Abstract
Background & objectives:
Emergence of antimicrobial resistance in Pseudomonas aeruginosa has led to the search for alternative agents for infections control. Natural products have been a good alternative to present antibiotics. The present study was undertaken to evaluate the effectiveness of cranberry in attenuation of virulence of P. aeruginosa in experimental urinary tract infection (UTI) in mouse model. Efforts were also directed to explore the action of cranberry towards virulence of P. aeruginosa through quorum sensing (QS) inhibition.
Methods:
Efficacy of cranberry was evaluated in an experimental UTI mouse model and on production of QS signals, alginate, pyochelin, haemolysin, phospholipase-C, cell-surface hydrophobicity, uroepithelial cell-adhesion assay and biofilm formation by already standardized methods.
Results:
Presence of cranberry showed significant decline in the production of QS signals, biofilm formation and virulence factors of P. aeruginosa in vitro (P<0.001). Further, cranberry was found to be useful in prevention of experimental UTI in mouse model as indicated by reduced renal bacterial colonization and kidney tissues destruction.
Interpretation & conclusions:
The findings of the present study indicated that cranberry inhibited QS and hence elaboration of virulence factors of P. aeruginosa. It also affected the adherence ability of this pathogen. This approach can lead to the discovery of new category of safe anti-bacterial drugs from dietary sources such as cranberry with reduced toxicity without the risk of antibiotic resistance.
Keywords: Biofilm, cranberry, P. aeruginosa, quorum sensing, virulence factors
Opportunistic pathogen Pseudomonas aeruginosa accounts for nearly 35 per cent of total nosocomial infections1. This organism produces several cell associated and extracellular virulence factors including alginate, protease, elastase, rhamnolipids, exotoxin A, exoenzyme S, siderophores (pyochelin, pyoverdin) and haemolysins. In addition, P. aeruginosa also forms biofilms on the catheter surfaces, which are usually resistant to conventional antibiotics, host immune defence mechanism, and hence needs special attention. Formation of biofilm and production of virulence factors are largely regulated by cell-to-cell communication, which is known as quorum sensing (QS). Without QS, the expression of virulence factor reduces significantly and results in less/no virulence of the organism2. In P. aeruginosa, two QS systems LasR–LasI and RhlR–RhlI function in a hierarchical manner through their cognate signal molecules N-(3-oxo-dodecanoyl)-L-homoserine lactone and N-butanoyl-L-homoserine lactone3,4. QS has been shown to be essential for the pathogenesis in a number of infections such as respiratory tract, burn wound, keratitis and urinary tract5,6,7,8. Nosocomial urinary tract infections (UTI) caused by P. aeruginosa are a significant health problem, which is usually associated with substantial morbidity and mortality1. Since virulence of this pathogen is under the regulation of QS, therefore, QS has been considered as ideal drug target for controlling Pseudomonas infections. Although, furanone compounds have been shown to inhibit QS in vitro9, but use of these compounds is also associated with possible toxicity in vivo. Therefore, research for alternative or adjunctive strategies to conventional antibiotic treatment, which could attenuate the virulence of P. aeruginosa, is required.
Cranberry (Vaccinium macrocarpon), is known to contain various phytochemicals like flavanoids, proanthocyanidins, anthocyanins, catechin, triterepenoids and organic acid. Naturally occurring cranberrys are acidic in nature, but in commercial preparation, this acidity is reduced because of dilution with water and addition of glucose and vitamin C, so as to make it acceptable and safe for oral consumption. Although cranberry juice has been shown to be beneficial in prevention of community acquired UTI caused by Escherichia coli10,11, but its role has not been evaluated in UTI caused by P. aeruginosa. Proanthocyanidins of cranberry have been shown to interfere QS of Vibrio harveyi12, but the effect of cranberry on QS in association with virulence potential of P. aeruginosa has not been explored. Therefore, the present study was carried out to determine the role and effect of cranberry on production of QS signals and virulence potential of P. aeruginosa. Further, cranberry was also evaluated as a non-antibiotic alternative agent in prevention of experimental UTI in mouse model.
Material & Methods
The study was conducted in the department of Microbiology, Panjab University, Chandigarh, India.
Bacterial strain: Standard strain of P. aeruginosa PAO1, obtained from Dr Barbara H. Iglewski, University of Rochester, New York (USA) was used as a standard reference strain. This strain is well characterized genetically and phenotypically for the elaboration of recognized virulence factors of P. aeruginosa.
Cranberry: Commercially available cranberry juice (Ocean Spray, Massachusetts, U.S.A.) containing 25 per cent of pure cranberry was used. The commercial juice contains 75 per cent water, glucose (100 g/l) and trace amount of vitamin C in addition to pure cranberry juice. Growth profile of P. aeruginosa PAO1 was carried out in presence of different concentrations of cranberry. Sub-inhibitory concentration showing no growth inhibition as compared to control, was selected for further experiments.
Biofilm generation: Biofilm was generated on sterile Foley's catheter (Rusch) under static conditions for seven days in presence and absence of sub-inhibitory concentration of cranberry. Catheter pieces were transferred to fresh medium after every 24 h. Each day catheter pieces in duplicate were removed, and rinsed thrice with phosphate buffer saline (PBS pH 7.2). Cells were scrapped from catheter surface with sterile scalpel blade and were sonicated at low-level. Dispersed sample was centrifuged and the pellet was suspended in 1 ml PBS. Biofilm cells were plated on nutrient agar plates to determine log cfu by standard tube dilution method13.
Acylhomoserine lactone (AHL) quantification: AHLs were extracted from the biofilm cells formed on catheter pieces in the presence and absence of cranberry. Briefly, catheter pieces were immersed in acidified ethyl acetate at 4o C overnight and ethyl acetate was used for β-galactosidase activity. Reporter strain (E. coli MG4) was grown in presence of extract at 37°C for 5½ h. After incubation, 1 ml of cells were diluted in Z buffer (Na2HPO4.7H20-0.06M, NaH2PO4.H20-0.04M, KCl-0.01M, MgSO4.7H2O-0.001M, β-mercaptoethanol-0.05M, pH-7.0) (1:1) and assayed for β-galactosidase activity using o-nitrophenyl-D-galactopyranoside (ONPG) as substrate14.
Scanning electron microscopy (SEM): SEM of biofilm cells grown on catheter surface in absence and presence of cranberry was performed15. Briefly, catheter pieces were fixed with 2.5 per cent glutaraldehyde overnight at 4°C followed by dehydration in gradient concentration of HPLC grade acetone. Catheter pieces were then stubbed with gold ions and observed with SEM model JSM-6100, SM-Jeol, 20 KV. All the chemicals used were purchased from M/s Fisher Scientifics (USA), M/s Sigma Aldrich Chemicals (USA) and M/s HiMedia Labs. Ltd. (India) and were of analytical grade.
Uroepithelial cell adhesion assay: UEC adhesion assay of biofilm cells (108 cfu/ml) grown in presence and absence of cranberry was performed16. Briefly, 1ml each of bacterial suspension and washed UECs (105 cells/ml) were incubated at 37°C for 1 h in shaker water bath. After that, cell mixture was centrifuged at 6,000 g for 10 min with washings of sterile PBS. Smears were prepared from cell pellet and were stained with Giemsa. Bacteria adhering to 30 UECs were counted thrice and average number of bacteria adhering per UEC was calculated.
Estimation of virulence factors in vitro: Different virulence factors elaborated by P. aeruginosa were estimated in the culture supernatant of biofilm cells grown in presence and absence of selected concentration of cranberry.
Alginate: Alginate was precipitated using an equal volume of culture supernatant and 2 per cent (w/v) cetylpyridium chloride followed by centrifugation at 6,000 g for 20 min17. Pellet was suspended and precipitated with 5 ml of iso-propanol followed by centrifugation at 6,000 g for 10 min. Pellet was suspended in 1 ml of normal saline. Alginate was determined using D-mannourate lactone to calibrate a standard curve.
Cell surface hydrophobicity: Cell culture supernatant was mixed with equal volume of p- xylene and after phase separation; optical density of aqueous phase was taken18. Surface hydrophobicity was expressed as percentage.
Pyochelin: Quantitation of pyochelin was done by mixing 1 ml each of cell free supernatant, 0.5N hydrochloric acid, nitrite molybdate reagent and 1N NaOH19. The final volume was made to 5 ml with distilled water. Absorbance was taken at 510 nm.
Haemolysin: Cell free and cell bound haemolysin was estimated in the absence and presence of cranberry.
Cell bound haemolysin: Human RBCs (2%) were prepared from 10 ml fresh blood collected from one healthy human and 1.5 ml of washed human RBC (2%) suspension was mixed with 1.5 ml of biofilm cells (1X108 cells/ml) and incubated at 37°C for 2 h. After incubation, mixture was centrifuged at 8,000 g for 15 min. Absorbance of supernatant was read at 545 nm. Haemolysin (mg/ml) was calculated by using lyophilized haemoglobin as standard reference.
Cell free haemolysin: Human RBC suspension (2%, 1.5 ml) was mixed with 1.5 ml of cell free supernatant and incubated at 37°C for 2 h. After incubation, assay mixture was centrifuged at 5,000 g for 5 min and absorbance was taken at 545 nm20.
Phospholipase-C (PLC): For PLC21, 50 per cent (v/v) egg yolk suspension was prepared in saline and mixed with pre-molten agar to a final concentration of 10 per cent. Sodium azide (0.1%) was added as preservative. Plates were prepared from mixed agar and wells were cut in agar plates. To these wells, 100 μl of culture supernatant was added and plates were incubated at 37°C for 24-48 h. Diameter of hydrolysis of egg yolk was measured.
Potential efficacy of cranberry in experimental UTI: Experimental UTI was induced in mice by standard method22. The study protocol was approved by the Panjab University Ethics Committee. Female LACA (Swiss Webster) mice, 6-8 wk old, free of bacteriurea, were used. Animals were divided into two major groups as control and test group. In test group, mice were given prophylactic treatment for 14 successive days with 1 ml of sub-inhibitory concentration of cranberry through oral feeding tube. This was followed by infection on day 15. In control, group 1 was given only infection without any treatment. In control group 2, mice were given prophylactic treatment with sterile saline orally, which was followed by infection. In control group 3, mice were given only cranberry treatment with no infection. Ten mice were used for each experimental group; 50 μl of bacterial inoculum (108 cfu/ml) was introduced slowly into the bladder of mice using a soft non radio-opaque polyethylene tubing (outer diameter 0.61mm, Clay Adams, USA).
Bacteriological examination: Animals were sacrificed on day 5 post infection. Aseptically removed individual kidney from each group was homogenized in 1 ml sterile saline and cfu/ml was estimated by standard dilution method for each kidney separately. Quantitative bacterial count was made per gram of kidney tissue.
Histopathological examination: Kidney tissues were fixed in 10 per cent buffered normal saline and dehydrated in 30-100 per cent gradient ethanol. Paraffin wax blocks were prepared and 5 μ thin sections were stained with haematoxylin and eosin (H&E). The medulla, cortex, calyx, and subcalyx of each kidney tissue were evaluated.
Malondialdehyde (MDA) estimation: Induction of pathology was evaluated based on the index of lipid peroxidation by estimating MDA23. Briefly, 0.5 ml of the tissue homogenate was added to 0.5 ml of Tris HCl (0.1 M, pH 7.4). Mixture was incubated at 37°C for 2 h followed by addition of 1 ml of ice-cold trichloroacetic acid (10% w/v). Samples were centrifuged at 1,000 g for 10 min. One ml of supernatant was mixed with 1 ml of thiobarbituric acid (0.67% w/v). Tubes were covered and kept in boiling water bath for 10 min. After cooling, absorbance was read at 532 nm.
Reactive nitrogen intermediates (RNI) estimation: Nitrite level was also estimated in renal tissues of infected mice24. Briefly, 100 μl of sample was mixed with 200 μl of Griess reagent (Sigma Chemicals, USA) followed by addition of 100 μl of 10 per cent trichloroacetic acid. Mixture was incubated for 20 min at room temperature. After centrifugation (5,000 g for 10 min), optical density of supernatants was read at 540 nm. The amount of nitrite was determined using standard curves of sodium nitrite.
Statistical analysis: All the experiments were repeated three times to validate the reproducibility of experiments. SigmaStat software, USA was used to analyze the results by using Student's t test.
Results
P. aeruginosa PAO1 was grown in presence of different concentrations of cranberry, and 15 per cent cranberry showing no growth inhibition as compared to control, was selected as sub-inhibitory concentration and was used for all in vitro and in vivo experiments (data not shown).
Effect of cranberry on virulence factors of P. aeruginosa in vitro: Biofilm was generated on the catheter surface in presence and absence of cranberry. In control, biofilm formation was maximum on 4th day (log cfu 11.4) with slight decline on day 5. Although, biofilm in the presence of cranberry also showed peak on 4th day (log cfu 4.2) but it was significantly (P<0.001) less than that of control (Fig. 1). For all further experiments, 4 day old biofilm was used since maximum biofilm mass was noted on 4th day. Production of QS signals was also declined significantly (P<0.001) in the presence of cranberry (Fig. 2). Scanning electron microscopy was performed to evaluate the structure of biofilm formed on catheter surface. In the absence of cranberry, bacterial cells were seen deeply enmeshed in extracellular glycocalyx and embedded in thick slimy matrix. On the other hand, inhibition of biofilm formation with significantly less exopolysaccharide was observed in presence of cranberry (Fig. 3). Production of virulence factors were observed on 4th day of biofilm formation in control as well as cranberry treated cells. On comparing with control, levels of alginate, cell surface hydrophobicity, pyochelin, haemolysin and PLC were reduced significantly in presence of cranberry (P<0.01, P<0.001, Table). Significant decrease in number of bacteria adhering to uroepithelial cells (UECs) was also observed in presence of cranberry (P<0.001, Table). Glucose and vitamin C present in juice in addition to cranberry were also evaluated for their effect and were observed not to affect the expression of any virulence factors (data not shown).
Fig. 1.
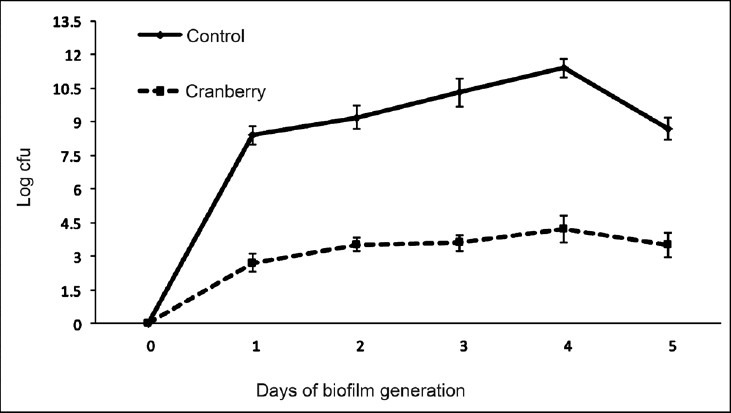
Generation of biofilm (expressed in log cfu) by Pseudomonas aeruginosa on catheter surface in absence and presence of sub-inhibitory concentration of cranberry from day 1 to day 5. Results are expressed as mean ± SD.
Fig. 2.
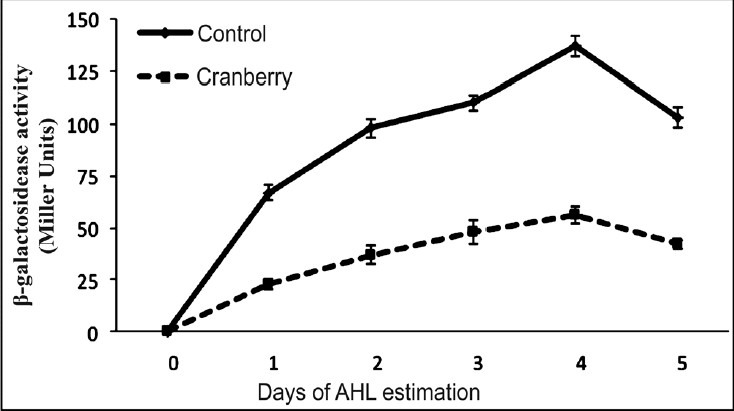
Production of quorum sensing signal molecules (in Miller Units) by biofilm cells of Pseudomonas aeruginosa in absence and presence of sub-inhibitory concentration of cranberry from day 1 to day 5. Results are expressed as mean ± SD.
Fig. 3.
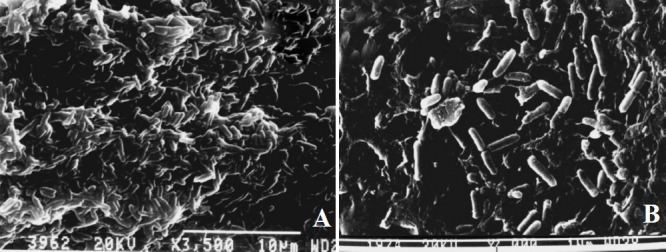
Photomicrograph showing the results of scanning electron microscopy of four day old biofilm grown on catheter surface by Pseudomonas aeruginosa in absence and presence of sub-inhibitory concentration of cranberry. Large amount of exopolysaccharide in control cells (A; X3500) and less amount of exopolysaccharide in cranberry treated and bacterial cells are clearly visible (B; X3500).
Table.
Production of different virulence factors and renal colonization by the biofilm cells of Pseudomonas aeruginosa in presence and absence of sub-inhibitory concentration of cranberry
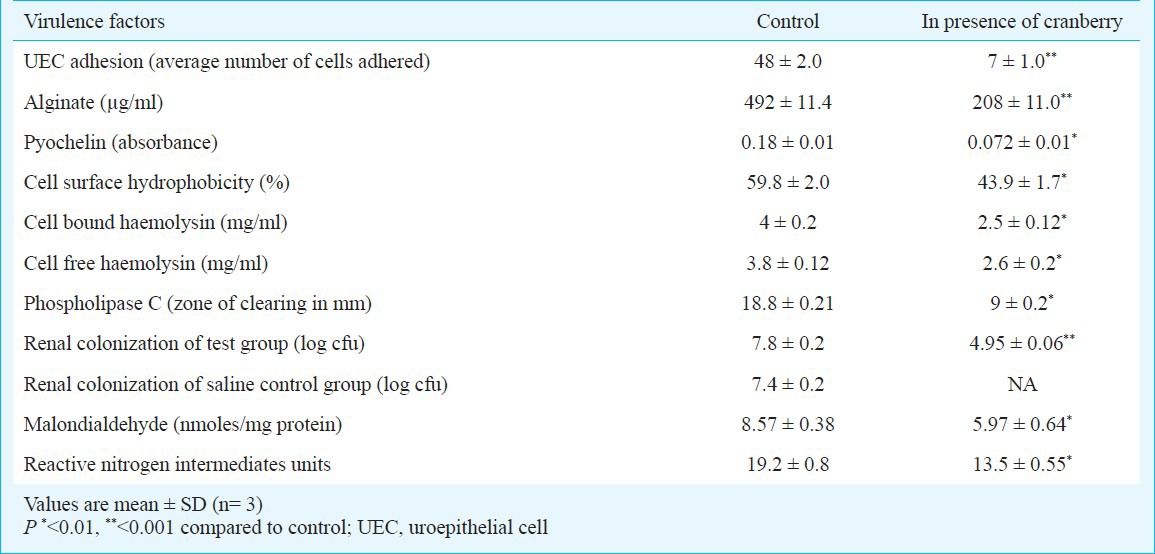
Effect of cranberry on experimental UTI: Efficacy of cranberry was evaluated in terms of prevention of experimental UTI in mouse model. Mice of all groups were sacrificed on day 5 post infection. Aseptically collected kidneys were subjected to bacteriological and pathological examinations. In control groups, mice given infection with P. aeruginosa without any treatment showed renal log cfu of 7.8 ± 0.2 while group under saline treatment showed renal log cfu of 7.4 ± 0.2. Group of mice undergone prophylactic treatment with cranberry showed significantly reduced renal colonization with log cfu of 4.95 ± 0.06 (Table, P<0.001). No bacteria were recovered from mice with cranberry treatment without infection. On histopathological investigation, renal tissue of mice without any treatment showed severe inflammation with infiltration of polymorphonuclear leukocytes in interstitium along with vascular permeability along with destruction of glomeruli and tubules. Saline treatment group also showed similar results. However, normal to mild inflammation was observed in cranberry treated group (Fig. 4A and B). Expression of renal pathology markers, MDA and RNI were also significantly reduced (P<0.01) in cranberry treated mice group as compared to control group suggesting reduced tissue destruction in the presence of cranberry (Table).
Fig. 4.
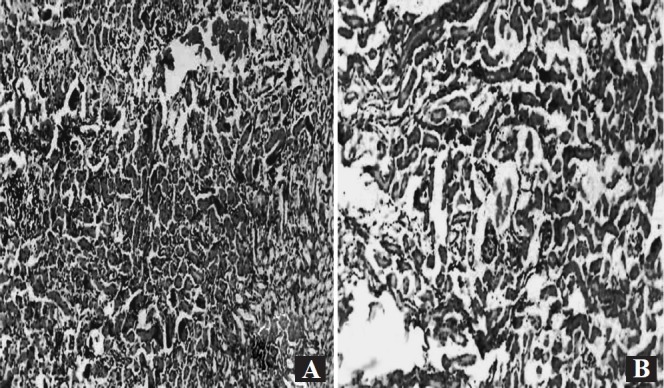
(A) Photomicrograph showing severe renal tissue inflammation with large number of polymorphonuclear leukocytes, along with destruction of glomeruli and tubules in renal tissue of control group of mice without treatment with cranberry (H & E X 100). (B) Photomicrograph showing mild renal tissue inflammation along with significantly less infiltration of ploymorphonuclear leukocytes in the renal tissue of group of mice undergone treatment with cranberry (H & E X 100).
Discussion
In P. aeruginosa, QS is known to regulate the virulence factors, biofilm formation and resistance to antimicrobials. Due to increased resistance to antimicrobial agents, non-antibiotic alternative measures using natural/herbal products, can be good option. In the present study, ability of cranberry to attenuate the virulence of P. aeruginosa was explored. Biofilm formed in the presence of cranberry showed significantly decreased log count as compared to control biofilm. Moreover, SEM revealed that biofilm in the presence of cranberry was thin and unstructured with scanty exopolysaccharide. On the other hand, bacterial cells were heavily embedded in exopolysaccharide forming thick biofilm in absence of cranberry indicating that cranberry inhibited the biofilm formation and exopolysaccharide production in P. aeruginosa. These results suggest that cranberry can inhibit the biofilm formation and hence may increase the susceptibility of biofilm cells to the action of antibiotics and hostile factors. Increased sensitivity of thin biofilm to antibiotics, may further lead to rapid clearing of infection25,26. Further, production of alginate, an important component of biofilm exopolysaccharide, was also reduced significantly in presence of cranberry. Alginate contributes to external morphology of biofilm and tissue colonization during infection25 and reduction in alginate production further indicates that cranberry may inhibit the biofilm formation of P. aeruginosa. A significant reduction in QS signal molecules was also observed in presence of cranberry. In P. aeruginosa QS regulates the virulence potential, hence inhibition of QS indicates the attenuation of Pseudomonas virulence in presence of cranberry.
P. aeruginosa showed significantly reduced ability to adhere to the UECs in the presence of cranberry as compared to control. Cell surface hydrophobicity, another mechanism of bacterial adherence, was also reduced significantly in the presence of cranberry. Significant reduction in adherence ability of P. aeruginosa in the presence of cranberry might result in the failure of establishment of infection in the urinary tract. Cranberry has been shown to be beneficial in the prevention of E. coli induced UTI10,11.
Though iron is present abundantly in tissues but is not available to the invading pathogen25. P. aeruginosa produces siderophore (pyochelin) to trap iron from the host tissues. Significant reduction in pyochelin level was observed in presence of cranberry, as compared to control. Haemolysins, another iron mopping mechanism, were also reduced significantly in presence of cranberry. Importance of siderophores and haemolysins has been demonstrated in UTI28,29. It has been observed that production of siderophore and haemolysin is also under the regulation of QS6,30 and reduction in their levels might have occurred due to QS inhibition. Significant reduction in the level of PLC, which helps pathogen to overcome the action of host defence and contributes towards tissue inflammation31, further supported that cranberry was effective in attenuating the virulence of P. aeruginosa.
To evaluate the efficacy of cranberry in attenuation of P. aeruginosa virulence in vivo, mice were given prophylactic treatment with cranberry followed by infection with P. aeruginosa. Establishment of UTI was evaluated based on bacteriological and pathology markers. Significantly, less renal bacterial colonization was observed in mice treated with cranberry as compared to control groups. Significant decrease in extent of tissue destruction, and lipid peroxidation (MDA level) was also observed in the presence of cranberry. This indicated the protective efficacy of cranberry in UTI. Additionally, cranberry may exhibit the synergistic effect by scavenging free radicals; thereby providing protection against oxidative damage. The estimation of nitrite is an indirect measure of nitric oxide content, which acts in many tissues to regulate diverse range of physiological process. Various clinical trials have shown that cranberry was effective in prevention of UTI caused by E. coli32.
In P. aeruginosa, virulence potential is strictly under the regulation of QS2. Therefore, instead of bactericidal or bacteriostatic strategies, attenuation of virulence via QS inhibition approach could be more effective. In the present study cranberry showed QS inhibitory properties, resulting in the attenuation of P. aeruginosa virulence in vitro and in vivo. A few previous studies have also shown that various phytochemicals have QS inhibitory properties12,33. Results of this study also suggest that phytochemicals can inhibit QS and its related virulence processes in pathogenic bacteria such as P. aeruginosa, and hence lead to the development of new safe anti-bacterial drugs from dietary sources with reduced toxicity and antibiotic resistance.
Conflicts of interests: The authors declare that they have no competing interests.
Acknowledgment
The authors acknowledge Dr Barbara H. Iglewski for providing standard strain of Pseudomonas aeruginosa PAO1.
Footnotes
Conflicts of interests: The authors declare that they have no competing interests
References
- 1.Leone M, Albanese J, Garnier F. Risk factors of nosocomial catheter associated urinary tract infection in a polyvalent intensive care unit. Intens Care Med. 2003;29:929–32. doi: 10.1007/s00134-003-1741-z. [DOI] [PubMed] [Google Scholar]
- 2.Wagner VE, Li LL, Isabella VM, Iglewski BH. Analysis of the hierarchy of quorum sensing regulation in Pseudomonas aeruginosa. Anal Bioanl Chem. 2007;387:469–79. doi: 10.1007/s00216-006-0964-6. [DOI] [PubMed] [Google Scholar]
- 3.McKnight SL, Iglewski BH, Pesci EC. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 2000;182:2702–8. doi: 10.1128/jb.182.10.2702-2708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diggle SP, Cornelis P, Williams P, Cámara M. 4-Quinolone signalling in Pseudomonas aeruginosa: old molecules, new perspectives. Int J Med Microbiol. 2006;296:83–91. doi: 10.1016/j.ijmm.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Rumbaugh KP, Griswold JA, Iglewski BH, Hamood AN. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect Immun. 1999;55:5854–62. doi: 10.1128/iai.67.11.5854-5862.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson JP, Feldman M, Iglewski BH, Prince A. Pseudomonas aeruginosa cell-to-cell signalling is required for virulence in a model of acute pulmonary infection. Infect Immun. 2000;68:4331–4. doi: 10.1128/iai.68.7.4331-4334.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu H, Bandara R, Conibear TC, Thuruthyil SJ, Rice SA, Kjelleberg S, et al. Pseudomonas aeruginosa with lasI quorum-sensing deficiency during corneal infection. Invest Ophthalmol Vis Sci. 2004;45:1897–903. doi: 10.1167/iovs.03-0980. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Chhibber S, Harjai K. Quorum sensing is necessary for the virulence of Pseudomonas aeruginosa during urinary tract infection. Kidney Int. 2009;76:286–92. doi: 10.1038/ki.2009.183. [DOI] [PubMed] [Google Scholar]
- 9.Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, Parsek MR, et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology. 2002;148:87–102. doi: 10.1099/00221287-148-1-87. [DOI] [PubMed] [Google Scholar]
- 10.Foo LY, Lu Y, Howell AB, Vorsa N. The structure of cranberry proantocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia in vitro. Phytochemistry. 2004;54:173–81. doi: 10.1016/s0031-9422(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 11.Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adherence activity. Phytochemistry. 2005;66:2281–91. doi: 10.1016/j.phytochem.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Feldman M, Weiss EI, Ofek I, Steinberg D. Interference of cranberry constituents in cell–cell signalling system of Vibrio harveyi. Curr Microbiol. 2009;59:469–74. doi: 10.1007/s00284-009-9462-3. [DOI] [PubMed] [Google Scholar]
- 13.Mittal R, Chhibber S, Sharma S, Harjai K. Effect of macrophage secretary products on elaboration of virulence factors by planktonic and biofilm cells of Pseudomonas aeruginosa. Comp Immunol Micro Infect Dis. 2006;29:12–26. doi: 10.1016/j.cimid.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Thuruthyil SJ, Willcox MD. Determination of quorum-sensing signal molecules and virulence factors of Pseudomonas aeruginosa isolates from contact lens-induced microbial keratitis. J Med Microbiol. 2002;51:1063–70. doi: 10.1099/0022-1317-51-12-1063. [DOI] [PubMed] [Google Scholar]
- 15.Nickel JC, Ruseska I, Wright JB, Costertorn JW. Tobramycin resistance of P. aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985;27:619–24. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma S, Garg UC, Ganguly NK, Sharma BK. Effect of iron on early changes in E. coli induced ascending pyelonephritis in rats. FEMS Microbiol Lett. 1987;41:295–8. [Google Scholar]
- 17.Mathee K, Ciofu O, Sternberg G, Campbell JIA. Mucoid conversion of Pseudomonas aeruginosa by H2O2: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology. 1999;145:1349–57. doi: 10.1099/13500872-145-6-1349. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg M, Guttinick D, Rosenberg E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. [Google Scholar]
- 19.Arnow LE. Calorimetric determination of the components of 3,4-dihydroxyphenylalanine tyrosine mixture. J Biol Chem. 1937;118:531–7. [Google Scholar]
- 20.Linkish PG, Vogt W. Direct hemolysin activity of phopholipase-A. Biochem Biophy Acta. 1972;270:241–50. doi: 10.1016/0005-2760(72)90235-4. [DOI] [PubMed] [Google Scholar]
- 21.Habermann E, Hardt KL. A sensitive and specific plate test for the quantitation of phospholipases. Anal Biochem. 1972;50:163–73. doi: 10.1016/0003-2697(72)90495-2. [DOI] [PubMed] [Google Scholar]
- 22.Mittal R, Chhibber S, Sharma S, Harjai K. Macrophage inflammatory protein-2, neutrophil recruitment and bacterial persistence in an experimental mouse model of urinary tract infection. Microbes Infect. 2004;6:1326–32. doi: 10.1016/j.micinf.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Anjaneyulu M, Chopra K. Effect of irbesartan on the antioxidant defence system and nitric oxide release in diabetic rat kidney. Am J Nephr. 2004;24:488–96. doi: 10.1159/000080722. [DOI] [PubMed] [Google Scholar]
- 24.Rockett KA, Awburn MM, Rockett EJ. Possible role of nitric oxide in malarial immunosuppression. Parasite Immunol. 1994;16:243–9. doi: 10.1111/j.1365-3024.1994.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 25.Shih PC, Huang CT. Effect of quorum-sensing deficiency on Pseudomonas aeruginosa on biofilm formation and antibiotic resistance. J Antimicrob Chemother. 2002;49:309–14. doi: 10.1093/jac/49.2.309. [DOI] [PubMed] [Google Scholar]
- 26.Skindersoe ME, Alhede M, Phipps R. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2008;52:3648–63. doi: 10.1128/AAC.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffiths E. Iron in biological systems. In: Bullen JJ, Griffiths, editors. Iron and infection: Molecular, physiological and clinical aspects. Chichester England: John Wiley and Sons Ltd; 1987. pp. 1–25. [Google Scholar]
- 28.Gabisoniia TG, Makaridze MR, Chanishvili TG, Iashvili BP. Antibiotic sensitivity and pathogenetic factors of hospital strains of Pseudomonas aeruginosa isolated from patients treated in a resuscitation unit. Antibiot Khimioter. 1992;37:17–8. [PubMed] [Google Scholar]
- 29.Visca P, Chiarini F, Mansi A, Vetriani C, Serino L, Orsi N. Metal regulation of siderophore synthesis in Pseudomonas aeruginosa and functional effects of siderophore-metal complexes. Appl Environ Microbiol. 1992;58:2886–93. doi: 10.1128/aem.58.9.2886-2893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesprit P, Faurisson F. Role of the quorum-sensing system in experimental pneumonia due to Pseudomonas aeruginosa in rats. Am J Respir Crit Care Med. 2003;167:1478–82. doi: 10.1164/rccm.200207-736BC. [DOI] [PubMed] [Google Scholar]
- 31.Meyers DJ, Berk RS. Characterization of phospholipase C from Pseudomonas aeruginosa as a potent inflammatory agent. Infect Immun. 1990;68:659–66. doi: 10.1128/iai.58.3.659-666.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiMartino P, Agniol R, David K. Reduction of Escherichia coli adherence to uroepithelial bladder cells after consumption of cranberry juice: a double- blind randomized placebo-controlled cross-over trial. World J Urol. 2006;24:21–7. doi: 10.1007/s00345-005-0045-z. [DOI] [PubMed] [Google Scholar]
- 33.Adonizio A, Kong KF, Mathee K. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother. 2008;52:198–203. doi: 10.1128/AAC.00612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


