Abstract
Since its advent in 1980, the scope of endoscopic ultrasound (EUS) has grown to include a wide range of indications, and it is now being incorporated as an integral part of everyday practice in the field of gastroenterology. Its use is extending from an adjuvant imaging aid to utilization as a therapeutic tool for various gastrointestinal disorders. EUS was first used to visualize remote organs, such as the pancreas and abdominal lymph nodes. When fine needle aspiration was introduced, the indications for EUS expanded to include tissue sampling for diagnostic purposes. At the same time, the needle can be used to convey a potential therapy to the internal organs, allowing access to remote sites. In this review, we aim to highlight the expanding spectrum of EUS indications and uses in the field of gastroenterology.
Keywords: Endoscopic ultrasound, Endoscopic ultrasound-guided fine needle aspiration, Ablation, Injection, Drainage, Immunohistochemistry, Gastroenterology
Core tip: Since its advent in 1980, the scope of endoscopic ultrasound has grown to include a wide range of indications, and it is now being incorporated as an integral part of everyday practice in the field of gastroenterology. Its use is extending from an adjuvant imaging aid to utilization as a therapeutic tool for various gastrointestinal disorders. In this review, we aim to highlight the expanding spectrum of endoscopic ultrasound indications and uses in the field of gastroenterology.
INTRODUCTION
Endoscopy, ultrasound, and computed tomography (CT) have revolutionized the field of clinical gastroenterology in recent decades. Despite their rapid development, these modalities do not allow a systematic assessment of the gastrointestinal tract (GIT) wall and its immediate surroundings. Awareness of this drawback prompted the development of a complementary procedure that would allow clinicians to overcome this problem. By combining a high-frequency ultrasound probe with an endoscope, the so-called “echoendoscope” or “endoscopic ultrasound (EUS)”, clearly detailed imaging of the structures close to the GIT wall was achieved. In 1980, the first mechanical radial EUS was applied clinically[1,2]. Since that date, the field of EUS has developed rapidly to encompass a wide range of indications, and expanded from diagnostic processes to therapeutic procedures. Here, we will try to highlight this revolution and to delineate the applications of EUS and its related practices.
EUS: DIAGNOSTIC INDICATIONS
The indications for EUS are determined by the anatomic conditions and the technical capabilities of the equipment. The high-resolution capacity and low penetration depth of EUS make it possible to obtain highly detailed images of the GIT wall and immediate surroundings to a depth of 4-5 cm[3]. The primary role of EUS is to delineate GIT lesions that are located beyond the gastric wall. The major indications are for GIT cancer staging, and delineation of masses. Table 1 summarizes these indications.
Table 1.
Endoscopic ultrasound: Diagnostic indications
| GIT cancer staging |
| Staging of gastroesophageal cancer |
| Staging of rectal cancer |
| Staging of ampullary cancer |
| Mass imaging and delineation |
| Pancreas |
| Gallbladder |
| Biliary tree |
| GIT submucosal lesions |
| Mediastinal and retroperitoneal mass |
GIT: Gastrointestinal tract.
GIT CANCER STAGING
Cancer staging was one of the earliest indications of EUS. Because it can delineate the component layers of the GIT wall, EUS is very well suited to classifying gastrointestinal cancers arising from the mucosa, using the widely accepted TNM classification. It is also useful for some extra-luminal malignancies, such as pancreatic cancer[4,5]. Also, one of the more recent indications of its use is in the characterization of a submucosal tumor that forms a bulge in conventional endoscopic images, and to determine its layer of origin, and, hence, its nature[2-5].
GIT MASS IMAGING AND DELINEATION
The advent of EUS enabled delineation of extra-luminal lesions with a high degree of accuracy, especially pancreatico-biliary lesions. The characterization of various pancreatic, biliary, and gallbladder (GB) lesions is now widely accepted and the use of EUS is considered an integral part of the diagnostic algorithm for these lesions[4,5]. New indications have become familiar to physicians in the field of gastroenterology, such as follow-up of intraductal papillary mucinous neoplasms of the pancreas, chronic pancreatitis, suspected pancreatic masses or cysts, and GB masses[3-5]. Use of EUS imaging also expanded to areas outside the GIT, e.g., assessment of mediastinal lymph nodes of unknown etiology[5].
CONTRAST-ENHANCED EUS
Contrast agents are new adjuvant tools in the field of ultrasound. They consist of gas-filled microbubbles encapsulated by a phospholipid or albumin shell that is injected intravenously, and its uptake-washout characteristics through a given lesion is captured by a specific color or the power Doppler mode of the ultrasound machine[6,7]. Therefore, by using contrast enhanced diffusion-EUS, clear differentiation of vascular-rich and hypovascular areas is possible. With development of harmonic imaging methods (contrast enhanced harmonic-EUS), it also became possible to obtain better visualization of the microcirculation and parenchymal perfusion, and better differentiation of tissue enhancement for more accurate classification[8,9].
EUS-ELASTOGRAPHY
Tissue elastic imaging represents a technical mode that allows calculation and visualization of tissue stiffness for non-invasive evaluation of fibrosis. The operating characteristics of the technique for detecting malignancy in pancreatic focal lesions yielded a sensitivity of 93%, and a specificity of 66%[10,11]. A further evaluation in the near future may enhance its diagnostic accuracy and help to avoid the need to obtain tissue for diagnosis.
SAFETY, COMPLICATIONS AND COST
The issue of safety and complications of diagnostic EUS has been extensively reviewed, and most of the relevant literature concluded that diagnostic EUS procedures were safe, and related complications were extremely low[12,13].
Regarding the cost-effectiveness, studies showed that EUS, when incorporated into a diagnostic algorithm, is cost-effective, especially in conjunction with fine needle guided procedures and when compared with other imaging modalities (e.g., CT and magnetic resonance imaging) and/or surgery[11-14]. However, the survey of Schembre and Lin, which evaluated the cost of EUS procedures, stated that maintenance and repair of EUS equipment is highly expensive, and should be taken into consideration when managing an EUS unit[15].
FORWARD-VIEWING EUS
Recently, forward-view EUS (FV-EUS) was developed to overcome the limitations of conventional oblique-view EUS; for example, the lack of on-procedure evaluation of the mucosa of the GIT wall, the difficult oblique approach to some target lesions, the diminished penetration force, and angling of the tip that reduces the possibility of deploying large bore devices in some positions[16] (Figure 1).
Figure 1.
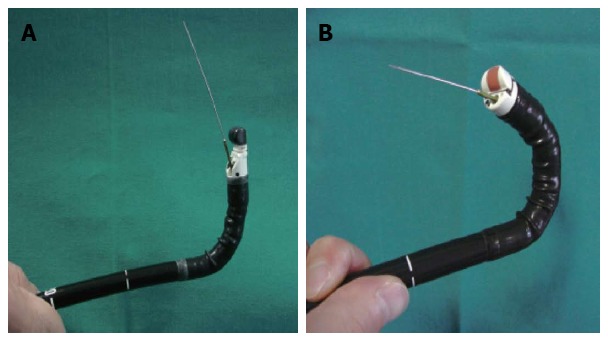
Difference between conventional oblique view and forward-view endoscopic ultrasound. A: Oblique-view endoscopic ultrasound (EUS). Note the maximum angulation and the needle direction; B: Forward-view EUS. Note the greater angle of retroflexion compared with conventional EUS.
Many recent studies and systematic reviews conducted to evaluate the capabilities of FV-EUS, in comparison with the conventional type, revealed a similarity in image quality and penetration force, in spite of a narrow imaging field. Moreover, the interventional procedures with FV-EUS were easier to perform compared with oblique-viewing EUS, and operators could reach difficult locations, such as the cecum. The main shortcomings reported were difficulties in intubating the esophagus or the second part of the duodenum in some patients and in aspirating pancreatic pseudocysts because of a lack of fixation of the guide-wire due to the absence of an elevator, but further comparative trials were recommended to investigate its usefulness in new indications[17,18].
EUS-GUIDED NEEDLE SAMPLING
The most fascinating advent in the field of EUS and its related interventions is needle-guided tissue sampling. Cytological or histological confirmation of a diagnosis is often required in order to distinguish between different scenarios. The idea of a biopsy needle first emerged in 1992 as a modification of those used for variceal injection[19], and the first reported case of EUS-guided biopsy was in a pancreatic lesion. Subsequently, EUS-guided needle sampling was studied for safety, accuracy and indications, with different needles and techniques being developed. Nowadays, the most widely used term for EUS-guided sampling is EUS-guided fine needle aspiration (EUS-FNA)[20].
EUS-GUIDED NEEDLE SAMPLING: INDICATIONS
A fundamental principle of EUS-FNA is to obtain information that would have the potential to affect patient management such as: (1) differentiating between benign and malignant lesions; (2) staging of cancer; and (3) obtaining histological evidence of malignancy before chemotherapy and/or radiotherapy, or even surgery[21]. Currently, most of the recent guidelines assign EUS-FNA as an integral part of sampling of the pancreas, mediastinal lymph nodes (esophageal/lung cancer), celiac lymph node, intra-abdominal lymph node, peri-rectal lymph node/mass, posterior mediastinal mass of unknown etiology, and intra-pleural/intra-abdominal fluid. In addition to these lesions, the indications for EUS-FNA have been expanded to submucosal tumors, small liver lesions, left adrenal masses, and suspected recurrent cancers in and adjacent to an anastomosis[9-22].
In spite of the growing list of EUS-FNA indications, a small list of contraindications should be considered. In general, EUS-FNA should not be used in situations in which FNA results would not affect the management strategy, the presence of bleeding diathesis, and if a high risk of tumor seeding is suspected. On-procedure, the inability to clearly visualize a lesion or a vessel interposed in the path between the needle and target might also be considered a contraindication[16-22].
EUS-GUIDED NEEDLE SAMPLING: SAFETY AND COMPLICATIONS
As an interventional modality, the possibility of complications from EUS-FNA must be taken into consideration. Many recent multicenter studies were designed to thoroughly evaluate this issue and their pooled conclusions considered that the complication rates from EUS-FNA in qualified institutes are quite rare[16-23]. In an interesting systematic review, the pooled rates of EUS-FNA complications in 51 articles (10941 patients) revealed an overall rate of morbidity of 0.98%, with pancreatitis and post-procedural pain being the most frequent[24]. However, some major complications were reported in the published guidelines, such as perforation, infection, and/or hemorrhage, but fortunately, these complications were extremely rare[16,18]. The use of careful add-on color Doppler-EUS examination reduced the risk of some potential complications especially the possibility of blood vessel injury[18-25]. An exception to this low rate of complications may be in cyst aspiration, where infection has been reported to occur in up to 15% of cases[26,27].
EUS-GUIDED NEEDLE SAMPLING: CYTO-PATHOLOGIC ISSUES
Generally speaking, the main role of EUS-FNA, and all other interventional biopsy techniques, is to obtain a sufficient tissue sample amenable to pathological examination and subsequently to formulation of a proper diagnosis. A smear slide is the standard method of preparing cells obtained from FNA, however, a “cell-block” which is a preparation of cells placed into a liquid medium or fixative, is the standard for hematoxylin and eosin (HE) staining[28].
One way to ensure the adequacy of materials obtained from the FNA procedure is the use of immediate cytologic evaluation (ICE) or that also known as rapid on-site cytologic evaluation (ROSE). The goal of this adjuvant option is to provide real-time feedback about the content and quality of the smears, in order to reduce the number of non-diagnostic or atypical biopsies and maximize the efficiency of the procedure. ICE or ROSE were more likely to result in a definitive diagnosis and less likely to result in an inadequate specimen[29-32].
Another adjuvant add-on staining procedure is the application of immunohistochemical stains for the identification of cytoplasmic or nuclear differentiation. Panels of immunoperoxidase stains can be used to identify a tumor type, characterize a lesion, or provide information for prognosis or treatment which enhances the EUS-FNA results[33].
EUS-GUIDED THERAPEUTIC IMPLICATIONS
Once a needle reaches a target, the spectrum of EUS-guided needle-related maneuvers can be expanded to endorse many therapeutic rather than diagnostic indications. EUS therapeutics are broadly classified into either EUS-guided fine needle injections (EUS-FNI) or EUS-guided drainage (EUS-FND). Table 2 summarizes these therapeutic indications.
Table 2.
Endoscopic ultrasound-guided therapeutic implications
| EUS-guided fine needle drainage |
| Biliary drainage |
| EUS-guided transpapillary rendezvous technique |
| Choledochoduodenostomy |
| Hepaticogastrostomy |
| EUS-guided cyst drainage |
| EUS-guided fine needle injection |
| Celiac plexus block and neurolysis |
| EUS-guided tattooing |
| EUS-guided ablation |
EUS: Endoscopic ultrasound.
EUS-FND
EUS-guided biliary drainage: The first reported EUS-guided-cholangiopancreatography was performed in 1996 by Wiersema et al[34], and subsequently, EUS-guided biliary drainage (EUS-BD) has been used for biliary decompression in patients with inoperable bile duct obstruction. EUS-BD has been reported as a salvage technique for failed conventional BD and, in general, it is indicated in circumstances in which conventional endoscopic retrograde cholangiopancreatography is difficult due to an altered anatomy or a tumor site that prevents access into the biliary tree[35-38].
To date, data for EUS-BD is still limited and most published trials are retrospective studies with small numbers of patients; however, the results are promising as its overall success rate was around 90% (range from 75% to 100%), and reported major complications, such as perforation, peritonitis and bleeding requiring surgery are uncommon[36-38]. There are three known techniques for EUS-guided BD: (1) EUS-guided transpapillary rendezvous; (2) EUS-guided choledochoduodenostomy (EUS-CDS); and (3) EUS-guided hepaticogastrostomy[37,38]. A brief summary of these procedures will be highlighted here. However, the detailed techniques of these maneuvers are beyond the scope of this review.
Through the EUS-guided transpapillary rendezvous technique, double-step endoscopies are performed to bypass biliary obstruction. EUS rendezvous is used solely to puncture the obstructed bile duct and pass a guide-wire in an antegrade manner through the native papilla to allow subsequent ERCP. The first step is to perform EUS-guided puncture of the bile duct through the stomach or duodenum. Then a contrast agent is injected to visualize the intra-hepatic and extra-hepatic bile ducts. After confirmation of bile duct puncture, a guide-wire is pushed through the obstructed segment across the papilla with fluoroscopic guidance to the duodenum. The next step is to remove the EUS scope leaving the guide-wire in place, then a duodenoscope is passed up to the papilla. Finally, the guide-wire is pulled back out the working channel of the duodenoscope for subsequent over-the-wire cannulation, and stent placement. The estimated overall success rate was about 80%, however, the guide-wire could be advanced across the obstruction in some difficult cases[33,38,39].
EUS-CDS was first reported by Horký et al[40] in 2001. Through a multistep technique, an EUS-guided biliary fistula is induced to connect the bile duct with the duodenum. However, a comparatively high complication rate (about 15%) has been reported, including biliary peritonitis and pneumoperitoneum[39-41].
The EUS-guided hepaticogastrostomy technique is broadly similar to EUS-CDS; in which a fine needle followed by a needle knife or cystotome, were used to puncture the intrahepatic bile ducts. The procedure was successful in almost all patients (> 96%)[33,42].
EUS-guided cyst/abscess drainage: Based on the same concept of introducing a needle into a known lesion, EUS-guided drainage of an abscess or cyst can be easily performed. Lesions such as pancreatic pseudocysts and intra-abdominal accumulations and abscesses are targets for this technique[43,44]. Many recent reports commented on the success rate of EUS-FND to drain remote abscesses and pseudopancreatic cysts and reported better outcomes[45,46].
EUS-FNI
EUS-guided FNI is a modified technique that utilized the concept of needle guidance to deliver a therapeutic target into a remote lesion/organ. In addition, this approach is effective in delivering concentrated drugs or chemotherapy[47].
The most exciting and promising subject in this context involves the delivery of antitumor agents into patients with locally advanced cancer, such as cancer of the pancreas, or esophagus. EUS-FNI has been used for precise delivery of antitumor agents into the targeted lesions to achieve a localized rather than a systemic delivery of the chemotherapeutic agents, which, in turn, may reduce systemic toxicities, maximize the delivered dose to the targeted lesions, and also reduce the cost[42,48,49]. This area of medical trials is still primitive and intense research efforts are needed.
Cystic lesions of the pancreas have also been treated with EUS-guided ablation through injection of sclerosing materials or absolute alcohol. However, this intervention remains investigational, although data have been encouraging[50].
Another use for EUS-FNI is the injection of ganglion blocking agents for celiac plexus neurolysis or block for pain relief in patients with pancreatic cancer. Celiac plexus block and neurolysis can improve pain and decrease the need for analgesics and opioids[51,52]. EUS provides a more direct and targeted approach secondary to better delineation of anatomic landmarks, close proximity of the transducer to the celiac plexus, and visualization of neural ganglionic structures that are not visible with other imaging modalities (Figure 2).
Figure 2.
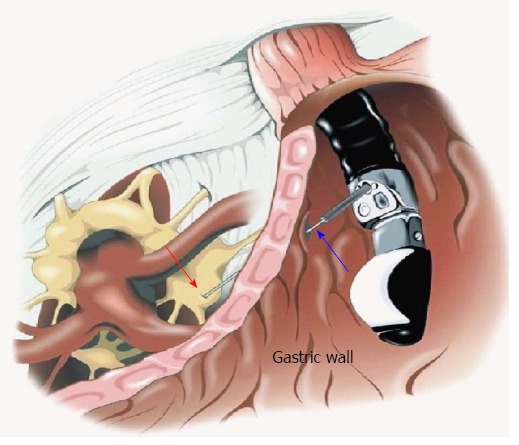
Endoscopic ultrasound-guided celiac plexus neurolysis. Red arrow: Celiac ganglion; Blue arrow: Endoscopic ultrasound needle transfixing the gastric wall.
EUS-guided vascular interventions: EUS has also been applied in the control of GIT bleeding. To date, most reports are of animal studies and small case reports/series[53]. New EUS-guided maneuvers have been reported for the management of upper GIT variceal and non-variceal bleeding, pseudo-aneurysm control, coil application and also embolization procedures, as well as the creation of intrahepatic portosystemic shunts and endoprostheses placement. However, these studies are in their early stages and a much research in the near future is expected[54,55].
EUS-guided photodynamic therapy: EUS-guided needle injection can also be used to deliver a photosensitizing drug, which induces targeted tissue necrosis on exposure to light of a specific wave length. The feasibility of this therapy was tested in a healthy swine model and further studies are examining its cost-effectiveness and biological side effects[30,56].
EUS-guided fiducial placement and brachytherapy: A fiduciary marker or fiducial is an object used as a point of reference in external beam radiation therapy. Gold fiducials are available to facilitate stereotactic body radiotherapy for the treatment of locally advanced pancreatic cancer[57,58]. Likewise, implanting radioactive seeds in the interstitial brachytherapy has shown some beneficial effects for the local control of malignant pancreatic tumors. These implants emit steady gamma rays that lead to local ablation[30,59-61]. Placement of such implants with the guide of EUS-FNI enables a precise targeting and avoids undue laparotomy. Investigations in this area reported successful placement in around 90% of patients.
CONCLUSION
EUS use is now considered a gold standard tool for many gastrointestinal diseases, especially pancreatico-biliary diseases, and adjuvant needle insertion allows access to remote lesions that were difficult to reach in the past. With the growing spectrum of indications, the clinical applicability of EUS has expanded to include therapeutic applications in addition to diagnostic uses, and some of these show great promise. Major breakthroughs in the technical advances of EUS technology were achieved in the last few decades, especially in scope design, accessory devices, and add-on facilities, which have placed EUS and its related maneuvers as a necessary procedure in many gastrointestinal indications.
Footnotes
P- Reviewers: Draganov P, Tandon RK, Tsuji Y S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Zhang DN
References
- 1.DiMagno EP, Buxton JL, Regan PT, Hattery RR, Wilson DA, Suarez JR, Green PS. Ultrasonic endoscope. Lancet. 1980;1:629–631. doi: 10.1016/s0140-6736(80)91122-8. [DOI] [PubMed] [Google Scholar]
- 2.Strohm WD, Phillip J, Hagenmüller F, Classen M. Ultrasonic tomography by means of an ultrasonic fiberendoscope. Endoscopy. 1980;12:241–244. doi: 10.1055/s-2007-1021752. [DOI] [PubMed] [Google Scholar]
- 3.Bhutani MS. Interventional endoscopic ultrasonography: state of the art at the new millenium. Endoscopy. 2000;32:62–71. doi: 10.1055/s-2000-139. [DOI] [PubMed] [Google Scholar]
- 4.Brugge WR. Endoscopic ultrasonography: the current status. Gastroenterology. 1998;115:1577–1583. doi: 10.1016/s0016-5085(98)70039-3. [DOI] [PubMed] [Google Scholar]
- 5.Byrne MF, Jowell PS. Gastrointestinal imaging: endoscopic ultrasound. Gastroenterology. 2002;122:1631–1648. doi: 10.1053/gast.2002.33576. [DOI] [PubMed] [Google Scholar]
- 6.Klibanov AL. Ultrasound molecular imaging with targeted microbubble contrast agents. J Nucl Cardiol. 2007;14:876–884. doi: 10.1016/j.nuclcard.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Reddy NK, Ioncică AM, Săftoiu A, Vilmann P, Bhutani MS. Contrast-enhanced endoscopic ultrasonography. World J Gastroenterol. 2011;17:42–48. doi: 10.3748/wjg.v17.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quaia E. Microbubble ultrasound contrast agents: an update. Eur Radiol. 2007;17:1995–2008. doi: 10.1007/s00330-007-0623-0. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa T, Itoh A, Kawashima H, Ohno E, Matsubara H, Itoh Y, Nakamura Y, Nakamura M, Miyahara R, Hayashi K, et al. Usefulness of EUS combined with contrast-enhancement in the differential diagnosis of malignant versus benign and preoperative localization of pancreatic endocrine tumors. Gastrointest Endosc. 2010;71:951–959. doi: 10.1016/j.gie.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Săftoiu A, Vilmann P. Differential diagnosis of focal pancreatic masses by semiquantitative EUS elastography: between strain ratios and strain histograms. Gastrointest Endosc. 2013;78:188–189. doi: 10.1016/j.gie.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Deprez PH. EUS elastography: is it replacing or supplementing tissue acquisition? Gastrointest Endosc. 2013;77:590–592. doi: 10.1016/j.gie.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 12.Yamao K, Sawaki A, Mizuno N, Shimizu Y, Yatabe Y, Koshikawa T. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): past, present, and future. J Gastroenterol. 2005;40:1013–1023. doi: 10.1007/s00535-005-1717-6. [DOI] [PubMed] [Google Scholar]
- 13.Al-Haddad M, Eloubeidi MA. Diagnostic and therapeutic applications of endoscopic ultrasound-guided punctures. Dig Dis. 2008;26:390–397. doi: 10.1159/000177030. [DOI] [PubMed] [Google Scholar]
- 14.Shetty D, Bhatnagar G, Sidhu HS, Fox BM, Dodds NI. The increasing role of endoscopic ultrasound (EUS) in the management of pancreatic and biliary disease. Clin Radiol. 2013;68:323–335. doi: 10.1016/j.crad.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Schembre D, Lin O. Frequency and costs of echo endoscope repairs: results of a survey of endosonographers. Endoscopy. 2004;36:982–986. doi: 10.1055/s-2004-825862. [DOI] [PubMed] [Google Scholar]
- 16.Imaizumi H, Irisawa A. Preliminary experience of a prototype forward-viewing curved linear array echoendoscope in a training phantom model. Dig Endosc. 2010;22 Suppl 1:S123–S127. doi: 10.1111/j.1443-1661.2010.00975.x. [DOI] [PubMed] [Google Scholar]
- 17.Kida M, Araki M, Miyazawa S, Ikeda H, Kikuchi H, Watanabe M, Imaizumi H, Koizumi W. Fine needle aspiration using forward-viewing endoscopic ultrasonography. Endoscopy. 2011;43:796–801. doi: 10.1055/s-0030-1256508. [DOI] [PubMed] [Google Scholar]
- 18.Iwashita T, Nakai Y, Lee JG, Park do H, Muthusamy VR, Chang KJ. Newly-developed, forward-viewing echoendoscope: a comparative pilot study to the standard echoendoscope in the imaging of abdominal organs and feasibility of endoscopic ultrasound-guided interventions. J Gastroenterol Hepatol. 2012;27:362–367. doi: 10.1111/j.1440-1746.2011.06923.x. [DOI] [PubMed] [Google Scholar]
- 19.Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–173. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 20.Hawes RH. The evolution of endoscopic ultrasound: improved imaging, higher accuracy for fine needle aspiration and the reality of endoscopic ultrasound-guided interventions. Curr Opin Gastroenterol. 2010;26:436–444. doi: 10.1097/MOG.0b013e32833d1799. [DOI] [PubMed] [Google Scholar]
- 21.Dumonceau JM, Polkowski M, Larghi A, Vilmann P, Giovannini M, Frossard JL, Heresbach D, Pujol B, Fernández-Esparrach G, Vazquez-Sequeiros E, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 22.Tarantino I, Barresi L. Interventional endoscopic ultrasound: Therapeutic capability and potential. World J Gastrointest Endosc. 2009;1:39–44. doi: 10.4253/wjge.v1.i1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polkowski M, Larghi A, Weynand B, Boustière C, Giovannini M, Pujol B, Dumonceau JM. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 24.Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, Li ZS. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283–290. doi: 10.1016/j.gie.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 25.Yamao K, Mizuno N, Takagi T, Hara K. How I do it and when I use (and do not use) EUS-FNA. Gastrointest Endosc. 2009;69:S134–S137. doi: 10.1016/j.gie.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Lim LG, Lakhtakia S, Ang TL, Vu CK, Dy F, Chong VH, Khor CJ, Lim WC, Doshi BK, Varadarajulu S, et al. Factors determining diagnostic yield of endoscopic ultrasound guided fine-needle aspiration for pancreatic cystic lesions: a multicentre Asian study. Dig Dis Sci. 2013;58:1751–1757. doi: 10.1007/s10620-012-2528-2. [DOI] [PubMed] [Google Scholar]
- 27.Oguz D, Öztaş E, Kalkan IH, Tayfur O, Cicek B, Aydog G, Kurt M, Beyazit Y, Etik D, Nadir I, et al. Accuracy of endoscopic ultrasound-guided fine needle aspiration cytology on the differentiation of malignant and benign pancreatic cystic lesions: a single-center experience. J Dig Dis. 2013;14:132–139. doi: 10.1111/1751-2980.12014. [DOI] [PubMed] [Google Scholar]
- 28.Jenssen C, Dietrich CF. Endoscopic ultrasound-guided fine-needle aspiration biopsy and trucut biopsy in gastroenterology - An overview. Best Pract Res Clin Gastroenterol. 2009;23:743–759. doi: 10.1016/j.bpg.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Logroño R, Waxman I. Interactive role of the cytopathologist in EUS-guided fine needle aspiration: an efficient approach. Gastrointest Endosc. 2001;54:485–490. doi: 10.1067/mge.2001.118445. [DOI] [PubMed] [Google Scholar]
- 30.Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–1294. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 31.da Cunha Santos G. ROSEs (Rapid on-site evaluations) to our patients: The impact on laboratory resources and patient care. Cancer Cytopathol. 2013;121:537–539. doi: 10.1002/cncy.21319. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt RL, Kordy MA, Howard K, Layfield LJ, Hall BJ, Adler DG. Risk-benefit analysis of sampling methods for fine-needle aspiration cytology: a mathematical modeling approach. Am J Clin Pathol. 2013;139:336–344. doi: 10.1309/AJCPEAKR4MO2GQBO. [DOI] [PubMed] [Google Scholar]
- 33.Noda Y, Fujita N, Kobayashi G, Itoh K, Horaguchi J, Takasawa O, Obana T, Koshita S, Kanno Y, Suzuki T, et al. Diagnostic efficacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound-guided fine-needle aspiration. J Gastroenterol. 2010;45:868–875. doi: 10.1007/s00535-010-0217-5. [DOI] [PubMed] [Google Scholar]
- 34.Wiersema MJ, Sandusky D, Carr R, Wiersema LM, Erdel WC, Frederick PK. Endosonography-guided cholangiopancreatography. Gastrointest Endosc. 1996;43:102–106. doi: 10.1016/s0016-5107(06)80108-2. [DOI] [PubMed] [Google Scholar]
- 35.Chavalitdhamrong D, Draganov PV. Endoscopic ultrasound-guided biliary drainage. World J Gastroenterol. 2012;18:491–497. doi: 10.3748/wjg.v18.i6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iqbal S, Friedel DM, Grendell JH, Stavropoulos SN. Outcomes of endoscopic-ultrasound-guided cholangiopancreatography: a literature review. Gastroenterol Res Pract. 2013;2013:869214. doi: 10.1155/2013/869214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahaleh M, Artifon EL, Perez-Miranda M, Gupta K, Itoi T, Binmoeller KF, Giovannini M. Endoscopic ultrasonography guided biliary drainage: summary of consortium meeting, May 7th, 2011, Chicago. World J Gastroenterol. 2013;19:1372–1379. doi: 10.3748/wjg.v19.i9.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkaria S, Lee HS, Gaidhane M, Kahaleh M. Advances in endoscopic ultrasound-guided biliary drainage: a comprehensive review. Gut Liver. 2013;7:129–136. doi: 10.5009/gnl.2013.7.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takikawa T, Kanno A, Masamune A, Hamada S, Nakano E, Miura S, Ariga H, Unno J, Kume K, Kikuta K, et al. Pancreatic duct drainage using EUS-guided rendezvous technique for stenotic pancreaticojejunostomy. World J Gastroenterol. 2013;19:5182–5186. doi: 10.3748/wjg.v19.i31.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horký J, Korístek V, Sumbera J, Novák M, Nová B. [2-stage surgery for congenital heart defects in children with pulmonary hypertension] Cesk Pediatr. 1975;30:162–163. [PubMed] [Google Scholar]
- 41.Hara K, Yamao K, Mizuno N, Hijioka S, Sawaki A, Tajika M, Kawai H, Kondo S, Shimizu Y, Niwa Y. Interventional endoscopic ultrasonography for pancreatic cancer. World J Clin Oncol. 2011;2:108–114. doi: 10.5306/wjco.v2.i2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hara K, Yamao K, Hijioka S, Mizuno N, Imaoka H, Tajika M, Kondo S, Tanaka T, Haba S, Takeshi O, et al. Prospective clinical study of endoscopic ultrasound-guided choledochoduodenostomy with direct metallic stent placement using a forward-viewing echoendoscope. Endoscopy. 2013;45:392–396. doi: 10.1055/s-0032-1326076. [DOI] [PubMed] [Google Scholar]
- 43.Fusaroli P, Kypraios D, Caletti G, Eloubeidi MA. Pancreatico-biliary endoscopic ultrasound: a systematic review of the levels of evidence, performance and outcomes. World J Gastroenterol. 2012;18:4243–4256. doi: 10.3748/wjg.v18.i32.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prasad GA, Varadarajulu S. Endoscopic ultrasound-guided abscess drainage. Gastrointest Endosc Clin N Am. 2012;22:281–90, ix. doi: 10.1016/j.giec.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Siddiqui AA, Dewitt JM, Strongin A, Singh H, Jordan S, Loren DE, Kowalski T, Eloubeidi MA. Outcomes of EUS-guided drainage of debris-containing pancreatic pseudocysts by using combined endoprosthesis and a nasocystic drain. Gastrointest Endosc. 2013;78:589–595. doi: 10.1016/j.gie.2013.03.1337. [DOI] [PubMed] [Google Scholar]
- 46.Gornals JB, De la Serna-Higuera C, Sánchez-Yague A, Loras C, Sánchez-Cantos AM, Pérez-Miranda M. Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. Surg Endosc. 2013;27:1428–1434. doi: 10.1007/s00464-012-2591-y. [DOI] [PubMed] [Google Scholar]
- 47.Verna EC, Dhar V. Endoscopic ultrasound-guided fine needle injection for cancer therapy: the evolving role of therapeutic endoscopic ultrasound. Therap Adv Gastroenterol. 2008;1:103–109. doi: 10.1177/1756283X08093887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carrara S, Petrone MC, Testoni PA, Arcidiacono PG. Tumors and new endoscopic ultrasound-guided therapies. World J Gastrointest Endosc. 2013;5:141–147. doi: 10.4253/wjge.v5.i4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee KH, Lee JK. Interventional endoscopic ultrasonography: present and future. Clin Endosc. 2011;44:6–12. doi: 10.5946/ce.2011.44.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeWitt J, McGreevy K, Schmidt CM, Brugge WR. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc. 2009;70:710–723. doi: 10.1016/j.gie.2009.03.1173. [DOI] [PubMed] [Google Scholar]
- 51.Nagels W, Pease N, Bekkering G, Cools F, Dobbels P. Celiac plexus neurolysis for abdominal cancer pain: a systematic review. Pain Med. 2013;14:1140–1163. doi: 10.1111/pme.12176. [DOI] [PubMed] [Google Scholar]
- 52.Doi S, Yasuda I, Kawakami H, Hayashi T, Hisai H, Irisawa A, Mukai T, Katanuma A, Kubota K, Ohnishi T, et al. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy. 2013;45:362–369. doi: 10.1055/s-0032-1326225. [DOI] [PubMed] [Google Scholar]
- 53.Magno P, Ko CW, Buscaglia JM, Giday SA, Jagannath SB, Clarke JO, Shin EJ, Kantsevoy SV. EUS-guided angiography: a novel approach to diagnostic and therapeutic interventions in the vascular system. Gastrointest Endosc. 2007;66:587–591. doi: 10.1016/j.gie.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Levy MJ, Wong Kee Song LM, Farnell MB, Misra S, Sarr MG, Gostout CJ. Endoscopic ultrasound (EUS)-guided angiotherapy of refractory gastrointestinal bleeding. Am J Gastroenterol. 2008;103:352–359. doi: 10.1111/j.1572-0241.2007.01616.x. [DOI] [PubMed] [Google Scholar]
- 55.Bokun T, Grgurevic I, Kujundzic M, Banic M. EUS-Guided Vascular Procedures: A Literature Review. Gastroenterol Res Pract. 2013;2013:865945. doi: 10.1155/2013/865945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsui Y, Nakagawa A, Kamiyama Y, Yamamoto K, Kubo N, Nakase Y. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas. 2000;20:14–20. doi: 10.1097/00006676-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Park WG, Yan BM, Schellenberg D, Kim J, Chang DT, Koong A, Patalano C, Van Dam J. EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest Endosc. 2010;71:513–518. doi: 10.1016/j.gie.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 58.Pishvaian AC, Collins B, Gagnon G, Ahlawat S, Haddad NG. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412–417. doi: 10.1016/j.gie.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 59.Sun S, Xu H, Xin J, Liu J, Guo Q, Li S. Endoscopic ultrasound-guided interstitial brachytherapy of unresectable pancreatic cancer: results of a pilot trial. Endoscopy. 2006;38:399–403. doi: 10.1055/s-2006-925253. [DOI] [PubMed] [Google Scholar]
- 60.Sun S, Qingjie L, Qiyong G, Mengchun W, Bo Q, Hong X. EUS-guided interstitial brachytherapy of the pancreas: a feasibility study. Gastrointest Endosc. 2005;62:775–779. doi: 10.1016/j.gie.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 61.Draganov PV, Chavalitdhamrong D, Wagh MS. Evaluation of a new endoscopic ultrasound-guided multi-fiducial delivery system: a prospective non-survival study in a live porcine model. Dig Endosc. 2013;25:615–621. doi: 10.1111/den.12046. [DOI] [PubMed] [Google Scholar]


