Abstract
A 63-year-old man with an isolated infarction of the corpus callosum developed expressive aphasia in addition to the deficits traditionally associated with a disconnection syndrome.
Background
We report a case of isolated corpus callosum infarction, made unusual by the presence of expressive aphasia in addition to the deficits traditionally associated with a disconnection syndrome.1 2 We review the speech deficits which have been reported following corpus callosum lesions, such as dysarthria and stuttering, and consider possible anatomical underpinnings of aphasia following a callosal lesion.
Case presentation
A 63-year-old right-handed man with a medical history significant for coronary artery disease and prior myocardial infarction experienced two episodes of systemic hypotension in the days after coronary artery bypass surgery. Afterwards, neurological examination was notable for poor initiation of speech, decreased verbal fluency, word-finding difficulty, impaired repetition, dysprosody and hypophonia, but with intact comprehension. He also exhibited many aspects of the callosal disconnection syndrome, including left hemifield paralexia, left ideomotor apraxia, left-hand agraphia, left tactile anomia, intermanual conflict, left visual extinction, left tactile extinction, blinded finger anomia and bilateral agraphaesthesia. He exhibited anosognosia for all deficits except for a vague notion of ‘problem speaking’.
Investigations
MRI of the brain revealed acute ischaemia (indicated by the presence of hyperintense signal on diffusion-weighted imaging sequence with correlated apparent diffusion coefficient (ADC) hypointensity) in part of the genu and almost the entirety of the right body of the corpus callosum (figure 1), in addition to two punctate areas of diffusion restriction in the right medial frontal lobe and right precentral gyrus. The rostrum and splenium of the corpus callosum were minimally affected, and cortical language areas were intact. Magnetic resonance angiography of the head and neck was significant for diffuse intracranial atherosclerosis with extensive stenosis of multiple intracranial vessels. Specifically, there was narrowing of the supraclinoid segment of the right internal carotid artery, with narrow and irregular calibre of the proximal A1 segment and incomplete visualisation of the distal A1 of the right anterior cerebral artery (ACA). Thus the proposed mechanism of injury was hypoperfusion in the territory of the severely stenosed right ACA, namely in its right pericallosal artery territory, during the two episodes of systemic hypotension following coronary bypass surgery.
Figure 1.
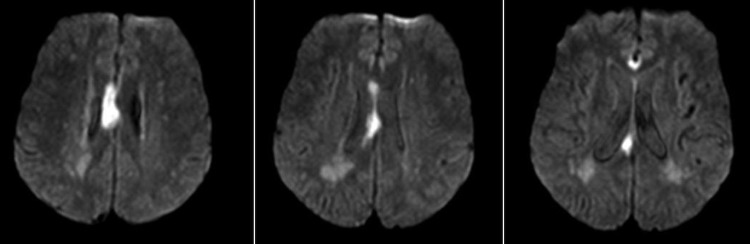
MRI of the brain showing restricted diffusion in the right body of the corpus callosum, with relative sparing of the genu and the splenium.
Treatment
The patient spent 2 weeks in an inpatient neurorehabilitation unit undergoing daily physical, occupational and speech therapy. Care was taken to provide specialised therapeutic strategies, including double simultaneous presentation of visual and tactile stimuli, that catered to his specific deficits. These included training in visual scanning, attentional training and perceptual retraining that are conventionally used in patients with unilateral neglect.3
Outcome and follow-up
Over the subsequent weeks, there was significant improvement in all of the patient's symptoms. At 4 weeks after the onset of the initial neurological deficit, verbal fluency and repetition had improved, as had the ideomotor apraxia, intermanual conflict and left hemipseudoneglect. However, the paucity of spontaneous speech, hypophonia and dysprosody remained.
Discussion
Peculiar speech abnormalities have been described following corpus callosum infarction/injury, including stuttering.4 French accent5 dysarthria6 and verbal aspontaneity.7 Aphasia as a symptom of corpus callosum infarction is rare. In one series, only 4 of 36 patients with callosal infarcts had decreased verbal fluency, but these all had left-sided lesions, and all but one of these had significant cortical involvement as well.8 In another series of 48 consecutive patients with anterior cerebral artery infarctions, transcortical motor aphasia was found in 10 of 30 patients with left-sided infarcts and 1 of 16 with a right-sided infarct, all of whom had involvement of the supplementary motor area or the subcortical white matter underlying it.9 A recent report described a case of expressive aphasia following infarct of the right body of the corpus callosum, associated with dysprosody and hypophonia.10 The proposed mechanism of aphasia was transcallosal diaschisis in the primary language cortex, which was supported by a relative hypoperfusion of the left frontotemporal junction on single-photon emission CT, despite normal MRI appearance of that area. In our patient with aphasia and no structural abnormalities in the primary language areas of the left hemisphere or the supplementary motor area in either hemisphere, a similar mechanism can be hypothesised. Alternatively, the aetiology of the language deficit may be due to damage to fibres from the inferior frontal lobe language area which pass through the genu and rostrum of the corpus callosum.11 However, the genu and splenium were relatively spared, making this less likely.
Many of the deficits observed in our patient were manifestations of a verbal–motor disconnection: verbal commands that are comprehended by the left hemisphere are unable to cross into the right hemisphere to direct the appropriate movements of the left hand.12 Aspects of recovery over the period of weeks following the infarct suggest that the right hemisphere regained access to language comprehension. This may occur through the pathways that cross through the splenium, which was spared in this patient, or through strengthening of subcallosal interhemispheric pathways.13 Through observation of another patient with an infarct of the body and the splenium with expressive aphasia that rapidly improved, it has been postulated that semantic memory of objects and shapes is bilaterally represented, and may only require training to gain access to these faculties.14
Learning points.
Isolated infarction of the corpus callosum is rare.
Expressive aphasia may be part of the callosal disconnection syndrome.
Most deficits following callosal injury improve significantly and rapidly with therapy.
Footnotes
Contributors: SS prepared initial draft of case report and SB was involved in editing of case report and choice of images.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Heilman KM, Bowers D, Watson RT. Pseudoneglect in a patient with partial callosal disconnection. Brain 1984;107(Pt 2):519–32 [DOI] [PubMed] [Google Scholar]
- 2.Feinberg TE, Schindler RJ, Flanagan NG, et al. Two alien hand syndromes. Neurology 1992;42:19–24 [DOI] [PubMed] [Google Scholar]
- 3.Lin KC. Right-hemispheric activation approaches to neglect rehabilitation poststroke. Am J Occup Ther 1996;50:504–15 [DOI] [PubMed] [Google Scholar]
- 4.Hamano T, Hiraki S, Kawamura Y, et al. Acquired stuttering secondary to callosal infarction. Neurology 2005;64:1092–3 [DOI] [PubMed] [Google Scholar]
- 5.Hall DA, Anderson CA, Filley CM, et al. A French accent after corpus callosum infarct. Neurology 2003;60:1551–2 [DOI] [PubMed] [Google Scholar]
- 6.Chung SJ, Kim JH, Ahn HJ, et al. Callosal dysarthria. Clin Neurol Neurosurg 2013;115:1173–6 [DOI] [PubMed] [Google Scholar]
- 7.Chang CC, Lee YC, Lui CC, et al. Right anterior cingulate cortex infarction and transient speech aspontaneity. Arch Neurol 2007;64:442–6 [DOI] [PubMed] [Google Scholar]
- 8.Alonso A, Gass A, Rossmanith C, et al. Clinical and MRI patterns of pericallosal artery infarctions: the significance of supplementary motor area lesions. J Neurol 2012;259:944–51 [DOI] [PubMed] [Google Scholar]
- 9.Kumral E, Bayulkem G, Evyapan D, et al. Spectrum of anterior cerebral artery territory infarction: clinical and MRI findings. Eur J Neurol 2002;9:615–24 [DOI] [PubMed] [Google Scholar]
- 10.Ishizaki M, Ueyama H, Nishida Y, et al. Crossed aphasia following an infarction in the right corpus callosum. Clin Neurol Neurosurg 2012;114:161–5 [DOI] [PubMed] [Google Scholar]
- 11.Caille S, Sauerwein HC, Schiavetto A, et al. Sensory and motor interhemispheric integration after section of different portions of the anterior corpus callosum in nonepileptic patients. Neurosurgery 2005;57:50–9; discussion 50–9 [DOI] [PubMed] [Google Scholar]
- 12.Watson RT, Heilman KM. Callosal apraxia. Brain 1983;106(Pt 2):391–403 [DOI] [PubMed] [Google Scholar]
- 13.Funnell MG, Corballis PM, Gazzaniga MS. Cortical and subcortical interhemispheric interactions following partial and complete callosotomy. Arch Neurol 2000;57:185–9 [DOI] [PubMed] [Google Scholar]
- 14.Marangolo P, De Renzi E, Di Pace E, et al. Let not thy left hand know what thy right hand knoweth. The case of a patient with an infarct involving the callosal pathways. Brain 1998;121(Pt 8):1459–67 [DOI] [PubMed] [Google Scholar]


