Abstract
Purple toe syndrome is a recognised adverse effect of warfarin therapy. The literature has described resolution of the ischaemic symptoms on withdrawal of the warfarin and switching to a low molecular weight heparin alternative. We present a case of an 82-year-old man with bilateral blanching vivacious toes and a livedo-reticularis type rash developing 2 weeks after being loaded with warfarin for first detected atrial fibrillation. Vascular surgical review and haematology thrombotic screen did not yield any other pathology and a diagnosis of purple toe syndrome due to warfarin was carried out. The warfarin was stopped and oral anticoagulation started with an oral factor Xa inhibitor, apixaban with resolution of his symptoms. This is the first case report of one of the novel oral anticoagulants being used to treat purple toe syndrome.
Background
Warfarin is used extensively in medical practice globally. Purple toe syndrome is a rare complication of warfarin therapy. Clinicians need to be aware as the ischaemic changes can have a significant impact on morbidity, but are completely reversible if the condition is recognised early, and warfarin is substituted early for alternative anticoagulation. Patients often have an ongoing need for anticoagulation; previous literature has only described subcutaneous low molecular weight heparin as a treatment strategy. With increasing awareness and use of novel oral anticoagulants, our case suggests that these can successfully be used as a more convenient alternative in such a situation.
Case presentation
An 82-year-old man with a medical history of hypertension, gout and eczema was loaded on warfarin (10, 10, and 5 mg respectively) after he presented to primary care with new-onset atrial fibrillation. Two weeks later, he returned to his general practitioner with discoloured and painful toes. The patient reported of bilateral thigh pain and swelling, and a rash spreading across both shins. He was struggling to weight bear and required a walking stick. The patient was referred to hospital for further assessment.
On examination, the calves were neither warm nor swollen, but a livedo reticularis like rash was seen spreading upwards from the feet to cover the anterior aspect of both shins. His bilateral blanching vivacious big toes (figure 1) were cold and extremely painful on palpation. No gangrene was present.
Figure 1.
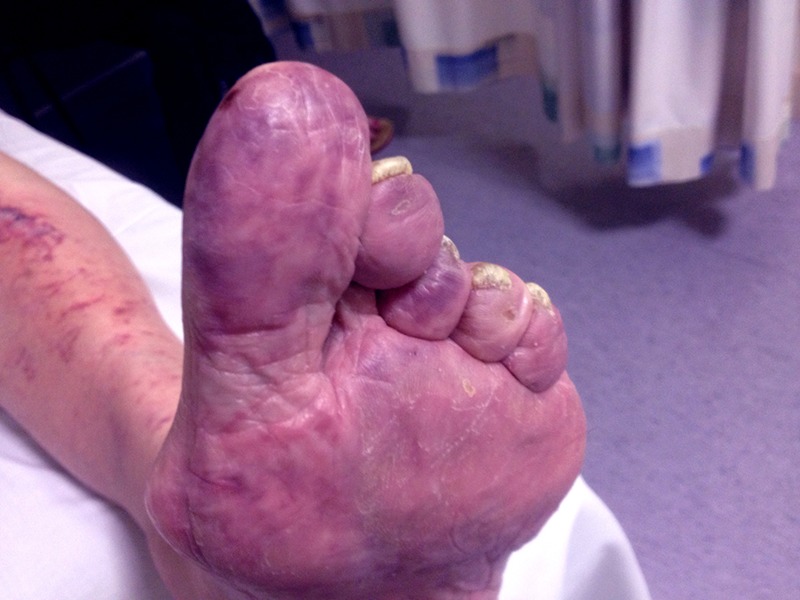
Purple toe of right foot.
Investigations
Blood results showed a normal activated partial thromboplastin time, normal prothrombin time and normal platelet count. The ECG confirmed his diagnosis of atrial fibrillation. A vascular surgical review was requested but this did not suggest a decrease in arterial flow, as the posterior tibialis and dorsalis pedis pulses were palpable bilaterally. The patient's case was reviewed with the haematologists, a prothrombotic screen was sent, but this did not show any abnormalities.
Differential diagnosis
A diagnosis of purple toe syndrome was carried out on the history (recent warfarin loading, no trauma), clinical signs (distal localised ischaemia) and exclusion of other pathology (impeded arterial or venous flow).
Treatment
The warfarin was stopped, and anticoagulation was achieved with oral apixaban 5 mg BD.
Outcome and follow-up
At outpatient review, his symptoms had settled and his anticoagulation was maintained within therapeutic range on apixaban.
Discussion
First described in 1961, purple toe syndrome is a rare adverse effect of warfarin therapy affecting 1 in 5000 patients; however, this risk increases to 3% if the patient is also protein C deficient.1 The pathogenesis was thought to be due to a direct and toxic insult to the capillaries, resulting in increased dilation and permeability of the vasculature.2 More recent theories describe cholesterol microemboli forming secondary to the anticoagulation insult itself, or indirectly via bleeding into atherosclerotic plaques.3 The subsequent effect depends on the location and size of the arteriole occluded by the microemboli. It is a diagnosis of exclusion; there must be an absence of obvious trauma, serious cold-induced injury or generalised cyanosis. Other causes of ischaemia must be ruled out, for example decreased arterial flow, impaired venous outflow and other prothrombotic states. It is most commonly documented to occur 3–8 weeks after starting anticoagulation, but has been noted to occur from 8 h to a year after starting therapy.4
If recognised early, complete resolution can be achieved by simply substituting warfarin for a different anticoagulant. This has been reported with other anticoagulants,4 but not yet with apixaban. As thromboembolic protection is still required for the underlying condition, a substitute for warfarin is usually necessary. In this case apixaban, an oral, direct and highly selective active site inhibitor of factor Xa was used as it has been licensed to be effective in patients with non-valvular atrial fibrillation.5
Learning points.
Purple toe syndrome is a rare complication of warfarin therapy.
The ischaemic changes can have a significant impact on morbidity, but are completely reversible if the condition is recognized early.
Oral factor Xa inhibitors are an alternative anticoagulant for use in patients with atrial fibrillation who have developed this unusual complication due to warfarin.
Footnotes
Contributors: HEC and JPC were involved with clinical care of the patient. HEC, HMK and PRG wrote the original manuscript and approved the final manuscript as submitted. JPC critically reviewed the manuscript and approved the final manuscript as submitted.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gallerani M, Manfredini R. Non haemorrhagic adverse reactions of oral anti coagulant therapy. Int J Cardiol 1995:49:1–7 [DOI] [PubMed] [Google Scholar]
- 2.Feder W, Auerbach R. "Purple toes": an uncommon sequela of oral coumarin drug therapy. Ann Intern Med 1961:55:911–17 [DOI] [PubMed] [Google Scholar]
- 3.Hyman BT, Landas SK, Ashman RF, et al. Warfarin-related purple toes syndrome and cholesterol microembolization. Am J Med 1987:82:1233–7 [DOI] [PubMed] [Google Scholar]
- 4.Rindone JP, Mellen CK, Eliason JD. Late onset purple toe syndrome with warfarin successfully treated with fondaparinux. Am J Ther 2011:18:e277–9 [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence. Apixaban for preventing stroke and systemic embolism in people with nonvalvular atrial fibrillation. NICE technology appraisal guidance 275. London: National Institute for Health and Care Excellence, 2013 [Google Scholar]


