Abstract
Background
It was previously thought that persons with genetic predispositions to vitiligo develop the condition after exposure to various precipitating environmental factors. However, in many cases, the aggravating factors of vitiligo have not been clearly identified.
Objective
To identify the aggravating factors of vitiligo in the working environment and daily life.
Methods
A total of 489 vitiligo patients were recruited from 10 institutions in South Korea; patients were provided with a questionnaire about environmental factors and behavior patterns in the workplace and in daily life, and their association with vitiligo.
Results
Ninety-five of the 470 enrolled patients (20.2%) answered that environmental risk factors in daily life and in the workplace affected the development of vitiligo. The most frequently attributed causes were trauma and burn (13.6%), followed by sunlight (12.8%), stress (12.8%), cleaning products/disinfectant/chemicals (4.9%), and hair dye (2.1%).
Conclusion
Vitiligo of the hand and foot was associated with frequent exposure to aggravating materials and overexposure to sunlight, along with frequent trauma of these areas, all of which could be considered important risk factors of vitiligo. The development of vitiligo could potentially be controlled through the early detection of aggravating factors.
Keywords: Environment, Occupations, Provoking factor, Risk factors, Vitiligo
INTRODUCTION
Vitiligo is an acquired depigmentation disorder characterized by a progressive loss of functioning epidermal melanocytes. The pathogenesis of vitiligo has not been clearly identified; however, various pathogenetic mechanisms have been suggested, including genetic association, cellular and humoral autoimmunity, oxidative stress, neurohormonal imbalance, abnormalities in the microenvironment surrounding the melanocytes, and intrinsic defects of melanocytes1,2,3,4,5. Additionally, the combination of several of these pathomechanisms has given rise to a convergence theory6. As a result, vitiligo is thought to develop in persons with a genetic predisposition that determines melanocyte fragility and susceptibility to precipitating environmental factors7. Although genetic backgrounds in patients with vitiligo are predetermined and cannot be controlled, the effort to avoid precipitating factors, which can be controlled, is important for the prevention of the development and aggravation of the condition.
Precipitating factors associated with the development of vitiligo include sunburn, stress, pregnancy, and exposure to cytotoxic compounds8. Although various environmental factors that could affect the development of vitiligo are encountered in everyday life, in many cases, the initiation factors and aggravating causes have not been clearly identified8,9. In addition, the development of vitiligo may be influenced by diverse occupational exposures such as chemical exposure, frequent physical trauma, or sun exposure8,10.
In occupational vitiligo, the disease is initiated after exposure to chemicals that are toxic to melanocytes, after which the condition progresses into generalized vitiligo8. Phenolic/catechol derivatives are major chemicals known to be associated with vitiligo, and could induce the condition8,11,12,13,14. A variety of allergens that cause allergic contact dermatitis (ACD) might also be initiating factors of contact/occupational vitiligo15,16,17,18,19,20. However, the frequency of vitiligo occurrence in patients of particular occupations, and the associated environmental and occupational exposures have not yet been reported.
Our study was designed to identify the provoking factors of vitiligo, especially in the work environment. The survey was conducted by using a questionnaire that consisted of questions about risk factors and exposures to the provoking materials mentioned above. Because these aggravating factors are present in daily life and in the occupational environment, potential exposure to these factors in both settings was investigated in the survey.
MATERIALS AND METHODS
Patients
This multicenter study was conducted with vitiligo patients who were examined in the dermatology clinics of 10 institutions in South Korea. The diagnosis of vitiligo was confirmed on the basis of Wood's light examination and a thorough physical examination by dermatologists affiliated with the Korean Society of Vitiligo. The study was approved by the institutional review board of each institution (IRB number of principal investigator's institution: 4-2011-0745).
Four hundred and eighty-nine patients with vitiligo were invited to participate in the study by responding to a questionnaire, in order to identify the risk factors and associated aggravating factors of vitiligo. The aim of the study was explained to patients, and written consent was obtained before the survey was administered. Demographics and clinical information about the patients were collected, including age, sex, disease duration, time after aggravation, and involved body site. Well-trained dermatologists classified the enrolled patients into 3 subtypes of vitiligo according to clinical manifestations: focal, segmental, and nonsegmental.
Questionnaire and assessment
The questionnaire investigated the following areas: disease extent (involved body site), disease duration and time after aggravation, occupation and associated working environment, and materials frequently exposed to at work or in daily life. The patients were also asked about the most likely causes among several known aggravating factors, including hair dye, trauma, scrubbing during bath, and sun exposure.
The occupations of the enrolled patients were categorized as follows: office job, housewife, student, health-care system, production (metal, manufacturing, electronic, chemical, and assembling), athlete, transportation, construction, agriculture, mining, or others. Concerning the work environment, we investigated the frequently exposed body sites, frequently traumatized body sites, wearing of protective devices and body site of their application, contact history with chemicals and materials, ventilation, radical change in temperature or humidity, and frequencies of dermatitis or vitiligo among coworkers in the same workplace.
Materials to which the patients were frequently exposed were categorized into 13 groups: nickel (accessories, watches, glasses frames, coins, keys, belts), chrome (cement, glass, chrome leather, plating), cobalt (alloy, paint, ceramics), rubber (rubber shoes, rubber gloves, surgical gloves), leather (leather processing chemicals, leather glue), cosmetics (base, preservative, antimicrobial ointment, antifungal ointment), cleaning products (dishwashing cleaner, bleach, soap, shampoo, conditioner), synthetic resins (epoxy, adhesive), mercury, hair dye, clothing, shoes, and agricultural pesticide. The association between these materials and their relation to the development of vitiligo was examined.
Statistical analysis
All statistical tests were carried out by using PASW software version 18 for Windows (IBM Co., Armonk, NY, USA). Risk factors or aggravating factors of vitiligo according to sex, subtype of vitiligo, and hand/foot or facial involvement were examined by using the χ2 test and logistic regression. In addition, we tried to identify, through logistic regression, the most probable risk factors among the patients who thought that the surrounding environment in their daily life and workplace affected the development of their vitiligo. We considered p-values <0.05 to be statistically significant.
RESULTS
Demographics and clinical characteristics of the study groups
A total of 470 vitiligo patients answered the questionnaire. The demographics of the patients are summarized in Table 1. The mean age was 47.6±14.4 years (range, 20~84 years). Two hundred and two patients (43.0%) were men and 268 patients (57.0%) were women. The mean disease duration was 92.8±119.1 months. Seventy-eight patients (16.6%) had the focal type, 37 patients (7.9%) the segmental type, and 354 patients (75.3%) the nonsegmental type of vitiligo. One hundred ninety-three patients (41.1%) had hand or foot involvement, and 259 patients (55.1%) had facial involvement.
Table 1.
Patient demographics
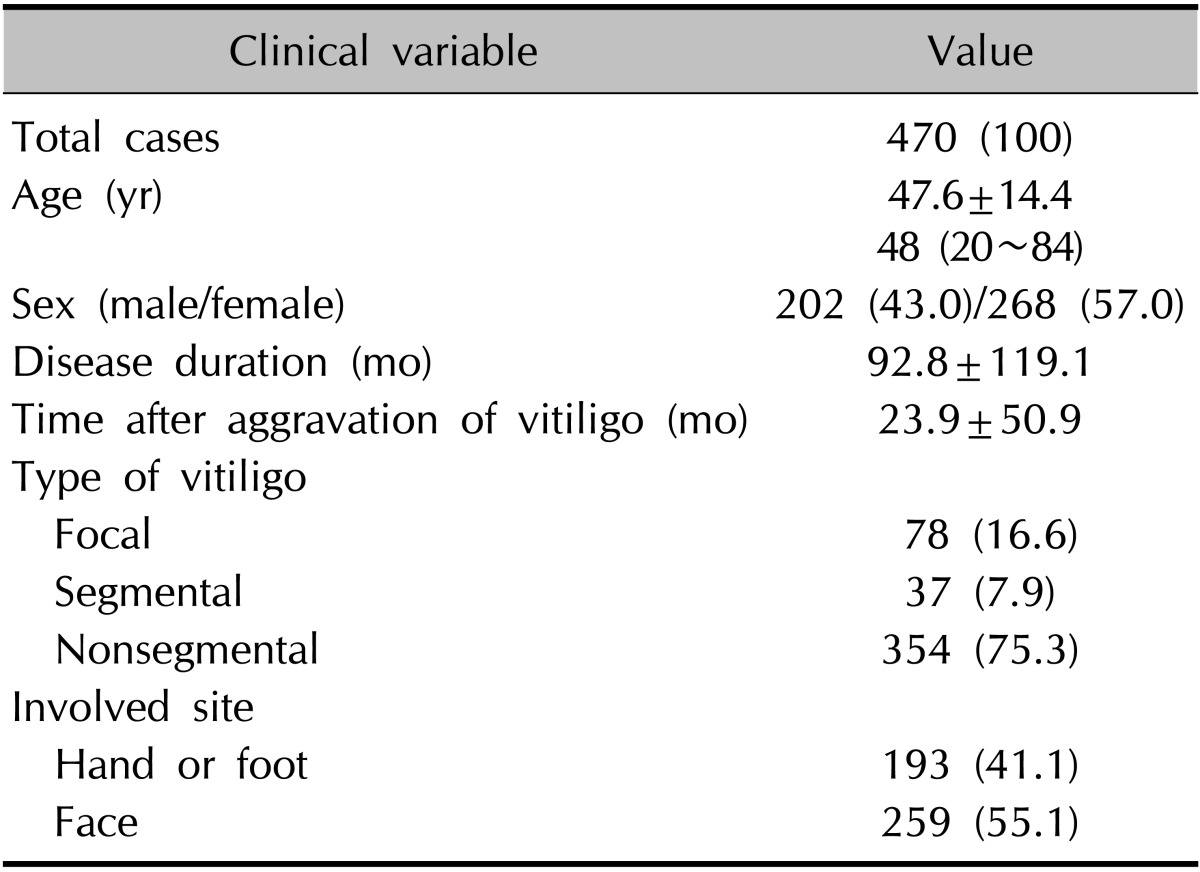
Values are presented as number (%), mean±standard deviation, or median (range).
Working environment of patients with vitiligo
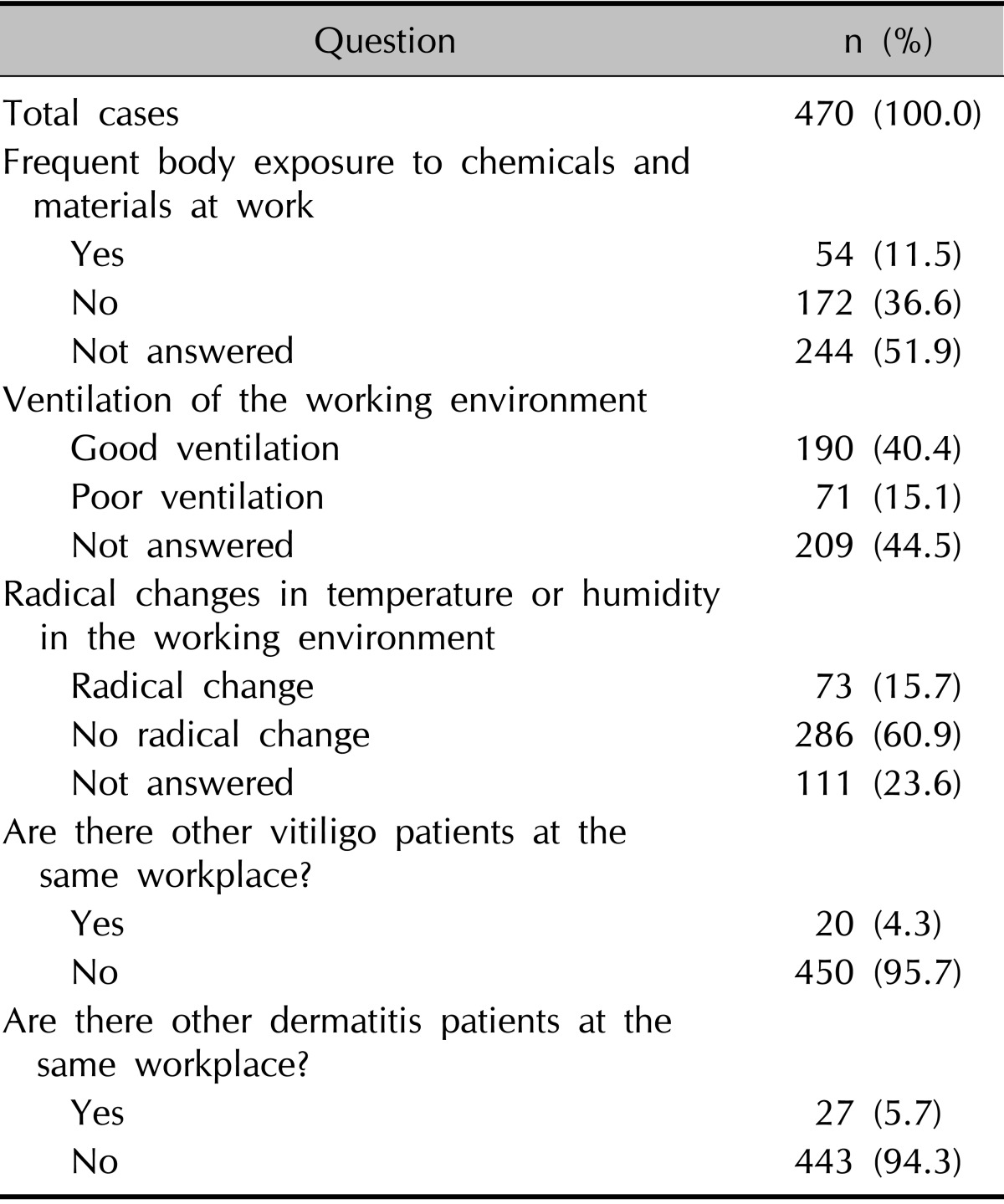
The patients' responses are summarized in Fig. 1 and Table 2. The most frequent occupation was housewife (33.0%), followed by office job (30.4%), production (7.4%), student (6.8%), private business (4.3%), health care/medical (3.2%), agriculture (2.6%), construction (2.1%), and transportation (1.7%). Beauty shop, fishery, mining/manufacturing, athlete, soldier, and painter were combined into 'others' (Fig. 1). Fifty-four patients (11.5%) answered that their body areas have been frequently exposed to chemicals or materials at work through air or direct contact. Seventy-one patients (15.1%) answered that their working environments are not well ventilated. Seventy-three patients (15.7%) answered that there are abrupt changes in temperature or humidity in their working environment. Twenty patients (4.3%) answered that they have coworkers with vitiligo at the same workplace, and 27 patients (5.7%) answered that they have coworkers with dermatitis (Table 2).
Fig. 1.
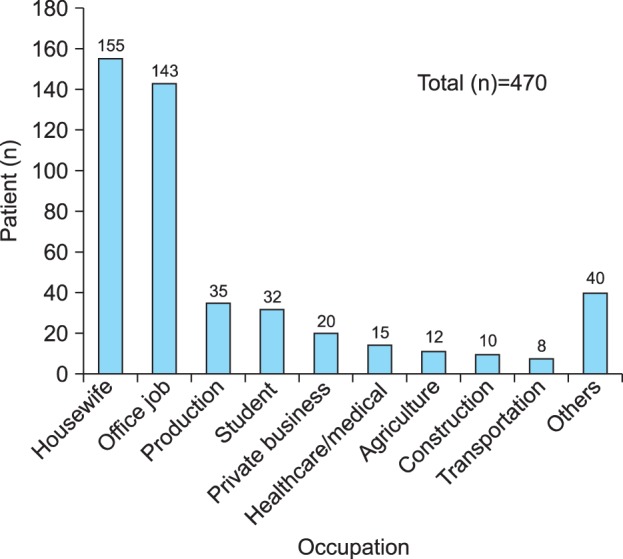
Occupations of patients with vitiligo. The most frequent occupation was housewife, followed by office job, working in production, and student.
Table 2.
Working environment of patients with vitiligo
Exposed and traumatized body sites and the use of protective devices
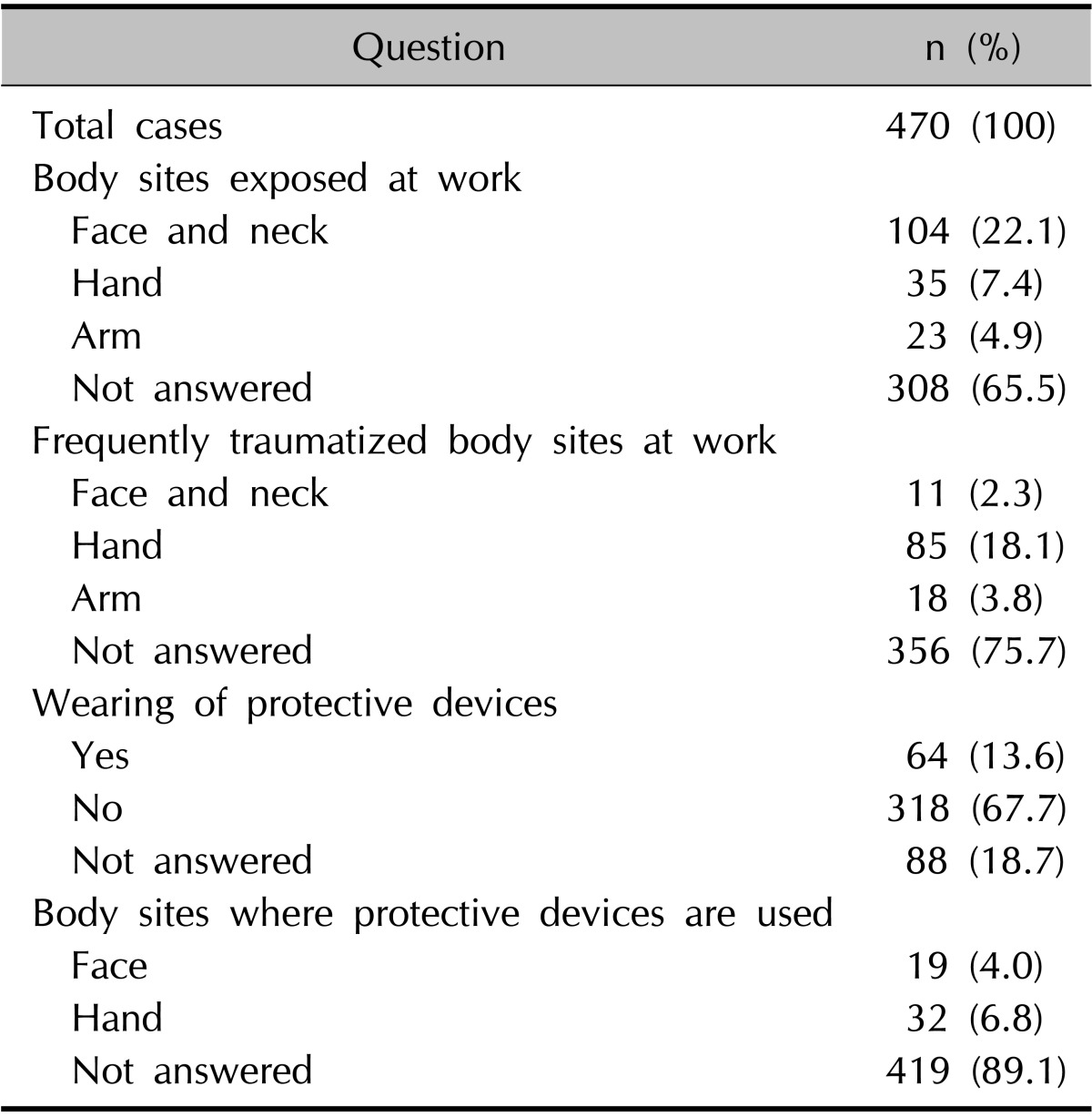
The patients' responses are summarized in Table 3. The face and neck were the body sites most commonly exposed to materials, followed by the hand and arm. The hand was the most frequently traumatized body site at work, followed by the arm, face, and neck. Sixty-four patients (13.6%) wore protective devices when working. The hand and face were the most common sites where protective devices were worn. Nevertheless, only 6 patients reported having complete protection; 14 patients complained that their faces were still exposed and another 14 patients complained that their hands were exposed despitewearing protective devices (data not shown).
Table 3.
Exposed and frequently traumatized body sites and the use of protective devices at work
Materials to which patients were frequently exposed in daily life, and vitiligo
The material to which the patients were most frequently exposed at home and in the workplace was cleaning products (30.0%), followed by cosmetics (17.0%), hair dye (11.4%), nickel (11.2%), rubber (7.3%), and clothing (5.5%) (Fig. 2A). Housewife and office job, which were the most commonly reported professions included in our study, showed similar exposure frequencies for cleaning products, cosmetics, hair dye, and nickel. However, vitiligo patients working in production companies showed a somewhat different exposure profile, reporting more frequent exposure to cleaning products (20.0%), synthetic resin (17.1%), rubber (14.3%), and cobalt/nickel/hair dye (11.4%).
Fig. 2.
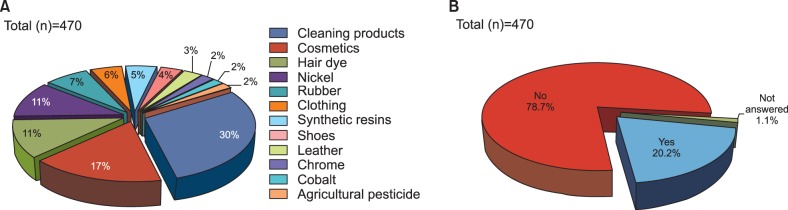
Frequent exposure to materials and their influence on the development of vitiligo. (A) The materials to which patients were most frequently exposed in the home and workplace were cleaning products (30.0%), followed by cosmetics (17.0%), hair dye (11.4%), nickel (11.2%), rubber (7.3%) and clothing (5.5%). (B) Ninety-five patients (20.2%) reported that frequent exposure to certain materials and environments in daily life affected the development of their vitiligo.
Ninety-five patients (20.2%) answered that frequent chemical and environmental exposures in their daily life affected the development of vitiligo (Fig. 2B). The most frequently reported causes associated with vitiligo occurrence were trauma and burn (13.6%), followed by sunlight (12.8%), stress (12.8%), cleaning products/disinfectant/chemicals (4.9%), and hair dye (2.1%) (Fig. 3).
Fig. 3.
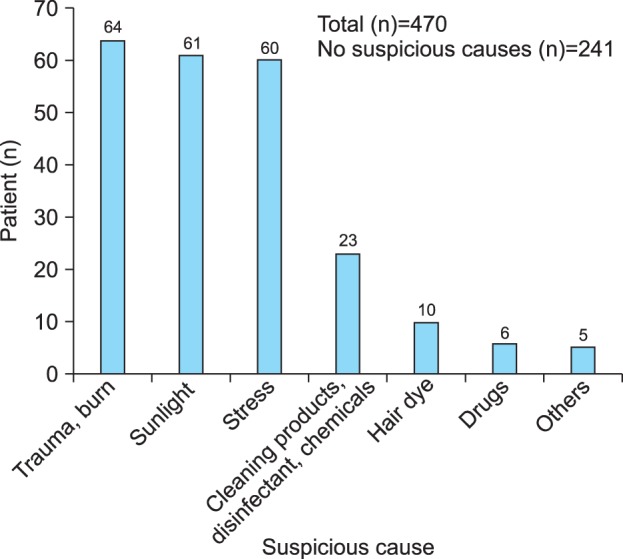
Most frequently reported causes associated with the occurrence of vitiligo. The most frequently reported causes associated with vitiligo occurrence were trauma and burn (13.6%), followed by sunlight (12.8%), stress (12.8%), cleaning products/disinfectant/chemicals (4.9%), and hair dye (2.1%).
Forty-three patients (9.1%) reported experiencing ACD after exposure to hair dye, but only 16 patients (3.4%) answered that hair dye aggravated their vitiligo (Table 4). Among these 16 patients, only 5 patients (50%) reported ACD from hair dye (data not shown).
Table 4.
Hair dye, trauma/scrubbing during bath, sunlight, and vitiligo
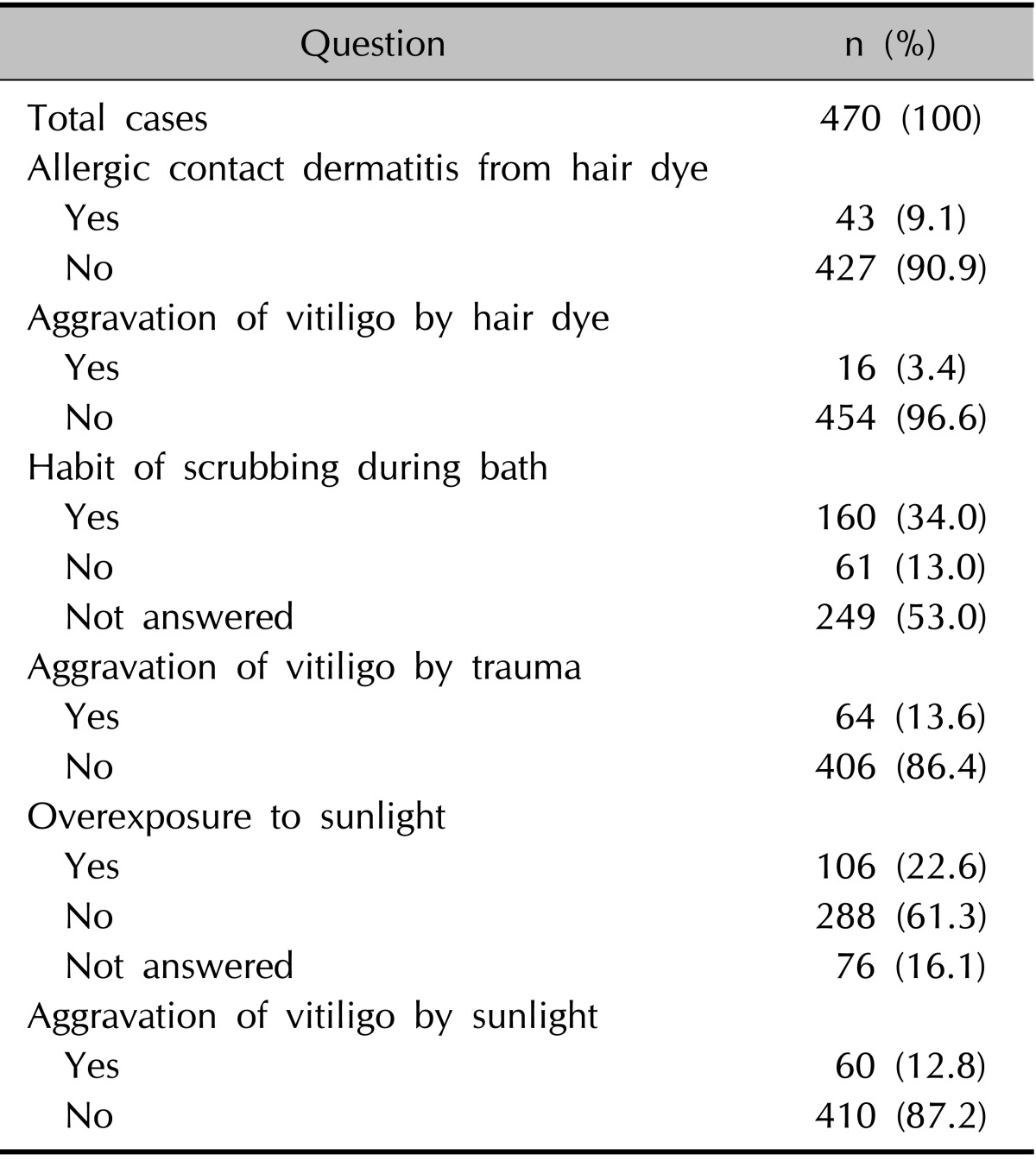
Trauma/scrubbing during bath and sunlight, and their effect on the aggravation of vitiligo
The patients' responses are summarized in Table 4. One hundred sixty patients (34.0%) reported scrubbing during bath. Sixty-four patients (13.6%) answered that vitiligo was aggravated by trauma. Among these 64 patients, 16 (25.0%) answered that vitiligo was aggravated by scrubbing during bath and 55 (85.9%) answered that vitiligo was aggravated by trauma other than scrubbing during bath.
One hundred and six patients (22.6%) answered that they had been overexposed to sunlight. The exposure mainly occurred during exercise and leisure in 81 patients (77.4%), and during work in 58 patients (54.7%). Of 106 patients overexposed to sunlight, 40 patients (37.7%) used sunscreen. Sixty of the total 470 patients (12.8%) thought that sunlight aggravated their vitiligo, but only 16 of these 60 patients (26.7%) used sunscreen.
Risk factors of vitiligo according to sex, subtype of vitiligo, and hand/foot or facial involvement
Risk factors of vitiligo related to sex, disease duration, time after aggravation, and subtype of vitiligo were not found to be statistically significant in univariate analysis.
Factors associated with hand or foot involvement were frequent facial exposure to chemicals and materials at work (β=1.950, p=0.003), frequent hand exposure to chemicals and materials at work (β=3.022, p=0.002), frequent physical trauma to the hands at work (β=2.147, p=0.002), and wearing of protective devices on the hands (β=4.029, p=0.001). In multivariate analysis, frequent hand exposure to chemicals and materials at work (β=2.996, p=0.018) and wearing of protective devices on the hands (β=2.649, p=0.046) were associated with hand and foot involvement in vitiligo.
Facial involvement in vitiligo was associated with working in construction (β=8.434, p=0.043) and frequent physical trauma to the face at work (β=9.315, p=0.033) in univariate analysis. In multivariate analysis, facial involvement was closely associated with frequent physical trauma to the face at work (β=9.121, p=0.036) and overexposure to sunlight (β=1.629, p=0.040).
Risk factors in patients whose environment in daily life and the workplace affected the development of vitiligo
In univariate analysis, patients who reported that environmental and occupational exposures affected the development of vitiligo more frequently reported frequent physical trauma to the hand at work (β=1.785, p=0.036), aggravation of vitiligo by physical trauma (β=1.926, p=0.028), and aggravation of vitiligo by sunlight exposure (β=2.455, p=0.014), and were associated with working in mining or manufacturing (β=2.287, p=0.029). In multivariate analysis, aggravation of vitiligo by sunlight exposure was more commonly observed in patients who suspected a close relation between their environment and the development of their vitiligo (β=2.995, p=0.028).
DISCUSSION
In vitiligo, as in many conditions, identifying risk factors and educating patients on how to avoid these risk factors are important in preventing disease progression. Various therapeutic approaches to vitiligo have been tested, but more effective treatments are still being sought. Therefore, prevention through the early detection of risk factors could be considered more important in vitiligo than treatment itself. Although the aggravating factors of vitiligo have been noted in many reports, only a few studies have analyzed these factors in both the daily life and occupational settings8,21. The purpose of this study was to identify the risk factors and associated aggravating factors of vitiligo in the working environment and in daily life.
Recently, provoking factors of vitiligo have been reported by using a questionnaire similar to that used in our study21; the subjects reported that emotional stress (55.4%) was the most frequent provoking factor, followed by sunburn (28.8%), mechanical factors (19.2%), and chemical factors (16.4%). However, the overall rate of vitiligo patients who mentioned provoking factors were lower in the present study than in Vrijman et al.'s21 study, and the prevalence of particular aggravation factors reported by enrolled patients with vitiligo was different, although the items associated with the aggravation of the disease were similar. This variation between the 2 studies might be explained by differences in ethnicity, Fitzpatrick skin types, and occupations and working environment of the patients participating in the survey.
Trauma and burn, which were listed as the most likely aggravating factors in our study, are associated with Koebner's phenomenon (KP). KP has been described as 'the development of lesions at sites of specifically traumatized uninvolved skin in patients with cutaneous diseases,' and has been observed in vitiligo, psoriasis, and other skin diseases10,22. In nonsegmental vitiligo, the incidence of KP is highly variable according to the reports, from 15% up to 70%23. KP in vitiligo can be used as a clinical parameter predicting prognosis24. Although the pathogenesis of KP in vitiligo remains unclear, multiple factors induced by trauma might be associated with the aggravation of vitiligo, including inflammatory cytokines, increased oxidative stress, defective melanocyte adhesion, and melanocyte growth factors5,11,25,26,27. Although occupations associated with frequent mechanical trauma among enrolled patients were not specifically reported in our study, in univariate analysis patients working in production or mining/manufacturing, which could be expected to have frequent mechanical trauma, more commonly reported that their workplace environment affected the development of vitiligo, as compared with vitiligo patients that had other jobs. These results suggest the possibility that frequent mechanical trauma during duty hours might affect the development of vitiligo. Therefore, vitiligo patients with KP initiated by trauma should consider an occupational change to avoid the aggravation of vitiligo.
Currently, contact/occupational vitiligo needs a clearer definition as it has not yet been included in the classification of vitiligo28. However, contact with chemicals and allergens has been reported to induce vitiligo lesions. Etiological agents have been reported in 864 cases of chemical leukoderma in India29. In the study, hair dye (27.4%) was the most frequently reported causative agent, followed by deodorant/perfume (21.6%) and detergent/cleaner (15.4%). Vrijman et al.21 reported that among 29 patients who reported chemical provoking factors, presumed vitiligo-inducing chemicals such as captan, paratertiary butyl phenol (PTBP), and diphencyprone were detected in 4 patients. The most common contributory chemical agent was PTBP, which represented 50.7% of the causative agents. Even if detailed information about specific contributory chemicals in vitiligo patients was not gathered from our study because of the limitations of the questionnaire, the materials to which patients were most frequently exposed in daily life were cleaning products (30.0%), followed by cosmetics (17.0%), hair dye (11.4%), and nickel (11.2%). However, only 23 patients (4.9%) mentioned that these materials were suspected causes of vitiligo occurrence. Among 16 patients who answered that hair dye aggravated their vitiligo, only 8 patients reported ACD to hair dye. Therefore, ACD to hair dyes could not be a prerequisite for development of vitiligo.
In our study, the face and neck were the most frequent areas exposed to vitiligo-provoking factors at work. Frequent physical trauma to the face and neck at work and working in construction were associated with facial involvement in vitiligo in univariate analysis. Because construction often occurs outdoors, workers are exposed to sunlight and prone to frequent trauma. In multivariate analysis, overexposure to sunlight was also a risk factor of facial involvement in vitiligo. From previous research, ultraviolet (UV) radiation has been considered a main culprit of koebnerization despite its usefulness for the treatment of vitiligo. The mechanism by which UV aggravates vitiligo has not been clearly determined; however, fragile melanocytes in patients with vitiligo are thought to be easily destroyed through apoptosis and necrosis after overexposure to UV. In our study, only 26.7% of patients who experienced aggravation of vitiligo by sunlight used sunscreen in daily life. This emphasizes how particular attention to the prevention of excessive sun exposure should be incorporated into patient education to prevent the aggravation of vitiligo and make the contrast between normal and depigmented skin less noticeable.
In addition, the hand was the second most commonly exposed area at work and the most frequently traumatized area. Hand and foot involvement in vitiligo was associated with frequent facial exposure to chemicals and materials at work, frequent hand exposure to chemicals and materials at work, and wearing of protective devices on the hands. It should be investigated how frequent facial exposure, which seems irrelevant, could increase the risk of hand and foot involvement in vitiligo. Acrofacial vitiligo is a subtype of nonsegmental vitiligo in which the lesions are usually confined to the face, hands, and feet28. This clinical association might have driven the observed statistical association in our study.
The finding that wearing protective devices on the hands increases hand involvement in vitiligo was contrary to expectations but can be explained in several ways. First, contact with the protective devices themselves could act as an aggravating factor through friction and irritation. Second, the necessity of wearing a protective device at work could reflect a heightened expectation of frequent exposure to chemicals or physical trauma in the working environment, more so than in working environments that do not require wearing of protective devices. Lastly, even if the protective device is worn at work, the protection may be inadequate. In reality, among 64 patients who reported wearing protective devices at work, only 6 patients stated that the protective device ensured complete protection.
The major limitation of this study was that the collection of data relied on the patients' memories and subjective interpretations. The frequency and degree of exposure to chemicals, sunlight, and trauma and their associations with vitiligo were not easily quantified, and it was difficult to clearly assess the relation between exposure and outcome. Therefore, the results from our survey could be affected by recall bias and subjective judgment. Furthermore the cases of suspected occupational vitiligo were not cross-checked by an occupational medicine specialist. However, this was the first multicenter epidemiologic study of vitiligo in Asia, using data from a large number of patients, to identify the risk factors and aggravating factors of vitiligo in the work place and daily life.
In conclusion, frequent exposure to certain materials, frequent trauma, and overexposure to sunlight could be associated with the development of vitiligo. Compared with the previous study in Netherlands, the overall rate of provoking factors was lower, and the prevalence of aggravation factors was different in the present study. Hopefully, this knowledge can be used to help prevent the development or aggravation of vitiligo through early detection and avoidance of aggravating factors.
References
- 1.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 2.Kemp EH, Waterman EA, Weetman AP. Autoimmune aspects of vitiligo. Autoimmunity. 2001;34:65–77. doi: 10.3109/08916930108994127. [DOI] [PubMed] [Google Scholar]
- 3.Dell'anna ML, Picardo M. A review and a new hypothesis for non-immunological pathogenetic mechanisms in vitiligo. Pigment Cell Res. 2006;19:406–411. doi: 10.1111/j.1600-0749.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 4.Haycock JW, Rowe SJ, Cartledge S, Wyatt A, Ghanem G, Morandini R, et al. Alpha-melanocyte-stimulating hormone reduces impact of proinflammatory cytokine and peroxide-generated oxidative stress on keratinocyte and melanoma cell lines. J Biol Chem. 2000;275:15629–15636. doi: 10.1074/jbc.275.21.15629. [DOI] [PubMed] [Google Scholar]
- 5.Lee AY, Kim NH, Choi WI, Youm YH. Less keratinocyte-derived factors related to more keratinocyte apoptosis in depigmented than normally pigmented suction-blistered epidermis may cause passive melanocyte death in vitiligo. J Invest Dermatol. 2005;124:976–983. doi: 10.1111/j.0022-202X.2005.23667.x. [DOI] [PubMed] [Google Scholar]
- 6.Le Poole IC, Das PK, van den Wijngaard RM, Bos JD, Westerhof W. Review of the etiopathomechanism of vitiligo: a convergence theory. Exp Dermatol. 1993;2:145–153. doi: 10.1111/j.1600-0625.1993.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 7.Hochstein P, Cohen G. The cytotoxicity of melanin precursors. Ann N Y Acad Sci. 1963;100:876–886. [PubMed] [Google Scholar]
- 8.Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208–214. doi: 10.1111/j.1600-0749.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 9.Cummings MP, Nordlund JJ. Chemical leukoderma: fact or fancy. Am J Contact Dermat. 1995;6:122–126. [Google Scholar]
- 10.Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16:241–248. doi: 10.1046/j.1473-2165.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 11.Westerhof W, d'Ischia M. Vitiligo puzzle: the pieces fall in place. Pigment Cell Res. 2007;20:345–359. doi: 10.1111/j.1600-0749.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- 12.Toosi S, Orlow SJ, Manga P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol. 2012;132:2601–2609. doi: 10.1038/jid.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thörneby-Andersson K, Sterner O, Hansson C. Tyrosinase-mediated formation of a reactive quinone from the depigmenting agents, 4-tert-butylphenol and 4-tert-butylcatechol. Pigment Cell Res. 2000;13:33–38. doi: 10.1034/j.1600-0749.2000.130107.x. [DOI] [PubMed] [Google Scholar]
- 14.Yang F, Sarangarajan R, LePoole IC, Medrano EE, Boissy RE. The cytotoxicity and apoptosis induced by 4-tertiary butylphenol in human melanocytes are independent of tyrosinase activity. J Invest Dermatol. 2000;114:157–164. doi: 10.1046/j.1523-1747.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 15.Jappe U, Geier J, Hausen BM. Contact vitiligo following a strong patch test reaction to triglycidyl-p-aminophenol in an aircraft industry worker: case report and review of the literature. Contact Dermatitis. 2005;53:89–92. doi: 10.1111/j.0105-1873.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 16.Silvestre JF, Albares MP, Escutia B, Vergara G, Pascual JC, Botella R. Contact vitiligo appearing after allergic contact dermatitis from aromatic reactive diluents in an epoxy resin system. Contact Dermatitis. 2003;49:113–114. doi: 10.1111/j.0105-1873.2003.0128l.x. [DOI] [PubMed] [Google Scholar]
- 17.Bhushan M, Beck MH. Allergic contact dermatitis from primula presenting as vitiligo. Contact Dermatitis. 1999;41:292–293. doi: 10.1111/j.1600-0536.1999.tb06167.x. [DOI] [PubMed] [Google Scholar]
- 18.Riordan AT, Nahass GT. Occupational vitiligo following allergic contact dermatitis. Contact Dermatitis. 1996;34:371–372. doi: 10.1111/j.1600-0536.1996.tb02233.x. [DOI] [PubMed] [Google Scholar]
- 19.Silvestre JF, Botella R, Ramón R, Guijarro J, Betlloch I, Morell AM. Periumbilical contact vitiligo appearing after allergic contact dermatitis to nickel. Am J Contact Dermat. 2001;12:43–44. [PubMed] [Google Scholar]
- 20.Trattner A, David M. Hair-dye-induced contact vitiligo treated by phototherapy. Contact Dermatitis. 2007;56:115–116. doi: 10.1111/j.1600-0536.2007.00989.x. [DOI] [PubMed] [Google Scholar]
- 21.Vrijman C, Hosseinpour D, Bakker JG, Wolkerstorfer A, Bos JD, van der Veen JP, et al. Provoking factors, including chemicals, in Dutch patients with vitiligo. Br J Dermatol. 2013;168:1003–1011. doi: 10.1111/bjd.12162. [DOI] [PubMed] [Google Scholar]
- 22.Miller RA. The Koebner phenomenon. Int J Dermatol. 1982;21:192–197. doi: 10.1111/j.1365-4362.1982.tb02070.x. [DOI] [PubMed] [Google Scholar]
- 23.Gauthier Y, Benzekri L. The Koebner's phenomenon. In: Picardo M, TaÏeb A, editors. Vitiligo. Berlin Heidelberg: Springer-Verlag; 2010. pp. 167–173. [Google Scholar]
- 24.van Geel N, Speeckaert R, De Wolf J, Bracke S, Chevolet I, Brochez L, et al. Clinical significance of Koebner phenomenon in vitiligo. Br J Dermatol. 2012;167:1017–1024. doi: 10.1111/j.1365-2133.2012.11158.x. [DOI] [PubMed] [Google Scholar]
- 25.Gauthier Y, Cario-Andre M, Lepreux S, Pain C, TaÏeb A. Melanocyte detachment after skin friction in non lesional skin of patients with generalized vitiligo. Br J Dermatol. 2003;148:95–101. doi: 10.1046/j.1365-2133.2003.05024.x. [DOI] [PubMed] [Google Scholar]
- 26.Jin Y, Birlea SA, Fain PR, Gowan K, Riccardi SL, Holland PJ, et al. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med. 2010;362:1686–1697. doi: 10.1056/NEJMoa0908547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Geel N, Speeckaert R, Taieb A, Picardo M, Böhm M, Gawkrodger DJ, et al. VETF members. Koebner's phenomenon in vitiligo: European position paper. Pigment Cell Melanoma Res. 2011;24:564–573. doi: 10.1111/j.1755-148X.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- 28.Ezzedine K, Lim HW, Suzuki T, Katayama I, Hamzavi I, Lan CC, et al. Vitiligo Global Issue Consensus Conference Panelists. Revised classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res. 2012;25:E1–E13. doi: 10.1111/j.1755-148X.2012.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinico-aetiological study of 864 cases in the perspective of a developing country. Br J Dermatol. 2009;160:40–47. doi: 10.1111/j.1365-2133.2008.08815.x. [DOI] [PubMed] [Google Scholar]


