Abstract
Clonorchiasis, which has been an important public health problem in China, is caused by ingestion of raw or undercooked fish contaminated by live metacercaria. Therefore, preventing fish from infecting is of great significance for controlling the disease. SERPINs (serine protease inhibitors) are well known as negative regulators of hemostasis, thrombolysis, and innate immune responses. In the present study, two full-length sequences encoding SERPIN were identified from metacercaria cDNA library of Clonorchis sinensis (C. sinensis) and were denominated as CsSERPIN and CsSERPIN3, respectively. Bioinformatics analysis showed that the two sequences shares 35.9% identity to each other. Both of the sequences have SERPIN domain and the greatest difference between the two domains is the reactive centre loop. Transmembrane region was found in CsSERPIN3 while not in CsSERPIN. The expression of the two CsSERPINs was significantly higher at the life stage of metacercaria than that of adult. The transcription levels of CsSERPIN and CsSERPIN3 at metacercaria stage were 3.249- and 11.314-fold of that at adult stage, respectively. Furthermore, the expression of CsSERPIN was 4.32-fold of that of CsSERPIN3 at metacercaria stage. Immunobiochemistry revealed that CsERPIN was dispersed at subtegument and oral sucker of metacercaria, while CsSERPIN3 localized intensely in the tegument of metacercaria of C. sinensis inside of the cyst wall. All these indicated that the CsSERPINs play important roles at metacercaria stage of the parasite. CsSERPIN may take part in regulation of endogenous serine proteinase and CsSERPIN3 may be involved in immune evasion and be a potential candidate for vaccine and drug target for clonorchiasis.
Keywords: Clonorchis sinensis, Serpin, Serine proteinase inhibitor, Metacercaria, Gene expression, Immunolocalization
Introduction
Clonorchiasis, caused by the liver fluke Clonorchis sinensis (C. sinensis), is one of the most prevalent parasitic diseases distributed in China, Korea, and Japan. As an important food-borne parasite, C. sinensis has afflicted more than 35 million people in Asia and approximately 15 million in China, creating a socio-economic burden in epidemic regions.1,2 Current evidences from experimental and epidemiological investigations have confirmed the association between C. sinensis and cholangiocarcinoma.2–4 Moreover, long-term infections by liver flukes lead to other hepatobiliary diseases such as pyogenic cholangitis, cholelithiasis, cholecystitis, and hepatic fibrosis.5,6 The consumption of raw or uncooked fish containing infectious metacercariae of C. sinensis is considered to be the major source of infection. Therefore, preventing fish from infection may be the best way of controlling C. sinensis infection.
Serpins are well known as negative regulators of haemostasis, thrombolysis, and innate immune responses.6 In our previous study, one novel serpin named CsSERPIN was identified and characterized.7,8 It is significantly highly expressed in metacercaria stage. The biochemical activities showed that rCsproSERPIN could significantly inhibit trypsin, chymotrypsin, and thrombin, indicating that it may play an important role in the information of metacercariae. In the present study, another serpin gene was identified and expressed. The two sepins were compared by bioinformatics, real-time PCR, localization in metacercariae, which might lay some cornerstone for evaluating CsSERPINs as potential candidate for vaccine against clonorchiasis.
Materials and Methods
Animals
Six-week-old male Sprague–Dawley rats were purchased from the animal centre of Sun Yat-Sen University for experiments. The rats were housed in aseptic cages and fed with pelleted food and water. All experiments were performed on the basis of guidelines approved by the international animal ethical standard.
Bioinformatics analysis of two cDNA sequence encoding C. sinensis serpin (Csserpin)
From the cDNA library of C. sinensis metacercaria, two different insert sequence, 133f12 and 84g02, were predicted of encoding homologue of serpins by BLASTx. The complete encoding sequence of CsSERPIN was analysed by Open Reading Frame Finder program at NCBI. The deduced protein sequences and the identities of them were analysed by Vector NTI suite software. Protein analysis was done with Proteomics and sequence analysis tools (http://ca.expasy.org/tools).
Quantitative PCR analysis of two CsSERPINs at stage of adult and metacercaria
Adult worms were harvested from biliary tracts of infected cats and metacercariae were isolated from Pseudorasbora parva by pepsin digestion. Total RNA was extracted from adults and metacercariae, respectively, after being washed three times in phosphate-buffered saline (PBS) (pH 7.4). TRIZOL reagent (Invitrogen) was applied for extraction according to manufacturer’s instructions and treated with DNase (Promega). cDNA was generated from total RNA with a PrimeScriptTM RT-PCR Kit (Perfect Real Time) from TaKaRa using 1–2 μg of total RNA as template. Real-time PCR was performed using the SYBR® Premix ExTaqTM kit (TaKaRa, Tokyo, Japan) according to the manufacturer’s protocol. The amplification conditions were optimized for LightCycler 480 (Roche, Switzerland). Using Primer primier 5.0 software, the primers for CsSERPIN were designed as 5′-AGT AAC GAT GCG AAG CGA GAG C-3′, 5′-ATA GAA CCC GAA GGA AGG AGG C-3′; for CsSERPIN3, they were 5′-CCA AAA TGT ATT CAT CTC CCC CC-3′, 5′-GGC AAT GTG GCT TCT TCT AAC TC-3′. Beta-actin (GenBank accession no. EU109284, 323 bp) was amplified as the endogenous control9 using the primers 5′-GGT GAC GCT GAA GTA TCC TAT TGA-3′, 5′-CCA AAG CAT AGC CCT CGT AGA T-3′, respectively. Data were analysed using LightCycler 480 Software and using the 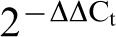 method.10
method.10
Cloning, expression and purification of rCsproSERPIN in Escherichia coli
To get the recombinant plasmid of CsSERPIN3, the gene-encoding sequence was amplified by using specific primers as follows: sense, 5′-CGG CAT ATG ATG GAG AGT GAA ATG GAT T-3′ and antisense, 5′-AAA CTC GAG CTA CAA GAC CTC AGG TTC-3′ with Nde I/Xho I as restriction enzyme sites (underlined), respectively. The purified PCR product was cloned into the corresponding restriction sites of pET28a(+) (Novagen, USA) vector and then the recombinant plasmid was transformed into E. coli BL21(DE3) (Promega). Recombinant C. senensis serpin protein (rCsproSERPIN3) was expressed by inducing with isopropyl-beta-D-thiogalactopyranoside at a final concentration of 0.2 mM at 30°C for 4 hours. The bacterial cells were harvested by centrifuging at 4°C for 15 minutes at 8000g and suspended in lysis buffer (0.5 M NaCl, 20 mM Tris–HCl, 5 mM imidazole, pH 8.0), sonicated on ice, and centrifuged at 10 000g for 15 minutes at 4°C. The recombinant fusion protein was batch-purified with His Bind Purification kit (Novagen) according to the manufacturer’s instructions. The eluted fractions were pooled and dialyzed with PBS (10 mM phosphate buffer, 27 mM KCl, 137 mM NaCl, pH 7.4). The purification and purity of the recombinant protein was monitored by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE, 12% gel) coloured with Coomassie blue, and protein concentration was estimated using Bradford assay. rCsproSERPIN was achieved as previously described.7
Acquirement of rat anti-rCsSERPIN immune serum and detection of IgG titre
For production of polyclonal antiserum for rCsproSERPIN and rCsproSERPIN3, Sprague–Dawley rat were immunized with purified rCsproSERPIN (100 μg) or rCsproSERPIN3 (100 μg) three times at 2-week intervals. Anti-sera were collected at two weeks after the second boost and the IgG titre was determined by enzyme-linked immunosorbent assay. Briefly, the plates were coated with 5 μg/ml purified rCsproSERPIN or rCsproSERPIN3 in coating buffer (0.05 M carbonate-bicarbonate, pH 9.6) and incubated at 4°C overnight, followed by blocked with 5% skimmed milk in PBS containing 0.05% Tween 20 (pH 7.4) for 2 hours at 37°C. After washing three times, the plates were incubated with different dilutions of corresponding immune sera. Rat sera immunized with PBS was measured as negative controls. Then they were washed and incubated with horseradish peroxidase-conjugated IgG (1∶20 000 dilutions in 0.1% BSA–PBST) for 1 hour. After washing, the enzyme reaction was developed with TMB substrate solution (BD Biosciences) and terminated by 2 M H2SO4. The absorbance was measured at 450 nm. Finally, they were incubated with the supernatant of E. coli BL21(DE3) containing pET28a(+) overnight at a ratio of 1∶9 and centrifuged. The immune sera were stored at −80°C for use.
Western blotting analysis of CsSERPIN and CsSERPIN3
Crude soluble antigens (CSAs) of adult worms and metacercariae were prepared as previously described. The rCsproSERPIN (5 μg), rCsproSERPIN3 (5 μg), or CSA (20 μg) were subjected to SDS–PAGE (12% gel) and then electrotransferred to membrane (Millipore). The polyvinylidene difluoride membranes were probed with naive rat serum, anti-rCsproSERPIN and anti-rCsproSERPIN3 rat serum (1:4000 dilutions), rat serum infected with C. sinensis (1∶400 dilution), normal human serum, and serum from human infected with C. sinensis, respectively. After washed three times, membranes were incubated with horseradish horseradish-conjugated anti-rat immunoglobulin G (Boster, 1∶2000 dilution) for 1 hour at room temperature (RT). After washed again, membranes were visualized with diaminobenzidine showing colour reagent (Boster) as substrate.
Immunolocalization of CsSERPIN at metacercaria of C. sinensis
Living metacercariae were washed three times in PBS (pH 7.4) and fixed in 4% paraformaldehyde in PBS (pH 7.2), embedded with paraffin, then sliced into section of 4–5 μm in thickness. The slides were blocked with normal goat sera containing 1% BSA for 2 hours at RT then incubated in a humid atmosphere at RT for 2 hours with serum from rat immunized in PBS (negative contrast), anti-rCsSERPIN rat serum and anti-rCsSERPIN3 rat serum which were diluted (0.1% BSA in PBST) to 1∶200. After washed five times with PBST, the slides were incubated further with fluorescein isothiscyanate-conjugated goat anti-rat IgG (1∶400 with 0.1% BSA in PBST; Invitrogen) for 1 hour at RT in dark, washed five times with PBST in dark again and subsequently observed under fluorescence microscope.
Results
Sequence analysis of CsSERPIN and CsSERPIN3
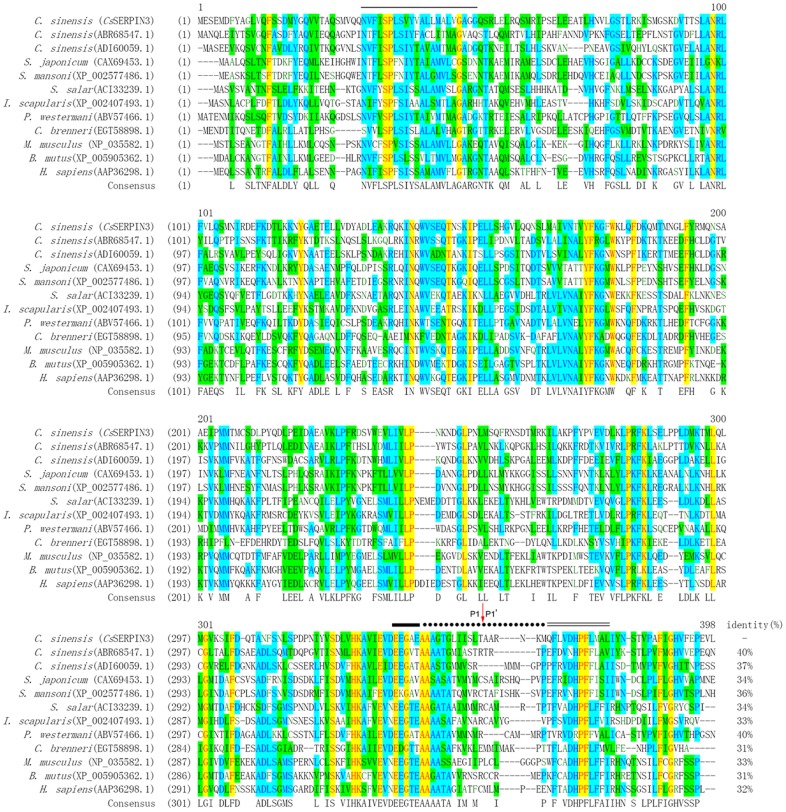
The inserted sequence of 133f12 was 1149 bp in length and 84g02 was 1161 bp. They encoded proteins of 382 amino acids (aa) and 386 aa, respectively, which dominated as CsSERPIN and CsSERPIN3. The calculated molecular weight of them is 42.2 and 43.5 kDa. Neither amino-acid sequence of CsSERPIN and CsSERPIN3 has N-terminal signal peptide. Both sequences have SERPIN domain and the greatest difference between the two domains is the reactive centre loop. Transmembrane region was found in CsSERPIN3 while not in CsSERPIN. The deduced amino-acid sequence of CsSERPIN3 was highly homologous to serpins from other species. It has been deposited to GenBank database with accession number KJ489357 (Fig. 1).
Figure 1.
Multiple sequence alignment. The deduced amino-acid sequence of CsSERPIN3 (GenBank accession number: KJ489357) from C. sinensis was aligned with those of presently known serpin. Gaps are introduced into the sequences for optimal alignment. The serpin motif is indicated as a single bold line, the reactive site loop shown as a dotted line, and the serpin signature indicated as a double thin line, and the transmembrane region of CsSERPIN3 presented as a single thin line. The scissile bond is marked with a red arrow.
Expression, purification, and characterization of rCsproSERPIN and rCsproSERPIN3
The two rCsproSERPINs could express as soluble fusion protein in E. coli BL21/DE with 6× His-tag at the N-terminals. The yield of purified rCsproSERPIN in soluble cell was 7.5 mg/l of E. coli, and that of rCsproSERPIN3 was 2 mg/l. The concentration of purified rCsproSERPIN and rCsproSERPIN3 was 3000 and 800 μg/ml, respectively (Fig. 2). The titre of the two rat anti-rCsSERPIN sera is above 51 200. The two kinds of rCsproSERPIN could be probed by mouse anti-His-tag monoclonal antibody at 44.5 and 45.8 kDa, respectively, which could also be recognized by the corresponding rat anti-rCsSERPIN serum, rat and human serum infected with C. sinensis. A pale band in the normal human sera could be found upon closer examination7 (Fig. 3).
Figure 2.
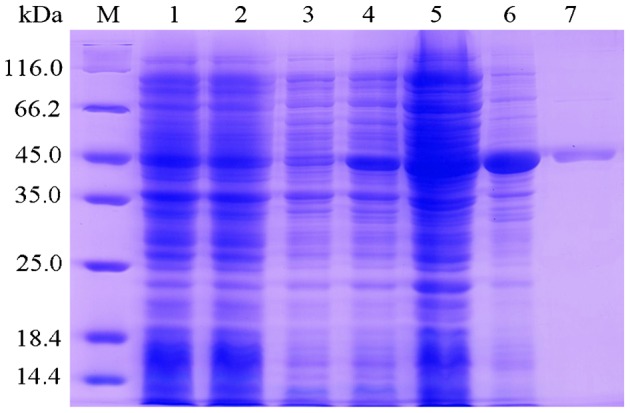
Expression and purification of rCsproSERPIN3 by 12% SDS–PAGE stained with Coomassie brilliant blue. Protein molecular weight markers (M), lysate of E. coli with pET28a(+) before induction (lane1) and after induction (lane 2), lysate of E. coli with pET28a(+)-CsSERPIN3 before induction (lane 3) and after induction (lane 4), supernatant (lane 5), and precipitant (lane 6) of lysate of E. coli with pET28a(+)-CsSERPIN3 after induction; lane 7, rCsproSERPIN3 after purification.
Figure 3.
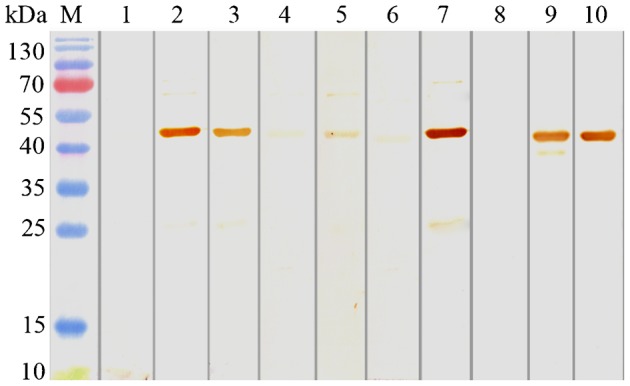
Western blot analysis of rCsproSERPIN3. Protein molecular weight markers; rCsproSERPIN3 probed with naive rat serum (lane 1), with anti-rCsproSERPIN3 rat serum (lane 2), with serum from rat infected with C. sinensis (lane 3), with serum from normal human (lane 4), with serum from human infected with C. sinensis (lane 5); lysate of E. coli with pET28a(+)-CsSERPIN3 before induction (lane 6) and after induction (lane 7) probed with anti-rCsproSERPIN3 rat serum; lysate of E. coli with pET28a(+)-CsSERPIN3 before induction (lane 8) and after induction (lane 9) probed with His monoclonal antibody of mouse; rCsproSERPIN3 after purification probed with His monoclonal antibody of mouse (lane 10).
Expression of CsSERPIN and CsSERPIN3 at life stage of metacercaria and adult of C. sinensis
The transcription of the two CsSERPINs could be detected at both metacercaria and adult stage. Compared with the mRNA level at adult stage, the transcription at metacercaria stage of CsSERPIN and CsSERPIN3 was 3.249 and 11.314 times higher with C. sinensis beta-actin as internal standard. Furthermore, the expression of CsSERPIN was 4.32-fold of that of CsSERPIN3 at metacercaria stage (Fig. 4). By Western-blot analysis, CSA of metacercariae can be obviously probed by anti-rCsproSERPIN and anti-rCsproSERPIN3 rat serum at about 45 kDa, while corresponding band could hardly be observed with CSA of adult probed by the immune serum (Fig. 5).
Figure 4.
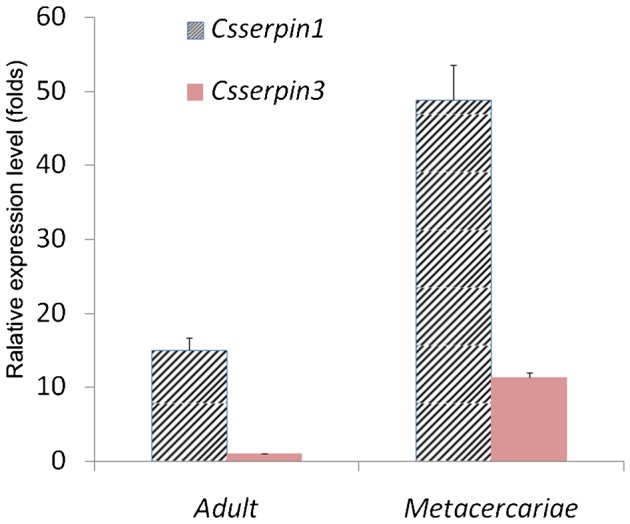
Transcript analysis of CsSERPIN and CsSERPIN3 at the stage of adult and metacercaria of Clonorchis sinensis. Beta-actin of C. sinensis (accession number: EU109284) was used as the transcription control. The ΔCt values of each adult and metacercaria were calculated by deducting the values of beta-actin, and the –ΔΔCt values were figured out according to the 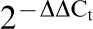 method. The amplification of CsSERPIN3 of adult worms was employed as the calibrator to evaluate relative expression levels of the others. Each bar represented the mean value from three experiments with standard deviation (*P<0.05).
method. The amplification of CsSERPIN3 of adult worms was employed as the calibrator to evaluate relative expression levels of the others. Each bar represented the mean value from three experiments with standard deviation (*P<0.05).
Figure 5.
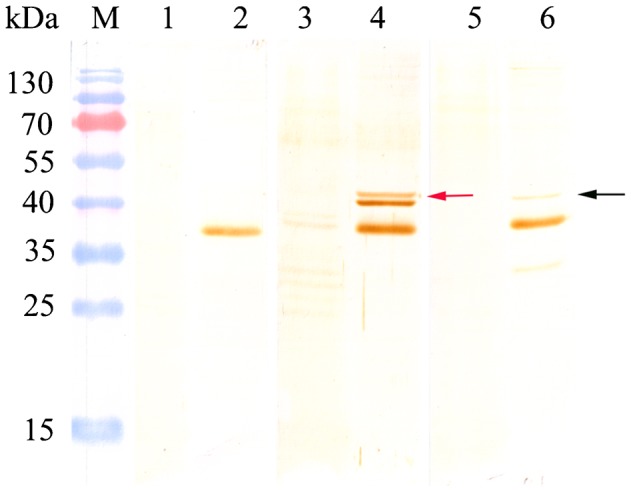
Western blot analysis of rCsproSERPIN and rCsproSERPIN3 in adult and metacercaria. Lane 1, soluble antigen of C. sinensis adult probed with naive rat serum; lane 2, soluble antigen of C. sinensis metacercaria probed with naive rat serum; lane 3, soluble antigen of C. sinensis adult probed with anti-rCsproSERPIN rat serum; lane 4, soluble antigen of C. sinensis metacercaria probed with anti-rCsproSERPIN rat serum; lane 5, soluble antigen of C. sinensis adult probed with anti-rCsproSERPIN3 rat serum; lane 6, soluble antigen of C. sinensis metacercaria probed with anti-rCsproSERPIN3 rat serum.
Immunolocalization of CsSERPIN and CsSERPIN3 in metacercaria of C. sinensis
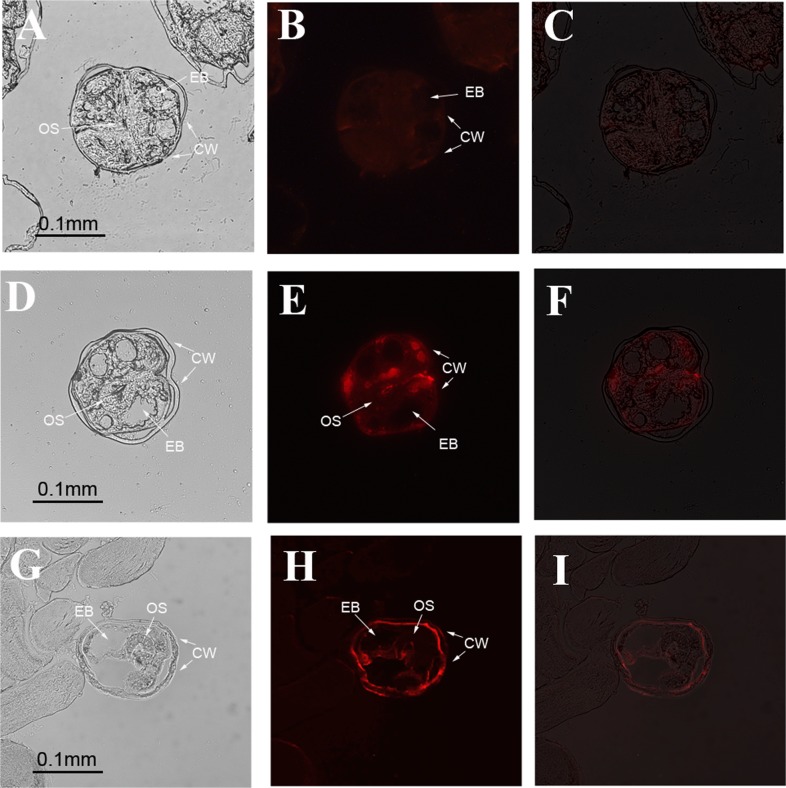
By immunofluorescence localization with anti-CsSERPIN and anti-CsSERPIN3 rat serum, the deposition of CsSERPIN and CsSERPIN3 in metacercariae was detected. The results showed that CsERPIN was dispersed at subtegument and oral sucker of metacercaria, while CsSERPIN3 localized mostly in the tegument of metacercaria of C. sinensis inside of the cyst wall (Fig. 6).
Figure 6.
Immunolocalization of CsSERPIN and CsSERPIN3 in metacercariae of C. sinensis. A–C were probed with serum from rats immunized with PBS (negative control); D–F were probed with serum from rats immunized with anti-rCsproSERPIN; G–I were probed with serum from rats immunized with anti- rCsproSERPIN3. B, E, and H are fluorescence microscopy images; A, D, and G are corresponding optical images of B, E, and H. C, F and I are superposed images (C, F, and I) of the previous two. OS, oral sucker; EB, excretory bladder; CW, cyst wall.
Discussion
In our previous study, a serpin of C. sinensis named CsSERPIN was acquired and characterized. It was highly expressed at the stage of metacercaria, which suggested that it played an important role in the stage of C. sinensis metacercaria. Another novel sequence encoding serpin named CsSERPIN3 was identified and characterized in this study. The cDNA sequence is 1161 bp in length which encoded a protein of 386 aa. It shares typical serpin motif, reactive centre loop, and serpin signature, which is well conserved in the serpin superfamily. Bioinformatics analysis identified that the greatest difference between CsSERPIN and CsSERPIN3 is the reactive centre loop. Neither of the two sequences was found containing characteristics N-terminal signal peptide cleavage sites. Transmembrane region was found in CsSERPIN3 while not in CsSERPIN. The transcription and expression of both CsSERPINs was much higher at the stage of metacercariae than that at adult. CsSERPIN was mostly deposited in oral sucker and some were dispersed at subtegument, while CsSERPIN3 localized in the tegument of metacercaria of C. sinensis inside of the cyst wall. Besides, soluble rCsproSERPIN and rCsproSERPIN3 were obtained and the recombinant protein could be probed by sera from infected rats or patients.
Some studies showed a serine proteinase-mediated encystation of Acanthamoeba and chemically synthesized serine proteinase inhibitors significantly reduced the encystation efficiency of Acanthamoeba.11 Both encystment and excystment of Acanthamoeba castellanii were found to be dependent on serine protease function.12 However, the function of serine protease should be tightly regulated by its inhibitor. In fact, the concept that parasites may utilize proteinase inhibitors to survive within the host which has been with us for many years.13,14 Many evidence demonstrated that parasite could encode serpins to inhibit the hosts’ serine proteinases and block their immune systems. A number of serpins have now been identified in parasitic helminths with putative involvement in immune regulation and in parasite survival through interference with the host immune response.15 A novel serpin expressed by blood-borne microfilariae of the parasitic nematode Brugia malayi inhibited human neutrophil serine proteinases.16,17 Serpins isolated from adult Schistosoma mansoni and Schistosoma haematobium possibly play a dual role of both in parasite physiology and in interactions with their hosts.18,19
The roles of serpins in parasites are usually closely related with their locations. The intracellular serpins are believed to be the protection of cells from superfluous proteolytic damage and the maintenance of cellular homeostasis.20 Schistosoma japonicum (S. japonicum) serpin is located on the tegument in S. japonicum adult worms and is speculated to be a vaccine candidate or a novel target for anti-schistosome drugs.19 Parasite-derived serpins with signal peptide are suggested to play essential roles in the survival of the parasites in the hosts via host immune evasion and protection from exogenous host proteases.13
The expression of the CsSERPIN and CsSERPIN3 at metacercariae stage was significantly high than that at adult stage (see supplementary material online for this article www.maneyonline.com/doi/suppl/10.1179/2047773214Y.0000000138), which gives us a clue that the two CsSERPINs play an important role in metacercariae. Although neither of them has signal peptide, there was great difference between CsSERPIN and CsSERPIN3 at the reactive centre loop. Transmembrane region was found in CsSERPIN3 and immunofluerence also showed that CsSERPIN3 localized in the tegument of metacercaria inside of the cyst wall, indicating that it should be a tegument-anchored protein. Moreover, rCsproSERPIN3 can be probed by the sera from patients infected with C. sinensis. The most likely reason for the band in the nomal human sera may be that the human was infected subclinical previously and there was a little cross-reactivity. All these manifested that CsSERPIN3 may be a candidate of vaccine and drug target for clonorchiasis. CsSERPIN did not contain any transmembrane region and it was mostly deposited in oral sucker and some were dispersed at subtegument, which implied that CsSERPIN was an intracellular serpin and not a secreted or membrane-bound protein. In view that it was highly expressed at the stage of metacercaria and the expression was 4.32 times of that of CsSERPIN3, the role of CsSERPIN may be the regulation the activities of intracellular serine proteinase of the parasite. Studies also displayed that rCsproSERPIN could be recognized by the sera of rabbits immunized with excretory/secretory products and may be involved in immunoregulation of host by inducing Th1-biased type cytokines in rats.8
Taken together, two different serpins which were both highly expressed at the stage of metacercaria were compared in this study. Their different locations may result in different functions. CsSERPIN probably plays an important role in regulation of endogenous serine proteinase and CsSERPIN3 is a potential candidate for vaccine or drug target. Further studies will be assessing the specific immune responses against CsSERPINs in intermediate host.
Disclaimer Statements
Contributors None.
Funding This work was supported by the National Key Basic Research and Development Project (973 Project; No. 2010CB530000) and National Natural Science Foundation of China (Nos. 81101270 and 81171602).
Conflicts of interest We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service, and/or company that could be construed as influencing the position presented in, or the review of the manuscript.
Ethics approval This study was prospectively performed and approved by the institutional Ethics Committees of Sun Yat-Sen University and conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
References
- 1.Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, et al. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31–41. doi: 10.1016/S1473-3099(04)01252-6. [DOI] [PubMed] [Google Scholar]
- 2.Young ND, Campbell BE, Hall RS, Jex AR, Cantacessi C, Laha T, et al. Unlocking the transcriptomes of two carcinogenic parasites, Clonorchis sinensis and Opisthorchis viverrini. PLoS Negl Trop Dis. 2010;4:e719. doi: 10.1371/journal.pntd.0000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, et al. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579–85. doi: 10.1111/j.1349-7006.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pak JH, Moon JH, Hwang SJ, Cho SH, Seo SB, Kim TS. Proteomic analysis of differentially expressed proteins in human cholangiocarcinoma cells treated with Clonorchis sinensis excretory-secretory products. J Cell Biochem. 2009;108:1376–88. doi: 10.1002/jcb.22368. [DOI] [PubMed] [Google Scholar]
- 5.Fried B, Reddy A, Mayer D. Helminths in human carcinogenesis. Cancer Lett. 2011;305:239–49. doi: 10.1016/j.canlet.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, Hu D, Wang L, Liang C, Hu X, Wang X, et al. Molecular cloning and characterization of a novel serpin gene of Clonorchis sinensis, highly expressed in the stage of metacercaria. Parasitol Res. 2009;106:221–5. doi: 10.1007/s00436-009-1654-z. [DOI] [PubMed] [Google Scholar]
- 8.Lei H, Tian Y, Chen W, Wang X, Li X, Mao Q, et al. The biochemical and immunological characterization of two serpins from Clonorchis sinensis. Mol Biol Rep. 2013;40:3977–85. doi: 10.1007/s11033-012-2475-1. [DOI] [PubMed] [Google Scholar]
- 9.Yoo WG, Kim TI, Li S, Kwon OS, Cho PY, Kim TS, et al. Reference genes for quantitative analysis on Clonorchis sinensis gene expression by real-time PCR. Parasit Res. 2009;104:321–8. doi: 10.1007/s00436-008-1195-x. [DOI] [PubMed] [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Moon EK, Chung DI, Hong YC, Kong HH. Characterization of a serine proteinase mediating encystation of Acanthamoeba. Eukaryot Cell. 2008;7:1513–7. doi: 10.1128/EC.00068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley R, Alsam S, Khan NA. The role of proteases in the differentiation of Acanthamoeba castellanii. FEMS Microbiol Lett. 2008;286:9–15. doi: 10.1111/j.1574-6968.2008.01249.x. [DOI] [PubMed] [Google Scholar]
- 13.Knox DP. Proteinase inhibitors and helminth parasite infection. Parasite Immunol. 2007;29:57–71. doi: 10.1111/j.1365-3024.2006.00913.x. [DOI] [PubMed] [Google Scholar]
- 14.Rascon AA, Jr, McKerrow JH. Synthetic and natural protease inhibitors provide insights into parasite development, virulence and pathogenesis. Curr Med Chem. 2013;20:3078–102. doi: 10.2174/0929867311320250005. [DOI] [PubMed] [Google Scholar]
- 15.Molehin AJ, Gobert GN, McManus DP. Serine protease inhibitors of parasitic helminths. Parasitology. 2012;139:681–95. doi: 10.1017/S0031182011002435. [DOI] [PubMed] [Google Scholar]
- 16.An C, Hiromasa Y, Zhang X, Lovell S, Zolkiewski M, Tomich JM, et al. Biochemical characterization of Anopheles gambiae SRPN6, a malaria parasite invasion marker in mosquitoes. PloS ONE. 2012;7:e48689. doi: 10.1371/journal.pone.0048689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham EG, Pinto SB, Ghosh A, Vanlandingham DL, Budd A, Higgs S, et al. An immune-responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proc Natl Acad Sci USA. 2005;102:16327–32. doi: 10.1073/pnas.0508335102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quezada LA, McKerrow JH. Schistosome serine protease inhibitors: parasite defense or homeostasis? An Acad Bras Cienc. 2011;83:663–72. doi: 10.1590/s0001-37652011000200025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan Y, Liu S, Song G, Xu Y, Dissous C. Characterization of a novel vaccine candidate and serine proteinase inhibitor from Schistosoma japonicum (Sj serpin). Vet Parasitol. 2005;131:53–60. doi: 10.1016/j.vetpar.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 20.Kang JM, Sohn WM, Ju JW, Kim TS, Na BK. Identification and characterization of a serine protease inhibitor of Clonorchis sinensis. Acta Trop. 2010;116:134–40. doi: 10.1016/j.actatropica.2010.06.007. [DOI] [PubMed] [Google Scholar]