Abstract
Background
Disodium ethylene diamine tetraacetic acid (EDTA) reduced adverse cardiac outcomes in a factorial trial also testing oral vitamins.
Objective
This report describes the intent-to-treat comparison of the 4 factorial groups overall and in patients with diabetes.
Methods
Double-blind placebo-controlled 2 × 2 factorial multicenter randomized trial of 1708 post-MI patients ≥ 50 years and creatinine ≤2.0 mg/dL randomized to receive 40 EDTA chelation or placebo infusions plus 6 caplets daily of a 28-component multivitaminmultimineral mixture or placebo. Primary endpoint was a composite of total mortality, MI, stroke, coronary revascularization, or hospitalization for angina.
Results
Median age was 65 years, 18% female, 94% Caucasian, 37% diabetic, 83% prior coronary revascularization, and 73% on statins. Five-year Kaplan-Meier estimates for the primary endpoint in the chelation + high-dose vitamin group was 31.9%, in the chelation + placebo vitamin group 33.7%, in the placebo infusion + active vitamin group 36.6%, and in the placebo infusions + placebo vitamin group 40.2 %. The reduction in primary endpoint by double active treatment compared with double placebo was significant (HR 0.74, 95% CI (0.57,0.95); p=0.016). In patients with diabetes, the primary endpoint reduction of double active compared with double placebo was more pronounced (HR 0.49, 95% CI (0.33,0.75), p<0.001).
Conclusions
In stable post- MI patients on evidence-based medical therapy, the combination of oral high-dose vitamins and chelation therapy compared with double placebo reduced clinically important cardiovascular events to an extent that was both statistically significant and of potential clinical relevance.
INTRODUCTION
Chelation therapy with ethylenediamine tetra acetic acid (EDTA) has long been used to treat atherosclerotic coronary and peripheral artery disease.1,2 The Trial to Assess Chelation Therapy (TACT)3 and found that this treatment reduced clinical events in post-myocardial infarction patients, particularly in patients with diabetes.4 Chelation therapy is often administered in conjunction with a regimen of oral high-dose vitamins and minerals,5 notwithstanding that he results of clinical trials of lower dose vitamin therapy have generally been negative.6,7 Nonetheless, chelation practitioners argued forcefully during the design phase of TACT for the inclusion of an adjunctive high dose vitamin and mineral regimen. Thus, a 2 × 2 factorial design (intravenous chelation versus placebo plus oral vitamins versus placebo) was selected in order to control for the use of vitamins, study the effects of chelation with versus without high-dose vitamins and thereby eliminate potential confounding due to uncontrolled vitamin use by study participants.8
The clinical safety and efficacy of the TACT vitamin regimen has been reported.9 These analyses demonstrated a non-significant, 11% reduction in the risk of the primary combined endpoint. The purpose of this paper is to describe the results across the 4 factorial groups in the 1708 randomized patients and among the 633 with diabetes.
METHODS
Overview
TACT, ClinicalTrials.gov identifier NCT00044213, was a double-blind 2 × 2 factorial trial in which patients were randomized to four groups:
Active IV chelation infusions + active oral vitamins
Active IV chelation infusions + placebo oral vitamins
Placebo IV chelation infusions + active oral vitamins
Placebo IV chelation infusions + placebo oral vitamins
The design and organizational aspects of TACT have been published previously.8 The National Heart, Lung, and Blood Institute (NHLBI), grant # U01 HL92607 and the National Center for Complementary and Alternative Medicine (NCCAM), grant # U01AT001156 provided funding and oversight to support the research and creation of the paper. The institutional review board at each clinical site approved the study, and patients provided written informed consent. A Data and Safety Monitoring Board (DSMB) monitored the study. The authors are solely responsible for the design and conduct of the study, all study analyses, the drafting and editing of the paper and its final contents.
Study population
Patients were at least 50 years of age and had sustained a myocardial infarction 6 weeks or more prior to enrollment. Patients were ineligible if they were women of childbearing potential, had a serum creatinine >2.0 mg/dL, or had other exclusion criteria as previously reported8. Patients were enrolled at a total of 134 sites in the United States and Canada.
Subgroup with diabetes
The study protocol called for examination of various pre-specified subgroups, diabetes among them. Therefore we also report exploratory analyses of the 4 factorial groups in patients with diabetes.
Treatment
The contents of the preparation and administration of the EDTA and placebo EDTA infusion treatments used in TACT have been described8 (eTable1). Intravenous treatment consisted of 40 infusions of disodium EDTA-based chelation therapy or a normal saline placebo administered as 30 weekly infusions followed by 10 maintenance infusions 2 to 8 weeks apart. The active oral high-dose vitamin treatment was a 28-component mixture to be taken as 3 caplets twice daily until the end of follow-up. The components and dosing of the oral vitamins were developed with the assistance of chelation practitioners to reflect their standard practice (eTable 2).
Follow-up
Patients were seen at baseline, and at each chelation infusion visit. Following the infusion phase, patients were called quarterly, attended annual clinic visits, and were seen at the end of the trial or at the 5 year follow-up, whichever was first. Vitamin/placebo caplets were distributed on a 3 to 6 monthly basis. Unused pills were returned to the site in order to assess compliance.
Endpoints
The primary endpoint was a composite of death from any cause, reinfarction, stroke, coronary revascularization, or hospitalization for angina. The composite of cardiovascular death, reinfarction, or stroke was a prespecified key secondary endpoint. A blinded independent clinical events committee adjudicated all non-procedural components of the primary end-point. The data coordinating center (DCC) verified the occurrence of coronary revascularizations using patient medical records.
Safety
Safety monitoring included periodic physical examination and laboratory assessments. A masked Medical Monitor at the DCC reviewed all serious adverse events.
Pre-Specified Subgroups
TACT pre-specified several subgroups for analyses. The present report restricts itself to an analysis of the factorial groups in patients with diabetes prior to randomization, as previously defined.4
Statistical analysis
As previously reported3, TACT enrolled 1708 patients, with a length of follow-up selected to provide 85% power for detecting a 25% relative reduction in the primary endpoint for each treatment factor, assuming a 2.5-year event rate in the placebo arm of 20% and a significance level of 0.05.
The TACT statistical analysis plan pre-specified that the factorial groups would be analyzed for the overall study, in order to assess any interaction of chelation therapy with oral vitamins. The analyses of the 4 factorial groups in the diabetes subgroup was not pre-specified and, as such, should be considered an exploratory analysis.
Randomization and treatment comparisons have been previously described. 3 The log-rank test10 was used for the statistical comparison of treatment groups. Cumulative event rates were calculated according to the Kaplan-Meier method. 11 Relative risks were expressed as hazard ratios (HR) with associated confidence intervals (CI), and were calculated using the Cox proportional hazards model.12 Outcomes were compared across the factorial groups, both in the overall population as well as for the population of patients with diabetes. Comparisons of treatment groups with respect to adherence and safety were performed using the chi-square test. Continuous variables are expressed as medians and interquartile ranges (IQRs) unless otherwise specified. Statistical analyses were performed using SAS software, versions 8.2 and 9.2 (SAS Institute, Cary NC).
RESULTS
Between September 10, 2003 and October 4, 2010, 1708 patients were randomized, 421 to EDTA chelation infusions+ high dose oral multivitamins, 418 to EDTA chelation infusions+ oral placebo, 432 to placebo infusions + high-dose oral multivitamins, and 437 to placebo infusions + oral placebo. The median duration of follow-up was 55 months (IQR 26,60) overall. There was no significant difference in length of follow-up across all 4 groups.
Baseline characteristics
Baseline characteristics were similar among the 4 randomized factorial groups (Table 1). Patients were 65 (59,72) years old, 18% female, and 9% minority. The qualifying myocardial infarction had occurred 4.6 (1.6, 9.2) years prior to enrollment. There was a high prevalence of diabetes (37%), of prior coronary revascularizations (83%), and post-infarct, guideline-recommended medication use of aspirin (84%), beta-blocker (72%), and statin (73%).
Table 1.
Baseline Characteristics of Patients for All 4 Factorial Groups
| EDTA Chelation and High-Dose Vitamins (N=421) | EDTA Chelation and Placebo Vitamins (N=418) | Placebo Infusions and High-dose Vitamins (N=432) | Placebo Infusions and Placebo Vitamins (N=437) | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 64.9 (58.8, 71.4) | 65.2 (59.7, 71.6) | 65.6 (58.7, 72.2) | 65.5 (59.2, 71.9) |
| Female | 70 (17%) | 82 (20%) | 77 (18%) | 70 (16%) |
| Caucasian | 397 (94%) | 393 (94%) | 400 (93%) | 415 (95%) |
| BMI | 29.2 (26.5, 33.4) | 30.0 (26.6, 33.9) | 29.7 (25.9, 33.4) | 29.9 (27.0, 33.8) |
| Blood Pressure | ||||
| Systolic | 130.0 (118.0, 140.0) | 130.0 (120.0, 140.0) | 130.0 (119.0, 140.0) | 130.0 (120.0, 140.0) |
| Diastolic | 76.0 (70.0, 80.0) | 76.0 (70.0, 80.0) | 76.0 (68.0, 82.0) | 76.0 (70.0, 80.0) |
| History | ||||
| Hypercholesterolemia | 337 (81%) | 339 (83%) | 343 (81%) | 351 (82%) |
| Hypertension | 280 (67%) | 288 (69%) | 294 (68%) | 307 (70%) |
| Former cigarette smoker | 236 (56%) | 231 (55%) | 251 (58%) | 237 (54%) |
| Angina pectoris | 226 (54%) | 235 (56%) | 221 (51%) | 244 (56%) |
| Anterior MI | 174 (41%) | 163 (39%) | 167 (39%) | 170 (39%) |
| Diabetes | 159 (38%) | 163 (39%) | 164 (38%) | 147 (34%) |
| Congestive heart failure | 68 (16%) | 86 (21%) | 69 (16%) | 84 (19%) |
| Peripheral vascular disease | 60 (14%) | 66 (16%) | 65 (15%) | 77 (18%) |
| Stroke | 28 (7%) | 29 (7%) | 28 (6%) | 26 (6%) |
| Time from qualifying MI to randomization (years)* | 4.3 (1.7, 9.0) | 4.3 (1.8, 9.3) | 4.8 (1.4, 10.2) | 4.8 (1.6, 8.5) |
| NYHA Functional Class | ||||
| No heart failure or Class I | 389 (92%) | 375 (90%) | 397 (92%) | 398 (91%) |
| Coronary revascularization | ||||
| Either CABG or PCI | 350 (83%) | 344 (82%) | 355 (82%) | 365 (84%) |
| PCI | 238 (57%) | 253 (61%) | 246 (57%) | 270 (62%) |
| CABG | 198 (47%) | 186 (44%) | 192 (44%) | 198 (45%) |
| Concomitant Medications | ||||
| Aspirin, warfarin or clopidogrel | 386 (93%) | 382 (92%) | 395 (91%) | 389 (89%) |
| Aspirin* | 365 (87%) | 352 (84%) | 364 (84%) | 346 (79%) |
| Beta-blocker | 293 (70%) | 318 (76%) | 309 (72%) | 306 (70%) |
| Statin | 310 (74%) | 305 (73%) | 319 (74%) | 314 (72%) |
| ACE or ARB | 256 (61%) | 269 (64%) | 273 (63%) | 286 (65%) |
| Clopidogrel | 101 (25%) | 111 (28%) | 99 (24%) | 114 (27%) |
| Warfarin | 28 (7%) | 45 (11%) | 32 (8%) | 43 (10%) |
| Diabetes medication | ||||
| Oral hypoglycemic | 103 (25%) | 88 (22%) | 104 (25%) | 85 (20%) |
| Insulin* | 25 (6%) | 48 (12%) | 46 (11%) | 41 (10%) |
| Laboratory Examinations | ||||
| Total cholesterol (mg/dL) | 164.0 (139.0, 193.0) | 161.5 (141.0, 192.0) | 163.5 (141.5, 194.0) | 169.0 (144.0, 202.5) |
| Triglycerides (mg/dL) | 138.0 (99.0, 203.0) | 131.0 (91.0, 193.0) | 145.0 (101.0, 206.0) | 147.0 (99.0, 210.0) |
| Glucose (mg/dL) | 103.0 (92.0, 120.0) | 102.5 (92.0, 123.0) | 102.0 (91.0, 124.0) | 103.0 (93.0, 119.0) |
| LDL (mg/dL) | 88.0 (66.5, 113.5) | 86.0 (66.0, 111.0) | 87.5 (66.0, 112.5) | 93.0 (71.0, 122.0) |
| HDL (mg/dL) | 43.0 (36.0, 52.0) | 43.0 (36.4, 52.0) | 43.0 (37.0, 51.0) | 41.0 (36.0, 50.0) |
| Creatinine (mg /dL) | 1.1 (0.9, 1.2) | 1.1 (0.9, 1.2) | 1.1 (0.9, 1.2) | 1.1 (0.9, 1.2) |
P-value is < 0.05.
Factorial Treatment Comparisons (overall group)
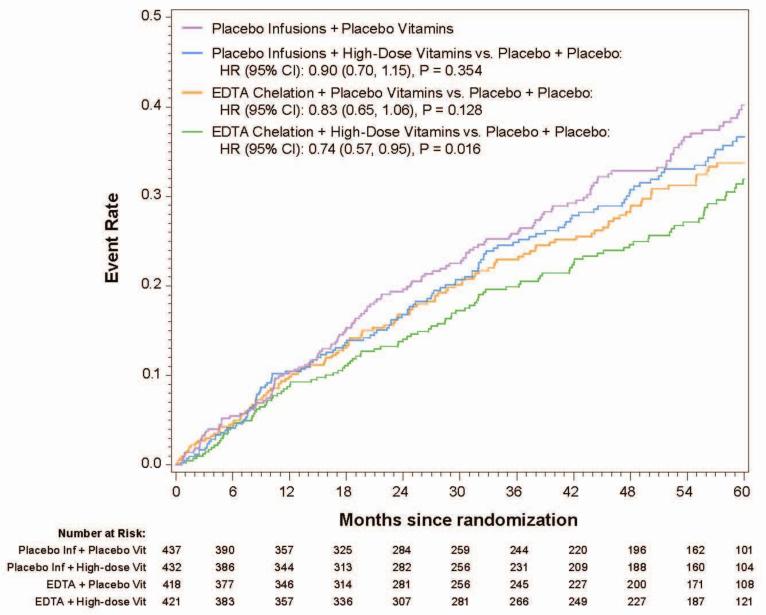
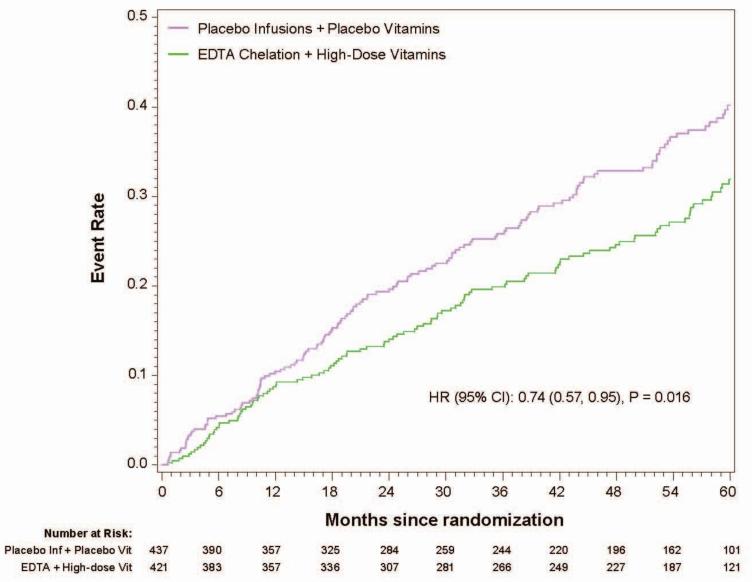
The 5-year Kaplan-Meier event rate estimate for the primary endpoint in the chelation + high-dose vitamin group was 31.9%, in the chelation + placebo vitamin group 33.7%, in the placebo infusion + active vitamin group 36.6%, and in the placebo infusions + placebo vitamin group 40.2 % (Figure 1a, Table 2,). The primary endpoint by treatment group occurred in 139 (32%) of the placebo infusion + placebo vitamin group, and 108 (26%) of patients in the chelation + high-dose vitamin group (Figure 1b). The groups with one active therapy had an intermediate number of events and were not statistically significantly different from the placebo-placebo group. The comparison of active infusion + active vitamins with placebo infusions + placebo vitamins was significant (HR 0.74, 95% CI (0.57,0.95); p=0.016). The absolute difference in 5-year Kaplan-Meier estimated event rates between placebo-placebo and active-active groups was 8.3% and the number needed to treat (NNT) to prevent 1 event over 5 years was 12.
Figure 1a.
Kaplan-Meler curves(4 groups, 10 endpoint, factorial)
Table 2.
Primary and Secondary Endpoint Components for all Four Factorial Groups
| EDTA Chelation and High-Dose Vitamins (N=421) | EDTA Chelation and Placebo Vitamins (N=418) | Placebo Infusions and High-dose Vitamins (N=432) | Placebo Infusions and Placebo Vitamins (N=437) | P-value* | |
|---|---|---|---|---|---|
| Primary Endpoint | |||||
| All-cause mortality, myocardial infarction, stroke, coronary revascularization or hospitalization for angina | 108 (26%) | 114 (27%) | 122 (28%) | 139 (32%) | 0.016 |
| Death | 43 (10%) | 44 (11%) | 44 (10%) | 49 (11%) | 0.490 |
| Myocardial infarction | 23 (5%) | 29 (7%) | 35 (8%) | 32 (7%) | 0.207 |
| Stroke | 4 (1%) | 6 (1%) | 4 (1%) | 9 (2%) | 0.161 |
| Coronary revascularization | 60 (14%) | 70 (17%) | 72 (17%) | 85 (19%) | 0.017 |
| Hospitalization for angina | 6 (1%) | 7 (2%) | 6 (1%) | 12 (3%) | 0.147 |
| Secondary Endpoint | |||||
| Cardiovascular death, myocardial infarction or stroke | 39 (9%) | 57 (14%) | 55 (13%) | 58 (13%) | 0.045 |
| Cardiovascular death | 19 (5%) | 31 (7%) | 26 (6%) | 25 (6%) | 0.355 |
Log-rank 1 df p-values. This is a comparison of the active-active vs. placebo-placebo cells only.
Figure 1b.
Kaplan-Meier curves placebo/placebo vs. active/active (10 endpont, factorial)
The principal secondary endpoint, cardiovascular death, MI, or stroke occurred in 58 (13%) of the placebo infusions + placebo vitamin group, 57 (14%) of the chelation + placebo vitamin group, 55 (13%) of the placebo infusion + active vitamin group, and 39 (9%) of patients in the chelation + high-dose vitamin group. The comparison of active infusion + active vitamins with placebo infusions + placebo vitamins favored chelation + vitamins (HR 0.66, 95% CI 0.44,0.99, p=0.046). The groups with one active therapy had an intermediate number of events, and were not statistically significantly different from the placebo-placebo group.
Treatment adherence
There were no significant differences in adherence to IV infusions or to oral vitamins between groups (Table 3). Consent withdrawal at some point during the trial was reported in 289 patients. A greater frequency of consent withdrawals occurred among patients randomized to placebo infusions.
Table 3.
Patient Status by all Treatment Arms
| EDTA Chelation & High-Dose Vitamins (N= 421) | EDTA Chelation & Placebo Vitamins (N= 418) | Placebo Infusions & High-Dose Vitamins (N= 432) | Placebo Infusions & Placebo Vitamins (N= 437) | P-value | |
|---|---|---|---|---|---|
| Patient Status | |||||
| Number of infusions | 40 (32, 40) | 40 (31, 40) | 40 (26, 40) | 40 (30, 40) | 0.401 |
| Discontinued infusions | 114 (27%) | 119 (28%) | 146 (34%) | 135 (31%) | 0.152 |
| Completed 30 infusions | 324 (77%) | 323 (77%) | 319 (74%) | 329 (75%) | 0.622 |
| Completed 40 infusions | 283 (67%) | 282 (67%) | 267 (62%) | 285 (65%) | 0.275 |
| Discontinued vitamins | 185 (44%) | 185 (44%) | 209 (48%) | 205 (47%) | 0.503 |
| Continued vitamins for at least 1 year | 328 (78%) | 321 (77%) | 317 (73%) | 325 (74%) | 0.384 |
| Continued vitamins for at least 3 years | 210 (50%) | 216 (52%) | 190 (44%) | 210 (48%) | 0.135 |
| Consent withdrawal | 50 (12%) | 65 (16%) | 91 (21%) | 83 (19%) | 0.002 |
Safety
Serious adverse events were documented in 55 patients (13%) of the EDTA chelation and high-dose vitamin group, 45 (11%) of the EDTA chelation and placebo vitamin group, 69 (16%) of the placebo infusion and high-dose vitamin group, and 58 (13%) of the placebo infusion and placebo vitamin group (p= 0.170).
Diabetes analyses
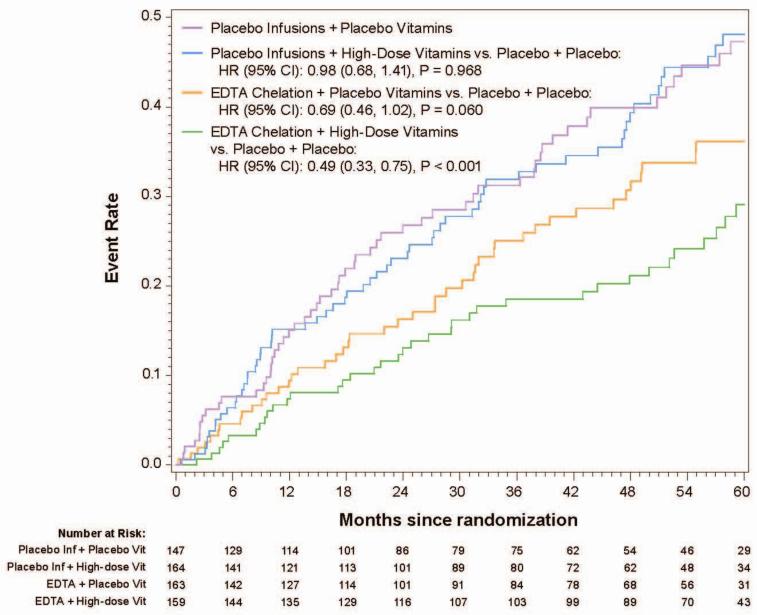
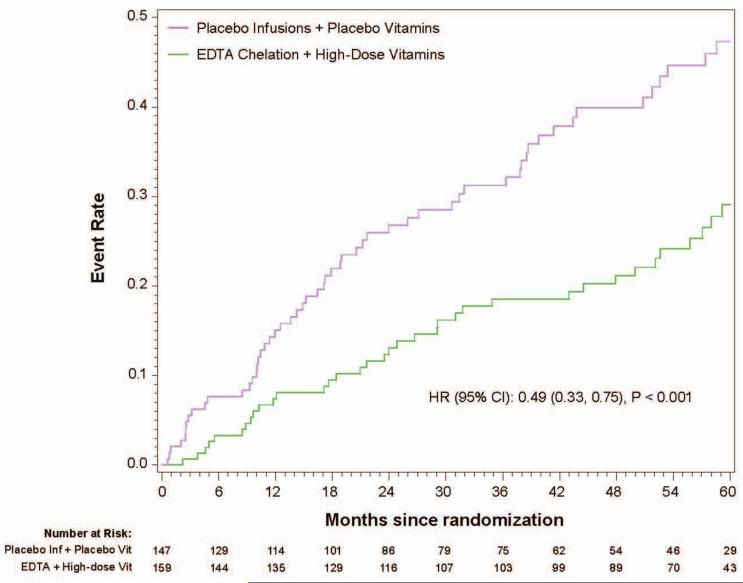
In the 633 patients with diabetes, the 5-year Kaplan-Meier event rate estimates for the primary endpoint in the chelation + high-dose vitamin group was 29.1%, in the chelation + placebo vitamin group 36.1%, in the placebo infusion + active vitamin group 48.1%, and in the placebo infusions + placebo vitamin group 47.3% (Figure2a). The primary endpoint by treatment group occurred in 56 (38%) of the placebo infusion + placebo vitamin group, and 36 (23%) of patients in the chelation + high-dose vitamin group (HR 0.49, 95% CI (0.33,0.75) p<0.001, 5-year NNT=5.5, Figure 2b). The factorial groups receiving only one active treatment had intermediate treatment benefit, not statistically significantly different from double placebo.
Figure 2a.
Kaplan-Meier curves(4groups, 10 endpoint, diabetes)
Figure 2b.
Kaplan-Meier curves placebo/placebo vs. active/active(10 endpoint, diabetes)
DISCUSSION
TACT was designed as a factorial trial of intravenous EDTA-based chelation and high-dose oral vitamins, to reflect chelation practice in the community, and control for confounding. Thus, we pre-specified an analysis of the 4 groups of the factorial treatment allocation. The analyses demonstrated a stepwise gradient in benefit, with highest risk accrued by patients on standard post-MI care, but neither chelation nor vitamins; intermediate risk by patients receiving only one intervention; and lowest risk by patients receiving both chelation and vitamins. When compared with patients receiving placebo only, the hazard ratio of patients receiving both the study interventions was 0.74 (95%CI 0.57, 0.95, p=0.016), with the 5-year NNT for the primary endpoint of 12. This compares with the 5-year NNT of 16 for statin therapy for secondary prevention.13 These effects were observed against a background of modern, evidence-based treatments for post-MI patients, including statins in 73% of patients, with a median LDL cholesterol of 89 mg/dL. Moreover, the benefit of combined therapy in patients with diabetes was greater, with a 5-year NNT for the primary endpoint of 5.5, again with a background of statin therapy in 76% of the diabetic patients.
Others have reported epidemiological14,15,16 and experimental findings 17 18 19 that may explain benefits of metal chelation in cardiovascular disease. Lead and cadmium are associated with myocardial infarction, stroke, hypertension, and death. Mechanisms include individual toxicities for each metal ion, but also a class-specific action on the body's defenses against oxidant stress. EDTA chelates environmental contaminants like lead, cadmium, antimony, tungsten, and many others20. In diabetic patients, copper and iron, both chelated by EDTA, are tightly linked to non-enzymatic catalytic oxidation of glucose, leading to the formation of advanced glycation end products. Other metals,21 also chelated by EDTA, may be involved with these redox reactions in diabetic patients, accounting for yet another mechanism of action for EDTA. The xenobiotic metal hypothesis is particularly appealing because the clinical benefits of chelation persist even after the infusions stop, with continued late separation of event curves.
There are other potential explanations for the observed treatment effect. The chelation solution contains a high dose of vitamin C, an antioxidant vitamin that may help reverse some forms of endothelial dysfunction.22 Whether repetitive infusions of vitamin C could lead to the persistent effect observed in TACT after infusions stop, however, seems doubtful.
Vitamin therapy has been exhaustively studied in clinical trials as primary prevention for coronary disease. Those trials, which have largely failed to detect any evidence of a treatment benefit, have almost all used one or a small numbers of single vitamins at modest doses.7,23 Thus, the lack of benefit of oral vitamins and minerals on cardiovascular events in prior studies should be recognized as pertaining to a different regimen than the high dose oral multivitamin and mineral regimen used here and a different (primary versus secondary prevention) study population.
The incremental benefit observed in the vitamin + chelation group calls for a methodological explanation. We reported that there was a non-significant, 11% reduction in the point estimate of the primary endpoint with oral vitamin therapy9 Our trial was not powered to detect an 11% difference between groups with sufficient precision to exclude the null effect. This small benefit of oral vitamin therapy, although not statistically significant by itself, may explain the incremental reduction in hazard ratio, from 0.823 to 0.74, we observed when patients receiving both active treatments were compared to the double placebo patients. A similar explanation applies to the large benefit observed in patients with diabetes treated with the double active regimen, compared with the double placebo.
Study caveats
Given the unexpected findings of TACT for practitioners of cardiovascular medicine, establishing the clinical and scientific significance of the TACT findings will require the performance of additional (i.e., more than one) high quality, adequately powered clinical trials, along with relevant laboratory studies to help identify mechanisms of benefit.
Noncompliance with randomized treatment likely reduced the power of the study to discern a difference between groups. The compliance issues have been reviewed in detail in prior publications, and the significance of chelation therapy benefit was maintained in conservative sensitivity analyses3,4 In addition, all patients had their death index status checked at the end of the study, and some patients withdrew after having sustained a primary endpoint, which mitigates some loss of data.
Conclusions
In stable post- MI patients on evidence-based medical therapy, the combination of oral high-dose vitamins and chelation therapy compared with double placebo reduced clinically important cardiovascular events to an extent that was both statistically significant and of potential clinical relevance.
Disclaimer
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Center for Complementary and Alternative Medicine, or the National Institutes of Health.
Acknowledgements
The authors gratefully acknowledge the organizational skills of Ana Mon MPH Project Leader at the Clinical Coordinating Center, Alyssa Cotler at NCCAM, Susan Dambrauskas (formerly at NHLBI), and Vivian Thompson at the DCRI for their competent professional assistance, and the Florida Heart Research Institute for supporting the pilot study. Gervasio Lamas MD reports that from 2000 to 2003 he served as a consultant to OmniComm, the electronic data capture company used in the trial. No funds were received, and all ties were severed as of 09/10/2003. The authors have no other conflicts to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clarke NE, Clarke CN, Mosher RE. Treatment of angina pectoris with disodium ethylene diamine tetraacetic acid. Am J Med Sci. 1956;232(6):654–666. doi: 10.1097/00000441-195612000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Grier MT, Meyers DG. So much writing, so little science: A review of 37 years of literature on Edetate Sodium chelation therapy. Ann Pharmacother. 1993;27:1504–1509. doi: 10.1177/106002809302701217. [DOI] [PubMed] [Google Scholar]
- 3.Lamas GA, Goertz C, Boineau R. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013 Mar 27;309(12):1241–50. doi: 10.1001/jama.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escolar E, Lamas GA, Mark DB. The Effect of an EDTA-based Chelation Regimen on Patients With Diabetes Mellitus and Prior Myocardial Infarction in the Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes. 2014;7:15–24. doi: 10.1161/CIRCOUTCOMES.113.000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozema TC. Special issue: protocols for chelation therapy. J Adv Med. 10:5–100. 997. [Google Scholar]
- 6.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. J Am Med Assoc. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 7.Sasso HD, Christen WG, Bubes V, et al. Multivitamins in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. Nov 7. 2012;308(17):1751–60. doi: 10.1001/jama.2012.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamas GA, Goertz C, Boineau R, et al. Design of the trial to assess chelation therapy (TACT). Am Heart J. 2012;163(1):7–12. doi: 10.1016/j.ahj.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamas G, Boineau R, Goertz C, et al. Oral High-Dose Multivitamins and Minerals After Myocardial Infarction. A Randomized, Controlled Trial. Ann Intern Med. 2013;159:797–804. doi: 10.7326/0003-4819-159-12-201312170-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Second Edition John Wiley & Sons, Inc; New York: 2002. [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–481. [Google Scholar]
- 12.Cox DR. Regression models and life-tables (with discussion). J Royal Statist Soc B. 1972;34:187–220. [Google Scholar]
- 13.Costa J, Borges M, David C, Vaz Carneiro A. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials. BMJ. 2006;332:1115–24. doi: 10.1136/bmj.38793.468449.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tellez-Plaza M, Guallar E, Fabsitz RR, et al. Cadmium exposure and incident peripheral arterial disease. Circ Cardiovasc Qual Outcomes. 2013;6:626–33. doi: 10.1161/CIRCOUTCOMES.112.000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menke A, Muntner P, Batuman VV, Silbergeld EK, Guallar E. Blood lead below 0.48 μmol/L (10 μg/dL) and mortality among US adults. Circulation. 2006;114(13):1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 16.Nawrot TS, Staessen JA. Low-level environmental exposure to lead unmasked as silent killer. Circulation. 2006;114:1347–9. doi: 10.1161/CIRCULATIONAHA.106.650440. [DOI] [PubMed] [Google Scholar]
- 17.Monnier VM. Transition metals redox: reviving an old plot for diabetic vascular disease. J Clin Invest. 2001;107:799–801. doi: 10.1172/JCI12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagai R, Murray DB, Metz TO, et al. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes. 2012;61:549–59. doi: 10.2337/db11-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frizzell N, Baynes JW. Chelation therapy: overlooked in the treatment and prevention of diabetes complications? Future Med Chem. 2013;5:1075–8. doi: 10.4155/fmc.13.73. [DOI] [PubMed] [Google Scholar]
- 20.Cranton EM, Liu ZX, Smith IM. Urinary Trace and Toxic Elements and Minerals in Untimed Urine Specimens. In Textbook on EDTA Chelation Therapy. 2nd Ed. Hampton Roads Publishing Co; Charlottesville, VA: 2001. pp. 503–539. [Google Scholar]
- 21.Agarwal S, Zaman T, Tuzcu EM, Kapadia SR. Heavy metals and cardiovascular disease: results from the National Health and Nutrition Examination Survey (NHANES) 1999-2006. Angiology. 2011;62(5):422–429. doi: 10.1177/0003319710395562. [DOI] [PubMed] [Google Scholar]
- 22.Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF, Jr, Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1996;93(6):1107–1113. doi: 10.1161/01.cir.93.6.1107. [DOI] [PubMed] [Google Scholar]
- 23.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261–6. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]