Abstract
Background & objectives:
Suppressed adrenal responses associated with inhaled steroid use have been reported in patients with bronchiectasis and have been shown to be associated with poor quality of life. This study was undertaken to examine the prevalence of suppressed cortisol responses in stable bronchiectasis and determine their correlation with the use of inhaled corticosteroids, radiologic severity of bronchiectasis and quality of life (QOL) scores.
Methods:
In this case-control study, cases were patients with bronchiectasis and suppressed cortisol responses and controls were healthy volunteers, and patients with bronchiectasis without suppressed cortisol responses. Symptoms, lung function test values, exercise capacity, HRCT severity scores for bronchiectasis, exacerbations, inhaled corticosteroid use and quality of life scores were compared between patients with and without suppressed cortisol values.
Results:
Forty consecutive patients with bronchiectasis and 40 matched controls underwent 1-μg cosyntropin testing. Baseline cortisol (mean difference -2.0 μg/dl, P=0.04) and 30-minute stimulated cortisol (mean difference -3.73 μg/dl, P=0.001) were significantly lower in patients with bronchiectasis. One patient had absolute adrenal insufficiency and 39.5 per cent (15/38) patients with bronchiectasis had impaired stimulated responses. Baseline and stimulated cortisol responses were unaffected by inhaled steroids (O.R 1.03, P=0.96). SGRQ scores were negatively correlated with body mass (r= -0.51, P=0.001) and bronchiectasis severity (r=0.37, P=0.019), but not related to baseline or stimulated cortisol responses.
Interpretation & conclusions:
Our results showed that the impaired adrenal responses to 1-μg cosyntropin were common in patients with bronchiectasis. This was not associated with the use of inhaled steroids or severity of bronchiectasis. Poor health status was associated with advanced disease and not with cortisol responses to the 1-μg cosyntropin test.
Keywords: Adrenal insufficiency, bronchiectasis, fatigue, St. George Respiratory Questionnaire, Synacten test
Bronchiectasis is characterized by non-reversible airway dilatation and a few evidence-based medical therapies exist for the treatment of non-cystic fibrosis (non-CF) bronchiectasis1. The use of inhaled corticosteroids (ICS) reduces sputum volume but does not reduce exacerbations or decline in lung function in bronchiectasis2. Fatigue, tiredness and weight loss are frequent in patients with bronchiectasis and these symptoms are often worsened during an exacerbation3. ICS treatment may cause a dose-dependent reduction in serum cortisol due to systemic absorption and this is associated with suppression of the hypothalamo-pituitary-adrenal (HPA) axis4. Patients with bronchiectasis have dilated and more permeable airways, with potential for greater systemic steroid absorption and also are affected with recurrent episodes of acute infection, both of which may blunt HPA responses. ICS use has been associated with impaired adrenal responses and poor health status in a series of patients with bronchiectasis using the 250 μg cosyntropin test in a previous study5. Compared to the western world, non-CF bronchiectasis is widely prevalent and more advanced at presentation6; also, large dose inhaled or nebulized steroids are frequently used in South Asia (personal observation). We conducted a case-control study to compare adrenal responses in patients with bronchiectasis with healthy matched controls using the 1 μg cosyntropin test and to assess the association of suppressed responses with symptoms of adrenal suppression, ICS use, pulmonary function, exercise testing, computed tomography (CT) scores of severity of bronchiectasis and quality of life (QoL) assessment by the St. George Respiratory Questionnaire (SGRQ).
Material & Methods
Patients: Consecutive patients evaluated in the outpatient clinic of the department of Chest Medicine, St. John's Medical College, Koramangala, Bangalore between March 2010 and February 2011 were eligible for enrollment. Inclusion criteria included the presence of bronchiectasis7 and stable lung function as defined by less than 20 per cent variability in 24 h sputum volume, forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) with no deterioration in respiratory symptoms from the baseline visit. Patients who had received more than three courses of oral steroids over the previous year, received oral or parenteral steroid over the previous six weeks prior to study enrollment and those on long-term nebulized steroids, had unreliable clinic attendance or declining informed consent were excluded from the study. Controls were healthy volunteers working in our hospital who were matched by age (± 5 yr) and gender to the cases. The study protocol was approved by the institutional ethics board.
Methodology: Patients without exacerbation at presentation were interviewed in a separate room by a single interviewer without prompting from relatives to complete the interview. Spirometry, post-bronchodilator forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) 15 min after 400 μg inhaled salbutamol were performed as per American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines8 using Powercube spirometer (Gangshorn Medizin electronics, Germany) and patients were evaluated for enrollment if no deterioration was noted as compared to a prior study. If no prior lung function data were available, another assessment was made at least one week after the first study. All patients also completed St. George Respiratory Questionnaire (SGRQ) in English or in previously validated local language (Kannada, Tamil and Hindi). The primary physician determined treatment for bronchiectasis, including the use of inhaled steroid. High-resolution computed tomography (HR) CT was performed using GE Bright Speed 16 slice (Fairfield, Connecticut, USA) by axial acquisition at 1 mm sections every 10 mm during a six-minute breath hold and the initial clinical assessment was performed within two weeks of the HRCT. The HRCT images were retrieved and severity scoring was performed according to a CT-measure of severity9 by two independent observers; any discrepancies were resolved by consensus. The extent of bronchiectasis in each lobe (subdivided into proximal, middle and distal using defined criteria) was scored on a 5-point scale based on severity of bronchiectasis, bronchial wall thickening, atelectasis, air trapping and mucoid impaction. The maximum possible score for each lobe was 40, with a total theoretical maximum of 240 per patient. Further, the extent of bronchiectasis alone was also semi-quantitatively scored as mild (Grade 1 bronchiectasis), moderate (Grade 2 bronchiectasis) and severe (cystic or saccular bronchiectasis). All newly diagnosed patients with bronchiectasis also underwent transthoracic echocardiography to look for evidence and severity of pulmonary artery hypertension, where present. Sub-maximal functional assessment was also performed at baseline in all patients by six-minute walk testing as per ATS guidelines10; patients who had a resting saturation of <92 per cent or exercise desaturation (>4% desaturation on testing) underwent arterial blood gas analysis to see if they fulfilled criteria for long-term oxygen therapy.
Controls were healthy hospital employees or students who were screened for absence of any medical complaints or medication intake and sequentially enrolled by matching for age (± 5 yr) and gender (Fig. 1). All enrolled subjects were evaluated following an overnight fast, having discontinued inhaled steroids for at least 24 h. Blood pressure was measured while supine, and at 1 and 3 min after standing. Postural hypotension was defined as a fall in systolic blood pressure of greater than 20 mmHg and a fall in diastolic blood pressure of greater than 10 mmHg upon standing. Intravenous access was established with a 20-guage cannula with a 10 centimeter extension and a 3-way stopcock. Blood was sampled at 0800 h for basal cortisol. Synthetic1-24 ACTH (adrenocorticotropin) cosyntropin 250 μg (1 milliliter, Synacthen®, Novartis, UK) was diluted in 499 ml of normal saline. Two milliliters of the reconstituted solution containing 1 μg cosyntropin was administered intravenously through the same cannula and flushed with ten milliliters of saline. Blood was drawn at exactly 30 min after administration of 1 μg cosyntropin for incremental cortisol response. Samples were processed using immunochemiluminometric assay (Beckmann-coulter AG).
Fig. 1.
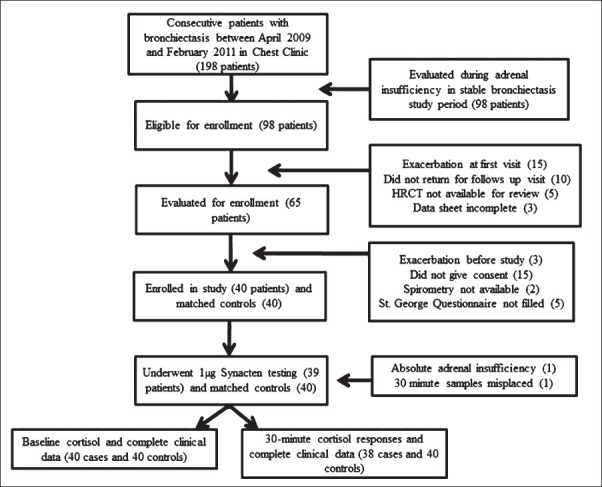
Flowchart of the patients enrolled in a case-control study of adrenal insufficiency in non-CF bronchiectasis.
Statistical analysis: Sample size calculation for the study was performed using the nMaster v1.0 program (CMC, Vellore, India). Using the published prevalence of suppressed responses in stable bronchiectasis and the prevalence with and without ICS use5, the estimated sample size was 38 patients to detect a 10 per cent difference with 80 per cent power and a type 1 error of 0.05.
The statistical package SPSS 16, USA (Statistical package for Social Sciences) was used to perform the statistical analysis. Epidemiological and outcome parameters of the patients are presented in a descriptive fashion (mean ± SD or median with IQR). Continuous variables were compared by the independent sample t test or Mann Whitney test and frequency proportions by the Chi-square test or Fisher's exact test. Odds ratio were calculated for the relationship between inhaled steroids and adrenal responses and cor-relation analyses were done for the presence of suppressed adrenal responses. All statistical tests were two-sided; P<0.05 was considered significant.
Results
We enrolled 40 consecutive patients with bronchiectasis and 40 matched healthy controls. One patient with tuberculosis-related bronchiectasis had absolute adrenal insufficiency (symptoms and baseline cortisol of 1.6 μg/dl) and was started on replacement steroids. Of the remaining 39 patients, all underwent 1 μg Synacten testing and their baseline data are provided in Table I. The study cohort had advanced bronchiectasis [70% grade 4 bronchiectasis, CT severity score 87.37 ± 34.46, (maximum possible score 240)] with frequent exacerbations [median 3; Interquartile range (IQR) 2-5.75 per year]. Desaturation on exercise (52.5%, 21/40) and pulmonary artery hypertension was common (27.5%, 11/40); no patient was on domiciliary oxygen therapy. 46.1 per cent (18/39) of the patients were on inhaled steroids and received inhaled budesonide (median dose 400 μg/day; range 200-800 μg/day) prior to enrollment (median 1.2 yr; IQR 8-24 months). The 30-minute cortisol value of one patient was unavailable and was excluded for analysis. Baseline cortisol was 10.45 and 12.46 μg/dl respectively for cases and controls (mean difference -2.01 μg/dl, P=0.04). The 30-minute stimulated cortisol response was 17.56 and 21.29 μg/dl respectively for cases and controls (mean difference -3.23 μg/dl, P<0.001). Using the lower 2.5th percentile cortisol response to cosyntropin as the cut point defining impaired adrenal reserve, 39.5 per cent (15/38) patients with bronchiectasis had impaired stimulated responses to 1 μg cosyntropin. Patients on inhaled steroids had a longer duration of symptoms [15.89 ± 1.98 (S.E) versus 9.25 ± 1.78 years (S.E), P=0.012] but lung function, exercise capacity, SGRQ scores, baseline and stimulated cortisol scores were similar (Table II). When patients with normal and suppressed cortisol responses were examined (Table III), there was no significant difference in lung function, CT severity scores, ICS use (OR 0.61, P=0.46), self-reported exacerbation rates and/or admissions in the last six months or SGRQ scores. The symptoms score of SGRQ was significantly correlated negatively with body mass index [(BMI), r= -0.51, P=0.001] and the impacts and total SGRQ scores were significantly correlated negatively with BMI (r= -0.46, P=0.03 and r =-0.48, P=0.02, respectively) and with severity of bronchiectasis as measured by HRCT score (r=0.37, P=0.019 and r=0.33, P=0.036, respectively) but not with baseline (r=0.12, P=0.45) or stimulated cortisol responses (r=0.15, P=0.38, Fig. 2).
Table I.
Baseline characteristics of patients undergoing 1μg Synacten test (N=40)

Table II.
Differences in characteristics among patients who used inhaled corticosteroids (ICS) and those who did not
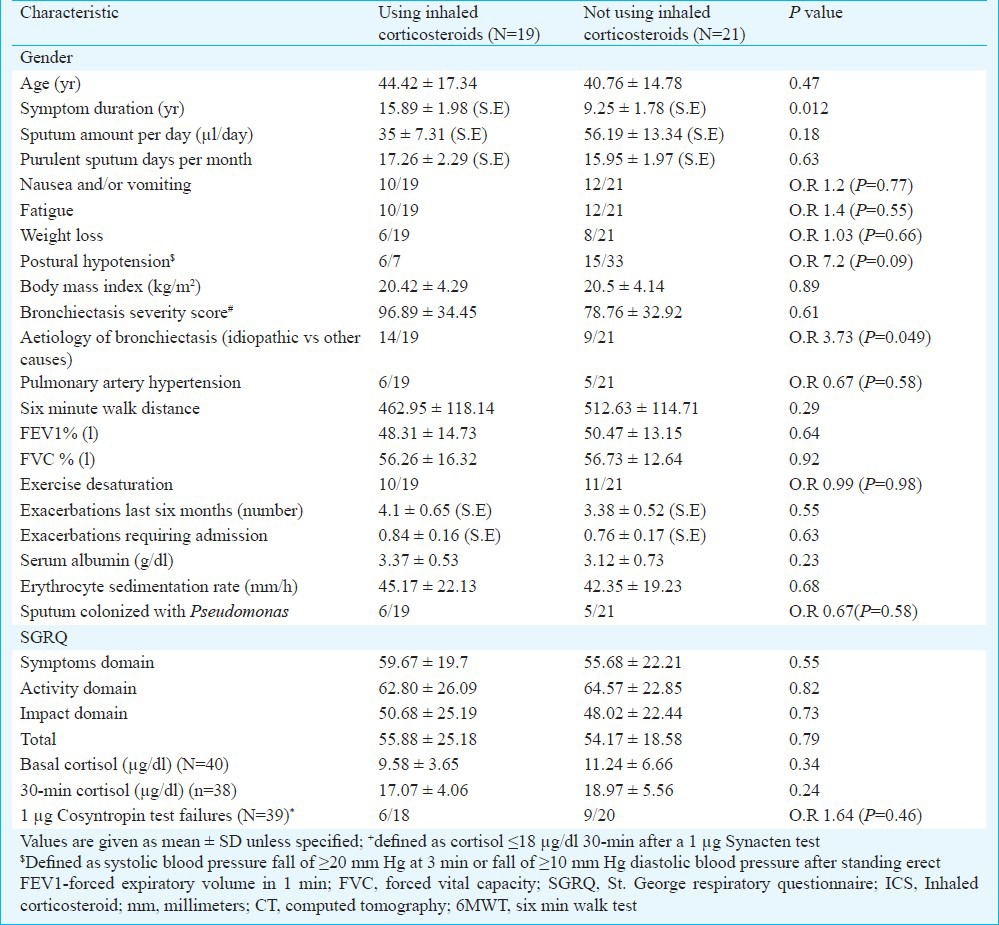
Table III.
Comparison of characteristics among patients with and without suppressed 30-minute cortisol responses by 1 μg cosyntropin test*
Fig. 2.
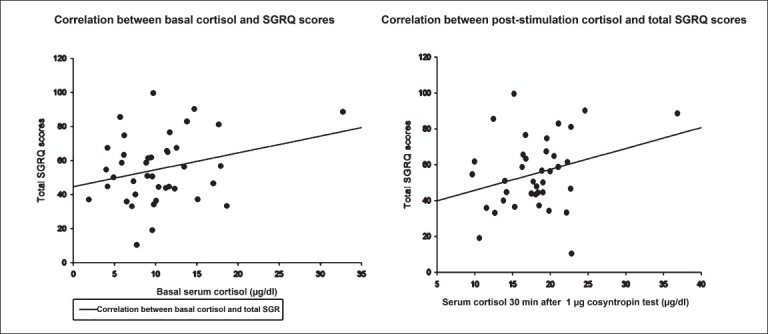
Scatter-plot with regression showing the lack of correlation of basal (left) and 30-min post-1 μg cosyntropin stimulation with total St. George Respiratory Questionnaire (SGRQ) scores.
Discussion
Inhaled corticosteroids are widely used to reduce sputum volume, purulence, exacerbations and quality of life in patients with non-CF bronchiectasis11,12,13; however, evidence suggests that the use of ICS does not improve spirometry measures of lung function and exacerbations in non-CF bronchiectasis2. High doses (≥800 μg budesonide equivalent) are often used clinically and in clinical trials of non-CF bronchiectasis11,13. This may be associated with significant long-term side effects that may be underestimated as most studies are either short-term (≤ 1 yr) in duration or used self-reported endpoints for measurement of side effects.
Concern about the use of ICS in non-CF bronchiectasis was also raised by finding suppressed cortisol responses using the 250 μg Synacten test5. An association with poor quality of life was also reported5. The greater systemic absorption through the dilated, permeable airways of patients with bronchiectasis5 and chronic systemic inflammation14 associated with repeated infections and exacerbations have been found to be related with the more frequent suppressed responses in bronchiectasis. The association of suppressed cortisol responses15 and size of adrenal glands16 with mortality have been reported in patients with septic shock. The present study was designed to investigate and compare the cortisol responses of patients with non-CF bronchiectasis with healthy controls. The impairment of adrenal reserve in bronchiectasis is likely to be mild and further, in a given individual it is difficult to predict the site of the abnormality in the HPA axis or its time of onset. The 1 μg cosyntropin test was used because it is more sensitive test than the 250 μg test17, is appropriate for patients with recent onset and partial secondary adrenal insufficiency18,19.
Our study showed that patients with non-CF bronchiectasis had unrecognized impaired adrenal response to cosyntropin when compared to matched controls. However, we could not demonstrate an association of the baseline, 30 min or incremental cortisol responses with the use of ICS, aetiology of bronchiectasis, defined radiologic measures of severity of bronchiectasis or quality of life. We also could not find an association with the number of self-reported exacerbations and hospitalizations over a 12-month period and cortisol responses. On exploratory analysis of the SGRQ scores in our study group, it was found that total SGRQ scores were closely linked to body weight, severity scores of bronchiectasis and quantity of sputum production.
We found a similar proportion of suppressed responses to those reported with high-dose cosyntropin testing, suggesting that suppressed responses are frequent in non-CF bronchiectasis5. A matched control group was used in the present study to account for the reported variability in responses to 1 μg cosyntropin testing and lower 2.5th percentile responses were obtained20,21. The strengths of the present study included the inclusion of consecutive patients from a prospective large well-defined cohort of non-CF bronchiectasis and matched controls to 1 μg cosyntropin testing. Also, the patients included had a long duration of symptoms, poor lung function and long duration of ICS use. The availability of data of additional functional testing and CT-scores of severity of bronchiectasis for cor-relation removed any confounding between ICS use, severity of bronchiectasis and SGRQ scores.
The limitations of the study included the small number of patients enrolled and the absence of information about the secular natural history of patients with suppressed responses to 1 μg cosyntropin and during exacerbations. Also, we measured total cortisol and not free cortisol and did not measure ACTH values; however, a previous study has suggested that abnormalities in cortisol-binding protein does not account for the suppressed responses5.
In conclusion, cortisol responses were frequently suppressed in patients with non-CF bronchiectasis by 1 μg-cosyntropin testing when compared to match controls. Suppressed cortisol responses were not associated with the use of ICS, aetiology of bronchiectasis, severity scores of bronchiectasis, number of self-reported exacerbations or SGRQ scores. Quality of life scores were negatively associated with body weight and CT-severity scores of bronchiectasis.
More data are needed about longitudinal cortisol responses and about the relation of these with prospectively collected data on exacerbations, decline in lung function and mortality.
Acknowledgment
Authors acknowledge Dr P.J. Jones, for providing permission to use the St. George Respiratory Questionnaire in English and local languages.
References
- 1.Pasteur MC, Bilton D, Hill AT British Thoracic Society Non CF Bronchiectasis Guideline Group. British thoracic society guideline for non-CF bronchiectasis. Thorax. 2010;65:577. doi: 10.1136/thx.2010.142778. [DOI] [PubMed] [Google Scholar]
- 2.Kapur N, Bell S, Kolbe J, Chang AB. Inhaled steroids for bronchiectasis. Cochrane Database Syst Rev. 2009;1:CD000996. doi: 10.1002/14651858.CD000996.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Hester KL, Macfarlane JG, Tedd H, Jary H, McAlinden P, Rostron L, et al. Fatigue in bronchiectasis. QJM. 2012;105:235–40. doi: 10.1093/qjmed/hcr184. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly R, Williams KM, Baker AB, Badcock CA, Day RO, Seale JP. Effects of budesonide and fluticasone on 24-hour plasma cortisol. A dose-response study. Am J Respir Crit Care Med. 1997;156:1746–51. doi: 10.1164/ajrccm.156.6.9703003. [DOI] [PubMed] [Google Scholar]
- 5.Holme J, Tomlinson JW, Stockley RA, Stewart PM, Barlow N, Sullivan AL. Adrenal suppression in bronchiectasis and the impact of inhaled corticosteroids. Eur Respir J. 2008;32:1047–52. doi: 10.1183/09031936.00016908. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal R, Gupta D, Aggarwal AN, Behara D, Jindal SK. Allergic bronchopulmonary aspergillosis: lessons from 126 patients attending a chest clinic in north India. Chest. 2006;130:442–8. doi: 10.1378/chest.130.2.442. [DOI] [PubMed] [Google Scholar]
- 7.McGuinness G, Naidich DP, Leitman BS, McCauley DI. Bronchiectasis: CT evaluation. AJR Am J Roentgenol. 1993;160:253–9. doi: 10.2214/ajr.160.2.8424327. [DOI] [PubMed] [Google Scholar]
- 8.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 9.Lynch DA, Newell J, Hale V, Dyer D, Corkery K, Fox NL, et al. Correlation of CT findings with clinical evaluations in 261 patients with symptomatic bronchiectasis. AJR Am J Roentgenol. 1999;173:53–8. doi: 10.2214/ajr.173.1.10397099. [DOI] [PubMed] [Google Scholar]
- 10.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 11.Tsang KW, Tan KC, Ho PL, Ooi GC, Ho JC, Mak J, et al. Inhaled fluticasone in bronchiectasis: a 12 month study. Thorax. 2005;60:239–43. doi: 10.1136/thx.2002.003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsang KW. Inhaled corticosteroids in COPD. Thorax. 1999;54:186. [PubMed] [Google Scholar]
- 13.Martinez-Garcia MA, Perpina-Tordera M, Roman-Sanchez P, Soler-Cataluna JJ. Inhaled steroids improve quality of life in patients with steady-state bronchiectasis. Respir Med. 2006;100:1623–32. doi: 10.1016/j.rmed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Crofford LJ. The hypothalamic-pituitary-adrenal axis in the pathogenesis of rheumatic diseases. Endocrinol Metab Clin North Am. 2002;31:1–13. doi: 10.1016/s0889-8529(01)00004-4. [DOI] [PubMed] [Google Scholar]
- 15.Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283:1038–45. doi: 10.1001/jama.283.8.1038. [DOI] [PubMed] [Google Scholar]
- 16.Jung B, Nougaret S, Chanques G, Mercier G, Cisse M, Aufort S, et al. The absence of adrenal gland enlargement during septic shock predicts mortality: a computed tomography study of 239 patients. Anesthesiology. 2011;115:334–43. doi: 10.1097/ALN.0b013e318225cfd7. [DOI] [PubMed] [Google Scholar]
- 17.Nye EJ, Grice JE, Hockings GI, Strakosch CR, Crosbie GV, Walters MM, et al. Comparison of adrenocorticotropin (ACTH) stimulation tests and insulin hypoglycemia in normal humans: low dose, standard high dose, and 8-hour ACTH-(1-24) infusion tests. J Clin Endocrinol Metab. 1999;84:3648–55. doi: 10.1210/jcem.84.10.6062. [DOI] [PubMed] [Google Scholar]
- 18.Streeten DH, Anderson GH, Jr, Bonaventura MM. The potential for serious consequences from misinterpreting normal responses to the rapid adrenocorticotropin test. J Clin Endocrinol Metab. 1996;81:285–90. doi: 10.1210/jcem.81.1.8550765. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi PG, Shah NS, Khandelwal AG, Chauhan P, Menon PS. Evaluation of low dose ACTH stimulation test in suspected secondary adrenocortical insufficiency. J Postgrad Med. 2002;48:280–2. [PubMed] [Google Scholar]
- 20.Fleseriu M, Gassner M, Yedinak C, Chicea L, Delashaw JB, Jr, Loriaux DL. Normal hypothalamic-pituitary-adrenal axis by high-dose cosyntropin testing in patients with abnormal response to low-dose cosyntropin stimulation: a retrospective review. Endocr Pract. 2010;16:64–70. doi: 10.4158/EP09153.OR. [DOI] [PubMed] [Google Scholar]
- 21.Dickstein G. High-dose and low-dose cosyntropin stimulation tests for diagnosis of adrenal insufficiency. Ann Intern Med. 2004;140:312–3. doi: 10.7326/0003-4819-140-4-200402170-00026. [DOI] [PubMed] [Google Scholar]


