Abstract
We demonstrate the ultrasonic propulsion of rod-shaped nanomotors inside living HeLa cells. These nanomotors (gold rods ~ 300 nm in diameter and ~ 3 μm long) attach strongly to the external surface of the cells, and are readily internalized by incubation with the cells for periods longer than 24 h. Once inside the cells, the nanorod motors can be activated by resonant ultrasound operating at ~ 4 MHz, and show axial propulsion as well as spinning. The intracellular propulsion does not involve chemical fuels or high power ultrasound and the HeLa cells remain viable. Ultrasonic propulsion of nanomotors may thus provide a new tool for probing the response of living cells to internal mechanical excitation, for controllably manipulating intracellular organelles, and for biomedical applications.
Keywords: Nanomotors, Cell uptake, Ultrasonic propulsion, Cell mechanotrasduction, Metallic nanowires
Nano- and micromotors, a class of synthetic micromachines, have received increasing attention over the past decade.[1] Among the applications proposed and achieved for these small machines, those with the farthest-reaching impact involve bio-medical research and health care.[2] These include the isolation and transport of cells,[3] sensing trace amounts of chemicals,[4] and damaging and penetrating tissues for non-invasive surgeries.[5] So far, however, there have been no reports of self-propelled inorganic nano- or microparticles inside living cells, which is a critical subject for advancing this field.
In this communication we demonstrate that acoustically powered gold nanrods interact dynamically with living cells and can be propelled inside them. HeLa cervical cancer cells were chosen as a model system for this study because of their popularity in biomedical research, as well as their ease of handling and growth.[6] We found that gold rods can be internalized by HeLa cells if they are incubated together for 24 h or more, and that the motors remain active inside the cells. Gold rods also attach strongly and dynamically to the external surface of live HeLa cells and move between cells when powered acoustically.
Experiments were carried out using the same acoustic chamber reported previously.[7] Gold nanorods (~3 μm long × 300 nm diameter) were fabricated by electrodeposition in porous alumina membranes (see SI for synthetic details and representative SEM images). In water, these rods show fast autonomous axial propulsion (peak axial speed of ~ 200 μm/s) and rotation in the resonant acoustic chamber with ~ 4 MHz excitation. These modes of propulsion have been attributed to asymmetric scattering of sound waves at the concave and convex ends of the rods.[7–8]
In order to observe intracellular propulsion, the nanorods and HeLa cells were first incubated together for 12, 24 and 48 h (see SI for experimental details). We found that longer incubation time led to significant uptake of gold rods in HeLa cells. For example, few rods were internalized by HeLa cells after 12 h of incubation, while after 48 h most of the metallic rods were internalized.
Interestingly some of these gold rods, although trapped inside cells, were still very responsive to the acoustic fields and remained active. Both directional motion and spinning could be observed inside the HeLa cells (Fig. 1, Video S1). The activity of gold rods could also be manipulated by adjusting the ultrasonic power.
Figure 1.
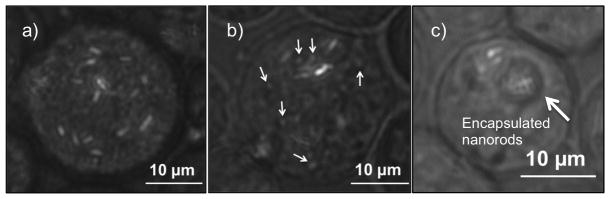
HeLa cells with gold rods internalized. a) one HeLa cell with many rods (light colored objects) inside. b) gray subcellular structures (a few are highlighted by arrows) can be seen interacting with active acoustic motors inside a HeLa cell. c) two vesicular structures in a HeLa cell contain many active but crowded acoustic motors
Several important observations were:
Uptake of gold rods differed greatly from one cell to another. It was possible to find cells containing more than 10 rods that were surrounded by cells with few or no rods inside (Video S1). This behavior has been previously reported in uptake studies of micron-size particles by cells.[9] Two possible explanations are that the rods may be unevenly distributed over the cells during the incubation period, or the intrinsic heterogeneity among individual HeLa cells may lead to uptake by some cells and not others.
Acoustic motors move more slowly inside cells (top speed ~ 60 μm/s) than outside the cells (~ 100 μm/s), indicating either a loss of acoustic power (possibly due to the damping by the cell), or, more likely, increased Stokes drag from the higher viscosity of cytoplasm (estimated to be 3–4 times that of water [10]).
There are microstructures in the cells - which appear as light gray against the darker background of the cytoplasm - that interacted with active gold rods in a manner similar to polystyrene microspheres of similar sizes (Fig. 1b) and were dragged by the vortex around spinning rods (Video S2). While not clearly identifiable, these objects appear to be subcellular organelles, such as the endoplasmic reticulum (ER). The strong coupling between the controlled motion of metallic rods and these structures provides a potentially useful way to agitate and probe cells from within.
Not all gold rods that were internalized could move freely. Many rods appeared to be either trapped in the membrane on their way into the cell, or hindered within the cell. There were also rare cases in which a number of gold rods were trapped inside a vesicle-like structure within the cell (Fig. 1c and Video S3). These observations may provide us opportunities to study subcellular structures and phagocytosis from a different perspective.
It is clear that the active acoustic motors inside cells are confined by the cell membrane. Conversely, acoustic motors outside cells cannot penetrate cell membranes at the power levels used in these experiments. Video S4 shows how a gold micro-rod (Fig. 2a) moved inside a HeLa cell and along the inner surface of its membrane. The trajectories of powered rods inside the cells was tracked, as shown in Fig. 2b for the rod imaged in Fig. 2a. This rod makes many sharp turns, and its speed also fluctuates (see histogram in Figure S5), suggesting frequent collisions inside the cell. To quantify this behavior, we calculated the directionality of motors moving inside and outside of cells (Fig. 2c). The directionality was defined as cosine of the angle between steps (see SI for details). Directionality is plotted as a function of length scale L, defined as L = vτ, where v is the average velocity in the medium (cellular cytoplasm or water) and τ is the time between measurements. In water, the axial motion of the rods is highly directional (cos θ ≤ 0.3 for L up to 5 μm). In contrast, the directionality of intracellular rods is low (cos θ ≥ 0.6) even at length scales down to 0.5 μm. This supports the conclusion that powered rods inside the cells undergo frequent collisions with subcellular structures.
Figure 2.
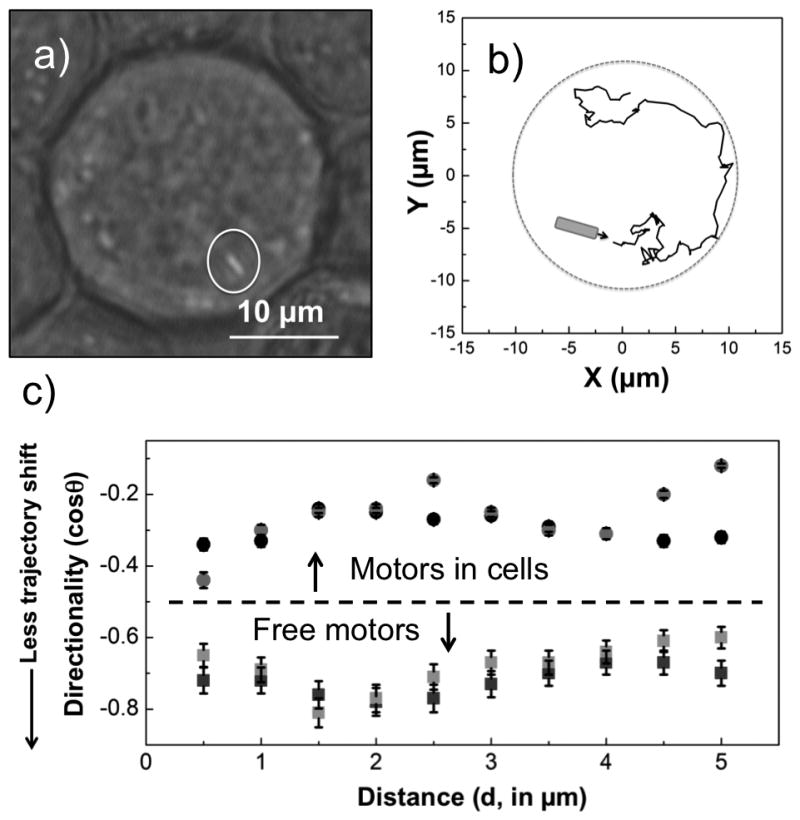
Tracking analysis of a gold rod inside a HeLa cell from a clip in Video S7. a) optical micrograph of the cell with two gold rods internalized (one rod is highlighted). b) the trajectory of the rod circled in a). c) the directionality of the gold rod (grey dot) and another gold rod trapped inside a HeLa cell (black dot, shown in Video S2), are compared to that of two motors moving freely in water (light grey and black squares). The error bars represent 95% confidence intervals.
Earlier papers have reported the internalization of micron-size particles by live HeLa cells, including nanowires similar to ours.[9, 11] Gratton and coworkers studied the cellular uptake of microparticles of different shapes and sizes and discovered that rod-shaped particles with relatively high aspect ratios were more prone to cell uptake.[11d] Chen and coworkers studied the cytotoxicity and uptake efficiencies of surface-modified gold nanowires (with lengths up to a few μm) by HeLa cells.[9] Our observations agree with these reports in that HeLa cells incubated with ~3 μm long gold rods showed significant uptake. Cell viability tests (see Supporting Information) indicated that most of the HeLa cells remained alive after uptake of nanowires and ultrasonic agitation. The mechanism of cellular uptake of microparticles has not been completely elucidated, although particles larger than 500 nm are commonly believed to be internalized primarily via phagocytosis.[12]
The uptake of acoustic motors in living HeLa cells has some implications for biophysical studies and biomedical applications. Synthetic nano- and micromotors have been studied for cell sorting, sensing, drug delivery, and tissue drilling, but they have not previously been implanted into living cells. There are many other kinds of particles that have been engineered for intracellular use, including nanoparticles with diagnostic and therapeutic applications,[13] magnetic beads commonly used for microrheology and mechanotransduction studies,[14] and magnetic nanowires for cell manipulation.[11b, 11c, 15] These particles, although immensely useful and widely studied, cannot be powered in situ to move autonomously. Instead, they rely on diffusion or convection (e.g., nanoparticles), or are collectively powered and controlled to move in concert (e.g., magnetic particles).
Acoustically propelled nano- and microwires may have several advantages over other kinds of particles for intracellular functions. First, because acoustic motors autonomously convert local acoustic energy into mechanical motion, each motor can move in a different direction and at its own speed. This is interesting for applications in which each active particle has its own target and functionality. Second, ultrasonically driven metallic rods have two modes of movement: axial propulsion and spinning about their axis, and these two modes can be switched at different ultrasonic frequencies. The two modes of motion in turn provide two ways to mechanically stimulate cells, either by shear stress induced by the rotating vortex, or by the axial force from the nanomotor. Third, the power (pressure larger than 10 Pa can be produced) and shape (sharp tips of as small as tens of nm can be fabricated) of the acoustic motors provide an opportunity to probe cellular structures that may not have been accessible with other particles. Fourth, by controlling the incubation time, nanomotors can be placed outside or inside living cells, enabling two distinct ways to manipulate and agitate the cells.
To investigate the interaction of gold nanomotors external to HeLa cells, they were mixed together in phosphate buffer saline (PBS) solution. Once ultrasound at the resonant frequency was applied, the gold rods and HeLa cells levitated to the mid-plane of the acoustic chamber where axial propulsion of the rods as well as slower propulsion of cells and rods towards in-plane nodes were observed. This is consistent with the behavior of magnetically steered nanowires in HeLa cell suspensions in an acoustic field.[16]
Under these conditions, the interaction between the co-suspended gold rods and HeLa cells was dominated by their surfaces (Fig. 3). Persistent attachment of rods to the HeLa cell surface was observed (Video S5). This attachment was fast, typically occurring immediately or shortly after the particles came into contact. The rods could attach at their tips or sides. In addition, the attachment of a gold rod to the surface of a HeLa cell did not noticeably affect the subsequent attachment of more rods. Although the mechanism of attachment remains unclear, both non-specific and specific chemical interactions may be involved. The cell and rod surfaces are negatively charged at neutral pH, but the high ionic strength (~ 0.2 mol/L) of the PBS buffer should collapse the electrical double layer and thus electrostatic repulsion between particles should be weak. Interactions with polar groups, especially amine and thiol groups on the cell surface, may lead to strong attachment to gold. We observe somewhat lower attachment probability of gold rods to dead HeLa cells, and ruthenium rods of similar shapes and sizes attach less persistently to the HeLa cells than gold rods.
Figure 3.
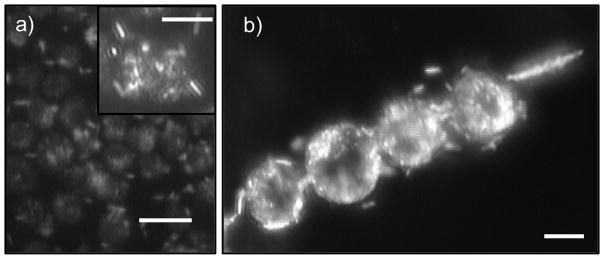
Gold rods attach strongly to the surface of HeLa cells when they are mixed together. a) optical micrograph of a dense aggregate of HeLa cells (dark spheres) with many rods (light particles) attached to the surface (scale bar: 20 μm). Inset: a magnified picture of the same aggregate showing one HeLa cell (center sphere) with many rods attached on the surface (scale bar: 10 μm). b) when both aligned at the acoustic nodal line, gold rods could induce fast rotation of HeLa cells to which they bound (scale bar: 10 μm). Figure 3 is a snapshot from Video S6. All images in this figure were taken in dark field.
When propelled by ultrasound, gold rods bound to the HeLa cell surface are intermittently released and move freely again until they attach to another cell (Figure. 4, Video S5). This attachment-release-attachment cycle can repeat many times, and represents an equilibrium between attractive binding forces and the propulsion force on the rods. There is no direct correlation between the speed of the motors and the duration of the attachment, and the duration was random even when a single motor was tracked (Fig. 4b). We also observed that HeLa cells with rods attached to their surfaces could rotate when they aligned at the nodal lines of the levitation plane, whereas unmodified HeLa cells did not (Fig. 3b and Video S6).
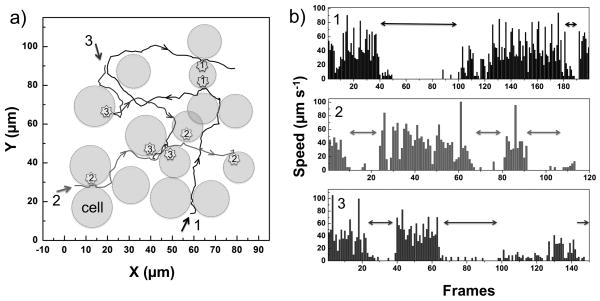
Figure 4.
Tracking analysis of three gold rods in a HeLa cell aggregate. a) the trajectory of three motors (motor #1, 2 and 3) among HeLa cells (represented as spheres). Arrows indicate the directions of motors, and stars indicate where the attachment of acoustic motors occurs, with numbering corresponding to each motor. b) the speeds of three motors (from top to bottom: motor #1, 2 and 3). Gaps (double arrows) indicate an attachment event that corresponds to a). The coordinates of each motor were tracked frame by frame at 30 frames per second (fps).
Because of the strong surface interaction, the activity of motors within a 2D matrix of HeLa cells is greatly limited (Fig. 3a and Fig. 4). Although the rods can be released from the cell surface, they are usually captured by nearby cells before they move far. Hopping between HeLa cells occurs intermittently, with rods spending most of their time bound to the cell surface. We note that reduced activity in a cancer cell matrix is a common problem for drug carriers, mostly due to the highly interconnected collagen fiber network and the elevated interstitial pressure of tumors.[18] Acoustic propulsion is therefore interesting in the context of drug delivery not only because the motors are actively propelled, but also because they can hop through a cell matrix, whereas passive particles typically lose their mobility once they attach to a cell surface.
To further investigate the effect of surface interactions on the movement of passive particles, gold rods were mixed with 10 μm diameter polystyrene (PS) spheres and human red blood cells (RBC). Very different interactions were observed. Gold rods, when approaching PS spheres or RBC, showed minimal surface attachment (more with RBC than PS), and altered their trajectory in a manner that resembled an elastic collision. The details of these experiments can be found in the SI and Video S7. These control experiments support the idea that specific chemical interactions control the adhesion of gold rods to HeLa cells.
In summary, HeLa cells readily take up gold nanorods when they are incubated together for a prolonged period of time (≥ 24 h). The rods remain acoustically active and undergo both directional motion and spinning inside the cells. Mechanical interactions between the gold rods and subcellular structures in HeLa cells are implicated by the tracking data. The introduction of synthetic nanomotors into living cells thus opens a new door to studying sub-cellular components and the response of cells to internal mechanical forces. In addition, functionalities such as sensing, molecule delivery, or photothermal excitation can be added to rod-shaped motors, enabling them to carry out different operations directly inside cells that may be interesting for biomedical applications.
Experimental Section
Gold nanorods were synthesized as previously described, and acoustic propulsion experiments were carried out in the same cylindrical cell used in earlier studies.[7] Full details are given in the Supporting Information.
Supplementary Material
Footnotes
W. W., S. A., T. J. H. and T. E. M. acknowledge support from the National Science Foundation under MRSEC grant DMR-0820404. S. X. and T. J. H. acknowledge support from the National Institute of Health (NIH) Director’s New Innovator Award (1DP2OD007209-01), NIH Clinical and Translational Science Award (UL1 TR000127) and Huck Innovative and Transformative Seed (HITS) Fund. Analytical instrumentation used in this work was supported by the Pennsylvania State University Materials Research Institute Nanofabrication Laboratory under National Science Foundation Cooperative Agreement No. ECS-0335765.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx. ((Please delete if not appropriate))
Contributor Information
Dr. Wei Wang, Department of Chemistry
Sixing Li, Department of Engineering Science and Mechanics, The Pennsylvania State University, University Park, PA 16802, USA.
Dr. Lamar Mair, Weinberg Medical Physics, LLC, Bethesda, MD, 20817, USA
Suzanne Ahmed, Department of Chemistry.
Prof. Tony Jun Huang, Email: junhuang@engr.psu.edu, Department of Engineering Science and Mechanics, The Pennsylvania State University, University Park, PA 16802, USA
Prof. Thomas E. Mallouk, Email: tem5@psu.edu, Department of Chemistry
References
- 1.a) Mallouk TE, Sen A. Sci Am. 2009:72–77. doi: 10.1038/scientificamerican0509-72. [DOI] [PubMed] [Google Scholar]; b) Wang W, Duan W, Ahmed S, Mallouk TE, Sen A. Nano Today. 2013;5:531–554. [Google Scholar]; c) Sengupta S, Ibele ME, Sen A. Angew Chem Int Edit. 2012;51:8434–8445. doi: 10.1002/anie.201202044. [DOI] [PubMed] [Google Scholar]; d) Ozin GA, Manners I, Fournier-Bidoz S, Arsenault A. Adv Mater. 2005;17:3011–3018. [Google Scholar]; e) Whitesides GM. Sci Am. 2001;285:78–83. doi: 10.1038/scientificamerican0901-78. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Gao W. ACS Nano. 2012;6:5745–5751. doi: 10.1021/nn3028997. [DOI] [PubMed] [Google Scholar]
- 3.a) Campuzano S, Orozco J, Kagan D, Guix M, Gao W, Sattayasamitsathit S, Claussen JC, Merkoçi A, Wang J. Nano Lett. 2011;12:396–401. doi: 10.1021/nl203717q. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sanchez S, Solovev AA, Schulze S, Schmidt OG. Chem Comm. 2011;2:698–700. doi: 10.1039/c0cc04126b. [DOI] [PubMed] [Google Scholar]
- 4.a) Wu J, Balasubramanian S, Kagan D, Manesh KM, Campuzano S, Wang J. Nat Commun. 2010;1:36. doi: 10.1038/ncomms1035. [DOI] [PubMed] [Google Scholar]; b) Kagan D, Calvo-Marzal P, Balasubramanian S, Sattayasamitsathit S, Manesh KM, Flechsig GU, Wang J. J Am Chem Soc. 2009;131:12082–12083. doi: 10.1021/ja905142q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Xi W, Solovev AA, Ananth AN, Gracias DH, Sanchez S, Schmidt OG. Nanoscale. 2013;5:1294–1297. doi: 10.1039/c2nr32798h. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kagan D, Benchimol MJ, Claussen JC, Chuluun-Erdene E, Esener S, Wang J. Angew Chem Int Edit. 2012;51:7519–7522. doi: 10.1002/anie.201201902. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Solovev AA, Xi W, Gracias DH, Harazim SM, Deneke C, Sanchez S, Schmidt OG. ACS Nano. 2012;6:1751–1756. doi: 10.1021/nn204762w. [DOI] [PubMed] [Google Scholar]
- 6.Syverton JT, Scherer WF. J Exptl Med. 1952;95:355–367. doi: 10.1084/jem.96.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Castro LA, Hoyos M, Mallouk TE. ACS Nano. 2012;6:6122–6132. doi: 10.1021/nn301312z. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Gradilla V, Orozco J, Sattayasamitsathit S, Soto F, Kuralay F, Pourazary A, Katzenberg A, Gao W, Shen Y, Wang J. ACS Nano. 2013;7:9232–9240. doi: 10.1021/nn403851v. [DOI] [PubMed] [Google Scholar]
- 9.Kuo CW, Lai JJ, Wei KH, Chen P. Adv Funct Mater. 2007;17:3707–3714. [Google Scholar]
- 104.Weiss M, Elsner M, Kartberg F, Nilsson T. Biophys J. 2004;87:3518–3524. doi: 10.1529/biophysj.104.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Prina-Mello A, Diao Z, Coey JM. J Nanobiotechnology. 2006;4:9. doi: 10.1186/1477-3155-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Tanase M, Felton EJ, Gray DS, Hultgren A, Chen CS, Reich DH. Lab Chip. 2005;5:598–605. doi: 10.1039/b500243e. [DOI] [PubMed] [Google Scholar]; c) Hultgren A, Tanase M, Felton EJ, Bhadriraju K, Salem AK, Chen CS, Reich DH. Biotechnol Progr. 2005;21:509–515. doi: 10.1021/bp049734w. [DOI] [PubMed] [Google Scholar]; d) Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. Proc Natl Acad Sci USA. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Alkilany AM, Murphy CJ. J Nanopart Res. 2010;12:2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Albanese A, Tang PS, Chan WCW. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]; c) Aderem A, Underhill DM. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]; d) Hu L, Mao ZW, Gao CY. J Mater Chem. 2009;19:3108–3115. [Google Scholar]
- 13.a) Gao JH, Xu B. Nano Today. 2009;4:281–281. [Google Scholar]; b) Davis ME, Chen Z, Shin DM. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 14.a) Wang N, Ingber DE. Biochem Cell Biol. 1995;73:327–335. doi: 10.1139/o95-041. [DOI] [PubMed] [Google Scholar]; b) de Vries AHB, Krenn BE, van Driel R, Kanger JS. Biophys J. 2005;88:2137–2144. doi: 10.1529/biophysj.104.052035. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Bausch AR, Möller W, Sackmann E. Biophys J. 1999;76:573–579. doi: 10.1016/S0006-3495(99)77225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Huang HD, Kamm RD, Lee RT. Am J Physiol-Cell Ph. 2004;287:C1–C11. doi: 10.1152/ajpcell.00559.2003. [DOI] [PubMed] [Google Scholar]
- 15.Safi M, Yan MH, Guedeau-Boudeville MA, Conjeaud H, Garnier-Thibaud V, Boggetto N, Baeza-Squiban A, Niedergang F, Averbeck D, Berret JF. ACS Nano. 2011;5:5354–5364. doi: 10.1021/nn201121e. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed S, Wang W, Mair LO, Fraleigh RD, Li S, Castro LA, Hoyos M, Huang TJ, Mallouk TE. Langmuir. doi: 10.1021/la403946j. in press. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Duan W, Sen A, Mallouk TE. Proc Natl Acad Sci USA. 2013;110:17744–17749. doi: 10.1073/pnas.1311543110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols JW, Bae YH. Nano Today. 2012;7:606–618. doi: 10.1016/j.nantod.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.