Abstract
Central blood pressure may be more closely associated with cardiovascular events than peripheral blood pressure. The aim of the present study was to investigate central blood pressure responses to exercise. Apparently healthy 18 subjects were enrolled in the study (38 ± 6 years) and changes in central and brachial blood pressure were recorded in response to ergometer and hand-grip exercises. Central blood pressure was estimated using an automated device (Omron HEM-9000AI). Systolic brachial blood pressure was increased after both ergometer (from 119 ± 10 to 172 ± 16 mmHg; P < 0.001) and hand-grip (from 118 ± 8 to 122 ± 9 mmHg; P = 0.001) exercises, but central systolic blood pressure was increased only after hand-grip exercise (from 117 ± 11 to 121 ± 12 mmHg; P = 0.002). The radial augmentation index was increased after hand-grip exercise, whereas ergometer exercise reduced this index. Heart rate was increased only after ergometer exercise. Thus, isometric, but not isotonic, exercise may increase central blood pressure in overall healthy subjects. The response of central blood pressure, which is a better index of cardiac load than peripheral blood pressure, to hand-grip exercise may be useful in evaluating cardiovascular risk.
A chronic increase in blood pressure is a major risk factor for cardiovascular disease, whereas reducing blood pressure reduces cardiovascular events1. Arterial pressure varies depending on the site in the arterial tree due to amplification of systolic blood pressure (SBP), and pulse pressure, from central to peripheral sites; thus, central blood pressure differs from peripheral blood pressure. Blood pressure measured over the brachial artery (peripheral blood pressure) is routinely used for individual risk evaluation and management of hypertension because it has been established as a powerful predictor of cardiovascular morbidity and mortality2,3,4,5. However, recent studies suggest that central blood pressure is more closely associated with target organ damage than peripheral blood pressure6,7,8. Furthermore, cardiovascular events are more closely associated with central rather than peripheral blood pressure9,10,11,12,13,14.
Most evidence on blood pressure as a surrogate marker of cardiovascular events was derived from blood pressures obtained at rest. However, blood pressure changes every moment in response to physical and mental stress, and peripheral blood pressure measured during exercise has been recognized as a marker of cardiovascular risk independent of resting peripheral blood pressure15,16,17,18. The risk related to a hypertensive response to exercise may be better assessed by central blood pressure given the greater impact of central compared with peripheral blood pressure on left ventricular afterload and myocardial oxygen consumption19. There have been a few studies investigating the effects of isotonic ergometer exercise on central blood pressure, but little is known about the effects of isometric exercise on central blood pressure. Hemodynamic responses to isometric exercises differ from those to isotonic exercises, and central blood pressure may respond differently to isometric and isotonic exercises. Thus, the aim of the present study was to compare the effects of isometric and isotonic exercise on central blood pressure. Drug therapy, especially for cardiovascular diseases, may modify the response of central and peripheral blood pressure to exercises and, thus, the present study included overall healthy subjects who were not taking any medication.
Results
The characteristics of the study subjects are given in Table 1. The blood pressure of all subjects in the study was within the normal range, indicating effective lifestyle modification. Central blood pressure tended to be lower than peripheral blood pressure measured over the brachium (P = 0.05), whereas late systolic blood pressure in the radial artery (SBP2) was lower than peripheral blood pressure (P < 0.0001).
Table 1. Characteristics of the study subjects.
| Age (years) | 37.8 ± 6.7 |
| Height (cm) | 171.8 ± 5.0 |
| Weight (kg) | 68.7 ± 9.0 |
| BMI (kg/m2) | 23.3 ± 3.2 |
| SBP (mmHg) | 119.4 ± 10.2 |
| DBP (mmHg) | 68.3 ± 10.3 |
| HR (b.p.m.) | 61.7 ± 11.3 |
| Central SBP (mmHg) | 114.4 ± 12.0 |
| SBP2 (mmHg) | 103.1 ± 11.2 |
| Radial AI (%) | 61.5 ± 11.6 |
| Serum creatinine (mg/dL) | 0.8 ± 0.1 |
| Uric acid (mg/dL) | 6.2 ± 0.8 |
| FPG (mg/dL) | 94.8 ± 9.0 |
| LDL-C (mg/dL) | 108.7 ± 22.8 |
| HDL-C (mg/dL) | 61.3 ± 12.1 |
| Triglyceride (mg/dL) | 105.4 ± 48.5 |
Data are given as the mean ±SD or as the number of subjects in a group with percentages in parentheses.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; AI, augmentation index; FPG, fasting plasma glucose; SBP2, late systolic pressure in the radial artery; HDL-C, high-density lipoprotein–cholesterol; LDL-C, low-density lipoprotein–cholesterol.
Ergometer exercise markedly increased peripheral systolic blood pressure (from 119 ± 10 to 172 ± 16 mmHg; Figure 1), but not diastolic blood pressure (from 68 ± 10 to 73 ± 13 mmHg; Figure 2). Heart rate was also increased by ergometer exercise (from 62 ± 11 to 118 ± 11 b.p.m.; Figure 3). The augmentation index (AI) obtained from the radial arterial pressure waveform was markedly reduced by isotonic exercise (from 61.4 ± 12.4% to 42.2 ± 7.7%; Figure 4). Ergometer exercise did not affect central blood pressure (from 114 ± 12 to 116 ± 13 mmHg; P = 0.39; Figure 5). Hand-grip exercise also increased peripheral systolic blood pressure (from 118 ± 8 to 122 ± 9 mmHg; Figure 1), but the increase was significantly smaller after hand-grip than ergometer exercise (4.0 ± 4.6 vs 54.5 ± 16.0 mmHg, respectively; P < 0.0001). Peripheral diastolic blood pressure was not altered by hand-grip exercise (from 74 ± 12 to 74 ± 12 mmHg; Figure 2). Furthermore, hand-grip exercise did not have any significant effect on heart rate (from 59 ± 9 to 59 ± 8 b.p.m.; Figure 3). In contrast to the response to ergometer exercise, hand-grip exercise slightly, but significantly increased both radial AI (from 66.4 ± 9.2 to 69.2 ± 9.4%; Figure 4) and central blood pressure (from 117 ± 11 to 121 ± 12 mmHg; Figure 5).
Figure 1. Peripheral systolic blood pressure responses, measured over the brachium, to ergometer and hand-grip exercises.
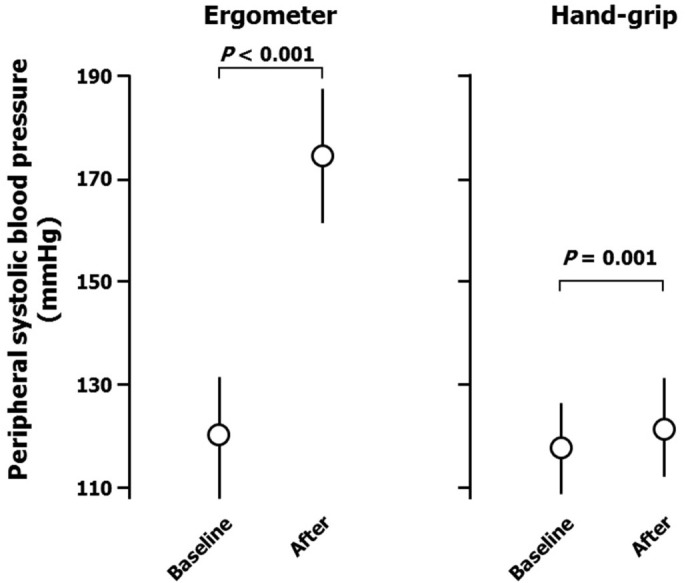
Data are the mean ± SD. The P-values shown were calculated by paired t-tests.
Figure 2. Peripheral diastolic blood pressure responses, measured over the brachium, to ergometer and hand-grip exercises.
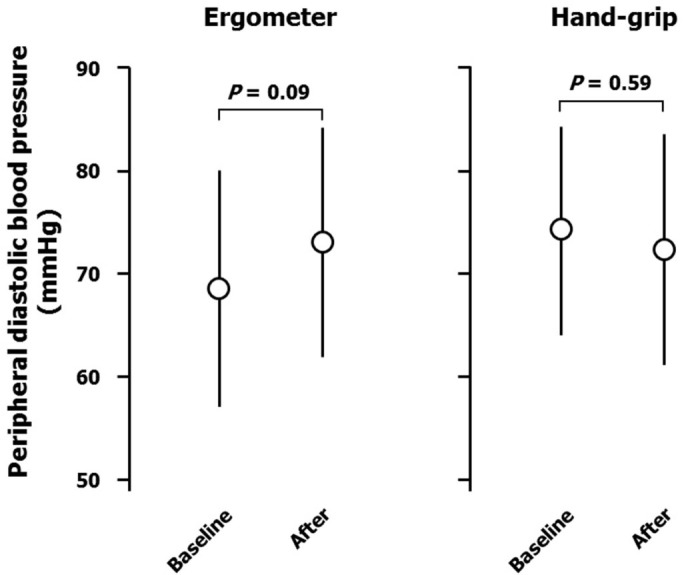
Data are the mean ± SD. The P-values shown were calculated by paired t-tests.
Figure 3. Heart rate responses to ergometer and hand-grip exercises.
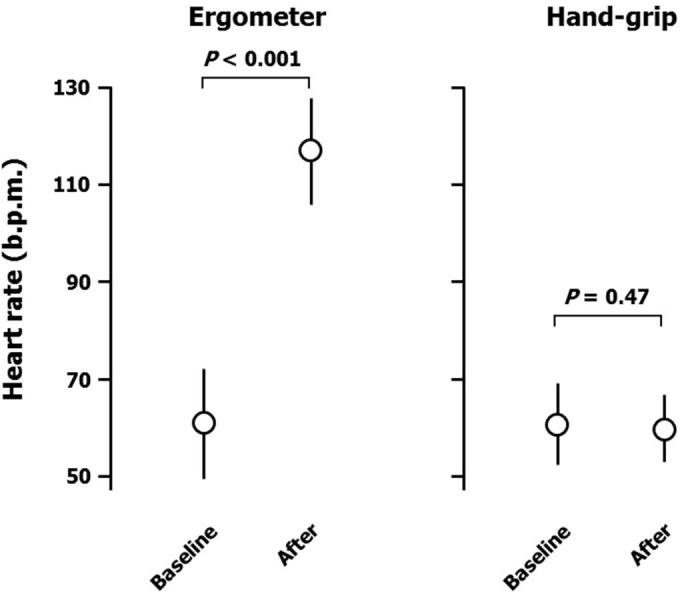
Data are the mean ± SD. The P-values shown were calculated by paired t-tests.
Figure 4. Central systolic blood pressure responses to ergometer and hand-grip exercises.
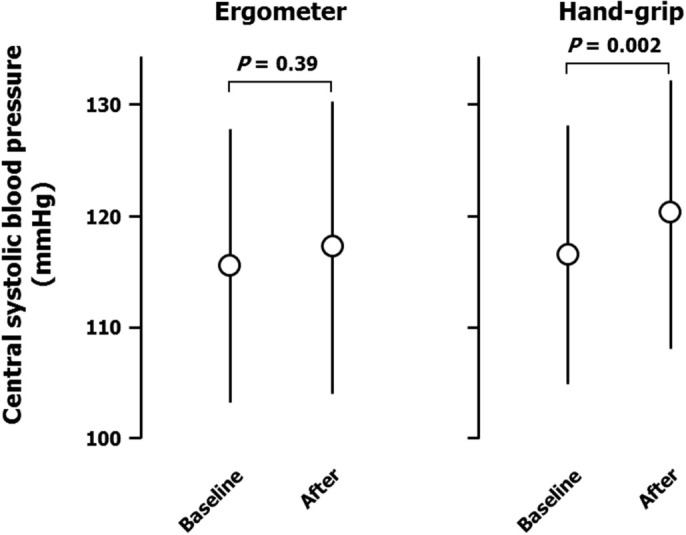
Data are the mean ± SD. The P-values shown were calculated by paired t-tests.
Figure 5. Response of augmentation index obtained from pressure waveform of the radial artery to ergometer and hand-grip exercises.
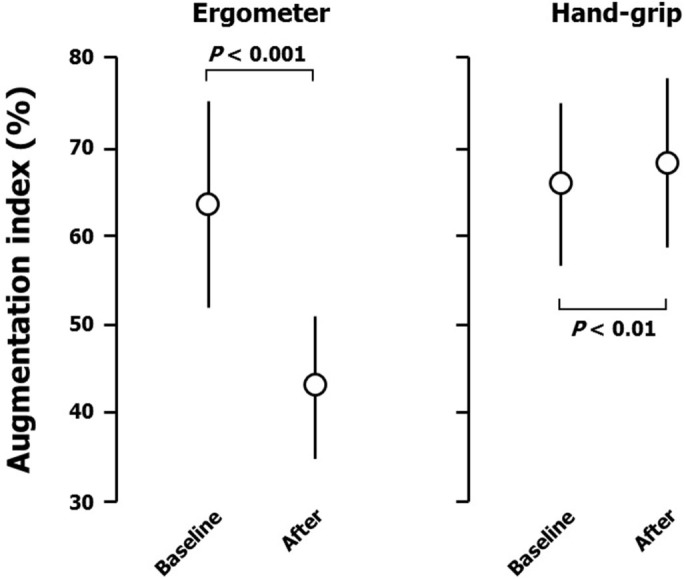
Data are the mean ± SD. The P-values shown were calculated by paired t-tests.
Central and peripheral blood pressures are different indices, but the two are closely associated in individuals. Thus, the ratio of central systolic blood pressure to peripheral systolic blood pressure was calculated in each individual and changes in this variable were compared after ergometer and hand-grip exercises (−0.29 ± 0.08 vs 0.00 ± 0.02, respectively; P < 0.0001). The results confirm the differential responses of central blood pressure to hand-grip and ergometer exercises.
Discussion
In the present study, central systolic blood pressure was increased after isometric hand-grip, but not isotonic ergometer, exercise. Because central blood pressure is a better index of cardiac load than peripheral blood pressure, the response of central blood pressure to hand-grip exercise may provide useful information when evaluating cardiovascular risk in overall healthy individuals.
The most important finding of the present study is that central blood pressure responded differently to ergometer and hand-grip exercises. The precise mechanism underlying the different responses of central blood pressure to isotonic and isometric exercises is not clear, but changes in the pressure waveform of the radial artery and peripheral blood pressure after the exercises provide some clues as to the mechanism involved. Ergometer exercise increased cardiac output and thereby increased the amplitude of the forward traveling wave. This may have resulted in an increase in the amplitude of the reflected wave. However, peripheral arterial dilatation caused by the isotonic exercise may have reduced the amplitude of the reflected wave26,27. Indeed, radial AI was markedly reduced although the amplitude of radial pulse pressure was increased after ergometer exercise. Central blood pressure may have been determined by the balance between an increase in cardiac output (increased forward traveling wave) and that in vascular relaxation (decreased reflected wave). Alternatively, the reduction in radial AI may be due to an increase in heart rate after exercise28. It is also possible that the elastic aorta buffered an increase in central aortic pressure caused by exercise. In contrast with the response to ergometer exercise, hand-grip exercise may have increased arterial resistance and thereby increased the amplitude of the radial reflected wave26,27. This is compatible with the finding that radial AI was augmented after hand-grip exercise. Moreover, increased stiffness in the conduit artery may have augmented pulse wave velocity, resulting in an increase in radial AI and premature return of the reflected wave in late systole in the central aorta. These responses after the isometric exercise may have been related to the increase in central blood pressure observed after hand-grip exercise. Cardiac output may have been increased after hand-grip exercise, but this effect was smaller than that after ergometer exercise because peripheral blood pressure showed only a mild increase. Changes observed after hand-grip exercise can be summarized as a product of arterial contraction caused by the isometric exercise.
In the present study, central blood pressure was estimated non-invasively using the Omron device. However, the superiority of central blood pressure over brachial blood pressure in the management of hypertension has been well established using the SphygmoCor device (AtCor Medical, Sydney, NSW, Australia), which records the radial pulse waveform and converts it to a central blood pressure waveform using a generalized transfer function10,11,29,30. Central blood pressure is then estimated by calibration against brachial blood pressure31,32. The Omron device records the radial pulse waveform and SBP2 is obtained by calibration against brachial blood pressure. Central systolic blood pressure is estimated using a regression equation21,25. Central systolic blood pressure values estimated by the Omron device are highly correlated with those estimated by a SphygmoCor device22,23,24 and these non-invasive estimations show close correlation with invasive measurements of central blood pressure21,24,25. However, both devices underestimate central systolic blood pressure, with the SphygmoCor device producing a larger deviation than the Omron device (average −15 vs −2 mmHg, respectively)24. Thus, the central blood pressure response observed in the present study may reflect the response of arterial pressure in the ascending aorta. The finding that the central blood pressure value estimated by the Omron device was not significantly lower than peripheral blood pressure measured over the brachium may sound strange. However, this is quite natural, because the measurement of peripheral blood pressure using a cuff over the brachial artery underestimates brachial arterial blood pressure33.
In contrast with the results of the present study, increases in central blood pressure after ergometer exercise were observed in a previous study in which a SphygmoCor device was used to estimate central blood pressure34. In the present study, central blood pressure was measured after ergometer exercise to minimize artifact due to exercise, whereas in the previous study the hemodynamic measurements were performed during exercise. This may explain, at least in part, the differences between these two studies. Alternatively, the discrepancy between the present and previous studies may be attributable to differences in the methods used to estimate central blood pressure.
Several studies have reported that peripheral blood pressure measured during exercise is a marker of cardiovascular risk independent of resting peripheral blood pressure15,16,17,18. However, central blood pressure has been shown to be a more important determinant of vascular function and cardiovascular risk than peripheral blood pressure. An increase in central blood pressure means increased pulsatile stress in the aorta, as well as increased left ventricular afterload and myocardial oxygen consumption, which can be detected only after measurement (estimation) of central blood pressure because marked differences exist between central and peripheral blood pressure20. Thus, evaluation of the response of central blood pressure to exercises is quite important and may provide useful information for risk assessment.
Interpretation of the results of the present study is limited by the following concerns. First, the accuracy of central blood pressure estimation using the Omron device during exercise has not been validated. Although central blood pressure was estimated after, but not during, exercise, in the present study, it is possible that a measurement artifact related to the exercise may have affected the results. Second, the intensity of the exercises was not considered in the present study although the response to exercise may vary depending on exercise intensity. Indeed, heart rate was not increased after hand-grip exercise. Third, the number of subjects included in the study was too small to enable definite conclusions to be drawn and, thus, this may be a hypothesis-generating study. Further studies with a greater number of subjects and different exercise intensities are needed to confirm the conclusions of the present study.
In conclusion, isometric, but not isotonic, exercise may increase central blood pressure in overall healthy subjects. Because central blood pressure is a better index of cardiac load than peripheral blood pressure, the response of central blood pressure to hand-grip exercise may be useful in evaluating cardiovascular risk.
Methods
Study population
Individuals were eligible for inclusion in the study if they: (1) were ≥25 years of age; (2) had normal blood pressure (<140/90 mmHg); (3) were not receiving any medication; and (4) were in a stable condition. All subjects had been diagnosed with essential hypertension based on clinical blood pressure measured on at least two different occasions and attained target blood pressure (<140/90 mmHg) following lifestyle modification without any antihypertensive medication. Blood pressure was measured by a doctor using a validated oscillometric technique (HEM-7070; Omron Healthcare, Kyoto, Japan) after subjects had been seated for 2 min with their back supported and their arms supported at heart level. Proper cuff size was determined based on arm circumference. Three consecutive blood pressure measurements were taken at 2-min intervals and the mean of the second and third measurements was recorded as the blood pressure. Exclusion criteria for the study were office blood pressure ≥140/90 mmHg while being untreated, and a history of target organ damage, cardiovascular disease, dyslipidemia, or impaired glucose tolerance.
The study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Nagoya City University Graduate School of Medical Sciences. All subjects provided written informed consent prior to participating in the study.
Study protocol
The overall healthy subjects were instructed to avoid heavy exercise for 24 h and to fast overnight before the exercise tests. Ergometer and hand-grip exercises were performed on different days in the morning in a quiet, temperature-controlled (22–25°C) room. The order of the exercises was random.
The ergometer test was started with 10 min rest in a supine position followed by 5 min rest sitting on the cycle ergometer. Subjects were first asked to pedal at 60 r.p.m. without any added load for 1 min before they were subjected to graded symptom-limited maximum exercise test (15 W/min ramping) on an electronically braked bicycle ergometer (STB-2400; Nihon Koden, Tokyo, Japan) until 80% of target heart rate ( = [220 – age] × 0.8) was achieved under a 12-lead electrocardiogram and peripheral blood pressure monitoring. Peripheral blood pressure was measured and central blood pressure was estimated before and after the exercise in seated subjects using an automated device (HEM-9000AI; Omron Healthcare).
On a separate day, subjects performed a hand-grip exercise using a custom-made hand-grip device. After 10 min rest, the hand-grip exercise was started with subjects in a supine position. The exercise protocol consisted of 90-s isometric exercise at 30% of the maximal grip, which had been predetermined. Peripheral blood pressure was measured and central blood pressure was estimated before and after the exercise in subjects in a supine position using the Omron device.
Estimation of central blood pressure
The detailed method for estimating central blood pressure has been published elsewhere20. Briefly, radial artery pressure waveforms and brachial blood pressure were recorded simultaneously using a fully automated device (HEM-9000AI; Omron Healthcare) to calculate late systolic pressure in the radial artery (SBP2) and to estimate central systolic blood pressure21,22,23,24,25. Brachial blood pressure was measured with an oscillometric manometer and radial pulse waveforms were recorded non-invasively using an applanation tonometer. Signals of the radial arterial pressure wave were low-pass filtered, first at a cut-off frequency of 105 Hz to remove high-frequency noise and then at 25 Hz to extract pressure waveforms. The radial arterial waveform obtained with this device is reportedly identical to the simultaneously and invasively measured intra-arterial pulse waveform of the opposite radial artery21.
Inflection points or peaks that corresponded to early and late systolic blood pressure were obtained by multidimensional derivatives of the original pulse pressure waveforms. The maximal systolic and diastolic pressures in the radial artery were calibrated with brachial systolic and diastolic blood pressure, respectively. SBP2 was calculated using the following equation:
 |
where P2 and PP are the height of the late systolic shoulder/peak pressure and the pulse pressure of the radial arterial pressure contour, respectively. Central systolic blood pressure was estimated using a regression equation with SBP2 as a major independent variable21,25. The radial augmentation index (AI) was calculated using the following equation:
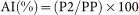 |
Statistics
All statistical analyses were performed using SPSS 19.0 (Chicago, IL, USA). Unless indicated otherwise, data in the text and tables are expressed as the mean ± SD. The significance of differences in variables before and after exercise was determined using a paired t-test. P < 0.05 was considered significant.
Author Contributions
Y.D. designed the study, S.T. collected and analyzed data, and S.T., T.S., S.Y. and Y.D. interpreted the data. S.T. drafted the manuscript and Y.D., G.K. and N.O. revised the manuscript. All authors reviewed the manuscript.
References
- Turnbull F. Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 362, 1527–1535 (2003). [DOI] [PubMed] [Google Scholar]
- Lewington S., Clarke R., Qizilbash N., Peto R. & Collins R. Prospective Studies Collaboration. Age-specific relevance of usual BP to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies [erratum published in Lancet 2003; 361: 1060]. Lancet 360, 1903–1913 (2002). [DOI] [PubMed] [Google Scholar]
- Chobanian A. V. et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42, 1206–1252 (2003). [DOI] [PubMed] [Google Scholar]
- Shimamoto K. et al. Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens. Res. 37, 253–387 (2014). [DOI] [PubMed] [Google Scholar]
- Mancia G. et al. Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 31, 1281–1357 (2013). [DOI] [PubMed] [Google Scholar]
- Boutouyrie P. et al. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation 100, 1387–1393 (1999). [DOI] [PubMed] [Google Scholar]
- Sharman J. E. et al. Left ventricular mass in patients with type 2 diabetes is independently associated with central but not peripheral pulse pressure. Diabetes Care 28, 937–939 (2005). [DOI] [PubMed] [Google Scholar]
- Subherwal S. et al. Central aortic pressure is independently associated with diastolic function. Am. Heart J. 159, 1081–1088 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar M. E. et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension 39, 735–738 (2002). [DOI] [PubMed] [Google Scholar]
- Williams B. et al. CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 113, 1213–1225 (2006). [DOI] [PubMed] [Google Scholar]
- Roman M. J. et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 50, 197–203 (2007). [DOI] [PubMed] [Google Scholar]
- Wang K. L. et al. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J. Hypertens. 27, 461–467 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. M. et al. Central versus ambulatory blood pressure in the prediction of all-cause and cardiovascular mortalities. J. Hypertens. 29, 454–459 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman J. E. & Laurent S. Central blood pressure in the management of hypertension: soon reaching the goal? J. Hum. Hypertens. 27, 405–411 (2013). [DOI] [PubMed] [Google Scholar]
- Filipovský J., Ducimetière P. & Safar M. E. Prognostic significance of exercise blood pressure and heart rate in middle-aged men. Hypertension 20, 333–339 (1992). [DOI] [PubMed] [Google Scholar]
- Singh J. P. et al. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham heart study. Circulation 99, 1831–1836 (1999). [DOI] [PubMed] [Google Scholar]
- Kurl S. et al. Systolic blood pressure response to exercise stress test and risk of stroke. Stroke 32, 2036–2041 (2001). [DOI] [PubMed] [Google Scholar]
- Schultz M. G. et al. Exercise-induced hypertension, cardiovascular events, and mortality in patients undergoing exercise stress testing: a systematic review and meta-analysis. Am. J. Hypertens. 26, 357–366 (2013). [DOI] [PubMed] [Google Scholar]
- Kelly R. P., Tunin R. & Kass D. A. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ. Res. 71, 490–502 (1992). [DOI] [PubMed] [Google Scholar]
- Takase H., Dohi Y. & Kimura G. Distribution of central blood pressure values estimated by Omron HEM-9000AI in the Japanese general population. Hypertens. Res. 36, 50–57 (2013). [DOI] [PubMed] [Google Scholar]
- Takazawa K., Kobayashi H., Shindo N., Tanaka N. & Yamashina A. Relationship between radial and central arterial pulse wave and evaluation of central aortic pressure using the radial arterial pulse wave. Hypertens. Res. 30, 219–228 (2007). [DOI] [PubMed] [Google Scholar]
- Richardson C. J. et al. Comparison of estimates of central systolic blood pressure and peripheral augmentation index obtained from the Omron HEM-9000AI and SphygmoCor systems. Artery Res. 3, 24–31 (2009). [Google Scholar]
- Kips J. G. et al. Comparison of central pressure estimates obtained from SphygmoCor, Omron HEM-9000AI and carotid applanation tonometry. J. Hypertens. 29, 1115–1120 (2011). [DOI] [PubMed] [Google Scholar]
- Ding F. H. et al. Validation of the noninvasive assessment of central blood pressure by the SphygmoCor and Omron devices against the invasive catheter measurement. Am. J. Hypertens. 24, 1306–1311 (2011). [DOI] [PubMed] [Google Scholar]
- Takazawa K. et al. Estimation of central aortic systolic pressure using late systolic inflection of radial artery pulse and its application to vasodilator therapy. J. Hypertens. 30, 908–916 (2012). [DOI] [PubMed] [Google Scholar]
- Izzo J. L. Jr et al. Relation of age and hypertension to the amplitude and timing of late systolic pressure augmentation waves. J. Am. Soc. Hypertens. 8 (Suppl A), A14 (2006). [Google Scholar]
- Hashimoto J., Westerhof B. E., Westerhof N., Imai Y. & O'Rourke M. F. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J. Hypertens. 26, 1017–1024 (2008). [DOI] [PubMed] [Google Scholar]
- Miyashita H. et al. Correction of heart rate dependency of radial artery augmentation index for clinical assessment of arterial stiffness. J. Hypertens. 24 (Suppl 4), S353 (2006). [Google Scholar]
- de Luca N., Asmar R. G., London G. M., O'Rourke M. F. & Safar M. E. REASON Project Investigators. Selective reduction of cardiac mass and central blood pressure on low-dose combination perindopril/indapamide in hypertensive subjects. J. Hypertens. 22, 1623–1630 (2004). [DOI] [PubMed] [Google Scholar]
- Wang K. L. et al. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension 55, 799–805 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauca A. L., O'Rourke M. F. & Kon N. D. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 38, 932–937 (2001). [DOI] [PubMed] [Google Scholar]
- Gallagher D., Adji A. & O'Rourke M. F. Validation of the transfer function technique for generating central from peripheral upper limb pressure waveform. Am J. Hypertens. 17, 1059–1067 (2004). [DOI] [PubMed] [Google Scholar]
- Ochiai H. et al. Assessment of the accuracy of indirect blood pressure measurements. Jpn. Heart J. 38, 393–407 (1997). [DOI] [PubMed] [Google Scholar]
- Casey D. P., Nichols W. W. & Braith R. W. Impact of aging on central pressure wave reflection characteristics during exercise. Am. J. Hypertens. 21, 419–424 (2008). [DOI] [PubMed] [Google Scholar]


