Abstract
Since the introduction of endoscopic ultrasound guided fine-needle aspiration (EUS-FNA), EUS has assumed a growing role in the diagnosis and management of pancreatic ductal adenocarcinoma (PDAC). The objective of this review is to discuss the various applications of EUS and EUS-FNA in PDAC. Initially, its use for detection, diagnosis and staging will be described. EUS and EUS-FNA are highly accurate modalities for detection and diagnosis of PDAC, this high accuracy, however, is decreased in specific situations particularly in the presence of chronic pancreatitis. Novel techniques such as contrast-enhanced EUS, elastography and analysis of DNA markers such as k-ras mutation analysis in FNA samples are in progress and might improve the accuracy of EUS in the detection of PDAC in this setting and will be addressed. EUS and EUS-FNA have recently evolved from a diagnostic to a therapeutic technique in the management of PDAC. Significant developments in therapeutic EUS have occurred including advances in celiac plexus interventions with direct injection of ganglia and improved pain control, EUS-guided fiducial and brachytherapy seed placement, fine-needle injection of intra-tumoral agents and advances in EUS-guided biliary drainage. The future role of EUS and EUS in management of PDAC is still emerging.
Keywords: Pancreatic ductal carcinoma, Pancreatic neoplasm, Endoscopic Ultrasound-Guided Fine Needle Aspiration, Endosonography, Endoscopic ultrasound guided fine-needle aspiration
Core tip: Applications of endoscopic ultrasound (EUS) in pancreatic cancer are emerging. We review the role of EUS in the detection, diagnosis and staging of pancreatic cancer. The introduction of recent novel techniques such as contrast-enhanced EUS, elastography and analysis of DNA markers in fine-needle aspiration samples might improve the accuracy of EUS. In addition, we review therapeutic application of EUS including celiac plexus interventions, fiducial and brachytherapy seeds placement, fine needle injection and EUS-guided biliary drainage.
INTRODUCTION
With the introduction of endoscopic ultrasound guided fine-needle aspiration of pancreatic masses by Vilmann et al[1] (EUS-FNA), endosonography has assumed an increasing role in the management of pancreatic ductal adenocarcinoma (PDAC). In this review, our objective is to discuss the various applications of EUS and EUS-FNA in PDAC. Initially, its use for detection, diagnosis and staging, including newly described techniques as contrast-enhanced EUS, elastography, and use of DNA markers will be described. Finally, the use of therapeutic EUS procedures including celiac plexus neurolysis and emerging therapies as fine-needle injection, implantation of fiducials and brachytherapy seeds will be discussed.
DETECTION AND DIAGNOSIS OF PANCREATIC CANCER
Detection of pancreatic cancer
EUS is the most sensitive nonoperative imaging test for the detection of malignant pancreatic lesions, with a reported sensitivity between 87%-100%[2-11]. EUS is markedly superior to transabdominal ultrasound (reported sensitivity between 64%-91%)[2-5,7] and has also been shown to be superior to computed tomography (CT) (sensitivity 66%-86%) for the detection of pancreatic masses in studies which compared both techniques[3-8,10,11]. EUS is clearly superior to conventional CT[3-5,8] and a few studies comparing EUS and multidetector-row CT (MDCT) for detection of pancreatic tumors demonstrated the superiority of EUS as compared to 4-row CT[10,11]. Agarwal et al[10] showed a sensitivity of 100% for EUS in the diagnosis of cancer compared to 86% for MDCT in a retrospective cohort of 81 patients with PDAC. DeWitt et al[11] reported similar findings in a prospective cohort of 80 patients with PDAC, showing that the sensitivity of EUS 98% statistically superior to MDCT 86% for detection of PDAC. There are scant comparisons between EUS and MRI for tumor detection with at least one study showing superiority of EUS[6] and one study showing superiority of magnetic resonance imaging (MRI)[9]. Future studies comparing EUS and 3.0 or higher Tesla MRI are necessary to further define the roles of each imaging modality in the diagnosis of pancreatic masses.
EUS is particularly useful for identification of small tumors that are not visualized by other imaging modalities[3,6,10-12] For tumors ≤ 30 mm in diameter, EUS was found to have a sensitivity of 93% compared to 53% for CT and 67% for MRI[6]. In a recent retrospective cohort by Wang et al[12], which included 116 patients with clinical presentation suspicious for PDAC and inconclusive MDCT findings, EUS showed a sensitivity of 87% and an accuracy of 92% in diagnosing pancreatic neoplasm. With thinner slice imaging and precisely timed contrast administration coupled with multiplanar reconstruction, pancreas protocol CT may now be able to identify small pancreatic masses that previously may have been undetected by conventional or even single detector dual-phase imaging[11]. EUS should be performed in all patients with obstructive jaundice or unexplained pancreatic and/or bile duct dilations in whom CT or MRI do not definitively identify a pancreatic lesion, both to detect any tumor and to exclude non-neoplastic diseases.
EUS may fail to identify a true pancreatic mass in patients with chronic pancreatitis, a diffusely infiltrating carcinoma, a prominent ventral/dorsal split or a recent episode (< 4 wk) of acute pancreatitis[13]. In a study of 80 patients with clinical suspicion of PDAC and a normal EUS, Catanzaro et al[14] found that no patient with a normal pancreatic EUS developed cancer during a follow-up period of 24 mo. Therefore, a normal pancreas by EUS examination essentially excludes PDAC although follow-up EUS or other studies should be done in the setting of chronic pancreatitis due to potentially impaired visualization. Acoustic shadowing caused by an indwelling biliary or pancreatic stent may also interfere with visualization of a small pancreatic mass. However, a recent retrospective study by Ranney et al[15] did not show any difference in the diagnostic yield or technical difficulty of EUS-FNA of visualized pancreatic masses in the presence of a biliary stent (plastic or metal).
Imaging-based technologies such as contrast-enhanced EUS (CE-EUS) may be used to differentiate PDAC from other benign or malignant lesions. In this procedure, an intravenous contrast agent is administered at time of EUS and microbubbles are detected in the microvasculature of pancreatic tumors during real-time evaluation. Adenocarcinomas show hypo-enhancement while neuroendocrine tumors and pseudotumoral chronic pancreatitis are iso- or hyper-enhancing. Numerous contrast agents are available including first generation contrast agents such as Levovist and second generation such as Sonovue and Sonazoid. In a recent meta-analysis including 1139 patients, the pooled sensitivity and specificity of CE-EUS for the differential diagnosis of pancreatic adenocarcinoma were 94% and 89%, respectively[16]. This study found that a hypoenhanced lesion by CE-EUS was a sensitive and accurate predictor of adenocarcinoma. In the United States, routine use of CE-EUS is limited by its high cost and the lack of both agent availability and expertise with this technique.
Another emerging technology used to differentiate benign from malignant masses is EUS elastography. This technology provides real-time evaluation of tissue stiffness and is based on the premise that there is less strain when hard tissues are compressed compared to soft tissues[17]. As malignant lesions are generally harder than normal adjacent tissue, measuring strain might aid classification of pancreatic masses. Results from 2 recent meta-analyses demonstrated a high pooled sensitivity of 95%-97% but a low pooled specificity of 67%-76%, respectively, for differential diagnosis of solid pancreatic masses[18,19]. Elastography might provide complementary information to EUS, potentially increasing the yield of EUS-FNA, and assist endosonographers to improve targeting of FNA[18]. Limitations of this technique include limited availability, difficulty controlling tissue compression by the endosonographer, presence of motion artifacts, and unclear stiffness cut-off values for pancreatic masses[19].
Elastography and contrast-enhanced imaging may be combined during the same procedure. Săftoiu et al[20] sequentially combined CE power Doppler with real-time elastography in 21 patients with chronic pancreatitis and 33 patients with PDAC undergoing EUS examination. The sensitivity, specificity, and accuracy of combined information provided by both tests to differentiate hypovascular hard masses suggestive of pancreatic carcinoma were 75.8%, 95.2%, and 83.3%, respectively, with a positive predictive value and negative predictive value of 96.2% and 71.4%, respectively.
Diagnosis of pancreatic cancer
EUS-FNA of a pancreatic mass was first described in 1992[1] and is currently the preferred method to sample pancreatic mass lesions, having largely replaced intraoperative sampling or biopsies under CT or US guidance. EUS-FNA is performed using the linear array echoendoscope, as the ultrasound transducer at its distal tip allows needle advancement under real-time guidance once the target is identified (Figure 1). EUS-FNA of a suspected metastatic site from PDAC (ascites, distant metastatic lymph node, omental nodule or a suspicious liver lesion) should be performed first. If those are negative for malignancy then either the suspected tumor or a regional lymph node may be sampled.
Figure 1.
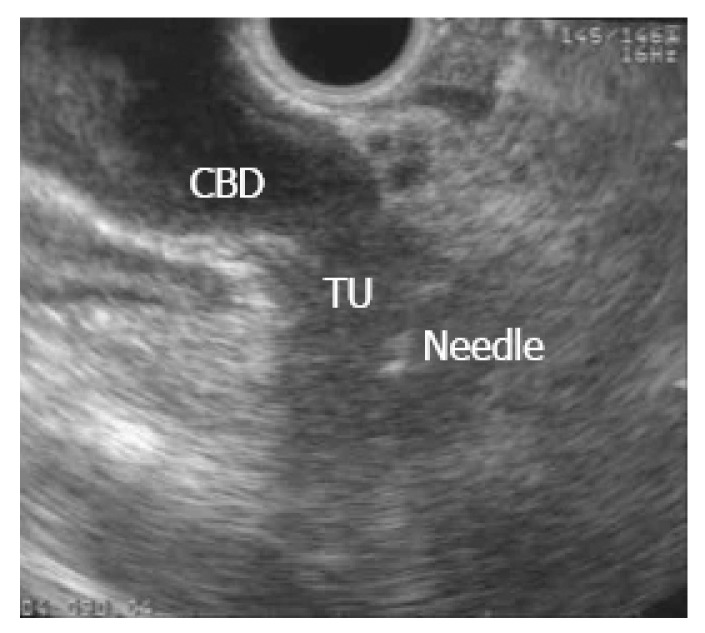
Endoscopic ultrasound guided fine-needle aspiration of pancreatic mass in a patient with painless jaundice.
EUS-FNA has excellent accuracy. Two recent meta-analyses reported a pooled sensitivity for the diagnosis of malignancy based on cytology of 85% and 89%, and a pooled specificity of 98% and 99%, respectively[21,22]. EUS-FNA of unresectable pancreatic cancer therefore is routinely performed where available but its use in patients with resectable pancreatic cancer remains controversial when neoadjuvant therapy is not planned.
EUS-FNA of pancreatic masses is overall a safe procedure. A recent systematic review by Wang et al[23] of 8246 patients with pancreatic lesions reported complications in 60 (0.82%) patients. Pancreatitis occurred in 36/8246 patients, of which 75% where mild. One patient with severe pancreatitis died, with an estimated pancreatitis-related mortality rate of 2.78%. The overall rate of pain, bleeding, fever and infection were 0.38%, 0.10%, 0.08% and 0.02% respectively.
Peritoneal seeding of tumor cells following EUS-FNA has been reported in up to 2.2% of patients but appears to be less than CT-guided FNA (16.3%)[24]. EUS-FNA did not increase the risk of peritoneal carcinomatosis in pancreatic masses in a comparison of 161 patients who underwent Endoscopic retrograde cholangiopancreatography (ERCP) alone with 56 who also underwent EUS-FNA[25]. Beane et al[26], compared overall and recurrence-free survival of patients with PDAC who underwent distal pancreatectomy, and found no difference between the 179 patients included who underwent preoperative EUS-FNA as compared with the 59 patients who did not. In addition, in a recent study, the risk of gastric/peritoneal recurrence after preoperative EUS-FNA was evaluated in 256 patients diagnosed with malignant pancreatic neoplasms who underwent surgery with curative intent, and it was found that EUS-FNA was not associated with increased needle track seeding[27].
Despite excellent accuracy and a low incidence of major complications, EUS-FNA of pancreatic masses has several limitations. Despite excellent sensitivity, negative predictive value of EUS-FNA for pancreatic tumor remains limited at 55%-65%[10,21]. Therefore, a negative or nondiagnostic FNA does not completely exclude the possibility of malignancy. Secondly, the presence of chronic pancreatitis decreases the diagnostic accuracy of EUS-FNA[28,29]. The presence of chronic pancreatitis may also hinder cytological interpretation of pancreatic FNA, decreasing sensitivity of EUS-FNA[30]. Third, EUS-FNA for pancreatic cancer has a false-positive rate of 1.1%, usually in patients with chronic pancreatitis[31].
The presence and experience of an on-site cytopathologist also impacts the accuracy of EUS-FNA[22,32] In a recent meta-analysis, which included 34 studies and 3644 patients, rapid on-site evaluation was a significant determinant of accuracy of EUS-FNA in the diagnosis of pancreatic masses[22]. The optimal number of EUS-FNA passes has been evaluated by 2 studies[32,33], which reported that at least 5-7 passes for pancreatic masses should be performed to maximize diagnostic yield. This information may prove helpful to endosonographers performing EUS-FNA when rapid pathology interpretation is unavailable.
A variety of commercially available FNA needles is available which range in size from 19 to 25 gauge (G). In a recent meta-analysis, 25-G needle was associated with a higher sensitivity but comparable specificity to the 22-G needle in 1292 patients with solid pancreatic lesions[34]. In another meta-analysis, 25-G needles appeared to have an advantage in adequacy of passes as compared to 22-G needles, without difference in accuracy, number of passes or complications[35]. Interestingly, 25-G needles were associated with less technical failures compared to 22-G needles when sampling pancreatic head and uncinate process lesions in some studies, and therefore should be considered first in those cases[36,37].
Due to its inherent rigidity, 19-G needles have been rarely used in the duodenum. Recently, a needle made of nitinol has been developed with enhanced flexibility to overcome these limitations (Flex 19, Boston Scientific, Natick, MA). The first report on the use of this needle included 38 patients, 32 of those with pancreatic head/uncinate lesions. Transduodenal FNA yielded adequate samples for cytological analysis in all 32 patients, without technical failures or procedure related complications[38].
EUS-FNA with use of DNA markers
In order to improve the diagnostic yield of EUS-FNA of pancreatic masses, analysis of abnormal genes in EUS-FNA samples is being investigated. The most studied marker is κ-ras. A prospective study including 394 pancreatic masses found that the combination of κ-ras mutation analysis with cytopathology increased the sensitivity of EUS-FNA from 87% to 93% and the accuracy from 89 to 94%[39]. Recently, a meta-analysis of 8 prospective studies (931 patients) assessing the accuracy of k-ras mutation analysis in the diagnosis of PDAC reported a pooled sensitivity and specificity of 77% and 93%, respectively. When combined with EUS-FNA alone, the addition of k-ras mutation testing improved sensitivity from 81% to 89% but decreased specificity from 97% to 92% for the diagnosis of PDAC. Among inconclusive EUS-FNA cases, k-ras mutation analysis reduced the false-negative rate by 56% and increased false positive rate by 11%[40]. The addition of other somatic mutations as p53 and p16 to K-ras mutation analysis has been shown to increase the sensitivity of PDAC detection to up to 100% in cases where FNA was inconclusive in one study[41]. Detection of chromosomal abnormalities by fluorescence in situ hybridization (FISH) analysis has also been recently investigated in the detection of pancreatic cancer. In combination with cytopathology, the use of FISH analysis to detect polysomy of chromosomes 3, 7, and 17 and deletion of 9p21 improves sensitivity of EUS-FNA from 61% to 85%[42]. Presently, in view of the high accuracy of standard FNA, together with elevated price and reduced availability of these genetic tests, it appears that its use in EUS-FNA samples should be limited to research protocols and in cases with inconclusive specimens.
EUS and staging of PDAC
Suspected malignant tumors of the pancreas should be assigned a TNM staging based on the most current American Joint Committee on Cancer staging classification, which describes the tumor extension (T), lymph node (N) and distant metastases (M) of tumors, respectively. If the tumor is limited to the pancreas, it is either a T1or T2 lesion. If the tumor is smaller than 2 cm it is a T1, if it is larger is a T2. In case the lesion extends beyond the pancreas, it is either a T3 or T4 lesion. Tumors extending to the celiac artery or superior mesenteric artery are considered T4 lesions, and tumors involving any other of the surrounding pancreatic structures as portal vein, ampulla or duodenal wall but the celiac or superior mesenteric artery are classified as T3. The distinction between T3 and T4 is important, as T4 lesions with involvement of celiac or superior mesenteric arteries is considered unresectable for curative intent. Reported accuracies of T staging by EUS range from 63%-94%[4-6,8,11,43-57]. Nodal (N) metastases are classified as absent (N0) or present (N1); including peripancreatic, gastro-hepatic or celiac malignant appearing lymph nodes. The accuracy of EUS for N-staging of pancreatic tumors ranges from 41%-86%[4-6,8,11,44,58]. Malignant echofeatures for detection of metastatic lymph nodes include size greater than 1 cm, hypoechoic echogenicity, sharp distinct margins, and round shape. If a lymph node has all four echofeatures, there is an 80%-100% chance of malignant invasion[59,60]. The sensitivity of EUS alone for the diagnosis of metastatic adenopathy in pancreatic PDAC is 28%-92%[5,6,44,49,51,52,54,55], however most report sensitivities under 65%. Metastatic lymph nodes that do not have all four endosonographic features described above[59] may therefore incorrectly assumed to be benign. Specificity of EUS alone for the diagnosis of metastatic adenopathy in PDAC is 26%-100%[5,6,44,49,51,52,54,55], however most report specificities above 70%. It is presumed that the addition of EUS-FNA of suspicious lymph nodes may increase specificity however there are little data that describe the impact of the addition of EUS-FNA to EUS alone. Routine EUS-FNA of peritumoral lymph nodes with pancreatic head cancers may not be necessary as those nodes are removed en-bloc with the surgical specimen. As presence of malignant celiac lymph nodes might preclude surgery, detailed survey of this region should be done at the time of preoperative EUS staging.
For detection of non-nodal metastatic cancer, CT and MRI are superior to EUS due to both anatomic limitations of normal gastrointestinal anatomy and the limited range of EUS imaging. Although the entire left and caudate hepatic lobes might be seen by EUS imaging in most patients, a portion of the right lobe may not be visualized by EUS. EUS clearly cannot replace but may supplement other modalities for staging of hepatic metastases. EUS might, however, detect and sample small hepatic lesions missed by other imaging modalities[61-63]. The sensitivity of EUS-FNA for benign and malignant liver masses reportedly ranges from 82%-94%[61,64] and the diagnosis of liver metastases from pancreatic cancer generally precludes surgical resection[64]. EUS may also identify and sample ascites either previously detected or undetected by other imaging studies[65,66]. Identification of malignant ascites and liver metastases by EUS-FNA is associated with poor survival following diagnosis[67]. Therefore, routine examination of the perigastric and duodenal spaces for ascites should be incorporated in the staging of every pancreatic mass.
THERAPEUTIC EUS APPLICATIONS
EUS and fiducials
Fiducials are inert radiographic markers implanted into a target tumoral lesion for both localization and tracking during image-guided radiation therapy (IGRT). This technique depends on reference points by which the lesion is identified and tracked during radiation therapy. Fiducials have been traditionally implanted by percutaneous or surgical approach. The use of EUS-guided fiducial placement was first described by Pishvaian et al[68] in a case series including 13 patients, 7 with PDAC. Technical success was achieved in 94%. Since then, several series reported successful EUS-guided implantation of fiducials in the pancreas (Figure 2), including more than 180 patients with technical success > 90%[38,69-73]. Reported complications were uncommon and included cholangitis in a case in which prophylactic antibiotics were not used), mild pancreatitis, minor bleeding and fiducial migration requiring repeat procedure.
Figure 2.
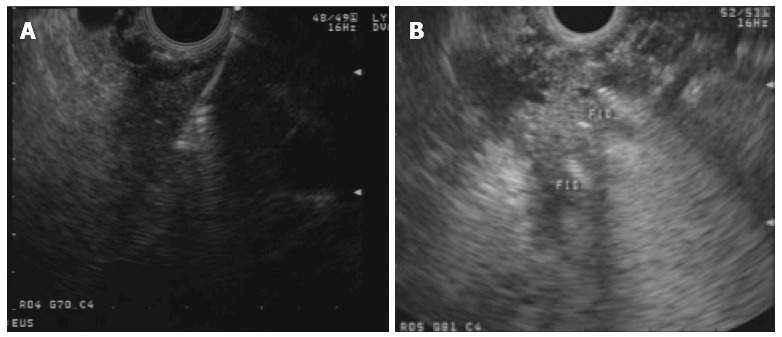
Endoscopic ultrasound guided deployment (A) and view (B) of fiducials in a pancreatic head mass.
Traditional fiducials are cylindrical gold seeds that can be loaded in a 19G needle. More modern coil design fiducials can be loaded into a 22G needle and in theory coil design might reduce migration. However, this was not confirmed in a recent retrospective series including 39 patients with PDAC (103 fiducials). In this study, comparison of both types of fiducials showed no difference in migration and in addition traditional cylindrical fiducials were significantly more visible during IGRT[73]. In summary, EUS placement of fiducials appears a feasible and safe technique; its practice, however, will depend on local availability of EUS expertise and IGRT.
EUS and brachytherapy
Instead of placing an inert radiologic marker, brachytherapy involves the insertion of a radioactive seed directly into the pancreatic tumor for localized therapy. Currently the most common radioactive seed used clinically is iodine-125, which has a half-time of 59.7 d and tissue penetration of 1.7 cm[74]. Currently there are only 3 case series reporting EUS-guided brachytherapy in PDAC. In the pilot study by Sun et al[75], in 15 patients with advanced PDAC median survival was 10.6 mo, with 27% partial response and a mean number of 22 iodine-125 seeds per patient. Technical success was 100%; local complications including pancreatitis and pseudocyst occurred in 3 patients, also hematological toxicity without clinical sequelae occurred in 3 patients. In the subsequent series by Jin et al[76], a median number of 10 seeds were placed in 22 patients with advanced PDAC. Dose calculation was based on tumor volume from reconstructed three dimensional CT images. Although placement under EUS guidance was successful in all patients with no major complications, only three achieved partial remission at 4 wk and no improvement in survival was shown. However, pain was significantly reduced 1 and 4 wk after the procedure. The most recent larger series by Du et al[77] included 100 patients with advanced PDAC who underwent brachytherapy with EUS guided implanted iodine-125 seeds. Pain scores dropped dramatically after one week post implantation, and maintained significant lower until the third month. The same group also used iodine-125 as a neurolytic agent in 23 patients undergoing EUS-guided CPN for unresectable PDAC[78]. At week 2.82% of patients had a reduction in pain score on a visual analogue scale and the mean narcotic consumption had decreased. This effect lasted until the study conclusion at 5-mo follow-up when only 2 patients were still alive. The authors postulate that iodine-125 may be a superior neurolytic agent compared to ethanol due to its longer half-life and deeper tissue penetration, although this has yet to be confirmed in a controlled clinical trial. The limited data so far for brachytherapy is encouraging, as it appears feasible and safe and might have some benefit in pain control in patients with locally advanced PDAC. Survival benefit, however, was not yet shown. Larger studies are needed to further evaluate this technique, including assessment of patient safety studies as well as safety of handling and storing radioactive material at endoscopy suites.
EUS-guided celiac plexus interventions
Patients with PDAC commonly develop abdominal pain that can be debilitating. Celiac plexus neurolysis (CPN) is a chemical splanchnicectomy of the celiac plexus that can be used to treat pain caused by PDAC. It can be performed by percutaneous, surgical or EUS-guided approach. EUS is well suited for identification of the celiac plexus due to the close approximation of the gastric wall with the origin of the celiac artery. EUS-CPN was first described in 1996 in 30 patients with intra-abdominal malignancy (25 with PDAC) who were treated with injection of bupivacaine and 98% absolute alcohol. Pain scores were significant lower compared with baseline at 2, 4, 8 and 12 wk after EUS-CPN[79]. Next, a prospective study including 58 patients with inoperable PDAC found that EUS-CPN provided significant decline in pain scores in 78% patients[80]. In a meta-analysis of randomized controlled trials of EUS-CPN for PDAC in 283 patients, Puli et al[81] reported 80% of patients experienced at least partial pain relief. Although the authors could not determine whether EUS-CPN reduced narcotic requirements due to heterogeneous reporting in the included studies, an earlier meta-analysis by Yan et al[82] reported a significant reduction in narcotic use with non-EUS guided CPN. Similar findings were reported in a more recent Cochrane meta-analysis which combined studies evaluating EUS-guided and percutaneous CPN[83]. In a double-blind, controlled trial by Wyse et al[84], which included 96 patients with advanced PDAC, early EUS-CPN provided greater pain relief as compared with conventional therapy at 1 mo and significantly greater at 3 mo. Morphine consumption was similar in both groups at 1 mo but tended toward lower consumption at 3 mo in the neurolysis group.
Over the past decade, advancements in echoendoscope designs have permitted the accurate identification of celiac ganglia and interest has developed in direct ganglia injection to improve the efficacy of CPN (Figure 3A and B)[85]. In a recent randomized controlled study, celiac ganglion neurolysis was more effective than celiac plexus neurolysis in relieving pain (73.5% vs 45.5%, respectively; P = 0.026)[86].
Figure 3.
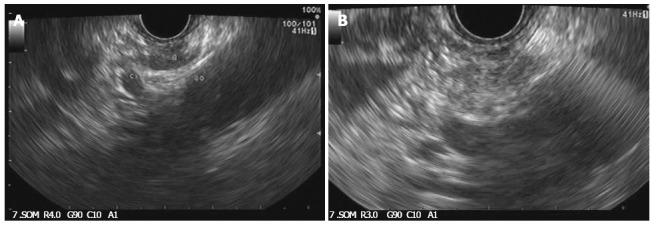
Celiac ganglia identified during endoscopic ultrasound (A) and injected during neurolysis under endoscopic ultrasound guidance (B).
EUS guided CPN is a safe procedure and complications are uncommon. Diarrhea (4%-15%) and orthostasis (1%) can occur due to disruption of the autonomic nervous system are usually mild and transient. A paradoxical increase in pain may occur in up to 9% of cases but generally resolves over several days[80]. Recently, serious complications including paralysis due to anterior spinal cord infarction[87,88], death from necrotic gastric perforation[89] or celiac artery thrombosis with infarction[90,91] have been reported.
EUS and fine needle injection
EUS-guided fine-needle injection (EUS-FNI) is an emerging method, which involves direct intra-tumoral delivery of therapeutic agents into pancreatic tumors under EUS guidance. This technique offers theoretic potential to deliver high dose concentration while minimizing systemic side effects.
In the pilot study by Chang et al[92], a single injection of allogeneic mixed lymphocyte culture (cytoimplant) was delivered by EUS-FNI in 8 patients with unresectable PDAC. The technique was feasible and not associated with substantial toxicity. In this series, median survival was 13.2 mo and two patients had partial response and one had a minor response. Subsequently, Hecht et al[93] reported the use of EUS-FNI of ONYX-015 (a gene-deleted replication-selective adenovirus that preferentially targets malignant cells) in 21 patients with locally advanced PDAC without significant liver metastasis. Patients underwent 8 sessions of EUS-FNI and the final treatments were given in combination with systemic gemcitabine. In this study, mean survival was 7.5 mo, and there were 2 partial regressions, 2 minor responses and 6 patients with stable disease. Nevertheless, there were serious complications including 2 duodenal perforations and 2 patients with sepsis, therefore limiting the use of EUS-FNI of this agent.
EUS-FNI of immature dendritic cells was reported by Irisawa et al[94] in a series with 7 patients with metastatic PDAC who previously failed gemcitabine. Mean survival was 9.9 mo. There were 3 partial responses, 2 patients with stable disease and no serious reported complications. Also Hanna et al[95] reported EUS-FNI of BC -819, a DNA plasmid that has the potential to treat PDAC that overexpresses H19 gene, in 6 patients with advanced PDAC. There were 3 partial responses and no serious reported complications.
TNFerade biologic is a replication-deficient adenoviral vector that expresses tumor necrosis factor α (TNF-α) under control of the Egr-1 promoter, which is inducible by chemotherapy and radiation In a phase I/II study, EUS or percutaneously guided intra-tumoral TNFerade biologic with 5-fluorouracil and radiotherapy was well tolerated and showed promising results in 50 patients with locally advanced PDAC[93]. Successively, a randomized multicenter trial, TNFerade biologic was compared with standard of care (SOC) in 304 patients with locally advanced PDAC. TNFerade was injected intratumorally by either EUS-guided approach or percutaneous transabdominal approach. Results showed that the addition of TNFerade to SOC was well tolerated, however did not prolong survival in patients with locally advanced PDAC. In addition, in the TNFerade arm of the study, multivariate analysis showed that TNFerade injection by EUS approach, rather than a percutaneous transabdominal approach was a risk factor for inferior progression- free survival. It is possible that greater variability existing in EUS operator skill across the participating institutions compared to the more straight forward percutaneous transabdominal approach technique might have resulted in reduced efficacy in the EUS group[96]. EUS-FNI although promising, up to now did not show noteworthy results in the treatment of PDAC.
EUS-guided biliary drainage
ERCP is the procedure of choice for bile duct stenting in obstructive jaundice in patients with advanced PDAC. When ERCP is not possible due to failed cannulation, altered upper gastrointestinal tract anatomy, a distorted ampulla, gastric outlet obstruction, a periampullary diverticulum or in situ enteral stents, EUS-guided biliary drainage (EGBD) has been used as a minimally invasive alternative to surgical biliary bypass or percutaneous transhepatic biliary drainage (PTBD)[97].
Two main approaches for EGBD have been used: direct transluminal stenting (hepaticogastrostomy or choledochoduodenoscopy, without accessing the papilla) and a rendezvous technique (wire placed into intrahepatic or extrahepatic biliary duct, passed through the papilla and retrieved by a duodenoscopy for biliary interventions). A third approach, EUS-guided antegrade transpapillary biliary stent placement, has also been described[98,99]. Case-series from expert tertiary centers suggest that EGBD can be performed with high therapeutic success (87%) but is associated with 10%-20% mild to moderate morbidity and rare serious adverse events[97,100-110]. Rendezvous technique appears to be the safest[110,111], however can only be attempted in whom the papilla or choledocho-enteric anastomosis is accessible by endoscopy. In addition, rendezvous biliary drainage either fail or is not possible in at least 25% of patients, is associated with prolonged procedure times and may lead to acute pancreatitis[97,102,107,108]. Transluminal stenting can be complicated by stent migration or occlusion, bile leak, cholangitis, hemobilia, pneumoperitoneum and bile peritonitis[104-106,111,112]. EUS-guided hepaticogastrostomy is potentially applicable to patients with duodenal obstruction or prior gastric surgery, however it can only be attempted when the left intra-hepatic system is dilated[111]. EUS-guided choledochoduodenostomy can be attempted only in patients with a native anatomy (intact duodenal bulb) and an intact biliary tree[112]. EGBD by using either rendezvous or directly transluminal technique requires needle puncture via an intrahepatic or an extrahepatic route in an non-obstructed patient with normal upper GI anatomy. It appears that extrahepatic route is preferable and safer than intrahepatic access, whether EGBD is performed by rendezvous or direct transluminal stenting[97,105,109,110].
Recently Park et al[110] reported a single-operator, non-randomized prospective study evaluating technical and functional success and adverse event rate of a treatment algorithm using a modified technique of “enhanced guidewire manipulation” for EGBD, performed at same-session after failed ERCP in 45 patients with malignant or benign biliary obstruction. Results of this approach showed a technical and functional success of 95% and overall adverse event rate of 11%, including pancreatitis, focal bile peritonitis, limited pneumoperitoneum, intraperitoneal stent migration and biloma.
Artifon et al[113] reported the first prospective randomized comparison between EGBD (choledochoduodenoscopy) and PTBD, in 25 patients with unresectable malignant biliary obstruction who failed ERCP (13 patients EGDB vs 12 patients in the PTBD group). In this small study, both groups had similar technical and clinical success, complication rate, cost and quality of life.
EGBD is a safe and effective alternative after failed ERCP, whether performed by rendezvous or direct luminal stenting. Although limited data suggests equivalency to PTBD, larger studies are needed to confirm those results. EGBD ideally should be performed by high skilled endoscopists trained in both ERCP and EUS, and should be limited to expert tertiary centers, where surgery and radiology back-up are available in case of adverse events.
CONCLUSION
EUS and EUS-FNA are highly accurate modalities for detection, diagnosis and staging of PDAC. This high accuracy is decreased, however in specific situations most notably in the presence of chronic pancreatitis. Newly techniques including contrast-enhanced EUS, elastography and detection of DNA markers are in progress and might improve the accuracy of EUS in the detection of PDAC in the setting of chronic pancreatitis. EUS and EUS FNA have recently progressed from a diagnostic to a therapeutic technique in the management of PDAC. Evolving therapeutic applications include celiac plexus interventions, fiducial and brachytherapy seeds placement, fine needle injection and EUS-guided biliary drainage. The future role of EUS and EUS in management of PDAC is still emerging.
Footnotes
P- Reviewers: Dai ZJ, Kawakubo K, Verbeke CS S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–173. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 2.Lin JT, Wang JT, Wang TH. The diagnostic value of endoscopic ultrasonography in pancreatic disorders. Taiwan Yi Xue Hui Za Zhi. 1989;88:483–487. [PubMed] [Google Scholar]
- 3.Rösch T, Lorenz R, Braig C, Feuerbach S, Siewert JR, Schusdziarra V, Classen M. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347–352. doi: 10.1016/s0016-5107(91)70729-3. [DOI] [PubMed] [Google Scholar]
- 4.Rösch T, Braig C, Gain T, Feuerbach S, Siewert JR, Schusdziarra V, Classen M. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology. 1992;102:188–199. doi: 10.1016/0016-5085(92)91800-j. [DOI] [PubMed] [Google Scholar]
- 5.Palazzo L, Roseau G, Gayet B, Vilgrain V, Belghiti J, Fékéte F, Paolaggi JA. Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma. Results of a prospective study with comparison to ultrasonography and CT scan. Endoscopy. 1993;25:143–150. doi: 10.1055/s-2007-1010273. [DOI] [PubMed] [Google Scholar]
- 6.Müller MF, Meyenberger C, Bertschinger P, Schaer R, Marincek B. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994;190:745–751. doi: 10.1148/radiology.190.3.8115622. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama M, Hagi H, Atomi Y, Saito M. Diagnosis of portal venous invasion by pancreatobiliary carcinoma: value of endoscopic ultrasonography. Abdom Imaging. 1997;22:434–438. doi: 10.1007/s002619900227. [DOI] [PubMed] [Google Scholar]
- 8.Gress FG, Hawes RH, Savides TJ, Ikenberry SO, Cummings O, Kopecky K, Sherman S, Wiersema M, Lehman GA. Role of EUS in the preoperative staging of pancreatic cancer: a large single-center experience. Gastrointest Endosc. 1999;50:786–791. doi: 10.1016/s0016-5107(99)70159-8. [DOI] [PubMed] [Google Scholar]
- 9.Ainsworth AP, Rafaelsen SR, Wamberg PA, Durup J, Pless TK, Mortensen MB. Is there a difference in diagnostic accuracy and clinical impact between endoscopic ultrasonography and magnetic resonance cholangiopancreatography? Endoscopy. 2003;35:1029–1032. doi: 10.1055/s-2003-44603. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal B, Abu-Hamda E, Molke KL, Correa AM, Ho L. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol. 2004;99:844–850. doi: 10.1111/j.1572-0241.2004.04177.x. [DOI] [PubMed] [Google Scholar]
- 11.DeWitt J, Devereaux B, Chriswell M, McGreevy K, Howard T, Imperiale TF, Ciaccia D, Lane KA, Maglinte D, Kopecky K, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753–763. doi: 10.7326/0003-4819-141-10-200411160-00006. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Shpaner A, Krishna SG, Ross WA, Bhutani MS, Tamm EP, Raju GS, Xiao L, Wolff RA, Fleming JB, et al. Use of EUS-FNA in diagnosing pancreatic neoplasm without a definitive mass on CT. Gastrointest Endosc. 2013;78:73–80. doi: 10.1016/j.gie.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Bhutani MS, Gress FG, Giovannini M, Erickson RA, Catalano MF, Chak A, Deprez PH, Faigel DO, Nguyen CC. The No Endosonographic Detection of Tumor (NEST) Study: a case series of pancreatic cancers missed on endoscopic ultrasonography. Endoscopy. 2004;36:385–389. doi: 10.1055/s-2004-814320. [DOI] [PubMed] [Google Scholar]
- 14.Catanzaro A, Richardson S, Veloso H, Isenberg GA, Wong RC, Sivak MV, Chak A. Long-term follow-up of patients with clinically indeterminate suspicion of pancreatic cancer and normal EUS. Gastrointest Endosc. 2003;58:836–840. doi: 10.1016/s0016-5107(03)02301-0. [DOI] [PubMed] [Google Scholar]
- 15.Ranney N, Phadnis M, Trevino J, Ramesh J, Wilcox CM, Varadarajulu S. Impact of biliary stents on EUS-guided FNA of pancreatic mass lesions. Gastrointest Endosc. 2012;76:76–83. doi: 10.1016/j.gie.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong TT, Hu DM, Zhu Q. Contrast-enhanced EUS for differential diagnosis of pancreatic mass lesions: a meta-analysis. Gastrointest Endosc. 2012;76:301–309. doi: 10.1016/j.gie.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 17.Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–134. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 18.Hu DM, Gong TT, Zhu Q. Endoscopic ultrasound elastography for differential diagnosis of pancreatic masses: a meta-analysis. Dig Dis Sci. 2013;58:1125–1131. doi: 10.1007/s10620-012-2428-5. [DOI] [PubMed] [Google Scholar]
- 19.Mei M, Ni J, Liu D, Jin P, Sun L. EUS elastography for diagnosis of solid pancreatic masses: a meta-analysis. Gastrointest Endosc. 2013;77:578–589. doi: 10.1016/j.gie.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Săftoiu A, Iordache SA, Gheonea DI, Popescu C, Maloş A, Gorunescu F, Ciurea T, Iordache A, Popescu GL, Manea CT. Combined contrast-enhanced power Doppler and real-time sonoelastography performed during EUS, used in the differential diagnosis of focal pancreatic masses (with videos) Gastrointest Endosc. 2010;72:739–747. doi: 10.1016/j.gie.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 21.Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319–331. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 22.Hébert-Magee S, Bae S, Varadarajulu S, Ramesh J, Frost AR, Eloubeidi MA, Eltoum IA. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013;24:159–171. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, Li ZS. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283–290. doi: 10.1016/j.gie.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 24.Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690–695. doi: 10.1016/s0016-5107(03)02009-1. [DOI] [PubMed] [Google Scholar]
- 25.Ikezawa K, Uehara H, Sakai A, Fukutake N, Imanaka K, Ohkawa K, Tanakura R, Ioka T, Tanaka S, Ishikawa O, et al. Risk of peritoneal carcinomatosis by endoscopic ultrasound-guided fine needle aspiration for pancreatic cancer. J Gastroenterol. 2013;48:966–972. doi: 10.1007/s00535-012-0693-x. [DOI] [PubMed] [Google Scholar]
- 26.Beane JD, House MG, Coté GA, DeWitt JM, Al-Haddad M, LeBlanc JK, McHenry L, Sherman S, Schmidt CM, Zyromski NJ, et al. Outcomes after preoperative endoscopic ultrasonography and biopsy in patients undergoing distal pancreatectomy. Surgery. 2011;150:844–853. doi: 10.1016/j.surg.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 27.Ngamruengphong S, Xu C, Woodward TA, Raimondo M, Stauffer JA, Asbun HJ, Wallace MB. Risk of gastric or peritoneal recurrence, and long-term outcomes, following pancreatic cancer resection with preoperative endosonographically guided fine needle aspiration. Endoscopy. 2013;45:619–626. doi: 10.1055/s-0033-1344216. [DOI] [PubMed] [Google Scholar]
- 28.Fritscher-Ravens A, Brand L, Knöfel WT, Bobrowski C, Topalidis T, Thonke F, de Werth A, Soehendra N. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768–2775. doi: 10.1111/j.1572-0241.2002.07020.x. [DOI] [PubMed] [Google Scholar]
- 29.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–36; quiz 751, 753. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz DA, Unni KK, Levy MJ, Clain JE, Wiersema MJ. The rate of false-positive results with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2002;56:868–872. doi: 10.1067/mge.2002.129610. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui AA, Kowalski TE, Shahid H, O’Donnell S, Tolin J, Loren DE, Infantolino A, Hong SK, Eloubeidi MA. False-positive EUS-guided FNA cytology for solid pancreatic lesions. Gastrointest Endosc. 2011;74:535–540. doi: 10.1016/j.gie.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 32.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–190. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 33.LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, Vallery S, DeWitt J, Sherman S, Collins E. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–481. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 34.Madhoun MF, Wani SB, Rastogi A, Early D, Gaddam S, Tierney WM, Maple JT. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: a meta-analysis. Endoscopy. 2013;45:86–92. doi: 10.1055/s-0032-1325992. [DOI] [PubMed] [Google Scholar]
- 35.Affolter KE, Schmidt RL, Matynia AP, Adler DG, Factor RE. Needle size has only a limited effect on outcomes in EUS-guided fine needle aspiration: a systematic review and meta-analysis. Dig Dis Sci. 2013;58:1026–1034. doi: 10.1007/s10620-012-2439-2. [DOI] [PubMed] [Google Scholar]
- 36.Sakamoto H, Kitano M, Komaki T, Noda K, Chikugo T, Dote K, Takeyama Y, Das K, Yamao K, Kudo M. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384–390. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 37.Camellini L, Carlinfante G, Azzolini F, Iori V, Cavina M, Sereni G, Decembrino F, Gallo C, Tamagnini I, Valli R, et al. A randomized clinical trial comparing 22G and 25G needles in endoscopic ultrasound-guided fine-needle aspiration of solid lesions. Endoscopy. 2011;43:709–715. doi: 10.1055/s-0030-1256482. [DOI] [PubMed] [Google Scholar]
- 38.Varadarajulu S, Bang JY, Hebert-Magee S. Assessment of the technical performance of the flexible 19-gauge EUS-FNA needle. Gastrointest Endosc. 2012;76:336–343. doi: 10.1016/j.gie.2012.04.455. [DOI] [PubMed] [Google Scholar]
- 39.Ogura T, Yamao K, Sawaki A, Mizuno N, Hara K, Hijioka S, Niwa Y, Tajika M, Kondo S, Shimizu Y, et al. Clinical impact of K-ras mutation analysis in EUS-guided FNA specimens from pancreatic masses. Gastrointest Endosc. 2012;75:769–774. doi: 10.1016/j.gie.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Fuccio L, Hassan C, Laterza L, Correale L, Pagano N, Bocus P, Fabbri C, Maimone A, Cennamo V, Repici A, et al. The role of K-ras gene mutation analysis in EUS-guided FNA cytology specimens for the differential diagnosis of pancreatic solid masses: a meta-analysis of prospective studies. Gastrointest Endosc. 2013;78:596–608. doi: 10.1016/j.gie.2013.04.162. [DOI] [PubMed] [Google Scholar]
- 41.Salek C, Benesova L, Zavoral M, Nosek V, Kasperova L, Ryska M, Strnad R, Traboulsi E, Minarik M. Evaluation of clinical relevance of examining K-ras, p16 and p53 mutations along with allelic losses at 9p and 18q in EUS-guided fine needle aspiration samples of patients with chronic pancreatitis and pancreatic cancer. World J Gastroenterol. 2007;13:3714–3720. doi: 10.3748/wjg.v13.i27.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubiliun N, Ribeiro A, Fan YS, Rocha-Lima CM, Sleeman D, Merchan J, Barkin J, Levi J. EUS-FNA with rescue fluorescence in situ hybridization for the diagnosis of pancreatic carcinoma in patients with inconclusive on-site cytopathology results. Gastrointest Endosc. 2011;74:541–547. doi: 10.1016/j.gie.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad NA, Lewis JD, Siegelman ES, Rosato EF, Ginsberg GG, Kochman ML. Role of endoscopic ultrasound and magnetic resonance imaging in the preoperative staging of pancreatic adenocarcinoma. Am J Gastroenterol. 2000;95:1926–1931. doi: 10.1111/j.1572-0241.2000.02245.x. [DOI] [PubMed] [Google Scholar]
- 44.Akahoshi K, Chijiiwa Y, Nakano I, Nawata H, Ogawa Y, Tanaka M, Nagai E, Tsuneyoshi M. Diagnosis and staging of pancreatic cancer by endoscopic ultrasound. Br J Radiol. 1998;71:492–496. doi: 10.1259/bjr.71.845.9691893. [DOI] [PubMed] [Google Scholar]
- 45.Buscail L, Pagès P, Berthélemy P, Fourtanier G, Frexinos J, Escourrou J. Role of EUS in the management of pancreatic and ampullary carcinoma: a prospective study assessing resectability and prognosis. Gastrointest Endosc. 1999;50:34–40. doi: 10.1016/s0016-5107(99)70341-x. [DOI] [PubMed] [Google Scholar]
- 46.Giovannini M, Seitz JF. Endoscopic ultrasonography with a linear-type echoendoscope in the evaluation of 94 patients with pancreatobiliary disease. Endoscopy. 1994;26:579–585. doi: 10.1055/s-2007-1009043. [DOI] [PubMed] [Google Scholar]
- 47.Grimm H, Maydeo A, Soehendra N. Endoluminal ultrasound for the diagnosis and staging of pancreatic cancer. Baillieres Clin Gastroenterol. 1990;4:869–888. doi: 10.1016/0950-3528(90)90024-b. [DOI] [PubMed] [Google Scholar]
- 48.Legmann P, Vignaux O, Dousset B, Baraza AJ, Palazzo L, Dumontier I, Coste J, Louvel A, Roseau G, Couturier D, et al. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol. 1998;170:1315–1322. doi: 10.2214/ajr.170.5.9574609. [DOI] [PubMed] [Google Scholar]
- 49.Midwinter MJ, Beveridge CJ, Wilsdon JB, Bennett MK, Baudouin CJ, Charnley RM. Correlation between spiral computed tomography, endoscopic ultrasonography and findings at operation in pancreatic and ampullary tumours. Br J Surg. 1999;86:189–193. doi: 10.1046/j.1365-2168.1999.01042.x. [DOI] [PubMed] [Google Scholar]
- 50.Mukai H, Nakajima M, Yasuda K, Cho E, Mizuma Y, Hayakumo T, Ashihara T, Mizuno S, Hirano S, Ikeda E. [Preoperative diagnosis and staging of pancreatic cancer by endoscopic ultrasonography (EUS)--a comparative study with other diagnostic tools] Nihon Shokakibyo Gakkai Zasshi. 1991;88:2132–2142. [PubMed] [Google Scholar]
- 51.Ramsay D, Marshall M, Song S, Zimmerman M, Edmunds S, Yusoff I, Cullingford G, Fletcher D, Mendelson R. Identification and staging of pancreatic tumours using computed tomography, endoscopic ultrasound and mangafodipir trisodium-enhanced magnetic resonance imaging. Australas Radiol. 2004;48:154–161. doi: 10.1111/j.1440-1673.2004.01277.x. [DOI] [PubMed] [Google Scholar]
- 52.Rivadeneira DE, Pochapin M, Grobmyer SR, Lieberman MD, Christos PJ, Jacobson I, Daly JM. Comparison of linear array endoscopic ultrasound and helical computed tomography for the staging of periampullary malignancies. Ann Surg Oncol. 2003;10:890–897. doi: 10.1245/aso.2003.03.555. [DOI] [PubMed] [Google Scholar]
- 53.Rösch T, Dittler HJ, Lorenz R, Braig C, Gain T, Feuerbach S, Höfler H, Siewert JR, Classen M. [The endosonographic staging of pancreatic carcinoma] Dtsch Med Wochenschr. 1992;117:563–569. doi: 10.1055/s-2008-1062347. [DOI] [PubMed] [Google Scholar]
- 54.Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Ginès MA, Real MI, Gilabert R, Quintó L, Trilla A, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492–501. doi: 10.1111/j.1572-0241.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 55.Tio TL, Sie LH, Kallimanis G, Luiken GJ, Kimmings AN, Huibregtse K, Tytgat GN. Staging of ampullary and pancreatic carcinoma: comparison between endosonography and surgery. Gastrointest Endosc. 1996;44:706–713. doi: 10.1016/s0016-5107(96)70056-1. [DOI] [PubMed] [Google Scholar]
- 56.Tio TL, Tytgat GN, Cikot RJ, Houthoff HJ, Sars PR. Ampullopancreatic carcinoma: preoperative TNM classification with endosonography. Radiology. 1990;175:455–461. doi: 10.1148/radiology.175.2.2183284. [DOI] [PubMed] [Google Scholar]
- 57.Yasuda K, Mukai H, Nakajima M, Kawai K. Staging of pancreatic carcinoma by endoscopic ultrasonography. Endoscopy. 1993;25:151–155. doi: 10.1055/s-2007-1010274. [DOI] [PubMed] [Google Scholar]
- 58.Ahmad NA, Lewis JD, Ginsberg GG, Rosato EF, Morris JB, Kochman ML. EUS in preoperative staging of pancreatic cancer. Gastrointest Endosc. 2000;52:463–468. doi: 10.1067/mge.2000.107725. [DOI] [PubMed] [Google Scholar]
- 59.Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474–479. doi: 10.1016/s0016-5107(97)70176-7. [DOI] [PubMed] [Google Scholar]
- 60.Catalano MF, Sivak MV, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442–446. doi: 10.1016/s0016-5107(94)70206-3. [DOI] [PubMed] [Google Scholar]
- 61.Hollerbach S, Willert J, Topalidis T, Reiser M, Schmiegel W. Endoscopic ultrasound-guided fine-needle aspiration biopsy of liver lesions: histological and cytological assessment. Endoscopy. 2003;35:743–749. doi: 10.1055/s-2003-41593. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen P, Feng JC, Chang KJ. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) of liver lesions. Gastrointest Endosc. 1999;50:357–361. doi: 10.1053/ge.1999.v50.97208. [DOI] [PubMed] [Google Scholar]
- 63.tenBerge J, Hoffman BJ, Hawes RH, Van Enckevort C, Giovannini M, Erickson RA, Catalano MF, Fogel R, Mallery S, Faigel DO, et al. EUS-guided fine needle aspiration of the liver: indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859–862. doi: 10.1067/mge.2002.124557. [DOI] [PubMed] [Google Scholar]
- 64.DeWitt J, LeBlanc J, McHenry L, Ciaccia D, Imperiale T, Chappo J, Cramer H, McGreevy K, Chriswell M, Sherman S. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: a large single-center experience. Am J Gastroenterol. 2003;98:1976–1981. doi: 10.1111/j.1572-0241.2003.07638.x. [DOI] [PubMed] [Google Scholar]
- 65.Chang KJ, Albers CG, Nguyen P. Endoscopic ultrasound-guided fine needle aspiration of pleural and ascitic fluid. Am J Gastroenterol. 1995;90:148–150. [PubMed] [Google Scholar]
- 66.Nguyen PT, Chang KJ. EUS in the detection of ascites and EUS-guided paracentesis. Gastrointest Endosc. 2001;54:336–339. doi: 10.1067/mge.2001.117544. [DOI] [PubMed] [Google Scholar]
- 67.DeWitt J, Yu M, Al-Haddad MA, Sherman S, McHenry L, Leblanc JK. Survival in patients with pancreatic cancer after the diagnosis of malignant ascites or liver metastases by EUS-FNA. Gastrointest Endosc. 2010;71:260–265. doi: 10.1016/j.gie.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 68.Pishvaian AC, Collins B, Gagnon G, Ahlawat S, Haddad NG. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412–417. doi: 10.1016/j.gie.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 69.Sanders MK, Moser AJ, Khalid A, Fasanella KE, Zeh HJ, Burton S, McGrath K. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc. 2010;71:1178–1184. doi: 10.1016/j.gie.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 70.Park WG, Yan BM, Schellenberg D, Kim J, Chang DT, Koong A, Patalano C, Van Dam J. EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest Endosc. 2010;71:513–518. doi: 10.1016/j.gie.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 71.DiMaio CJ, Nagula S, Goodman KA, Ho AY, Markowitz AJ, Schattner MA, Gerdes H. EUS-guided fiducial placement for image-guided radiation therapy in GI malignancies by using a 22-gauge needle (with videos) Gastrointest Endosc. 2010;71:1204–1210. doi: 10.1016/j.gie.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Ammar T, Coté GA, Creach KM, Kohlmeier C, Parikh PJ, Azar RR. Fiducial placement for stereotactic radiation by using EUS: feasibility when using a marker compatible with a standard 22-gauge needle. Gastrointest Endosc. 2010;71:630–633. doi: 10.1016/j.gie.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 73.Khashab MA, Kim KJ, Tryggestad EJ, Wild AT, Roland T, Singh VK, Lennon AM, Shin EJ, Ziegler MA, Sharaiha RZ, et al. Comparative analysis of traditional and coiled fiducials implanted during EUS for pancreatic cancer patients receiving stereotactic body radiation therapy. Gastrointest Endosc. 2012;76:962–971. doi: 10.1016/j.gie.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin Z, Chang KJ. Endoscopic ultrasound-guided fiducial markers and brachytherapy. Gastrointest Endosc Clin N Am. 2012;22:325–31, x. doi: 10.1016/j.giec.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 75.Sun S, Xu H, Xin J, Liu J, Guo Q, Li S. Endoscopic ultrasound-guided interstitial brachytherapy of unresectable pancreatic cancer: results of a pilot trial. Endoscopy. 2006;38:399–403. doi: 10.1055/s-2006-925253. [DOI] [PubMed] [Google Scholar]
- 76.Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40:314–320. doi: 10.1055/s-2007-995476. [DOI] [PubMed] [Google Scholar]
- 77.Du Y, Jin Z, Meng H, Zou D, Chen J, Liu Y, Zhan X, Wang D, Liao Z, Li Z. Long-term effect of gemcitabine-combined endoscopic ultrasonography-guided brachytherapy in pancreatic cancer. J Interv Gastroenterol. 2013;3:18–24. [Google Scholar]
- 78.Wang KX, Jin ZD, Du YQ, Zhan XB, Zou DW, Liu Y, Wang D, Chen J, Xu C, Li ZS. EUS-guided celiac ganglion irradiation with iodine-125 seeds for pain control in pancreatic carcinoma: a prospective pilot study. Gastrointest Endosc. 2012;76:945–952. doi: 10.1016/j.gie.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 79.Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656–662. doi: 10.1016/s0016-5107(96)70047-0. [DOI] [PubMed] [Google Scholar]
- 80.Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316–324. doi: 10.1067/mge.2001.117515. [DOI] [PubMed] [Google Scholar]
- 81.Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330–2337. doi: 10.1007/s10620-008-0651-x. [DOI] [PubMed] [Google Scholar]
- 82.Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol. 2007;102:430–438. doi: 10.1111/j.1572-0241.2006.00967.x. [DOI] [PubMed] [Google Scholar]
- 83.Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011;(3):CD007519. doi: 10.1002/14651858.CD007519.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541–3546. doi: 10.1200/JCO.2010.32.2750. [DOI] [PubMed] [Google Scholar]
- 85.Levy MJ, Topazian MD, Wiersema MJ, Clain JE, Rajan E, Wang KK, de la Mora JG, Gleeson FC, Pearson RK, Pelaez MC, et al. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct Ganglia neurolysis and block. Am J Gastroenterol. 2008;103:98–103. doi: 10.1111/j.1572-0241.2007.01607.x. [DOI] [PubMed] [Google Scholar]
- 86.Doi S, Yasuda I, Kawakami H, Hayashi T, Hisai H, Irisawa A, Mukai T, Katanuma A, Kubota K, Ohnishi T, et al. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy. 2013;45:362–369. doi: 10.1055/s-0032-1326225. [DOI] [PubMed] [Google Scholar]
- 87.Fujii L, Clain JE, Morris JM, Levy MJ. Anterior spinal cord infarction with permanent paralysis following endoscopic ultrasound celiac plexus neurolysis. Endoscopy. 2012;44 Suppl 2 UCTN:E265–E266. doi: 10.1055/s-0032-1309708. [DOI] [PubMed] [Google Scholar]
- 88.Mittal MK, Rabinstein AA, Wijdicks EF. Pearls & amp; oy-sters: Acute spinal cord infarction following endoscopic ultrasound-guided celiac plexus neurolysis. Neurology. 2012;78:e57–e59. doi: 10.1212/WNL.0b013e318248df51. [DOI] [PubMed] [Google Scholar]
- 89.Loeve US, Mortensen MB. Lethal necrosis and perforation of the stomach and the aorta after multiple EUS-guided celiac plexus neurolysis procedures in a patient with chronic pancreatitis. Gastrointest Endosc. 2013;77:151–152. doi: 10.1016/j.gie.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 90.Gimeno-García AZ, Elwassief A, Paquin SC, Sahai AV. Fatal complication after endoscopic ultrasound-guided celiac plexus neurolysis. Endoscopy. 2012;44 Suppl 2 UCTN:E267. doi: 10.1055/s-0032-1309709. [DOI] [PubMed] [Google Scholar]
- 91.Jang HY, Cha SW, Lee BH, Jung HE, Choo JW, Cho YJ, Ju HY, Cho YD. Hepatic and splenic infarction and bowel ischemia following endoscopic ultrasound-guided celiac plexus neurolysis. Clin Endosc. 2013;46:306–309. doi: 10.5946/ce.2013.46.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang KJ, Nguyen PT, Thompson JA, Kurosaki TT, Casey LR, Leung EC, Granger GA. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer. 2000;88:1325–1335. doi: 10.1002/(sici)1097-0142(20000315)88:6<1325::aid-cncr8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 93.Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–561. [PubMed] [Google Scholar]
- 94.Irisawa A, Takagi T, Kanazawa M, Ogata T, Sato Y, Takenoshita S, Ohto H, Ohira H. Endoscopic ultrasound-guided fine-needle injection of immature dendritic cells into advanced pancreatic cancer refractory to gemcitabine: a pilot study. Pancreas. 2007;35:189–190. doi: 10.1097/01.mpa.0000250141.25639.e9. [DOI] [PubMed] [Google Scholar]
- 95.Hanna N, Ohana P, Konikoff FM, Leichtmann G, Hubert A, Appelbaum L, Kopelman Y, Czerniak A, Hochberg A. Phase 1/2a, dose-escalation, safety, pharmacokinetic and preliminary efficacy study of intratumoral administration of BC-819 in patients with unresectable pancreatic cancer. Cancer Gene Ther. 2012;19:374–381. doi: 10.1038/cgt.2012.10. [DOI] [PubMed] [Google Scholar]
- 96.Herman JM, Wild AT, Wang H, Tran PT, Chang KJ, Taylor GE, Donehower RC, Pawlik TM, Ziegler MA, Cai H, et al. Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J Clin Oncol. 2013;31:886–894. doi: 10.1200/JCO.2012.44.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khashab MA, Dewitt J. EUS-guided biliary drainage: is it ready for prime time? Yes! Gastrointest Endosc. 2013;78:102–105. doi: 10.1016/j.gie.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 98.Nguyen-Tang T, Binmoeller KF, Sanchez-Yague A, Shah JN. Endoscopic ultrasound (EUS)-guided transhepatic anterograde self-expandable metal stent (SEMS) placement across malignant biliary obstruction. Endoscopy. 2010;42:232–236. doi: 10.1055/s-0029-1243858. [DOI] [PubMed] [Google Scholar]
- 99.Artifon EL, Safatle-Ribeiro AV, Ferreira FC, Poli-de-Figueiredo L, Rasslan S, Carnevale F, Otoch JP, Sakai P, Kahaleh M. EUS-guided antegrade transhepatic placement of a self-expandable metal stent in hepatico-jejunal anastomosis. JOP. 2011;12:610–613. [PubMed] [Google Scholar]
- 100.Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52–59. doi: 10.1016/j.gie.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 101.Maranki J, Hernandez AJ, Arslan B, Jaffan AA, Angle JF, Shami VM, Kahaleh M. Interventional endoscopic ultrasound-guided cholangiography: long-term experience of an emerging alternative to percutaneous transhepatic cholangiography. Endoscopy. 2009;41:532–538. doi: 10.1055/s-0029-1214712. [DOI] [PubMed] [Google Scholar]
- 102.Kim YS, Gupta K, Mallery S, Li R, Kinney T, Freeman ML. Endoscopic ultrasound rendezvous for bile duct access using a transduodenal approach: cumulative experience at a single center. A case series. Endoscopy. 2010;42:496–502. doi: 10.1055/s-0029-1244082. [DOI] [PubMed] [Google Scholar]
- 103.Fabbri C, Luigiano C, Fuccio L, Polifemo AM, Ferrara F, Ghersi S, Bassi M, Billi P, Maimone A, Cennamo V, et al. EUS-guided biliary drainage with placement of a new partially covered biliary stent for palliation of malignant biliary obstruction: a case series. Endoscopy. 2011;43:438–441. doi: 10.1055/s-0030-1256097. [DOI] [PubMed] [Google Scholar]
- 104.Komaki T, Kitano M, Sakamoto H, Kudo M. Endoscopic ultrasonography-guided biliary drainage: evaluation of a choledochoduodenostomy technique. Pancreatology. 2011;11 Suppl 2:47–51. doi: 10.1159/000323508. [DOI] [PubMed] [Google Scholar]
- 105.Park do H, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276–1284. doi: 10.1016/j.gie.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 106.Hara K, Yamao K, Niwa Y, Sawaki A, Mizuno N, Hijioka S, Tajika M, Kawai H, Kondo S, Kobayashi Y, et al. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol. 2011;106:1239–1245. doi: 10.1038/ajg.2011.84. [DOI] [PubMed] [Google Scholar]
- 107.Iwashita T, Lee JG, Shinoura S, Nakai Y, Park DH, Muthusamy VR, Chang KJ. Endoscopic ultrasound-guided rendezvous for biliary access after failed cannulation. Endoscopy. 2012;44:60–65. doi: 10.1055/s-0030-1256871. [DOI] [PubMed] [Google Scholar]
- 108.Shah JN, Marson F, Weilert F, Bhat YM, Nguyen-Tang T, Shaw RE, Binmoeller KF. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest Endosc. 2012;75:56–64. doi: 10.1016/j.gie.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 109.Dhir V, Bhandari S, Bapat M, Maydeo A. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos) Gastrointest Endosc. 2012;75:354–359. doi: 10.1016/j.gie.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 110.Park do H, Jeong SU, Lee BU, Lee SS, Seo DW, Lee SK, Kim MH. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video) Gastrointest Endosc. 2013;78:91–101. doi: 10.1016/j.gie.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 111.Savides TJ, Varadarajulu S, Palazzo L. EUS 2008 Working Group document: evaluation of EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2009;69:S3–S7. doi: 10.1016/j.gie.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 112.Itoi T, Yamao K. EUS 2008 Working Group document: evaluation of EUS-guided choledochoduodenostomy (with video) Gastrointest Endosc. 2009;69:S8–12. doi: 10.1016/j.gie.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 113.Artifon EL, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, Otoch JP, Gupta K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768–774. doi: 10.1097/MCG.0b013e31825f264c. [DOI] [PubMed] [Google Scholar]


