Abstract
Few data are available on the epidemiology of soil-transmitted helminths (STHs) in indigenous populations of the Peruvian Amazon. While albendazole is being increasingly used in deworming campaigns, few data exist on the impact of mass drug administration in isolated populations. We studied the prevalence of STHs, anemia, and malnutrition in a Matsigenka ethnic group from the Peruvian Amazon. Participants had received two doses of albendazole on consecutive days, 3 months before and again 2 weeks before data collection. Overall, 290 subjects were included. Most were female (53.7%) and 63.9% were ≤19 years old. Half of the participants had helminth infections. Trichiuris (30.2%), hookworm (19.1%), Ascaris (17.7%), and Strongyloides (5.6%) were the most common helminths. Other helminth ova included Capillaria hepatica and Fasciola-like eggs. Subjects of 5–19 years (51.8 %) and 20–35 years (68.6 %) old had helminths more often than those under 5 years (38%) and older than 35 years (41.5%) (P = 0.02). Anemia was detected in 41% of children and this was more common in children under 5 years that in those of 5–19 years [odd ratio (OR) = 5.68; 95% CI: 2.71–11.88]. Overall, 72.1% of children were malnourished. Stunting was common in children (70.7%), but wasting was not (2.9%). Despite repeated albendazole administration, this population continued to have a high prevalence of STHs, anemia, and malnutrition. In addition, we detected unusual organisms and organisms that do not respond to albendazole. Further studies are needed to assess the rationale and efficacy of mass chemotherapy for STHs in the Amazon.
Keywords: Soil-transmitted helminths, Strongyloides, Mass drug administration, Manu jungle, Amazon, Peru, Albendazole
Background
Soil-transmitted helminths (STHs) are a major health problem in resource-poor countries.1 The number of Ascaris lumbricoides, Tricuris trichuria, and hookworm infections worldwide were estimated to be over 1.7 billion in 2010.2 In Peru, the prevalence of STHs in school-aged children is quite variable (ranging between 1.6% and 78.9%).3 Larocque et al. reported a prevalence of A. lumbricoides, T. trichuria, and hookworm of 64%, 82%, and 47%, respectively, in pregnant women from Iquitos in the northern Amazon basin of Peru.4 In contrast, Machicado et al. reported a markedly lower prevalence of A. lumbricoides (5%), T. trichiura (5%), and hookworm (14%) in Shipibo-Conibo indigenous groups from the southern Peruvian Amazon jungle.5 Briones-Chavez et al. reported significantly higher risk for STHs in settlers than in the indigenous population in a remote region of the Peruvian Amazon.6 Similar variations are evident in other regions of the Amazon.7–9 Prevalence reports of STH infections among indigenous populations of the Brazilian Amazon vary between 38% and 80%.10–13 These findings suggest that infection prevalence may vary significantly depending on groups tested and geographic area. In addition, they highlight the lack of information on factors associated with STHs and their impact in remote Amazon communities. Mass deworming campaigns are being performed in these communities. However, there are limited data on which to assess the rationale or impact of these efforts.
Pre-school and school-aged children are prone to have high worm burdens making them more vulnerable to short and long term sequellae. These include malnutrition, anemia, and impaired cognitive development.1 In order to tackle this problem; the World Health Organization (WHO) recommends periodic administration of anthelminthic drugs.14 In Peru, it is estimated that three million children between 1 and 15 years of age are in need of STHs mass chemotherapy.15 However, only 11.2% of Peruvian school-aged children at risk received treatment in 2010.15 Furthermore, some studies have demonstrated low efficacy of single-dose benzimidazole therapy in hookworm and Trichuris infections, suggesting either emergence of drug resistance or rapid re-infection after treatment. Either of these possibilities would compromise the efficacy of mass drug administration.1,16–18
An integrated approach including health education, sanitation, and antihelminthics has controlled STHs in some highly endemic regions.1,18,19 An important obstacle to the success of these efforts is the lack of accurate epidemiological descriptions of STH infections in different geographical and ecological regions.20 The prevalence of STHs and effectiveness of current WHO control recommendations in remote communities in the Peruvian Amazon have largely been ignored. To generate preliminary data on the rationale and impact of mass drug treatment, we assessed the prevalence of STHs in a Matsigenka community in the Manu area of the Peruvian Amazon after mass administration of albendazole.
Methods
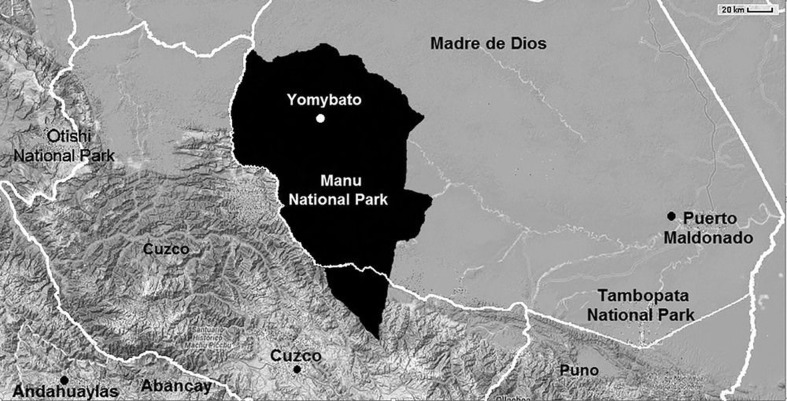
We performed a cross-sectional study during a health intervention in Yomybato community (S: 11.76085, W: 71.87385) in the Madre de Dios region of Peru (Fig. 1). This community is inside the Manu National Park in a remote area of the Southern Peruvian rain-forest only accessible by boat. The population targeted consisted of subjects living in Yomybato community (n = 225) and surrounding family clans. They belong almost exclusively to the Matsigenka ethnic group living in voluntary isolation with no commercial exchange with outside communities. Houses were made of wood and palm leafs with dirt floors and no sewage or treated water. Children and women walked bare feet. Female and male subjects from all ages were included in the health intervention organized by ‘House of the Children’ non-profit organization in November 2012. The non-profit organization personnel were healthcare workers familiar with the area and trained extensively for data collection. Anthropometric measurements, capillary blood, and stool samples were collected from participants by these personnel. These data were used to assess the prevalence of malnutrition, anemia, and STH infections. Health authorities had unexpectedly administered mass chemotherapy to the entire population approximately 3 months and again 2 weeks before specimen collection using two doses of albendazole 400 mg given on consecutive days. No baseline STH prevalence data are available from before mass chemotherapy.
Figure 1.
Geographic location of Yomybato within Manu National Park (adapted from Google maps).
Information on age, sex, weight, and height was used to evaluate the nutritional status of children. The WHO open access Anthroplus software was used to calculate the z-scores for weight for age, height for age, and Body Mass Index for age as appropriate for each age group. Undernutrition and stunting were defined using the 2007 WHO growth references standards.21 HemoCue® Hemoglobin tests (HemoCue AB, Ängelholm, Sweden) were used on site to assess the presence of anemia. WHO age- and sex-adjusted hemoglobin cutoff values were used for comparison.22 One stool sample per subject was collected and immediately preserved in 10% formalin for transport to Cusco, where they were processed. Owing to the logistic difficulties of working in these highly isolated areas, obtaining additional specimens was not feasible. All stool samples were tested using direct and rapid sedimentation for the detection of intestinal parasites. A modified Kato–Katz test using formalin preserved stools was performed. The quantitative results from this test were excluded since the Kato–Katz is not standardized for preserved stools. All positive tests were confirmed by a second observer.
The data on demographics, anthropometry, and hemoglobin levels were extracted from the non-profit organization records using standardized forms. Data on stool test results were obtained from our laboratory records. The Statistical Package for the Social Science version 18.0 was used for data analysis (SPSS, Inc., Chicago, IL, USA). The prevalence of malnutrition, anemia, and STH infections were calculated. Means (±SD) were calculated for continuous variables and compared using t-tests. The Chi square test and Odds ratios were used to evaluate categorical variables. A P≤0.05 was considered statistically significant.
The Universidad Peruana Cayetano Heredia Institutional Review Board approved the use of de-identified subjects’ information for this study.
Results
Two hundred and ninety subjects from 52 family clans were included in the study. The mean number of subjects per family was 5.5 (±2.6 subjects). Two hundred and fifteen (74.1%) subjects provided a stool sample and 225 (77.5%) provided a blood sample for hemoglobin testing. Table 1 shows the demographic characteristics of the population. Most (63.2%, 136/215) participants had at least one parasite other than B. hominis and 49.7% (107/215) were infected by at least one helminth parasite. Table 2 shows the total and age-stratified prevalence of intestinal parasites in the population studied.
Table 1. Demographic characteristics of the participants.
| Characteristic | % (n) | Median (interquartile range) | |
| Sex (n = 287) | Female | 53.7 (154) | … |
| Male | 46.3 (133) | ||
| Age | … | 13.9 years (5.9–27.5 years) | |
| Age groups (n = 280) | <5 years | 22.1 (62) | … |
| 5–19 years | 41.8 (117) | ||
| 20–35 years | 19.3 (54) | ||
| >35 years | 16.8 (47) | ||
| Women of reproductive age (n = 280) | Si | 36.1 (101) | … |
| No | 63.9 (179) | ||
| Number of subjects per clan (n = 290) | … | 5 subjects (3–7.25) |
Table 2. Age stratified prevalence of intestinal parasites in Yomybato.
| Prevalence, % (n) | |||||
| <5 years (n = 50) | 5–19 years (n = 83) | 20–35 years (n = 35) | >35 years (n = 34) | Total* (n = 215) | |
| Helminths | |||||
| Ascaris lumbricoides | 12 (6) | 16.9 (14) | 28.6 (10) | 20.6 (7) | 17.7 (38) |
| Trichuris trichura | 20 (10) | 34.9 (29) | 40 (14) | 17.6 (6) | 30.2 (65) |
| Hookworm | 10 (5) | 16.9 (14) | 37.1 (13) | 20.6 (7) | 19.1 (41) |
| Strongyloides stercoralis | 6 (3) | 4.8 (4) | 2.9 (1) | 8.8 (3) | 5.6 (12) |
| Hymenolepsis nana | 0 (0) | 2.4 (2) | 2.9 (1) | 0 (0) | 1.4 (3) |
| Enterobius vermicularis | 6 (3) | 1.2 (1) | 8.6 (3) | 5.9 (2) | 4.2 (9) |
| Capillaria hepatica | 0 (0) | 1.2 (1) | 2.9 (1) | 5.9 (2) | 2.3 (5) |
| Fasciola-like eggs | 0 (0) | 3.6 (3) | 5.7 (2) | 0 (0) | 2.3 (5) |
| Any helminth infection | 38 (19) | 51.8 (43) | 68.6 (24) | 41.5 (14) | 49.7 (107) |
| Protozoa | |||||
| Giardia intestinalis | 34 (17) | 38.8 (33) | 17.1 (6) | 8.8 (3) | 28.4 (61) |
| Blastocystis hominis | 34 (17) | 53.7 (44) | 51.4 (18) | 41.2 (14) | 46.3 (99) |
Note: *Numbers in the rows do not sum the total because information about age was missing in 13 subjects.
P<0.05 for G. intestinalis, hookworm, and any helminth infection.
Subjects aged 5–19 years (43/83, 51.8%) and 20–35 years (24/35, 68.6%) had helminths more often than those under 5 years (19/31, 38%) or older than 35 years (14/34, 41.2%) (P = 0.03). When comparing all age groups significant differences were found in the prevalence of hookworm (P = 0.01), but not in the prevalence of Trichuris (P = 0.05), Ascaris (P = 0.2), or Strongyloides (P = 0.7) (Table 2). Of the children 19 years old and younger who provided a stool sample for testing, 46% (61/132) were infected with at least one helminth. Those under 5 years were less likely than children of 5–19 years old to have T. trichura [odds ratio (OR) = 0.4; 95% CI: 0.17–0.94]. Among children with complete anthropometric data, underweight was encountered in 28.7% (27/94) of children of 0–10 years old, stunting in 70.7% (99/140) of children, and wasting in 2.9% (4/140). Overall, 72.1% (101/140) of children had some kind of malnutrition. Malnutrition was associated with family clan (P<0.01), but not with sex (P = 0.5), age group (P = 0.1), or helminth infections (P = 0.2).
Of those children that provided a blood sample, 41% (60/146) had anemia. Anemia was more commonly diagnosed in children under 5 years old than in children of 5–19 years (67.3% versus 26.6%, OR = 5.68; 95% CI: 2.71–11.88). Children that were underweight had anemia more often than children without it (OR = 2.87; 95% CI: 0.99–8.45). Anemia was not associated with helminths infection (P = 0.7) or with any specific species. Anemia did not vary with sex (P = 0.1) or family clan (P = 0.1).
Discussion
In this study, we documented the prevalence of STHs in an isolated indigenous population of the southern Peruvian Amazon after mass chemotherapy. Owing to logistical difficulties, we were only able to test a single stool sample and did not use optimal methods for the diagnosis of Strongyloides or Enterobius infections, which were likely under diagnosed. Nevertheless, half of those studied had documented helminth infections, despite recent albendazole treatment. We documented anemia and malnutrition as important health problems in the area. In other studies, helminth infection is a major risk factor for both anemia and malnutrition.4,23
This study was designed to assess baseline prevalence of infection with STHs before chemotherapy. However, the ministry of health independently performed two rounds of mass drug administration prior to data collection. The post-treatment prevalences of Ascaris (18%), Trichuris (30%), and hookworm (20%) found in our study are difficult to evaluate as no pre-treatment data were collected. A study in the Northern Peruvian Amazon used single-dose albendazole for deworming children and assessed the prevalence of STHs 2 weeks after treatment. This study documented a post-treatment prevalence of 2% for Ascaris, 67% for Trichuris, and 6% for hookworm which sharply contrast with the higher prevalence of Ascaris and hookworm found in Yomybato.24 Pre-treatment studies including indigenous populations in the Amazon show a wide variation in prevalence.7–13 A study of the Achuars in the northern Peruvian Amazon found a prevalence of Ascaris and Trichuris of 92% and 96%, respectively.25 Markedly different pre-treatment results were reported in a study of the Shipibo-Conibo in the Madre de Dios region of Peru. The latter showed a prevalence of STHs lower than our post-treatment results (5% for Ascaris and Trichuris and 15% for hookworm).5 Studies in Brazil among untreated indigenous groups from Para and Mato Grosso states reported the prevalence of Ascaris ranging from 25% to 35% and of hookworm ranging from 19% to 33%. Of note, Trichuris prevalence was very low ranging from 0.6% to 1.1%.10,12,13 Interestingly, the pre-treatment prevalence of STHs in indigenous groups from Brazil was comparable to the post-treatment prevalence in Yomybato.10,13 The STHs prevalence found in our study 2 weeks after treatment with two doses of albendazole suggests a high pre-treatment prevalence and/or infection intensity of STHs in Yomybato.
The prevalence of Ascaris in our population was almost 18% after repeated albendazole treatment before stool sample collection. Other studies have documented reductions in Ascaris prevalence of >90% after a single dose of albendazole.24,26,27 Steinmann et al. documented a reduction of Ascaris prevalence of 96% after a single dose of albendazole.27 The prevalence of Ascaris decreased from 70% to 2% 2 weeks after treatment with single dose albendazole in another part of the Peruvian Amazon.24 We do not believe that the observed prevalence of Ascaris was due to re-infection because the last dose of albendazole was given to subjects 2 weeks before collection of stools. In addition, repeated doses (four in a 3-month period) should have been effective even with rapid reinfection and with the limited albendazole activity in juvenile parasite forms.28–30 Cure rates with albendazole are influenced by the tests used to diagnose the infection, the pre-treatment intensity of infection, and undernutrition.16,30 The single-stool samples tests used in our study are not particularly sensitive to detect STHs. Thus, from our data, we can only ascertain that undernutrition was common and might have affected the cure rates. It is important to note that cure rates are not the best parameter to assess the efficacy of albendazole based programs.30 The goal of these programs is not to achieve cure, but rather to limited the morbidity associated with high intensity infections. From our data, we cannot draw conclusions about egg reduction rates or albendazole efficacy. However, the high prevalence of infection detected with insensitive techniques suggests that the current regimen may be suboptimal in this population. Clearly, further research on the effects of mass albendazole in highly vulnerable isolated populations from the Amazon is warranted.
There was a high prevalence of helminths other than Ascaris, hookworm, and Trichuris. Strongyloides stercoralis infection was demonstrated in 6% of individuals. This is almost certainly an underestimation since the methods used are not sensitive enough to reliably detect Strongyloides larvae.31 Similar high prevalence rates of strongyloidiasis have been noted elsewhere in the Peruvian Amazon.32 The health impact and consequences of the infection with Strongyloides have largely been ignored in STHs control programs.23,33 For example, one or two doses of albendazole monotherapy, as used here, have limited impact on Strongyloides prevalence or worm burden. Despite the likelihood of significant morbidity and mortality caused by Strongyloides, albendazole mass chemotherapy programs continue to be widely used.33
Five individuals had Capillaria hepatica eggs detected in their stool. Eggs of this parasite are only rarely found in human stool samples. The parasite infects the liver parenchyma, mainly of animals. Subjects passing C. hepatica eggs in the stool often have eaten animal liver harboring eggs that are then just passed in the feces. These eggs are not infectious as they require several days in the environment to embryonate.34 Similar prevalence rates of Capillaria have been reported in indigenous groups from Brazil.10 We also documented eggs that resembled Fasciola hepatica. Autochthonous fascioliasis has not been described from the Amazon in Peru. Neither the snail intermediate hosts nor the typical mammalian definitive hosts are reported in the area. Thus, additional studies are needed to confirm the exact identity of these parasites, and molecular studies are in process.
In our study, there was a high prevalence of malnutrition and anemia in children compared to studies among indigenous populations in the Ecuadorian and Bolivian Amazon,7–9 but comparable to those described in Brazil.35 While STHs were not significantly associated with anemia and malnutrition in our population, our study design, the small sample size, and the administration of albendazole before our study may have masked a real association. There are clear associations of high burdens of helminths with malnutrition, anemia, and cognitive impairment in other settings, but the health benefits of mass chemotherapy have been difficult to demonstrate. A recent Cochrane review noted better evidence for treatment of infected individuals than for mass chemotherapy.36 For example, a massive cluster-randomized trial in India failed to demonstrate any clinical benefit of mass chemotherapy.37 In the current population, albendazole use for mass chemotherapy is further complicated by the presence of helminths that would not be expected to respond to the current regimen (e.g. Strongyloides and Fasciola). Thus, there is a need to obtain more data on the efficacy of this approach in the Amazon.
In summary, we have documented a high post-treatment prevalence of helminth infections in an isolated indigenous population of the Peruvian Amazon. This population suffers from a high prevalence of anemia and malnutrition, suggesting a heavy impact of helminths on the population’s health. The prevalence of STHs was high despite previous rounds of chemotherapy, suggesting a need to study the factors influencing the response to mass chemotherapy among high-risk isolated populations with limited access to health care in the Amazon.
Disclaimer Statements
Contributors MMC wrote the project, collected the data, analyzed the data, and wrote the manuscript. ML and EA assisted in drafting the project, performed the laboratory work, revised and approved the manuscript. ACW assisted in writing the project and analyzing the data, revised and approved the manuscript.
Funding Universidad Peruana Cayetano Heredia and University of Texas Medical Branch Collaborative Research Center.
Conflicts of interest The authors have no conflicts of interest to declare.
Ethics approval The Universidad Peruana Cayetano Heredia Institutional Review Board approved the use of de-identify subjects’ information for this study.
Acknowledgments
The authors would like to thank Dr Mark Eberhard from the Center for Disease Control and Prevention for the assistance in the final identification of helminth parasites. Special thanks to Ms Nancy Santullo and Mr Caleb Matos from ‘House of the Children’ non-profit organization for providing the dataset for the present study.
References
- 1.Tchuem Tchuenté LA. Control of soil-transmitted helminths in sub-Saharan Africa: diagnosis, drug efficacy concerns and challenges. Acta Trop. 2011;120(Suppl 1):S4–11. doi: 10.1016/j.actatropica.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saboyá MI, Catalá L, Ault SK, Nicholls RS. Prevalence and intensity of infection of soil-transmitted helminths in Latin America and the Caribbean Countries: mapping at second administrative level 2000–2010. Washington, DC: Pan American Health Organization; 2011. Available from: http://www.paho.org/hq/index.php?option = com_docman&task = doc_view&gid = 14335&Itemid = (accessed 15 May 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larocque R, Casapia M, Gotuzzo E, Gyorkos TW. Relationship between intensity of soil-transmitted helminth infections and anemia during pregnancy. Am J Trop Med Hyg. 2005;73:783–9. [PubMed] [Google Scholar]
- 5.Machicado JD, Marcos LA, Tello R, Canales M, Terashima A, Gotuzzo E. Diagnosis of soil-transmitted helminthiasis in an Amazonic community of Peru using multiple diagnostic techniques. Trans R Soc Trop Med Hyg. 2012;106:333–9. doi: 10.1016/j.trstmh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Briones-Chávez C, Torres-Zevallos H, Canales M, Stamato CM, O’Riordan TG, Terashima A. Differences in prevalence of geohelminth infections between indigenous and settler populations in a remote Amazonian region of Peru. Trop Med Int Heal. 2013;18:615–8. doi: 10.1111/tmi.12077. [DOI] [PubMed] [Google Scholar]
- 7.San Sebastian M, Santi S. The health status of rural school children in the Amazon basin of Ecuador. J Trop Ped. 1999;45:379–82. doi: 10.1093/tropej/45.6.379. [DOI] [PubMed] [Google Scholar]
- 8.Sackey ME, Weigel MM, Armijosb RX. Predictors and nutritional consequences of intestinal parasitic infections in rural Ecuadorian children. J Trop Ped. 2003;49:17–23. doi: 10.1093/tropej/49.1.17. [DOI] [PubMed] [Google Scholar]
- 9.Tanner S, Chuquimia-Choque ME, Huanca T, McDade TW, Leonard WR, Reyes-García V. The effects of local medicinal knowledge and hygiene on helminth infections in an Amazonian society. Soc Sci Med. 2011;72:701–9. doi: 10.1016/j.socscimed.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Santos RV, Coimbra CE, Flowers NM, Silva JP. Intestinal parasites in the Xavante Indians, Central Brazil. Rev Med Trop Sao Pab. 1995;32:135–8. doi: 10.1590/s0036-46651995000200009. [DOI] [PubMed] [Google Scholar]
- 11.Miranda RA, Xavier FB, Lima JR, Menezes RC. Prevalence of intestinal parasitism in Tembé tribe Indian settlements, Brazilian Eastern Amazon. Rev Soc Bra Med Trop. 1999;32:389–93. doi: 10.1590/s0037-86821999000400009. [DOI] [PubMed] [Google Scholar]
- 12.Miranda RA, Xavier FB, Menezes RC. Intestinal parasitism in a Parakanã indigenous community in southwestern Pará State, Brazil. Cad Saúde Púb Rio Jan. 1998;14:507–11. doi: 10.1590/s0102-311x1998000300007. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari JO, Ferreira MU, Aranha Camargo LM, Ferreira CS. Intestinal parasites among Karitiana Indians from Rondonia State, Brazil. Rev Inst Med Trop Sao Pab. 1992;34:223–5. doi: 10.1590/s0036-46651992000300007. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Soil-transmitted helminthiases: eliminating soil-transmitted helminthiases as a public health problem in children: progress report 2001–2010 and strategic plan, 2011–2020. Geneva: World Health Organization; 2012. Available from: http://www.wpro.who.int/mvp/topics/ntd/STH_stratplan.pdf (accessed 15 May 2014). [Google Scholar]
- 15.World Health Organization. Soil transmitted helminthiases: number of children (pre-SAC and SAC) requiring preventive chemotherapy for soil transmitted helminthiases. Geneva: World Health Organization; 2012. Available from: http://apps.who.int/neglected_diseases/ntddata/sth/sth.html (accessed 15 May 2014). [Google Scholar]
- 16.Vercruysse J, Albonico M, Behnke JM, Kotze AC, Prichard RK, McCarthy JS, et al. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int J Parasitol Drugs Drug Resist. 2011;1:14–27. doi: 10.1016/j.ijpddr.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vercruysse J, Levecke B, Prichard R. Human soil-transmitted helminths: implications of mass drug administration. Curr Opin Infect Dis. 2012;25:703–8. doi: 10.1097/QCO.0b013e328358993a. [DOI] [PubMed] [Google Scholar]
- 18.Jia TW, Melville S, Utzinger J, King CH, Zhou XN. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1621. doi: 10.1371/journal.pntd.0001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9:e1001162. doi: 10.1371/journal.pmed.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooker S, Clements AC, Bundy DA. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006;62:221–61. doi: 10.1016/S0065-308X(05)62007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Onis M, Onyango AW, Borghi E, Sydam A, Nishida C, Siekmann J. Development of a WHO growth reference for school aged children and adolescents. Bull World Health Organ. 2007;85:660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Iron deficiency anaemia assessment, prevention and control: a guide for programme managers. Geneva: World Health Organization; 2001. Available from: http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf (accessed 15 May 2014). [Google Scholar]
- 23.Verhagen LM, Incani RN, Franco CR, Ugarte A, Cadenas Y, Sierra-Ruiz CI, et al. High malnutrition rate in Venezuelan Yanomi compared to Warao Amerindians and creoles: Significant associations with intestinal parasites and anemia. PLoS One. 2013;8:e77581. doi: 10.1371/journal.pone.0077581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gyorkos TW, Maheu-Giroux M, Blouin B, Saavedra L, Casapia M. Eficacia del albendazole en dosis única sobre las infecciones por helmintos transmitidos por el suelo en escolares de una comunidad de Iquitos, Peru. Rev Peru Med Exp Salud Publica. 2013;30:601–7. [PubMed] [Google Scholar]
- 25.Kroeger A, Schulz S, Witte B, Skewes-Ramm R, Etzler A. Helminthiasis and cultural change in the Peruvian rainforest. J Trop Med Hyg. 1992;95:104–13. [PubMed] [Google Scholar]
- 26.Steinmann P, Utzinger J, Du ZW, Jiang JY, Chen JX, Hattendorf J, et al. Efficacy of single-dose and triple-dose albendazole and mebendazole against soil-transmitted helminths and Taenia spp.: a randomized controlled trial. PLoS One. 2011;6:e25003. doi: 10.1371/journal.pone.0025003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vercruysse J, Behnke JM, Albonico M, Ame SM, Angebault C, Bethony JM, et al. Assessment of the anthelmintic efficacy of albendazole in school children in seven countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis. 2011;5:e948. doi: 10.1371/journal.pntd.0000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yap P, Du ZW, Wu FW, Jiang JY, Chen R, Zhou XN, et al. Rapid re-infection with soil-transmitted helminths after triple dose albendazole treatment of school-aged children in Yunnan, People’s Republic of China. Am J Trop Med Hyg. 2013;89:23–31. doi: 10.4269/ajtmh.13-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geerts S, Gryseels B. Antihelmintic resistance in human helminths: a review. Trop Med Int Health. 2001;6:915–21. doi: 10.1046/j.1365-3156.2001.00774.x. [DOI] [PubMed] [Google Scholar]
- 30.Montresor A. Cure rate is not a valid indicator for assessing drug efficacy and impact of preventive chemotherapy interventions against schistosomiasis and soil-transmitted helminthiasis. Trans R Soc Trop Med Hyg. 2011;105:361–3. doi: 10.1016/j.trstmh.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inês Ede J, Souza JN, Santos RC, Souza ES, Santos FL, Silva ML, et al. Efficacy of parasitological methods for the diagnosis of Strongyloides stercoralis and hookworm in faecal specimens. Acta Trop. 2011;120:206–10. doi: 10.1016/j.actatropica.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Yori PP, Kosek M, Gilman RH, Cordova J, Bern C, Chavez CB, et al. Seroepidemiology of strongyloidiasis in the Peruvian Amazon. Am J Trop Med Hyg. 2013;74:97–102. [PMC free article] [PubMed] [Google Scholar]
- 33.Krolewiecki AJ, Lammie P, Jacobson J, Gabrielli AF, Levecke B, Socias E, et al. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis. 2013;7:e2165. doi: 10.1371/journal.pntd.0002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabada MM, Lopez M, White AC. Capillaria hepatica pseudoinfection. Am J Trop Med Hyg. 2013;89:609. doi: 10.4269/ajtmh.13-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leite MS1, Cardoso AM, Coimbra CE, Jr, Welch JR, Gugelmin SA, Lira PC, et al. Prevalence of anemia and associated factors among indigenous children in Brazil: results from the First National Survey of Indigenous People’s Health and Nutrition. Nutr J. 2013;12:69. doi: 10.1186/1475-2891-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor-Robinson DC, Maayan N, Soares-Weiser K, Donegan S, Garner P. Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance. Cochrane Database Syst Rev. 2012;(11):CD000371. doi: 10.1002/14651858.CD000371.pub5. [DOI] [PubMed] [Google Scholar]
- 37.Awasthi S, Peto R, Read S, Richards SM, Pande V, Bundy D, et al. Population deworming every 6 months with albendazole in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet. 2013;381:1478–86. doi: 10.1016/S0140-6736(12)62126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]