Abstract
Breath tests are non-invasive tests and can detect H2 and CH4 gases which are produced by bacterial fermentation of unabsorbed intestinal carbohydrate and are excreted in the breath. These tests are used in the diagnosis of carbohydrate malabsorption, small intestinal bacterial overgrowth, and for measuring the orocecal transit time. Malabsorption of carbohydrates is a key trigger of irritable bowel syndrome (IBS)-type symptoms such as diarrhea and/or constipation, bloating, excess flatulence, headaches and lack of energy. Abdominal bloating is a common nonspecific symptom which can negatively impact quality of life. It may reflect dietary imbalance, such as excess fiber intake, or may be a manifestation of IBS. However, bloating may also represent small intestinal bacterial overgrowth. Patients with persistent symptoms of abdominal bloating and distension despite dietary interventions should be referred for H2 breath testing to determine the presence or absence of bacterial overgrowth. If bacterial overgrowth is identified, patients are typically treated with antibiotics. Evaluation of IBS generally includes testing of other disorders that cause similar symptoms. Carbohydrate malabsorption (lactose, fructose, sorbitol) can cause abdominal fullness, bloating, nausea, abdominal pain, flatulence, and diarrhea, which are similar to the symptoms of IBS. However, it is unclear if these digestive disorders contribute to or cause the symptoms of IBS. Research studies show that a proper diagnosis and effective dietary intervention significantly reduces the severity and frequency of gastrointestinal symptoms in IBS. Thus, diagnosis of malabsorption of these carbohydrates in IBS using a breath test is very important to guide the clinician in the proper treatment of IBS patients.
Keywords: Bacterial overgrowth, Breath test, Carbohydrate malabsorption, Irritable bowel syndrome, Lactulose breath test, Small intestine, Sorbitol breath test
Core tip: Bloating and distention are often attributed to dietary factors by patients with irritable bowel syndrome (IBS). Recently, small intestinal bacterial overgrowth (SIBO) has been advocated as a pathogenetic factor of IBS. Sugar malabsorption in the bowel can lead to bloating, cramps, diarrhea and other symptoms of IBS as well as affecting absorption of other nutrients. The breath test is now a well-established noninvasive test for assessing malabsorption of sugars in the small intestine. The glucose breath test has been reported as a better diagnostic method for determination of SIBO. Therefore, this review highlights the role of breath tests in diagnosis and management of IBS.
INTRODUCTION
Breath tests are inexpensive, simple and non-invasive, inexpensive tests which can be used for (1) detection of excess bacteria in the small intestine; (2) evaluation of carbohydrate maldigestion; and (3) estimation of intestinal transit time. In order to diagnose irritable bowel syndrome (IBS), all the above parameters should be ruled out.
In 1970s, breath hydrogen (H2) was used to estimate lactose malabsorption. Lactose malabsorption was also studied by Newcomer and associates[1] using 14CO2-labeled lactose, breath H2 and blood sugar changes. In 1978, it was observed that not all disaccharides were hydrolyzed and absorbed in the small intestine during the digestion of foods with help of breath H2[2].
Breath testing consists of measurement of H2/methane (CH4) produced by bacterial fermentation of unabsorbed carbohydrate that is ingested by subjects (Figure 1). Subsequent breath samples are collected atspecific time intervals (i.e., every 15 or 30 min) for 2-5 h. These breath samples are analyzed using the SC Microlyser (Figure 2) to measure amount of exhaled H2 and CH4. H2 and CH4 gases exhaled in the breath are generally the end result of fermentation of carbohydrate ingested by bacteria in intestine[3]. CO2 is produced by all cells during metabolism, but only bacteria produce H2 and CH4 as metabolic by-products. Thus, if either H2 and/or CH4 are produced in body, this proves that a substrate has been exposed to intestinal bacteria with leading tobacterial fermentation[4].
Figure 1.
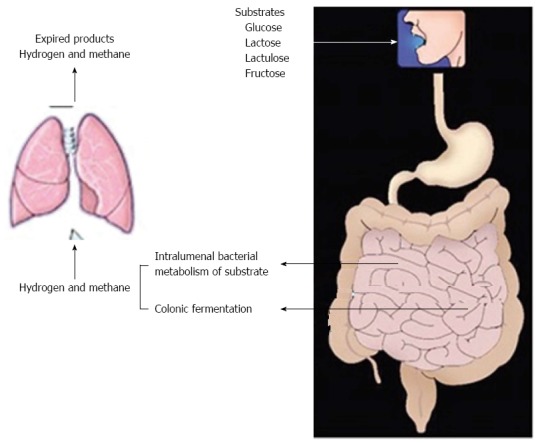
Principle of breath testing.
Figure 2.

Gases released can be detected by Breath analyzer.
TYPES OF BREATH TESTS
Breath tests are most frequently used for diagnosis of lactose, sorbitol and fructose malabsorption, the glucose breath test (GBT) for small intestinal bacterial overgrowth (SIBO) and the lactulose breath test for orocecal transit time.
GLUCOSE BREATH TEST
Under physiological conditions, glucose is straight away absorbed in the small intestine[5]. However, if there is bacterial overgrowth in small intestine, bacterial fermentation of glucose leading to production of H2 can take place prior to the absorption of glucose, which is measured by increase in H2/CH4 concentration. Thus, any increase ≥ 10 ppm in H2/CH4 concentration in two consecutive readings above the basal value is to be considered as significant and indicates about SIBO.
LACTULOSE BREATH TEST
Lactulose is a simple disaccharide. Generally, there is no lactulase enzyme in the small intestine to hydrolyze this sugar, therefore it is transported intact to the colon where it is metabolized by colonic bacteria. End products of its metabolism include H2 and CH4. The time interval between ingestion of lactulose and rise in breath H2/CH4 concentration ≥ 10 ppm in two consecutive readings above the basal value is measure of orocecal transit time.
LACTOSE BREATH TEST
Lactose intolerance is prevailing throughout the world. Subjects generally avoid milk and other dairy products to improve their symptoms. For effective utilization, lactose requires hydrolysis by the enzyme lactase. An increase in H2/CH4 concentration ≥ 20 ppm in two consecutive readings above the basal value is considered lactose intolerance. The breath test is now being considered to be the most practical and dependable method to diagnose malabsorption of lactose.
FRUCTOSE BREATH TEST
This test can help to determine if individual has any problem in fructose digestion. Individuals with fructose intolerance may show symptoms like gas, diarrhea, gas, bloating and cramping. Fructose occurs as simple sugar in fruits, vegetables, and honey. When fructose comes in contact with normal bacteria in the intestine, H2 and/or CH4 gas is expired. Usually, a dose of 25 g of fructose is used. An increase in H2/CH4 ≥ 20 ppm in two consecutive readings above the basal value indicates fructose intolerance.
SORBITOL BREATH TEST
Sorbitol is found in stone fruits, and also used as an artificial sweetener in sugar-free gum and mints. It is poorly absorbed in small intestine. Sorbitol breath test determines if an individual can absorb small amount of sorbitol. This can help to decide if dietary restriction of sorbitol can lead to improvement in gastrointestinal symptoms.
The various types of breath tests for H2/CH4 measurement are shown in Figure 3.
Figure 3.
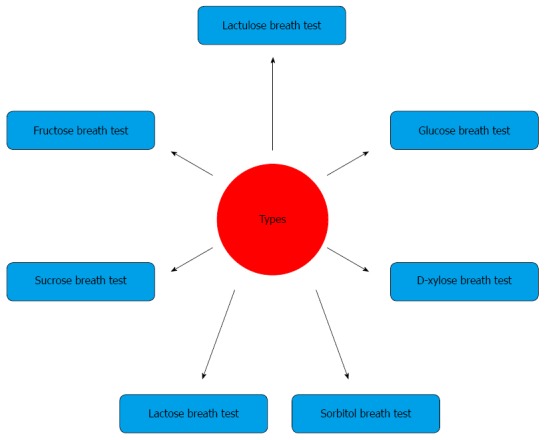
Types of breath tests.
ROLE OF BREATH TESTS IN IBS
IBS is incessant condition of intestine. According to the Rome III criteria[6], it is defined as recurrent abdominal pain or discomfort at least 3 d/mo in last 3 mo associated with two or more of the following: (1) improvement in abdominal pain with defecation; (2) onset associated with a change in frequency of defecation; and (3) onset associated with a change in form (appearance) of stools.
IBS is characterized by impaired defecation, abdominal discomfort and bloating. IBS is functional gastrointestinal disorders in which a variety of factors, including abnormal visceral sensation, psychosocial factors and altered motility interact to cause symptoms. Although mechanisms underlying IBS are not fully known, a best possible explanation of symptoms may be that 92% of IBS patients suffer from bloating[7]. Some investigators have reported increased H2 gas production following administration of fermentable substrates in subjects with IBS compared with healthy controls[8]. A possible explanation for these observations has been that certain individuals who meet diagnostic criteria for IBS may actually have SIBO, due to colonization of the proximal small bowel with fermenting bacteria or intolerance to a carbohydrate.
SIBO IN IBS PATIENTS
Exact prevalence of SIBO in newly diagnosed IBS is not known. Variable data are reported in the literature which reflect different sensitivity and specificity of methods, either biochemical or microbiological, used for diagnosis of SIBO. An exact estimation of SIBO prevalence should have important therapeutic implications as SIBO and symptoms related to it (i.e., abdominal bloating) can be successfully treated bynon-absorbable antibiotics[9,10]. Breath tests are not only easy to perform but are also non-invasive compared with jejunal aspiration. These also give quicker information in comparison to jejunal aspiration[11]. SIBO occurs in wavering frequencies in IBS[12,13]. It varies according criteria used to measure SIBO and geographical area. The GBT has been reported as a diagnostic test for SIBO[14,15]. It is the most extensively used test, as the substrate is inexpensive, and glucose is fermented by small intestinal bacteria into H2/CH4 and CO2. Kerlin and Wong[16], have reported that GBT performed for 2-h had a sensitivity of 93% and a specificity of 78% in SIBO identification against the gold standard of a jejunal aspirate. The jejunal aspirate culture has been used as the gold standard to diagnose SIBO but limitations of this test include the challenges posed by attempting to culture all strains and species, possibility of contamination and the most important being its invasiveness[17,18].Therefore, breath tests (lactulose or glucose breath tests) are most commonly used[19]. The different patterns observed in glucose and lactulose breath tests for detection of SIBO are shown in Figure 4.
Figure 4.
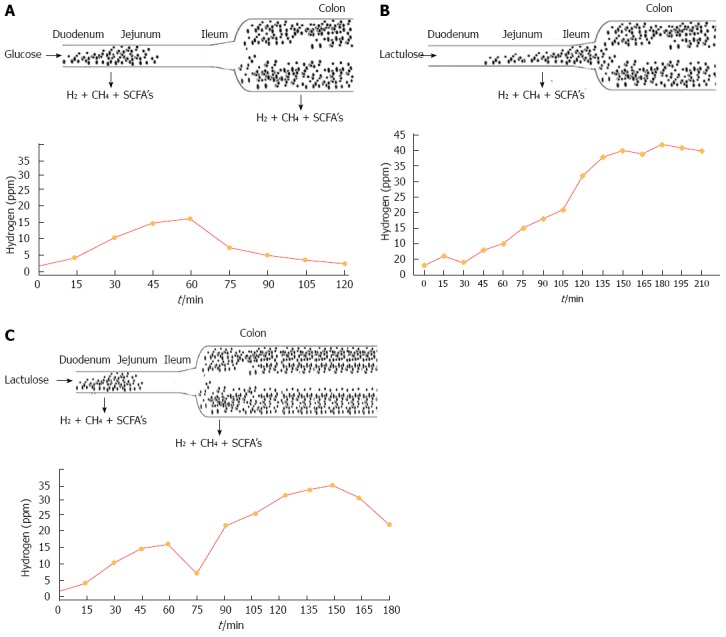
It shows pattern of breath test with bacterial overgrowth in duodenum and jejunum (A), more colonic type of bacteria in small Intestine (B) and more bacteria in duodenum, jejunum and colon showing 2 peaks with lactulose administration (C).
Prevalence of SIBO in IBS patients was found to be 4% (based on the definition of ≥ 105 CFU/mL of bacteria in jejunal aspirate) which is similar to that observed in healthy individuals[20]. However, Lupascu et al[21] observed that positive GBT was found in 31% (20/65) of IBS patients compared with 4% (4/102) in a control group. In comparison to this, a study was performed by Pimentel et al[22] in 111 IBS subjects using the lactulose breath test. He reported a prevalence of SIBO of 84% in IBS compared with 20% in healthy individuals. Additionally, the administration of neomycin significantly pacified IBS symptoms. The sensitivity and specificity of the GBT for SIBO were 62.5% and 82%, respectively, and of the lactulose breath test were 52% and 86%, respectively[23]. Another study also found a higher percentage of SIBO (76%) in IBS patients using the lactulose breath test[24]. The variation in lactulose and GBTs may be due to differences in the nature of the substrate and diagnostic method used. Another practice of breath sample analysis utilized substrates such as D-xylose or glycocholic acid labeled with 13C and 14C isotopes, followed by analysis by mass spectrography or scintillation counting of breath samples for isotopic CO2[25-27]. 14C-labeled substrate however are not applicable for testing children and pregnant women.
STUDIES RELATED TO SIBO IN IBS IN DIFFERENT POPULATIONS
Cuoco and Salvagnini[9] reported that 46% of 96 patients in North Italy with IBS had positive breath test after oral lactulose administration. European investigators reported increased gastrointestinal bacterial flora in 43% of IBS patients in comparison to 12% of controls[20]. United States-based clinicians have also reported positive test in around 80% of IBS patients[28-30]. In a study using a lactulose H2 breast test and whole-gut scintigraphy in IBS patients, radio-labeled material almost always reached cecum before H2 breath content rose by > 20 ppm[28,30]. This study provided convincing evidence that lactulose H2 breath testing reflects variations in orocecal transit time rather than a diagnosis of SIBO. A meta-analysis in patients with IBS found that prevalence of positive lactulose or glucose H2 breath test was 54% and 31%, respectively, with significant heterogeneity between studies[12]. Park et al[31] also observed that lactulose breath test was not useful for discriminating IBS patients from controls. A recent study by Meyrat et al[32] also observed a high percentage of positive lactulose breath tests among IBS patients (71%). IBS-associated symptoms improved following 2 wk of treatment with rifaximin. The authors concluded that rifaximin treatment pacifies symptoms in lactulose breath test-positive IBS patients. Similar results were observed by other authors in relation to SIBO and its treatment with rifaximin in IBS patients[33-37]. Law et al[38] observed that therapy with PPI did not affect production of H2 on lactulose breath tests in IBS patients. Parodi et al[39] showed that GBT is useful to identify a subgroup of IBS-like patients, whose symptoms are a result of SIBO. Normalization of the GBT after antibiotic therapy was found to be associated with a significant improvement in symptoms. In a study from Pakistan, the lactose H2 breath test was used to diagnose SIBO in IBS patients[40]. SIBO was observed by the lactose H2 breath test in 14% (32/234) cases. It was positive in 19% (22/119) diarrheal type IBS (IBS-D) patients, while 9% (10/115) patients had chronic non-specific diarrhea. In another study, sucrose was used as a substrate to diagnose SIBO[41]. The authors observed that 32.9% (52/158) patients with IBS had abnormal breath tests compared with 17.9% (6/34) of controls while SIBO+ve and SIBO-ve patients did not differ in prevalence of IBS subtypes. Sachdeva et al[42] also showed that SIBO was more prevalent in IBS patients 23.7% (14/59) than healthy controls [2.7% (1/37)] using GBT. Patients with D-IBS suffered from SIBO more frequently as compared with non-D-IBS patients [37% (10/27) vs 12.5% (4/32)]. Constipation-type IBS (C-IBS) had the lowest number of patients with SIBO (9%, 1/11) among all IBS subgroups. The prevalence of SIBO in children affected by IBS was studied by Scarpellini et al[43]. They observed that an abnormal lactulose breath test was significantly higher in IBS patients (65%, 28/43) than in control subjects (7%, 4/56). The study conducted in our laboratory on SIBO in IBS patients showed that the prevalence of SIBO in IBS patients from North India was approximately 11.1%[44], which is lower than the reported prevalence in Western countries[12]. GBT was found to be a more appropriate test for the SIBO detection than lactulose breath test as per thestudy performed in our laboratory. SIBO was positive in 34.3% (60/175) patients with lactulose and in 6.2% (11/175) patients using GBT. In controls, lactulose breath test was positive for SIBO in 30% (45/150) and in 0.66% (1/150) using GBT. It was also observed in this study that a positive lactulose breath test for SIBO was not significantly different in patients and controls; while using GBT, SIBO was significantly higher (P < 0.01) in patients than in controls. Thus, we concluded that the lactulose breath test was not a good test to discriminate SIBO in IBS patients from controls[45]. Various studies[46-48] have demonstrated the disadvantages of using lactulose in diagnosing SIBO, mainly because of the high rate of false positive results. Table 1 also clearly shows that the percentage of SIBO in IBS patients is high with the lactulose breath test compared with the GBT.
Table 1.
Comparison of glucose and lactulose breath tests for diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome
| Year | Ref. | Substrate | % age of SIBO + ve | Number of patients |
| 2005 | Lupascu et al[21] | Glucose | 31 | 65 |
| 2007 | Majewski et al[35] | Glucose | 46 | 204 |
| 2008 | Rana et al[45] | Glucose | 11.1 | 225 |
| 2009 | Parodi et al[39] | Glucose | 16 | 130 |
| 2010 | Reddymasu et al[15] | Glucose | 36 | 98 |
| 2011 | Sachdeva et al[42] | Glucose | 23.7 | 59 |
| 2012 | Rana et al[44] | Glucose | 6.2 | 175 |
| 2008 | Grover et al[41] | Sucrose | 32.9 | 158 |
| 2011 | Yakoob et al[40] | Lactose | 14 | 234 |
| 2003 | Pimentel et al[22] | Lactulose | 84 | 111 |
| 2005 | Nucera et al[79] | Lactulose | 65 | 98 |
| 2007 | Madrid et al[24] | Lactulose | 76 | 367 |
| 2008 | Bratten et al[47] | Lactulose | 67 | 264 |
| 2009 | Scarpellini et al[43] | Lactulose | 65 | 43 |
| 2009 | Peralta et al[33] | Lactulose | 56 | 97 |
| 2010 | Park et al[31] | Lactulose | 56.3 | 555 |
| 2012 | Meyrat et al[32] | Lactulose | 71 | 150 |
| 2013 | Scarpellini et al[36] | Lactulose | 66 | 50 |
SIBO: Small intestinal bacterial overgrowth.
By analyzing the above literature, it can be concluded that the GBT is a better diagnostic test for SIBO in IBS patients compared with the lactulose breath test, and that occurrences of SIBO in IBS patients varies among different populations.
LACTOSE INTOLERANCE AND IBS
Lactose intolerance has been known for over a century. Figure 5 explains the mechanism of lactose intolerance. The lactose H2 breath test[49] extensively used as test for lactose intolerance. Pattern of the breath test observed in lactose-tolerant and lactose-intolerant patients is shown in Figure 6A.
Figure 5.
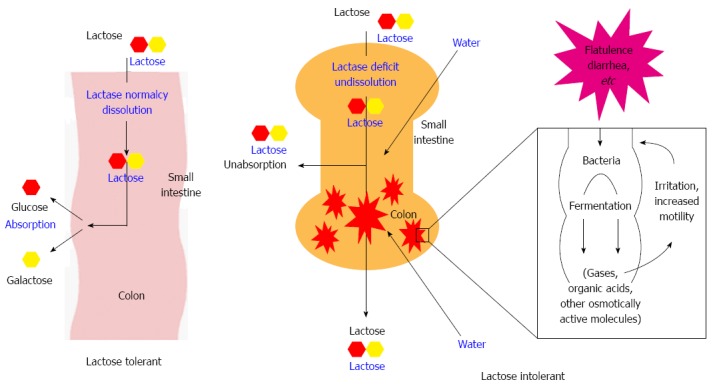
Mechanism of lactose intolerance.
Figure 6.
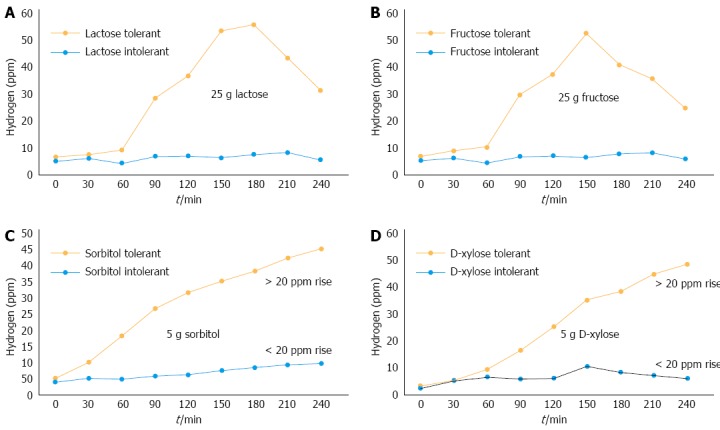
Pattern of lactose (A), fructose (B), sorbitol (C) and D-xylose (D) and tolerance and intolerance using lactose breath test.
The lactose H2 breath test is not sufficient for the diagnosis of lactose intolerance because lactose malabsorbers can also give negative H2 breath test. It has been observed that individuals with methanogenic flora, measurement of breath CH4 may improve accuracy of the lactose H2 breath test in analysing lactose malabsorption[50].
STUDIES SHOWING INTERDEPENDENCE OF IBS AND LACTOSE INTOLERANCE
IBS and lactose intolerance have similar symptoms and both of them are common all over the world[51,52]. It is approximated that 4%-74% of healthy individuals in different geographic regions[53,54] and 4%-78% IBS patients[55,56] may have lactose intolerance. Symptoms of LI may be influenced by the type of diet taken by an individual like the type and amount of polysaccharides, caffeines, intake of fluid and the type of gut flora of that individual[57]. Lactose intolerance patients are at more risk of developing IBS[52] as they have higher visceral sensitivity to effect of lactose in the luminal as compared with lactose-tolerant subjects[58]. Studies have shown that lactose maldigestion affected 24%-27% of IBS patients by lactose breath test[59,60]. In study by Alpers, it was documented that 45% of IBS patients have lactose intolerance. However, only 30% were able to relate their symptoms with milk and other dairy products[61]. Strikingly, some IBS patients who did not suffer from lactose maldigestion complained about symptoms of lactose intolerance. Thus, this shows that lactose intolerance should be measured in IBS patients.
Studies have revealed the presence of lactose malabsorption patients suspected with IBS by H2 breath testing[60-64]. One study observed that 23% (256/1122) patients with suspected IBS showed lactose malabsorption with 25 g of lactose[63]. In another study, 50 g of lactose was used to assess 186 patients with suspected IBS. They also observed that occurrence of LI in IBS was 25.8% (48/186)[64]. In a succeeding publication, authors showed that patients with lactose malabsorption had no significant relationship with their gastrointestinal (GI) symptoms compared with patients without lactose malabsorption[65]. Böhmer and Tuynman[56] also indicated similar lactose malabsorption i.e., 24.3% by H2 breath testing in IBS patients. In contrast to these findings, Tolliver et al[65] showed significant improvement in IBS symptom scores in 75% of IBS patients with lactose-intolerant after specific dietary intervention 5 years. In an North Indian study by Gupta et al[66], it was observed that persistence of lactose intolerance was similar IBS patients of IBS (72%, 89/124) and healthy controls (60%, 32/53). However, IBS patients more frequently complained about symptoms following lactose intake even though levels of breath H2 were similar to healthy individuals[66]. Prevalance of lactose intolerance in IBS-D patients was commensurable to that in patients with other types of IBS. Their results further advocated that self-reported milk intolerance has 81% positive and 23% low negative predictive values for lactose intolerance diagnosis. Therefore, absence of such self-reported lactose intolerance should not be used to exclude lactose intolerance in IBS patients. These results are in similar lines with previous report from Italy[67]. In this study, LI was analyzed by self-reported symptoms with positive and negative predictive values observed to be 75% and 31%, respectively. In a recent study[68], however, production of H2 and distention were similar among IBS patients and healthy controls using lactose breath test. However, lactose intolerance was more common in IBS (53.8%) than in controls (28.1%).
A study was also conducted in our laboratory to observe lactose intolerance in different types of IBS patients from north India[54]. 44% (11/25) patients were of D-IBS, 28% (7/25) patients of spastic and remaining seven (28%) patients had characteristics of both types of symptoms. Abnormal lactose H2 breath test was observed in 82% (9/11) D-IBS which was significantly higher than controls. Furthermore, patients with D-IBS had a higher incidence of lactose intolerance compared with patients with spastic type or features of both types. Furthermore, Yang et al[69] observed that malabsorption of 40 g lactose was observed in 93% of controls and 92% of patients with D-IBS. Fewer controls than D-IBS patients were intolerant to 10 g lactose (3% vs 18%), 20 g lactose (22% vs 47%), and 40 g lactose (68% vs 85%). Self-reported lactose intolerance was more frequently observed in D-IBS (63%) than controls (22%), and thus ateless dairy products.
In children, lactose intolerance was also found to be linked with IBS. Gremse et al[70] showed that lactose maldigestion may be an important contributory factor in IBS children.Lactose avoidance in these patients may reduce medication use to relieve symptoms.
The relationship of the lactose breath test with methanogenic flora has also been investigated in various studies. Vernia et al[71] showed that after an oral dose of lactose less H2 is excreted by patients with predominant fasting CH4 low CH4 producers (LMP). Lower prevalence of grave lactose intolerance and its symptoms during the test in predominant CH4 producers (PMP) may be associated with lower and slower H2 excretion. Thus, taking only H2 excretion as effective means to quantify carbohydrate malabsorption is unrelaible in PMP. CH4-producing patients are expected to have a increased false negative rate of lactose intolerance compared with LMP after lactose ingestion. As symptoms are related to the amount of gas produced in colon, lactose breath test recognizes patients with lactose intolerance irrespective of presence of lactose malabsorption and helps in predicting effect of a lactose-restricted diet. Similarly, we observed that lactose breath test was present in 50% (77/154) of IBS patients and in 49.6% (142/286) of controls. It was also observed that the lactose breath test was negative due to PMP in 6.49% (5/77) of IBS patients and in 20.14% (29/154) controls. The effect was more plausible in healthy subjects than in IBS patients[72]. However, in a recent study, Lee et al[73] observed that CH4 and H2 are not associated with specific symptoms in IBS patients.
Thus, it can be concluded that measurement of lactose intolerance using the lactose breath test is essential in IBS patients to modify their diet for improvement of symptoms. It also indicates the importance of CH4 measurement along with H2 gas to detect lactose intolerance.
CONTROVERSIAL STUDIES ON LACTOSE INTOLERANCE IN IBS PATIENTS
Farup et al[53] observed that IBS and lactose malabsorption were found to be unrelated disorders. A usual test for lactose malabsorption seems unnecessary in persons with IBS in an area with a low lactose malabsorptionprevalence. Milk-related symptoms and symptoms after lactose intake were inaccurate predictors for lactose malabsorption. In a study by Corlew-Roath et al[74], incidence of fructose and lactose malabsorption in populations with and without IBS was comparable. 33% of both groups had lactose malabsorption, fructose malabsorption or both. Both populations also had similar results with diets. IBS patients had 77% compliance and 72% in patients without IBS. However, patients without IBS showed improvement in symptoms with dietary changes than IBS patients. This advocates that IBS symptoms are not dependent on carbohydrate maldigestion, and dietary changes may not improve symptoms in patients with IBS.
COMBINATION OF SUBSTRATES AND IBS SYMPTOMS
Lactose[75], fructose[76] and sorbitol malabsorption[77,78] have also been blamed for symptoms present in IBS patients. In a study in IBS patients[79], SIBO was present in 65% (64/98) using the lactulose breath test. SIBO-positive patients further showed significantly higher prevalence of malabsorption by lactose breath test (83% vs 64%), fructose breath test (70% vs 36%) and sorbitol breath test (70% vs 36%) when compared with the SIBO negative IBS patients. SIBO eradication caused significant reduction in lactose, fructose and sorbitol positive breath tests.They concluded that SIBO positivity should always be assessed first, before analyzing for carbohydratemalabsorption and specific carbohydrateelimination diets in IBS patients. Fructose, sorbitol and lactose breath tests could become a useful diagnostic approach in SIBO-negative patients with refractory symptoms. Sugar malabsorption could be primary (congenital enzymatic/carrier deficiency) or acquired due to damage in intestine due to acute gastroenteritis, celiac disease, Crohn’s disease or due to medications[80]. When carbohydrates malabsorption occurs, their passage in bowel causes production of short chain fatty acids and gas with initiation of syndrome characterized by abdominal pain, diarrhea and meteorism, thus mimicking IBS symptoms. In a study by Moukarzel et al[81] breath H2 tolerance tests with lactose, sucrose and apple juice in the amount patients normally consumed were positive in 32%, 0%, and 50%, respectively. They concluded that some individuals with IBS have symptoms depending upon malabsorption of carbohydrates present in apple juice, pear nectar and may improve with correct choices of fruit juice. Moreover, in a recent study by Wilder-Smith et al[82], it was observed that intolerance due to fructose intolerance was more frequent than lactose intolerance in all subgroups of functional gastrointestinal disorders. However, in an IBS-constipation subgroup, lactose intolerance was found to be more common. Table 2 summarizes the incidence of lactose intolerance reported in IBS patients by various authors.
Table 2.
Lactose Intolerance in irritable bowel syndrome patients using lactose breath test
| Year | Ref. | % age of lactose intolerance | Number of patients |
| 1994 | Corazza et al[50] | 34.4 | 32 |
| 1994 | Tolliver et al[64] | 25.8 | 186 |
| 1998 | Vesa et al[63] | 23.0 | 1122 |
| 2001 | Böhmer et al[56] | 24.3 | 70 |
| 2001 | Rana et al[54] | 82.0 | 11 |
| 2002 | Moukarzel et al[81] | 32.0 | 28 |
| 2004 | Vernia et al[55] | 75.6 | 475 |
| 2006 | Alpers et al[61] | 45.0 | 150 |
| 2007 | Gupta et al[66] | 72.0 | 124 |
| 2009 | Rana et al[72] | 50.0 | 154 |
| 2009 | Corlew-Roath et al[74] | 33.0 | 66 |
| 2012 | Knudsen et al[67] | 64.7 | 406 |
| 2013 | Zhu et al[68] | 53.8 | 277 |
| 2013 | Yang et al[69] | 47.0 | 60 |
| 2013 | de Roest et al[105] | 37.8 | 90 |
FRUCTOSE INTOLERANCE AND IBS
It has been advocated that fructose malabsorption was present in 36% of European population[83]. The symptoms include both intestinal complaints as well as extraintestinal symptoms such as depression[84]. In studies with an uncontrolled diet, occurrence of malabsorption due to fructose was higher in IBS patients (30%-70%[85,86]) than in healthy subjects (0%-50%[87,88]). However, no difference was observed in a diet controlled study[89]. Goldstein et al[78] reported that, among patients with IBS or functional abdominal complaints, 44% suffered from fructose malabsorption based on consumption of 50 g fructose, and 56%-60% improved on a low-fructose diet. Improvement with a fructose-reduced diet has also been observed in other uncontrolled studies[90,91]. The association between IBS and fructose malabsorption is thus far from settled. Most likely, the diverging data can be explained by the fact that there is no general agreement on the criteria for diagnosis of fructose malabsorption. Finally, from a pathophysiological viewpoint, it would be matter of concern to further determine response to a fructose-restricted diet in IBS patients and the correlations with both the daily intake of fructose and the fructose absorption capacity of IBS patients. However, further studies are needed for validation. All data taken together indicate that fructose malabsorption should be kept in mind while managing IBS patients. A study by Reyes-Huerta et al[92] observed that 52% (13/25) IBS patients had fructose intolerance compared with 16% (4/25) control subjects (P = 0.01). They concluded that intolerance in fructose may be responsible for gastrointestinal symptoms in at least half of IBS patients, especially in the group of IBS-D patients. The pattern observed for fructose tolerance and intolerance using the fructose breath test is shown in Figure 6B.
80% of functional bowel disease patients suffered from fructose malabsorption. However, few randomized controlled studies advocated that there is lower prevalence of fructose malabsorption among IBS patients compared with healthy individuals[89,93]. The number of patients in these studies was small, but there was general agreement that IBS patients reported more frequently. This again highlights the problem with identifying specific diagnostic criteria with both positive breath test and symptoms for practical working definition. Effect of dietary treatment for fructose malabsorption in IBS patients is also very significant. Fernández-Bañares et al[94] reported that after fructose-free diet, symptom improvement was present at 1 mo and 12 mo in 81% and 76% of patients with Rome II criteria of functional abdominal bloating and gas-related symptoms. Shepherd and Gibson[95] advocated that 77% patients improved with restriction in diet. Better response was seen in in those that were adherent (85%) to diet restriction than non-adherent (36%). Another study on dietary restriction by Choi et al[91] observed significant improvement in belching, pain, fullness, bloating, diarrhea and indigestion with diet. However, Berg et al[96] observed that the fructose breath test did not discriminate between patients with and without a response to a diet restricted with fructose. Even in the group with a negative fructose breath test, a significant improvement in symptom scores was observed. A summary of fructose intolerance in IBS patients is presented in Table 3.
Table 3.
Fructose Intolerance in irritable bowel syndrome patients using fructose breath test
SORBITOL INTOLERANCE AND IBS
Sorbitol is not completely absorbed and lead to osmotic diarrhea if large amounts (20-50 g) are ingested. A positive breath test can be seen observed with a dose as small as 5 g in healthy subjects. Most participants experienced mild gastrointestinal symptoms after 10 g of sorbitol but after 20 g severe gastrointestinal symptoms[97]. In this method, H2 or CH4 are measured in end-expiratory breath samples every 30 min for 4 h. An increase ≥ 20 ppm in 2 consecutive readings is considered a positive test.
FRUCTOSE AND SORBITOL AS SUBSTRATE FOR IBS SYMPTOMS
Small bowel transit is accelerated due to mixture of fructose (25 g) and sorbitol (5 g)[98]. Precise mechanism of this phenomenon is not known but there is some evidence that bacterial fermentation products may lead to activation of feedback pathways that play a role in regulation of gut motility[99]. Limited data have suggested that SIBO and fructose malabsorption might have a bi-directional cause and effect relationship. On one hand, fructose may cause survival of intestinal bacteria in distal small intestine as easily available metabolic substrate for the synthesis of fructans as adherence factors. There is no direct evidence supporting or rejecting that these events occur in distal small intestine. By eliminating all potential metabolic substrates for bacteria by feeding patients with an elemental diet resulted in loss of features of SIBO along with improvement in symptoms of IBS[100]. On the other hand, patients with presumed SIBO abolished fructose malabsorption when treated with antibiotics along with reduction in associated symptoms[79].
Recently, Yao et al[101] observed that sorbitol was completely absorbed by similar proportion of IBS patients (40%) and healthy subjects (33%). Although IBS patients absorbed more mannitol (80% vs 43%). Production of breath H2 was similar in both groups after lactulose but it reduced in IBS patients after ingestion of both polyols. Overall GI symptoms significantly increased after consumption of both polyols in IBS patients only. However, symptoms were independent of malabsorption of both polyols.
Thus,data in literature shows possible association between fructose, sorbitol and lactose malabsorption with IBS, suggesting that an exclusion of appropriate carbohydrate from diet may improve symptoms in IBS patients who have positive breath test with respect to that specific carbohydrate.However, need for breath testing to recognize individuals with specific carbohydrate malabsorption prior to dietary changes has been debated.
D-XYLOSE INTOLERANCE AND IBS
When D-xylose is absorbed incompletely, enteric bacteria metabolize the non-absorbed D-xylose in the colon, or in the small bowel with bacterial overgrowth, yielding H2, which can be measured in the breath. The direct measurement of breath H2after oral intake of D-xylose avoids necessity of using radioactive tracers[14]. Most breath H2 is formed in colon due to carbohydrate fermentation by the indigenous flora, which allows measurement of intestinal transit[102]. Increased rates of H2 production occur in small intestine when bacterial overgrowth is present. Study by Lembcke et al[103], showed that H2 breath test with 25 g D-xylose was of no clinical relevance for diagnosis of celiac sprue. D-xylose tests were indicative of the IBS in 5 out of 10 (50%) patients. However, the diagnostic impact of this needs further investigation.
FERMENTABLE OLIGOSACCHARIDES, DISACCHARIDES, MONOSACCHARIDES AND POLYOLS IN IBS
It is apparent from the available literature that the consumption of fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) may result in symptoms in some IBS patients. In a study by Ong et al[104], breath test was performed after intake of a FODMAP diet. They observed that over the entire day with high FODMAP diet in volunteers and IBS patients, increased levels of H2 breath was produced. However, breath CH4 were reduced in 10 healthy subjects but not in patients of IBS. Thus, they concluded that FODMAPs in diet induce increased H2 production in intestine, influence CH4 production and thus, induce gastrointestinal symptoms in IBS patients. Similar observations were seen in a recent study by de Roest et al[105]. Fructose malabsorption (75.6%), lactose malabsorption (37.8%) and SIBO (13.3%) was present in patients in this study. 75.6% patients who were adherent to diet, showed improvement in IBS symptoms. They further concluded that diet with less FODMAP is better for IBS patients. Thus, the current techniques of testing breath and dietary advice forms a good basis to manage IBS patients.
The patterns observed for sorbitol and D-xylose intolerance during respective breath tests are shown in Figure 6C and D, respectively.
CH4 IN IBS PATIENTS
In humans, CH4 is mostly produced by Methanobrevibactersmithii (M. smithii) as a result of the conversion of 4 mol H2 and 1 mol CO2 to 1 mol CH4, competing for H2 with sulfate reducing bacteria. This process occurs mainly in the left colon[106,107]. It is an important reason for measuring both gases by breath tests (Figure 7). There is proof of slow transit time in CH4 producers[108]. In one study, it has been reported that mean of transit time in CH4 producers was 84.6 h and in non-producers was 48.6 h. Thus, indicating that some association may exist between delayed gut motility and CH4.
Figure 7.
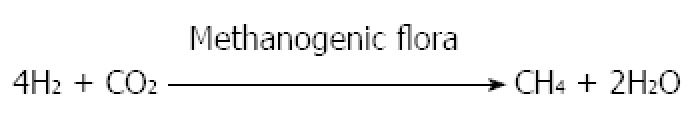
Production of methane by methanogenic flora.
CONSTIPATION-DIARRHEA-CH4: ANY RELATIONSHIP?
Studies have advocated that production of CH4 and constipation are strongly related. A study[109] showed that when patients with constipation and increased CH4 production at fasting state and after intake was glucose were treated with rifaximin, their breath CH4 levels were reduced and constipation symptoms were also improved.CH4 excretion mean was found to increase along with reduction in bowel movements in C-IBS patients using lactulose H2 breath test[110,111]. However, apprehension remains as to whether CH4 causes constipation or rather is result intestinal hypomotility. In contrast, patients suffering from diarrhea generally have higher excretion of breath H2, during fasting state and after glucose intake[13]. CH4 was observed to be associated with presence and degree of constipation in a study on 87 patients of IBS. 24% (20/87) produced CH4 in lactulose H2 breath test[112]. In a study by Kajs et al[113] it was found that low CH4 producers had a significantly higher breath H2 than high CH4 producers on consumption of basal diet and after ingestion of sorbitol (27.1 ± 2.7 ppm vs 15.8 ± 3.6 ppm) or oat fiber (13.1 ± 0.08 ppm vs 9.6 ± 1.2 ppm). Low producers of CH4 showed extremely increased cramping and bloating after ingestion of sorbitol and increased bloating after fiber ingestion. However, high CH4 producers showed no such symptoms. Thus, they concluded that methanogenic flora is linked with decreased symptomatic response to ingestion of non-absorbable, carbohydrates in healthy individuals. This indicates that normal flora manipulation could be of therapeutic value in non-methanogenic IBS patients.
Experiments in animals[114] have also suggested an active role for CH4 in affecting intestinal motility, while other human investigations have shown that slow transit may facilitate growth of methanogenic bacteria[115,116]. However, it cannot be excluded that methanogenic organisms lead to constipation indirectly through the modification of the luminal environment, by producing active substrates or by competing with other bacterial species[117-119]. Recent study has advocated that degree of CH4 production in breath testing may be related to constipation in IBS patients. Therefore, CH4 testing may be useful for identification of candidates with constipation for antibiotic treatment to pacify IBS symptoms[120]. Moreover in a Spanish study[121], it was observed that patients of IBS who had low production of H2 were 6 times more frequently constipated in lactulose breath test.In another study on subjects of IBS by Pimentel et al[114], fasting motility index in CH4-producing subjects was significantly increased compared with H2-producing subjects. Testing of H2 alone overlooks the importance of CH4 as a fermentation product[119]. 30%-50% of human population are producers of CH4. Synthesis of CH4 mostly consumes large amounts of H2, this may waiver diagnostic accuracy of breath testing when alone H2 is considered[122]. In a similar study by Lasa et al[123], it was observed that patients having low level of breath H2 excretion after lactulose ingestion had significantly greater abdominal bloating than those with increased level of breath H2 excretion. Kim et al[124] further observed in C-IBS patients with CH4 on breath testing, M. smithii is predominant methanogen.They reported that number and proportion of M. smithii in stool is well correlated with breath CH4 in their study.
It is apparent from the above-mentioned literature that CH4 should also be measured during breath testing in IBS patients so that manipulation of gut flora can be performed in these patients.
RECOMMENDATIONS FOR USE OF BREATH TESTS FOR IBS PATIENTS
On the basis of this review, it is apparent that breath tests are useful for the management of IBS patients: (1) breath tests can be useful in evaluating diarrhea, constipation, functional bloating and suspected malabsorption in IBS patients; (2) Breath test analyzing both H2 and CH4 has been shown to be of more importance than breath test using only H2 measurement for carbohydrate malabsorption and SIBO diagnosis; (3) GBT is a better diagnostic test for SIBO than the lactulose breath test, which gives false positive results; (4) breath tests are non-invasive, simple and safe alternatives to more invasive procedures such as obtaining aspirates for culturing and/or biopsies; (5) some errors may exist. In carbohydrate malabsorption false positive tests for SIBO may occur due to colonic fermentation and production of gas. In gastrointestinal motor disorders, delayed gastric emptying may cause false negative tests, and rapid transit through small bowel may result in false positive breath tests; (6) false positive results may also occur if the subject does not adhere to a low fiber diet the day before the test. Thus, patient is advised to reduce fiber intake prior to test, as this will effect a significant reduction in H2 production in the intestine, thus creating better testing environment; (7) accurate results are also not obtained if the patient has taken antibiotics, which change intestinal flora and are thus avoided within 4 wk prior to testing; and (8) laxatives and enemas also result in decreased transit time through the intestine, leading to reduced time for bacterial fermentation or loss of bacteria producing H2 or CH4.
CONCLUSION
This review summarizes the use of breath tests, not only to direct about dietary interventions but they also to provide prognostic information. These breath tests can help in the diagnosis of SIBO and carbohydrate malabsorption in IBS patients. Further studies analyzing H2 and CH4 concentrations in breath samples may improve diagnostic criteria for carbohydrate malabsorption in IBS patients. Moreover, area-under-the-curve analysis of the change in H2/CH4 concentration in breath samples over time after administering lactulose as a substrate may in future help to analyze the bacterial level in the bowel. Breath testing is also a useful to the low-FODMAP diet in IBS patients. In most cases of food intolerance, diagnosis is difficult. Thus, breath testing provides accurate, reliable and a non-invasive measure of absorption of a test sugar by assessment of breath H2/CH4 levels.Breath tests are performed to determine whether fructose and/or lactose and/or sorbitol are FODMAPs for an individual who has IBS symptoms. Thus, it can be shown whether an individual can or cannot completely digest fructose, lactose and sorbitol. This can be helpful to patients as well as physicians to formulate a particular diet which may help to reduce gastrointestinal symptoms present in IBS patients.
Footnotes
P- Reviewers: Bailey MT, Hughes PA, Quigley EMM, Wong RK S- Editor: Qi Y L- Editor: Cant MR E- Editor: Ma S
References
- 1.Newcomer AD, McGill DB, Thomas PJ, Hofmann AF. Prospective comparison of indirect methods for detecting lactase deficiency. N Engl J Med. 1975;293:1232–1236. doi: 10.1056/NEJM197512112932405. [DOI] [PubMed] [Google Scholar]
- 2.Bond JH, Levitt MD. Effect of dietary fiber on intestinal gas production and small bowel transit time in man. Am J Clin Nutr. 1978;31:S169–S174. doi: 10.1093/ajcn/31.10.S169. [DOI] [PubMed] [Google Scholar]
- 3.Urita Y, Ishihara S, Akimoto T, Kato H, Hara N, Honda Y, Nagai Y, Nakanishi K, Shimada N, Sugimoto M, et al. Seventy-five gram glucose tolerance test to assess carbohydrate malabsorption and small bowel bacterial overgrowth. World J Gastroenterol. 2006;12:3092–3095. doi: 10.3748/wjg.v12.i19.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamilton L. Breath tests and gastroenterology. 2nd ed. Milwaukee: QuinTron Instrument Company; 1998. [Google Scholar]
- 5.Bond JH, Levitt MD. Use of breath hydrogen (H2) to quantitate small bowel transit time following partial gastrectomy. J Lab Clin Med. 1977;90:30–36. [PubMed] [Google Scholar]
- 6.Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE, editors . Rome III. The Functional Gastrointestinal Disorders. 3rd ed. McLean, VA: Degnon Associates, Inc; 2006. [Google Scholar]
- 7.Ringel Y, Williams RE, Kalilani L, Cook SF. Prevalence, characteristics, and impact of bloating symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:68–72; quiz 3. doi: 10.1016/j.cgh.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 8.King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352:1187–1189. doi: 10.1016/s0140-6736(98)02146-1. [DOI] [PubMed] [Google Scholar]
- 9.Cuoco L, Salvagnini M. Small intestine bacterial overgrowth in irritable bowel syndrome: a retrospective study with rifaximin. Minerva Gastroenterol Dietol. 2006;52:89–95. [PubMed] [Google Scholar]
- 10.Majewski M, Reddymasu SC, Sostarich S, Foran P, McCallum RW. Efficacy of rifaximin, a nonabsorbed oral antibiotic, in the treatment of small intestinal bacterial overgrowth. Am J Med Sci. 2007;333:266–270. doi: 10.1097/MAJ.0b013e3180536784. [DOI] [PubMed] [Google Scholar]
- 11.King CE, Toskes PP. Breath tests in the diagnosis of small intestine bacterial overgrowth. Crit Rev Clin Lab Sci. 1984;21:269–281. doi: 10.3109/10408368409165785. [DOI] [PubMed] [Google Scholar]
- 12.Ford AC, Spiegel BM, Talley NJ, Moayyedi P. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1279–1286. doi: 10.1016/j.cgh.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 13.Ghoshal UC, Kumar S, Mehrotra M, Lakshmi C, Misra A. Frequency of small intestinal bacterial overgrowth in patients with irritable bowel syndrome and chronic non-specific diarrhea. J Neurogastroenterol Motil. 2010;16:40–46. doi: 10.5056/jnm.2010.16.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bond JH, Levitt MD. Use of pulmonary hydrogen (H 2 ) measurements to quantitate carbohydrate absorption. Study of partially gastrectomized patients. J Clin Invest. 1972;51:1219–1225. doi: 10.1172/JCI106916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddymasu SC, Sostarich S, McCallum RW. Small intestinal bacterial overgrowth in irritable bowel syndrome: are there any predictors? BMC Gastroenterol. 2010;10:23. doi: 10.1186/1471-230X-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerlin P, Wong L. Breath hydrogen testing in bacterial overgrowth of the small intestine. Gastroenterology. 1988;95:982–988. doi: 10.1016/0016-5085(88)90173-4. [DOI] [PubMed] [Google Scholar]
- 17.Lee KJ, Tack J. Altered intestinal microbiota in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:493–498. doi: 10.1111/j.1365-2982.2010.01482.x. [DOI] [PubMed] [Google Scholar]
- 18.Gasbarrini A, Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Ojetti V, Gasbarrini G. Small intestinal bacterial overgrowth: diagnosis and treatment. Dig Dis. 2007;25:237–240. doi: 10.1159/000103892. [DOI] [PubMed] [Google Scholar]
- 19.Ghoshal UC. How to interpret hydrogen breath tests. J Neurogastroenterol Motil. 2011;17:312–317. doi: 10.5056/jnm.2011.17.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posserud I, Stotzer PO, Björnsson ES, Abrahamsson H, Simrén M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802–808. doi: 10.1136/gut.2006.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lupascu A, Gabrielli M, Lauritano EC, Scarpellini E, Santoliquido A, Cammarota G, Flore R, Tondi P, Pola P, Gasbarrini G, et al. Hydrogen glucose breath test to detect small intestinal bacterial overgrowth: a prevalence case-control study in irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22:1157–1160. doi: 10.1111/j.1365-2036.2005.02690.x. [DOI] [PubMed] [Google Scholar]
- 22.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 23.Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978–2990. doi: 10.3748/wjg.v16.i24.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madrid AM, Defilippi C C, Defilippi G C, Slimming A J, Quera P R. [Small intestinal bacterial overgrowth in patients with functional gastrointestinal diseases] Rev Med Chil. 2007;135:1245–1252. [PubMed] [Google Scholar]
- 25.Swart GR, van den Berg JW. 13C breath test in gastroenterological practice. Scand J Gastroenterol Suppl. 1998;225:13–18. [PubMed] [Google Scholar]
- 26.King CE, Toskes PP. Comparison of the 1-gram [14C]xylose, 10-gram lactulose-H2, and 80-gram glucose-H2 breath tests in patients with small intestine bacterial overgrowth. Gastroenterology. 1986;91:1447–1451. doi: 10.1016/0016-5085(86)90199-x. [DOI] [PubMed] [Google Scholar]
- 27.Fromm H, Hofmann AF. Breath test for altered bile-acid metabolism. Lancet. 1971;2:621–625. doi: 10.1016/s0140-6736(71)80068-5. [DOI] [PubMed] [Google Scholar]
- 28.Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852–858. doi: 10.1001/jama.292.7.852. [DOI] [PubMed] [Google Scholar]
- 29.Pimentel M, Wallace D, Hallegua D, Chow E, Kong Y, Park S, Lin HC. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann Rheum Dis. 2004;63:450–452. doi: 10.1136/ard.2003.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Citters GW, Lin HC. Management of small intestinal bacterial overgrowth. Curr Gastroenterol Rep. 2005;7:317–320. doi: 10.1007/s11894-005-0025-x. [DOI] [PubMed] [Google Scholar]
- 31.Park JS, Yu JH, Lim HC, Kim JH, Yoon YH, Park HJ, Lee SI. [Usefulness of lactulose breath test for the prediction of small intestinal bacterial overgrowth in irritable bowel syndrome] Korean J Gastroenterol. 2010;56:242–248. doi: 10.4166/kjg.2010.56.4.242. [DOI] [PubMed] [Google Scholar]
- 32.Meyrat P, Safroneeva E, Schoepfer AM. Rifaximin treatment for the irritable bowel syndrome with a positive lactulose hydrogen breath test improves symptoms for at least 3 months. Aliment Pharmacol Ther. 2012;36:1084–1093. doi: 10.1111/apt.12087. [DOI] [PubMed] [Google Scholar]
- 33.Peralta S, Cottone C, Doveri T, Almasio PL, Craxi A. Small intestine bacterial overgrowth and irritable bowel syndrome-related symptoms: experience with Rifaximin. World J Gastroenterol. 2009;15:2628–2631. doi: 10.3748/wjg.15.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28–35; quiz 36. doi: 10.1038/ajg.2011.355. [DOI] [PubMed] [Google Scholar]
- 35.Majewski M, McCallum RW. Results of small intestinal bacterial overgrowth testing in irritable bowel syndrome patients: clinical profiles and effects of antibiotic trial. Adv Med Sci. 2007;52:139–142. [PubMed] [Google Scholar]
- 36.Scarpellini E, Giorgio V, Gabrielli M, Filoni S, Vitale G, Tortora A, Ojetti V, Gigante G, Fundarò C, Gasbarrini A. Rifaximin treatment for small intestinal bacterial overgrowth in children with irritable bowel syndrome. Eur Rev Med Pharmacol Sci. 2013;17:1314–1320. [PubMed] [Google Scholar]
- 37.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 38.Law D, Pimentel M. Proton pump inhibitor therapy does not affect hydrogen production on lactulose breath test in subjects with IBS. Dig Dis Sci. 2010;55:2302–2308. doi: 10.1007/s10620-009-1010-2. [DOI] [PubMed] [Google Scholar]
- 39.Parodi A, Dulbecco P, Savarino E, Giannini EG, Bodini G, Corbo M, Isola L, De Conca S, Marabotto E, Savarino V. Positive glucose breath testing is more prevalent in patients with IBS-like symptoms compared with controls of similar age and gender distribution. J Clin Gastroenterol. 2009;43:962–966. doi: 10.1097/MCG.0b013e3181a099a5. [DOI] [PubMed] [Google Scholar]
- 40.Yakoob J, Abbas Z, Khan R, Hamid S, Awan S, Jafri W. Small intestinal bacterial overgrowth and lactose intolerance contribute to irritable bowel syndrome symptomatology in Pakistan. Saudi J Gastroenterol. 2011;17:371–375. doi: 10.4103/1319-3767.87176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grover M, Kanazawa M, Palsson OS, Chitkara DK, Gangarosa LM, Drossman DA, Whitehead WE. Small intestinal bacterial overgrowth in irritable bowel syndrome: association with colon motility, bowel symptoms, and psychological distress. Neurogastroenterol Motil. 2008;20:998–1008. doi: 10.1111/j.1365-2982.2008.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sachdeva S, Rawat AK, Reddy RS, Puri AS. Small intestinal bacterial overgrowth (SIBO) in irritable bowel syndrome: frequency and predictors. J Gastroenterol Hepatol. 2011;26 Suppl 3:135–138. doi: 10.1111/j.1440-1746.2011.06654.x. [DOI] [PubMed] [Google Scholar]
- 43.Scarpellini E, Giorgio V, Gabrielli M, Lauritano EC, Pantanella A, Fundarò C, Gasbarrini A. Prevalence of small intestinal bacterial overgrowth in children with irritable bowel syndrome: a case-control study. J Pediatr. 2009;155:416–420. doi: 10.1016/j.jpeds.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 44.Rana SV, Sinha SK, Sikander A, Bhasin DK, Singh K. Study of small intestinal bacterial overgrowth in North Indian patients with irritable bowel syndrome: a case control study. Trop Gastroenterol. 2008;29:23–25. [PubMed] [Google Scholar]
- 45.Rana SV, Sharma S, Kaur J, Sinha SK, Singh K. Comparison of lactulose and glucose breath test for diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Digestion. 2012;85:243–247. doi: 10.1159/000336174. [DOI] [PubMed] [Google Scholar]
- 46.Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011;60:334–340. doi: 10.1136/gut.2009.205476. [DOI] [PubMed] [Google Scholar]
- 47.Bratten JR, Spanier J, Jones MP. Lactulose breath testing does not discriminate patients with irritable bowel syndrome from healthy controls. Am J Gastroenterol. 2008;103:958–963. doi: 10.1111/j.1572-0241.2008.01785.x. [DOI] [PubMed] [Google Scholar]
- 48.Walters B, Vanner SJ. Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: comparison with 14C-D-xylose and healthy controls. Am J Gastroenterol. 2005;100:1566–1570. doi: 10.1111/j.1572-0241.2005.40795.x. [DOI] [PubMed] [Google Scholar]
- 49.Eisenmann A, Amann A, Said M, Datta B, Ledochowski M. Implementation and interpretation of hydrogen breath tests. J Breath Res. 2008;2:046002. doi: 10.1088/1752-7155/2/4/046002. [DOI] [PubMed] [Google Scholar]
- 50.Corazza GR, Benati G, Strocchi A, Malservisi S, Gasbarrini G. The possible role of breath methane measurement in detecting carbohydrate malabsorption. J Lab Clin Med. 1994;124:695–700. [PubMed] [Google Scholar]
- 51.Matthews SB, Waud JP, Roberts AG, Campbell AK. Systemic lactose intolerance: a new perspective on an old problem. Postgrad Med J. 2005;81:167–173. doi: 10.1136/pgmj.2004.025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romagnuolo J, Schiller D, Bailey RJ. Using breath tests wisely in a gastroenterology practice: an evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol. 2002;97:1113–1126. doi: 10.1111/j.1572-0241.2002.05664.x. [DOI] [PubMed] [Google Scholar]
- 53.Farup PG, Monsbakken KW, Vandvik PO. Lactose malabsorption in a population with irritable bowel syndrome: prevalence and symptoms. A case-control study. Scand J Gastroenterol. 2004;39:645–649. doi: 10.1080/00365520410005405. [DOI] [PubMed] [Google Scholar]
- 54.Rana SV, Mandal AK, Kochhar R, Katyal R, Singh K. Lactose intolerance in different types of irritable bowel syndrome in north Indians. Trop Gastroenterol. 2001;22:202–204. [PubMed] [Google Scholar]
- 55.Vernia P, Marinaro V, Argnani F, Di Camillo M, Caprilli R. Self-reported milk intolerance in irritable bowel syndrome: what should we believe? Clin Nutr. 2004;23:996–1000. doi: 10.1016/j.clnu.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Böhmer CJ, Tuynman HA. The effect of a lactose-restricted diet in patients with a positive lactose tolerance test, earlier diagnosed as irritable bowel syndrome: a 5-year follow-up study. Eur J Gastroenterol Hepatol. 2001;13:941–944. doi: 10.1097/00042737-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Burden S. Dietary treatment of irritable bowel syndrome: current evidence and guidelines for future practice. J Hum Nutr Diet. 2001;14:231–241. doi: 10.1046/j.1365-277x.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- 58.Sciarretta G, Giacobazzi G, Verri A, Zanirato P, Garuti G, Malaguti P. Hydrogen breath test quantification and clinical correlation of lactose malabsorption in adult irritable bowel syndrome and ulcerative colitis. Dig Dis Sci. 1984;29:1098–1104. doi: 10.1007/BF01317083. [DOI] [PubMed] [Google Scholar]
- 59.Böhmer CJ, Tuynman HA. The clinical relevance of lactose malabsorption in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 1996;8:1013–1016. doi: 10.1097/00042737-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 60.Parker TJ, Woolner JT, Prevost AT, Tuffnell Q, Shorthouse M, Hunter JO. Irritable bowel syndrome: is the search for lactose intolerance justified? Eur J Gastroenterol Hepatol. 2001;13:219–225. doi: 10.1097/00042737-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Alpers DH. Diet and irritable bowel syndrome. Curr Opin Gastroenterol. 2006;22:136–139. doi: 10.1097/01.mog.0000208462.92136.02. [DOI] [PubMed] [Google Scholar]
- 62.Hamm LR, Sorrells SC, Harding JP, Northcutt AR, Heath AT, Kapke GF, Hunt CM, Mangel AW. Additional investigations fail to alter the diagnosis of irritable bowel syndrome in subjects fulfilling the Rome criteria. Am J Gastroenterol. 1999;94:1279–1282. doi: 10.1111/j.1572-0241.1999.01077.x. [DOI] [PubMed] [Google Scholar]
- 63.Vesa TH, Seppo LM, Marteau PR, Sahi T, Korpela R. Role of irritable bowel syndrome in subjective lactose intolerance. Am J Clin Nutr. 1998;67:710–715. doi: 10.1093/ajcn/67.4.710. [DOI] [PubMed] [Google Scholar]
- 64.Tolliver BA, Herrera JL, DiPalma JA. Evaluation of patients who meet clinical criteria for irritable bowel syndrome. Am J Gastroenterol. 1994;89:176–178. [PubMed] [Google Scholar]
- 65.Tolliver BA, Jackson MS, Jackson KL, Barnett ED, Chastang JF, DiPalma JA. Does lactose maldigestion really play a role in the irritable bowel? J Clin Gastroenterol. 1996;23:15–17. doi: 10.1097/00004836-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 66.Gupta D, Ghoshal UC, Misra A, Misra A, Choudhuri G, Singh K. Lactose intolerance in patients with irritable bowel syndrome from northern India: a case-control study. J Gastroenterol Hepatol. 2007;22:2261–2265. doi: 10.1111/j.1440-1746.2007.04986.x. [DOI] [PubMed] [Google Scholar]
- 67.Knudsen CD, Di Palma JA. Carbohydrate challenge tests: do you need to measure methane? South Med J. 2012;105:251–253. doi: 10.1097/SMJ.0b013e318252d428. [DOI] [PubMed] [Google Scholar]
- 68.Zhu Y, Zheng X, Cong Y, Chu H, Fried M, Dai N, Fox M. Bloating and distention in irritable bowel syndrome: the role of gas production and visceral sensation after lactose ingestion in a population with lactase deficiency. Am J Gastroenterol. 2013;108:1516–1525. doi: 10.1038/ajg.2013.198. [DOI] [PubMed] [Google Scholar]
- 69.Yang J, Deng Y, Chu H, Cong Y, Zhao J, Pohl D, Misselwitz B, Fried M, Dai N, Fox M. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:262–268.e1. doi: 10.1016/j.cgh.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 70.Gremse DA, Nguyenduc GH, Sacks AI, DiPalma JA. Irritable bowel syndrome and lactose maldigestion in recurrent abdominal pain in childhood. South Med J. 1999;92:778–781. doi: 10.1097/00007611-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 71.Vernia P, Camillo MD, Marinaro V, Caprilli R. Effect of predominant methanogenic flora on the outcome of lactose breath test in irritable bowel syndrome patients. Eur J Clin Nutr. 2003;57:1116–1119. doi: 10.1038/sj.ejcn.1601651. [DOI] [PubMed] [Google Scholar]
- 72.Rana SV, Sinha SK, Sharma S, Kaur H, Bhasin DK, Singh K. Effect of predominant methanogenic flora on outcome of lactose hydrogen breath test in controls and irritable bowel syndrome patients of north India. Dig Dis Sci. 2009;54:1550–1554. doi: 10.1007/s10620-008-0532-3. [DOI] [PubMed] [Google Scholar]
- 73.Lee KN, Lee OY, Koh DH, Sohn W, Lee SP, Jun DW, Lee HL, Yoon BC, Choi HS, Hahm JS. Association between symptoms of irritable bowel syndrome and methane and hydrogen on lactulose breath test. J Korean Med Sci. 2013;28:901–907. doi: 10.3346/jkms.2013.28.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corlew-Roath M, Di Palma JA. Clinical impact of identifying lactose maldigestion or fructose malabsorption in irritable bowel syndrome or other conditions. South Med J. 2009;102:1010–1012. doi: 10.1097/SMJ.0b013e3181b64c7f. [DOI] [PubMed] [Google Scholar]
- 75.Vernia P, Di Camillo M, Marinaro V. Lactose malabsorption, irritable bowel syndrome and self-reported milk intolerance. Dig Liver Dis. 2001;33:234–239. doi: 10.1016/s1590-8658(01)80713-1. [DOI] [PubMed] [Google Scholar]
- 76.Litschauer-Poursadrollah M, El-Sayad S, Wantke F, Fellinger C, Jarisch R. [Abdominal spasms, meteorism, diarrhea: fructose intolerance, lactose intolerance or IBS?] Wien Med Wochenschr. 2012;162:506–512. doi: 10.1007/s10354-012-0158-0. [DOI] [PubMed] [Google Scholar]
- 77.Ledochowski M, Widner B, Bair H, Probst T, Fuchs D. Fructose- and sorbitol-reduced diet improves mood and gastrointestinal disturbances in fructose malabsorbers. Scand J Gastroenterol. 2000;35:1048–1052. doi: 10.1080/003655200451162. [DOI] [PubMed] [Google Scholar]
- 78.Goldstein R, Braverman D, Stankiewicz H. Carbohydrate malabsorption and the effect of dietary restriction on symptoms of irritable bowel syndrome and functional bowel complaints. Isr Med Assoc J. 2000;2:583–587. [PubMed] [Google Scholar]
- 79.Nucera G, Gabrielli M, Lupascu A, Lauritano EC, Santoliquido A, Cremonini F, Cammarota G, Tondi P, Pola P, Gasbarrini G, et al. Abnormal breath tests to lactose, fructose and sorbitol in irritable bowel syndrome may be explained by small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2005;21:1391–1395. doi: 10.1111/j.1365-2036.2005.02493.x. [DOI] [PubMed] [Google Scholar]
- 80.Swagerty DL, Walling AD, Klein RM. Lactose intolerance. Am Fam Physician. 2002;65:1845–1850. [PubMed] [Google Scholar]
- 81.Moukarzel AA, Lesicka H, Ament ME. Irritable bowel syndrome and nonspecific diarrhea in infancy and childhood--relationship with juice carbohydrate malabsorption. Clin Pediatr (Phila) 2002;41:145–150. doi: 10.1177/000992280204100303. [DOI] [PubMed] [Google Scholar]
- 82.Wilder-Smith CH, Materna A, Wermelinger C, Schuler J. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013;37:1074–1083. doi: 10.1111/apt.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Born P, Zech J, Stark M, Classen M, Lorenz R. [Carbohydrate substitutes: comparative study of intestinal absorption of fructose, sorbitol and xylitol] Med Klin (Munich) 1994;89:575–578. [PubMed] [Google Scholar]
- 84.Skoog SM, Bharucha AE. Dietary fructose and gastrointestinal symptoms: a review. Am J Gastroenterol. 2004;99:2046–2050. doi: 10.1111/j.1572-0241.2004.40266.x. [DOI] [PubMed] [Google Scholar]
- 85.Choi YK, Johlin FC, Summers RW, Jackson M, Rao SS. Fructose intolerance: an under-recognized problem. Am J Gastroenterol. 2003;98:1348–1353. doi: 10.1111/j.1572-0241.2003.07476.x. [DOI] [PubMed] [Google Scholar]
- 86.Rumessen JJ, Gudmand-Høyer E. Functional bowel disease: malabsorption and abdominal distress after ingestion of fructose, sorbitol, and fructose-sorbitol mixtures. Gastroenterology. 1988;95:694–700. doi: 10.1016/s0016-5085(88)80016-7. [DOI] [PubMed] [Google Scholar]
- 87.Rumessen JJ, Gudmand-Høyer E. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut. 1986;27:1161–1168. doi: 10.1136/gut.27.10.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Truswell AS, Seach JM, Thorburn AW. Incomplete absorption of pure fructose in healthy subjects and the facilitating effect of glucose. Am J Clin Nutr. 1988;48:1424–1430. doi: 10.1093/ajcn/48.6.1424. [DOI] [PubMed] [Google Scholar]
- 89.Fernández-Bañares F, Esteve-Pardo M, de Leon R, Humbert P, Cabré E, Llovet JM, Gassull MA. Sugar malabsorption in functional bowel disease: clinical implications. Am J Gastroenterol. 1993;88:2044–2050. [PubMed] [Google Scholar]
- 90.Johlin FC, Panther M, Kraft N. Dietary fructose intolerance: diet modification can impact self-rated health and symptom control. Nutr Clin Care. 2004;7:92–97. [PubMed] [Google Scholar]
- 91.Choi YK, Kraft N, Zimmerman B, Jackson M, Rao SS. Fructose intolerance in IBS and utility of fructose-restricted diet. J Clin Gastroenterol. 2008;42:233–238. doi: 10.1097/MCG.0b013e31802cbc2f. [DOI] [PubMed] [Google Scholar]
- 92.Reyes-Huerta JU, de la Cruz-Patiño E, Ramírez-Gutiérrez de Velasco A, Zamudio C, Remes-Troche JM. [Fructose intolerance in patients with irritable bowel syndrome: a case-control study] Rev Gastroenterol Mex. 2010;75:405–411. [PubMed] [Google Scholar]
- 93.Skoog SM, Bharucha AE, Zinsmeister AR. Comparison of breath testing with fructose and high fructose corn syrups in health and IBS. Neurogastroenterol Motil. 2008;20:505–511. doi: 10.1111/j.1365-2982.2007.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fernández-Bañares F, Rosinach M, Esteve M, Forné M, Espinós JC, Maria Viver J. Sugar malabsorption in functional abdominal bloating: a pilot study on the long-term effect of dietary treatment. Clin Nutr. 2006;25:824–831. doi: 10.1016/j.clnu.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 95.Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106:1631–1639. doi: 10.1016/j.jada.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 96.Berg LK, Fagerli E, Martinussen M, Myhre AO, Florholmen J, Goll R. Effect of fructose-reduced diet in patients with irritable bowel syndrome, and its correlation to a standard fructose breath test. Scand J Gastroenterol. 2013;48:936–943. doi: 10.3109/00365521.2013.812139. [DOI] [PubMed] [Google Scholar]
- 97.Hyams JS. Sorbitol intolerance: an unappreciated cause of functional gastrointestinal complaints. Gastroenterology. 1983;84:30–33. [PubMed] [Google Scholar]
- 98.Madsen JL, Linnet J, Rumessen JJ. Effect of nonabsorbed amounts of a fructose-sorbitol mixture on small intestinal transit in healthy volunteers. Dig Dis Sci. 2006;51:147–153. doi: 10.1007/s10620-006-3100-8. [DOI] [PubMed] [Google Scholar]
- 99.Cherbut C, Aubé AC, Blottière HM, Galmiche JP. Effects of short-chain fatty acids on gastrointestinal motility. Scand J Gastroenterol Suppl. 1997;222:58–61. doi: 10.1080/00365521.1997.11720720. [DOI] [PubMed] [Google Scholar]
- 100.Pimentel M, Constantino T, Kong Y, Bajwa M, Rezaei A, Park S. A 14-day elemental diet is highly effective in normalizing the lactulose breath test. Dig Dis Sci. 2004;49:73–77. doi: 10.1023/b:ddas.0000011605.43979.e1. [DOI] [PubMed] [Google Scholar]
- 101.Yao CK, Tan HL, van Langenberg DR, Barrett JS, Rose R, Liels K, Gibson PR, Muir JG. Dietary sorbitol and mannitol: food content and distinct absorption patterns between healthy individuals and patients with irritable bowel syndrome. J Hum Nutr Diet. 2014;27 Suppl 2:263–275. doi: 10.1111/jhn.12144. [DOI] [PubMed] [Google Scholar]
- 102.Bond JH, Levitt MD, Prentiss R. Investigation of small bowel transit time in man utilizing pulmonary hydrogen (H2) measurements. J Lab Clin Med. 1975;85:546–555. [PubMed] [Google Scholar]
- 103.Lembcke B, Bornholdt C, Kirchhoff S, Lankisch PG. Clinical evaluation of a 25 g D-xylose hydrogen (H2) breath test. Z Gastroenterol. 1990;28:555–560. [PubMed] [Google Scholar]
- 104.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 105.de Roest RH, Dobbs BR, Chapman BA, Batman B, O’Brien LA, Leeper JA, Hebblethwaite CR, Gearry RB. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract. 2013;67:895–903. doi: 10.1111/ijcp.12128. [DOI] [PubMed] [Google Scholar]
- 106.Christl SU, Murgatroyd PR, Gibson GR, Cummings JH. Production, metabolism, and excretion of hydrogen in the large intestine. Gastroenterology. 1992;102:1269–1277. [PubMed] [Google Scholar]
- 107.Macfarlane GT, Gibson GR, Cummings JH. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol. 1992;72:57–64. doi: 10.1111/j.1365-2672.1992.tb04882.x. [DOI] [PubMed] [Google Scholar]
- 108.Lin HC, Pimentel M, Chen JH. Intestinal transit is slowed by luminal CH4. NeurogastroenterolMotil. 2002;14:437. [Google Scholar]
- 109.Ghoshal UC, Srivastava D, Verma A, Misra A. Slow transit constipation associated with excess methane production and its improvement following rifaximin therapy: a case report. J Neurogastroenterol Motil. 2011;17:185–188. doi: 10.5056/jnm.2011.17.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Le Nevé B, Posserud I, Böhn L, Guyonnet D, Rondeau P, Tillisch K, Naliboff B, Mayer EA, Simrén M. A combined nutrient and lactulose challenge test allows symptom-based clustering of patients with irritable bowel syndrome. Am J Gastroenterol. 2013;108:786–795. doi: 10.1038/ajg.2013.75. [DOI] [PubMed] [Google Scholar]
- 111.Pimentel M, Mayer AG, Park S, Chow EJ, Hasan A, Kong Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci. 2003;48:86–92. doi: 10.1023/a:1021738515885. [DOI] [PubMed] [Google Scholar]
- 112.Furnari M, Savarino E, Bruzzone L, Moscatelli A, Gemignani L, Giannini EG, Zentilin P, Dulbecco P, Savarino V. Reassessment of the role of methane production between irritable bowel syndrome and functional constipation. J Gastrointestin Liver Dis. 2012;21:157–163. [PubMed] [Google Scholar]
- 113.Kajs TM, Fitzgerald JA, Buckner RY, Coyle GA, Stinson BS, Morel JG, Levitt MD. Influence of a methanogenic flora on the breath H2 and symptom response to ingestion of sorbitol or oat fiber. Am J Gastroenterol. 1997;92:89–94. [PubMed] [Google Scholar]
- 114.Pimentel M, Lin HC, Enayati P, van den Burg B, Lee HR, Chen JH, Park S, Kong Y, Conklin J. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1089–G1095. doi: 10.1152/ajpgi.00574.2004. [DOI] [PubMed] [Google Scholar]
- 115.Fiedorek SC, Pumphrey CL, Casteel HB. Breath methane production in children with constipation and encopresis. J Pediatr Gastroenterol Nutr. 1990;10:473–477. doi: 10.1097/00005176-199005000-00010. [DOI] [PubMed] [Google Scholar]
- 116.Peled Y, Weinberg D, Hallak A, Gilat T. Factors affecting methane production in humans. Gastrointestinal diseases and alterations of colonic flora. Dig Dis Sci. 1987;32:267–271. doi: 10.1007/BF01297052. [DOI] [PubMed] [Google Scholar]
- 117.Pitcher MC, Cummings JH. Hydrogen sulphide: a bacterial toxin in ulcerative colitis? Gut. 1996;39:1–4. doi: 10.1136/gut.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gibson GR, Macfarlane GT, Cummings JH. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J Appl Bacteriol. 1988;65:103–111. doi: 10.1111/j.1365-2672.1988.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 119.Gibson GR, Cummings JH, Macfarlane GT. Competition for hydrogen between sulphate-reducing bacteria and methanogenic bacteria from the human large intestine. J Appl Bacteriol. 1988;65:241–247. doi: 10.1111/j.1365-2672.1988.tb01891.x. [DOI] [PubMed] [Google Scholar]
- 120.Kunkel D, Basseri RJ, Makhani MD, Chong K, Chang C, Pimentel M. Methane on breath testing is associated with constipation: a systematic review and meta-analysis. Dig Dis Sci. 2011;56:1612–1618. doi: 10.1007/s10620-011-1590-5. [DOI] [PubMed] [Google Scholar]
- 121.Dima G, Peralta D, Novillo A, Lasa J, Besasso H, Soifer L. [Predominance of constipation in subjects with hydrogen-consuming intestinal flora] Acta Gastroenterol Latinoam. 2012;42:182–185. [PubMed] [Google Scholar]
- 122.Cloarec D, Bornet F, Gouilloud S, Barry JL, Salim B, Galmiche JP. Breath hydrogen response to lactulose in healthy subjects: relationship to methane producing status. Gut. 1990;31:300–304. doi: 10.1136/gut.31.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lasa J, Peralta D, Dima G, Novillo A, Besasso H, Soifer L. Comparison of abdominal bloating severity between Irritable Bowel Syndrome patients with high and low levels of breath hydrogen excretion in a lactulose breath test. Rev Gastroenterol Mex. 2012;77:53–57. doi: 10.1016/j.rgmx.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 124.Kim G, Deepinder F, Morales W, Hwang L, Weitsman S, Chang C, Gunsalus R, Pimentel M. Methanobrevibacter smithii is the predominant methanogen in patients with constipation-predominant IBS and methane on breath. Dig Dis Sci. 2012;57:3213–3218. doi: 10.1007/s10620-012-2197-1. [DOI] [PubMed] [Google Scholar]


