein level in a cell type–dependent manner. Examples of cell lines able to upregulate ARNT under hypoxic conditions are listed in Table 1.
Table 1.
Cell lines that can upregulate ARNT in response to hypoxic conditions.
Furthermore, the latter study also identified two cell lines (that is, HepG2 and Kelly) that were not responding with ARNT elevation after 24 h of prolonged hypoxia (30). Therefore, these cells might be useful controls in future studies.
ROLE OF ARNT IN TUMOR DEVELOPMENT AND PROGRESSION
Because ARNT is generally considered to be constitutively expressed, the knowledge about its role in tumor biology is limited and underrepresented. However, recent findings that certain tumor cells are able to upregulate ARNT in response to oxygen deprivation suggest an advantage regarding cell proliferation and/or cell survival during tumourigenesis.
A few single nucleotide polymorphisms have been described within the arnt gene that occur at low frequencies among the population (2). Two-thirds of these mutational events affect the PAS and transactivation domains of the protein. There is evidence that a single point mutation within the PAS domain is sufficient to promote ARNT degradation, potentially lowering constitutive ARNT protein levels (2). In addition, mutations within the PAS domains of ARNT affect the recruitment of binding partners (2,31). However, the misregulation of bHLH-PAS proteins is considered to potentially promote tumor survival (1). Evidence for an important role of ARNT during tumor growth was provided by Shi et al. (32). Herein, the authors monitored the growth of Hepa-1 cells conditionally expressing arnt in a murine xenograft model. On the basis of the kinetic data, it was concluded that ARNT is especially required during early stages of tumor growth. Furthermore, the authors proposed ARNT as a preferable drug target compared with HIF-1α in certain tumors (32). The role of ARNT as a potential attack point for therapeutics in a subset of cancers was confirmed by a recent study that links the expression of this bHLH-PAS transcription factor to cisplatin resistance (33). The cisplatin resistance was mediated by the ARNT/Sp1-dependent transcription of the multidrug resistance-1 (MDR1) gene, which encodes an ATP-binding cassette (ABC) transporter promoting the efflux of this anticancer drug (33).
OPEN QUESTIONS
Hypoxia-dependent upregulation of ARNT is obviously a cell type–specific attribute (29,30). Therefore one might ask how these cells acquire the capability to elevate ARNT expression under oxygen deprivation. Mechanistic insights from recent studies suggest that different ways might exist (29,30). However, the regulation of ARNT or if it responds to stimulation is poorly understood (34). Regarding the expression of ARNT under hypoxic conditions, a pivotal role of HIF-1α was revealed in one cell line (29). Indeed, HIF-1α can perform several tasks independent of its binding partner ARNT and thereby contribute to cell-specific transcriptional responses to hypoxia (15). It was demonstrated that HIF-1α might act as a coactivator or repressor on certain genes. In addition, HIF-1, which is composed of HIF-1α and ARNT, can induce the expression of genes encoding other transcription factors or micro-RNAs (miR-NAs). These hypoxia-responsive transcription factors and miRNAs in turn can induce or repress the expression of secondary HIF-1 target genes (15). Both mechanisms might therefore account for the cell-specific upregulation of ARNT under oxygen deprivation (29). The concept of hypoxia-dependent up-regulation of ARNT and key questions regarding the underlying mechanisms are summarized in Figure 1B. Therefore, future studies are needed to address these issues. In general, projects aiming to investigate the HIF-α subunits should extend the focus and include ARNT into the setting. Because of the general notion that ARNT is unaffected by hypoxia, the role of this protein might not be considered as biologically relevant or influenced under certain experimental conditions. The additional effort to study ARNT as well will be less compared with the scientific benefit.
CONCLUSIONS
The capability of certain cells to elevate ARNT in response to hypoxia is an interesting new concept in HIF biology. Only a very limited number of studies exist that focus on the regulation of ARNT, especially under hypoxia.
According to an overwhelming quantity of reviews, ARNT is constitutively expressed (12,23,35–38), meaning that neither the arnt mRNA nor the ARNT protein level is influenced by hypoxia (5). By contrast, recent studies clearly demonstrate the existence of exceptions from this dogma (29,30). Therefore, the statement that ARNT is constitutively expressed should be revised and not generalized anymore. While studying the HIF pathway, putative treatment effects on ARNT should be considered and more often controlled in future studies. This step is of great importance when ARNT is intended to serve as a loading control or as a reference.
Uncovering the mechanisms of hypoxia-dependent upregulation of ARNT as well as the benefit of this capability for appropriate cells will close a gap of knowledge in HIF biology and might reveal new targets for anticancer therapeutics.
ACKNOWLEDGMENTS
The authors thank Wolfgang Jelkmann for support and helpful discussions. The authors are grateful to Gabriela Fletschinger for help with graphic design.
M Mandl is an associated member of the Institute of Physiology at the University of Lübeck.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Bersten DC, Sullivan AE, Peet DJ, Whitelaw ML. bHLH-PAS proteins in cancer. Nat Rev Cancer. 2013;13:827–41. doi: 10.1038/nrc3621. [DOI] [PubMed] [Google Scholar]
- 2.Urban JD, Budinsky RA, Rowlands JC. Single nucleotide polymorphisms in the human aryl hydrocarbon receptor nuclear translocator (ARNT) gene. Drug Metab Pharmacokinet. 2011;26:637–45. doi: 10.2133/dmpk.DMPK-11-SC-031. [DOI] [PubMed] [Google Scholar]
- 3.Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15:678–85. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Depping R, et al. Nuclear translocation of hypoxia-inducible factors (HIFs): involvement of the classical importin alpha/beta pathway. Biochim Biophys Acta. 2007;1783:394–404. doi: 10.1016/j.bbamcr.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–80. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem. 2010;391:1235–48. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- 8.Zagorska A, Dulak J. HIF-1: the knowns and unknowns of hypoxia sensing. Acta Biochim Pol. 2004;51:563–85. [PubMed] [Google Scholar]
- 9.Marinkovic N, Pasalic D, Ferencak G, Grskovic B, Stavljenic Rukavina A. Dioxins and human toxicity. Arh Hig Rada Toksikol. 2010;61:445–53. doi: 10.2478/10004-1254-61-2010-2024. [DOI] [PubMed] [Google Scholar]
- 10.Hankinson O. Why does ARNT2 behave differently from ARNT? Toxicol Sci. 2008;103:1–3. doi: 10.1093/toxsci/kfn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin XY, et al. siRNA-mediated knockdown of aryl hydrocarbon receptor nuclear translocator 2 affects hypoxia-inducible factor-1 regulatory signaling and metabolism in human breast cancer cells. FEBS Lett. 2011;585:3310–5. doi: 10.1016/j.febslet.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J. A dialogue between the hypoxia-inducible factor and the tumor microenvironment. Cancer Microenviron. 2008;1:53–68. doi: 10.1007/s12307-008-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–14. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obacz J, Pastorekova S, Vojtesek B, Hrstka R. Cross-talk between HIF and p53 as mediators of molecular responses to physiological and genotoxic stresses. Mol Cancer. 2013;12:93. doi: 10.1186/1476-4598-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–61. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 16.Kummar S, et al. Multihistology, target-driven pilot trial of oral topotecan as an inhibitor of hypoxia-inducible factor-1alpha in advanced solid tumors. Clin Cancer Res. 2011;17:5123–31. doi: 10.1158/1078-0432.CCR-11-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strofer M, et al. Stabilisation and knockdown of HIF: two distinct ways comparably important in radiotherapy. Cell Physiol Biochem. 2011;28:805–12. doi: 10.1159/000335794. [DOI] [PubMed] [Google Scholar]
- 18.Ban HS, Uto Y, Nakamura H. Hypoxia-inducible factor inhibitors: a survey of recent patented compounds (2004–2010) Expert Opin Ther Pat. 2011;21:131–46. doi: 10.1517/13543776.2011.547477. [DOI] [PubMed] [Google Scholar]
- 19.Guerin E, et al. In vivo topoisomerase I inhibition attenuates the expression of hypoxia-inducible factor 1alpha target genes and decreases tumor angiogenesis. Mol Med. 2012;18:83–94. doi: 10.2119/molmed.2011.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melillo G. Inhibiting hypoxia-inducible factor 1 for cancer therapy. Mol Cancer Res. 2006;4:601–5. doi: 10.1158/1541-7786.MCR-06-0235. [DOI] [PubMed] [Google Scholar]
- 21.Xie J, Huang X, Park MS, Pham HM, Chan WK. Differential suppression of the aryl hydrocarbon receptor nuclear translocator-dependent function by an aryl hydrocarbon receptor PAS-A-derived inhibitory molecule. Biochem Pharmacol. 2014;88:253–65. doi: 10.1016/j.bcp.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park EJ, et al. Targeting the PAS-A domain of HIF-1alpha for development of small molecule inhibitors of HIF-1. Cell Cycle. 2006;5:1847–53. doi: 10.4161/cc.5.16.3019. [DOI] [PubMed] [Google Scholar]
- 23.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–71. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–14. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253–9. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 26.Chilov D, et al. Induction and nuclear translocation of hypoxia-inducible factor-1 (HIF-1): heterodimerization with ARNT is not necessary for nuclear accumulation of HIF-1alpha. J Cell Sci. 1999;112:1203–12. doi: 10.1242/jcs.112.8.1203. [DOI] [PubMed] [Google Scholar]
- 27.Zhong H, Hanrahan C, van der Poel H, Simons JW. Hypoxia-inducible factor 1alpha and 1beta proteins share common signaling pathways in human prostate cancer cells. Biochem Biophys Res Commun. 2001;284:352–6. doi: 10.1006/bbrc.2001.4981. [DOI] [PubMed] [Google Scholar]
- 28.Vavilala DT, et al. Honokiol inhibits HIF pathway and hypoxia-induced expression of histone lysine demethylases. Biochem Biophys Res Commun. 2012;422:369–74. doi: 10.1016/j.bbrc.2012.04.143. [DOI] [PubMed] [Google Scholar]
- 29.Mandl M, Kapeller B, Lieber R, Macfelda K. Hypoxia-inducible factor-1beta (HIF-1beta) is upregulated in a HIF-1alpha-dependent manner in 518A2 human melanoma cells under hypoxic conditions. Biochem Biophys Res Commun. 2013;434:166–72. doi: 10.1016/j.bbrc.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 30.Wolff M, Jelkmann W, Dunst J, Depping R. The aryl hydrocarbon receptor nuclear translocator (ARNT/HIF-1beta) is influenced by hypoxia and hypoxia-mimetics. Cell Physiol Biochem. 2013;32:849–58. doi: 10.1159/000354487. [DOI] [PubMed] [Google Scholar]
- 31.Partch CL, Gardner KH. Coactivators necessary for transcriptional output of the hypoxia inducible factor, HIF, are directly recruited by ARNT PAS-B. Proc Natl Acad Sci U S A. 2011;108:7739–44. doi: 10.1073/pnas.1101357108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi S, Yoon DY, Hodge-Bell K, Huerta-Yepez S, Hankinson O. Aryl hydrocarbon nuclear translocator (hypoxia inducible factor 1beta) activity is required more during early than late tumor growth. Mol Carcinog. 2010;49:157–65. doi: 10.1002/mc.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan YY, Kalpana S, Chang WC, Chang WC, Chen BK. Expression of aryl hydrocarbon receptor nuclear translocator enhances cisplatin resistance by upregulating MDR1 expression in cancer cells. Mol Pharmacol. 2013;84:591–602. doi: 10.1124/mol.113.087197. [DOI] [PubMed] [Google Scholar]
- 34.van Uden P, et al. Evolutionary conserved regulation of HIF-1beta by NF-kappaB. PLoS Genet. 2011;7:e1001285. doi: 10.1371/journal.pgen.1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–13. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 36.Rapisarda A, Melillo G. Overcoming disappointing results with antiangiogenic therapy by targeting hypoxia. Nat Rev Clin Oncol. 2012;9:378–90. doi: 10.1038/nrclinonc.2012.64. [DOI] [PubMed] [Google Scholar]
- 37.Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci. 2008;33:526–34. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Legrand M, Mik EG, Johannes T, Payen D, Ince C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med. 2008;14:502–16. doi: 10.2119/2008-00006.Legrand. [DOI] [PMC free article] [PubMed] [Google Scholar]