Abstract
Preoperative staging of the axilla in women with invasive breast cancer using ultrasound-guided needle biopsy (UNB) identifies approximately 50% of patients with axillary nodal metastases prior to surgical intervention. Although moderately sensitive, it is a highly specific staging strategy that is rarely falsely-positive, hence a positive UNB allows patients to be triaged to axillary lymph-node dissection (ALND) avoiding potentially unnecessary sentinel node biopsy (SNB). In this review, we extend our previous work through an updated literature search, focusing on studies that report data on UNB utility. Based on data for 10,934 breast cancer patients, sourced from 35 studies, a positive UNB allowed triage of 1,745 cases (simple proportion 16%) to axillary surgical treatment: the utility of UNB was a median 19.8% [interquartile range (IQR) 11.6%-26.7%] across these studies. We also modelled data from a subgroup of studies, and estimated that amongst patients with metastases to axillary nodes, the odds ratio (OR) for high nodal disease burden for a positive UNB versus a negative UNB was 4.38 [95% confidence interval (95% CI): 3.13, 6.13], P<0.001. From this model, the estimated proportion with high nodal disease burden was 58.9% (95% CI: 50.2%, 67.0%) for a positive UNB, whereas the estimated proportion with high nodal disease burden was 24.6% (95% CI: 17.7%, 33.2%) if UNB was negative. Overall, axillary UNB has good clinical utility and a positive UNB can effectively triage to ALND. However, the evolving landscape of axillary surgical treatment means that UNB will have relatively less utility where surgeons have modified their practice to omission of ALND for minimal nodal metastatic disease.
KEYWORDS : Breast cancer, axillary staging, node metastases, test utility, ultrasound-guided needle biopsy (UNB)
Introduction
Surgical management of the axilla in women with invasive breast cancer has changed considerably in the last two decades: sentinel node biopsy (SNB) has replaced axillary lymph node dissection (ALND) as the primary surgical staging approach1,2 with selective ALND based on the status of the sentinel node(s). More recently, evidence from a landmark randomized trial (Z0011)3 has shown that omission of ALND may be appropriate in defined groups of patients (clinical stage T1-2 and N0 patients having breast conservation and whole-breast radiation) with minimal sentinel node disease burden. It is not surprising then that the evolution of surgical treatment of the axilla has shaped the role of preoperative staging, specifically the use of preoperative axillary ultrasound with selective ultrasound-guided needle biopsy (UNB). In this review, we estimate and discuss the utility of UNB highlighting the role of preoperative UNB and its consequences on surgical management of the axilla.
Background on clinical utility of a test
Test accuracy describes the ability of a test to rule in or out a disease or to assess disease severity. On the other hand clinical utility of a test represents the capacity to use the information from the test to enable a decision to adopt, or to reject, a therapy or an intervention. Test utility expresses4 to what extent testing contributes to improving health outcomes. Bossuyt et al.4 report that key features of test clinical utility are that use of the test improves health outcomes, and that the test forms part of a strategy whereby health outcomes are generated ‘not only by using the test but also by a management strategy that starts with testing but includes all downstream consequences of subsequent clinical management’4. In the specific scenario of the axilla in invasive breast cancer, knowledge of the status of axillary nodes prior to surgical intervention can affect treatment planning. Two meta-analyses5,6, each based on a large number of original studies and hence large datasets, have reported that a preoperative strategy of ultrasound with selective UNB of abnormal-appearing or suspicious axillary node(s) correctly identifies approximately 50% of breast cancer patients who have node metastases. Diepstraten et al.6 estimated this strategy to have a sensitivity of 50% [95% confidence interval (95% CI): 43%, 57%] and Houssami et al.5 reported it as a median 55.2% (IQR 41.8%-68.2%) across primary studies. On this basis exists the potential utility of preoperative UNB whereby ultrasound-directed needle biopsy can confirm metastases to the axillary nodes, enabling triage to ALND and a single axillary operation.
Background on ultrasound accuracy
Using ultrasound with selective UNB (based on ultrasound features of nodes) for preoperative staging of the axilla in newly diagnosed breast cancer patients has been practiced for many years5,7,8, however it is noteworthy that the progressive use of this approach is not solely related to accuracy. It has been partly due to the relative efficiency and modest cost of this combination strategy, the long-established use of ultrasound in breast diagnosis, and because breast ultrasound is a patient-friendly form of image-guided intervention. It should also be noted that ultrasound on its own yields moderate and variable accuracy: meta-analysis5 of data (4,313 subjects) from 21 studies8-28 found a median ultrasound sensitivity of 61.4% (IQR 51.2%-79.4%) and a median ultrasound specificity of 82.0% (IQR 76.9%-89.0%). Therefore the addition of UNB (directed by axillary ultrasound features) is intended to improve both the sensitivity and the specificity of preoperative staging, and in particular to substantially improve its specificity such that a positive UNB can be used to plan surgical treatment of the axilla. So, using data from the same meta-analysis, it can be shown that for the subset of 1,733 patients who were selected to UNB, the median UNB sensitivity is 79.4% (IQR 68.3%-88.9%) and the median UNB specificity is 100% (IQR 100%-100%)5. Various criteria have been used to define abnormal nodes, including morphologic features and/or node size (enlarged nodes), and to select patients to UNB; some of the most frequently reported morphologic features12,13,16,17,24,29-31 defining suspicious nodes are:
thickening of the cortex (primary studies have used various thresholds to define thickening, usually 2-3 mm, but some studies have used a wider mm threshold to define thickening); cortical thickening may be diffuse or focal;
cortex shape/appearance: eccentric or irregular; asymmetric; lobulated (uni- or multi-lobulation);
absence/loss of central fatty hilum (this criterion is predictive of metastases but it is not frequently present so may be insensitive);
rounded nodes (ratio of the longitudinal and transverse dimensions).
Review methods
We previously reported a systematic evidence review on the accuracy and utility of UNB5. In the present review, we extend our previous work focusing on studies that report data on UNB utility. Because the accuracy of ultrasound and UNB has been comprehensively reported in our meta-analysis5 and also in another more recent meta-analysis from Diepstraten et al.6, we will not repeat analyses of ultrasound and UNB accuracy. Instead, we present a summary table of findings from these previous meta-analyses (Table 1) to inform readers of reported accuracy estimates. For the present analysis, we extended our previous review by updating the literature search strategy described by Houssami et al.5, and performed this at January 2014 (Medline and Pre-Medline search). Studies were eligible for the updated review if they provided data on ultrasound-guided fine needle aspiration biopsy (FNAB) or core needle biopsy (CNB) of axillary nodes (collectively referred to as UNB) in women with invasive breast cancer, and if they provided data that quantify or allow estimation of clinical utility. We defined utility as the proportion of women triaged to axillary surgery or axillary treatment as previously defined5. Amongst all eligible studies (from the previous and the updated search) we also looked for data that would allow investigation of UNB results in relation to nodal disease burden.
Table 1. Accuracy of preoperative ultrasound & UNB for staging the axilla in invasive breast cancer based on two meta-analyses.
| Measures of accuracy or utility [number in analysis] | Summary statistic or estimate (95% CI or IQR)§ (%) |
|---|---|
| Accuracy | |
| Ultrasound alone [4,313]5 | Median sensitivity: 61.4 (IQR, 51.2-79.4); Median specificity: 82.0 (IQR, 76.9-89.0) |
| Ultrasound +/– UNB [9,212]6 | Pooled sensitivity: 50.0 (CI, 43.0, 57.0) |
| Ultrasound +/– UNB [9,212]6 | False negative rate*: 25 (CI, 24, 27) |
| Cases selected to UNB: UNB accuracy [2,805, excludes insufficient results]5 | Pooled sensitivity: 79.6† (CI, 74.1, 84.2) Pooled specificity: 98.3 (CI, 97.2, 99.0) |
| Cases selected to UNB: UNB predictive values [2,874]5 | Median PPV: 100 (IQR, 100-100) Median NPV: 67.4 (IQR, 60.0-76.2) |
§, 95% CI given for modeled (pooled) estimates, IQR given for median proportion of summarized data; *, Estimated proportion of women with a negative ultrasound +/– UNB (ultrasound with selective UNB) who are found to have axillary nodal metastases at SNB; †, Meta-analysis5 estimated the sensitivity of axillary UNB (for all breast cancer patients who had UNB) as 79.6% based on thirty studies, however recent work which confirms a similar sensitivity of 79% for all breast cancer patients also shows that UNB sensitivity of axillary nodes is much lower (33%) in the subgroup with invasive lobular histology32. UNB, ultrasound-guided needle biopsy; IQR, inter-quartile range; SNB, sentinel node biopsy; UNB, ultrasound-guided needle biopsy.
Statistical analysis
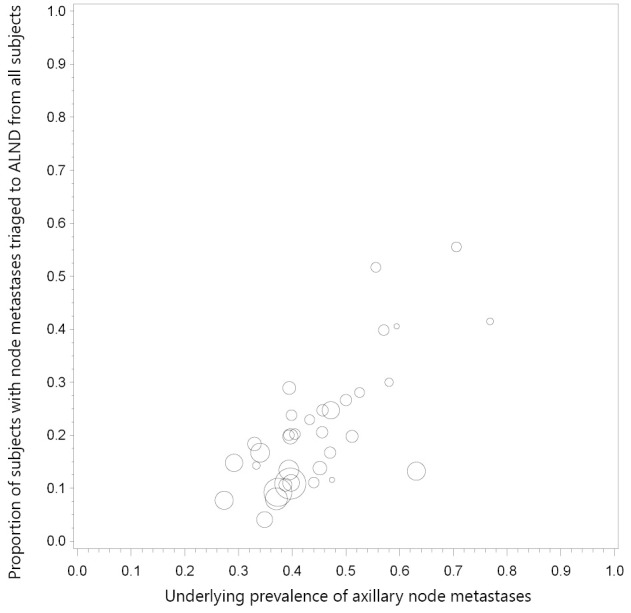
Descriptive statistics (median and IQR) were used to describe UNB utility, which was calculated as the simple proportion of women triaged to axillary surgery or axillary treatment, from all subjects included in the study (therefore the denominator for this calculation was not restricted to women who had UNB but included all cases). Because we previously found evidence of a positive linear correlation between UNB utility and the underlying prevalence (study-specific proportion) of axillary node metastases across studies, we also calculated descriptive statistics for underlying prevalence of axillary node metastases. We used a bubble plot to demonstrate the relationship between UNB utility and the underlying prevalence of axillary node metastases across all studies.
For the subset of studies that provided data on UNB results in relation to node disease burden, we used logistic regression modelling incorporating a random-effect for study to investigate nodal disease burden according to whether UNB was positive versus negative. Node disease burden was examined in the model by analysing the proportion of patients with high nodal disease burden (defined as >3 metastatic nodes in the majority of studies) from all patients with axillary node metastases (total of low and high node disease burden), by UNB result. Therefore the model estimated the odds ratio (OR) and corresponding 95% CI for high nodal disease burden in patients with a positive UNB versus those with a negative UNB.
Results
Our updated search yielded 35 eligible studies9,11-17,21-23,25-28,30,31,33-50 providing data on 10,934 patients with breast cancer in whom a positive UNB result allowed triage of 1,745 cases (simple proportion 16%) to axillary surgical treatment: the utility of UNB was a median 19.8% (IQR 11.6%-26.7%) across all studies9,11-17,21-23,25-28,30,31,33-50. Axillary treatment consisted of triage directly to ALND for the vast majority (and avoidance of SNB) but in some studies UNB was used to affect neoadjuvant therapy prior to ALND9,31,41,47. The median prevalence of node metastases (proportion of patients found to have node metastases on surgical histology) across the 35 studies was 43.2% (IQR 38.7%-51.2%)9,11-17,21-23,25-28,30,31,33-50. In Figure 1, the bubble plot (bubble size reflects study size) displays study-specific proportion of utility (proportion of subjects triaged to axillary surgery based on UNB result) in relation to study-specific underlying prevalence of node metastases.
Figure 1.
Bubble plot shows study-specific UNB utility (proportion of subjects triaged to axillary surgery based on UNB result) in relation to underlying prevalence of node metastases.
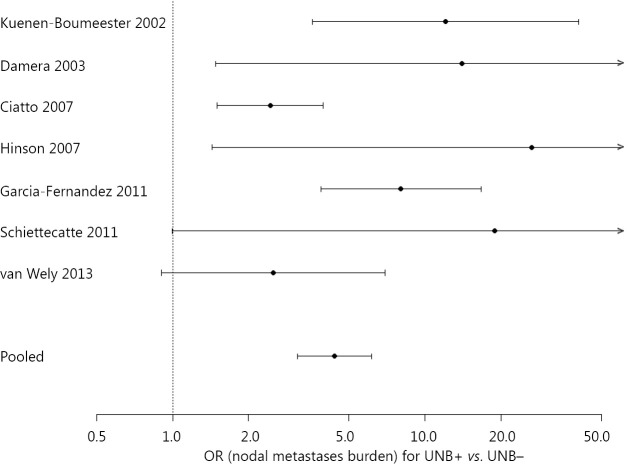
Seven studies20,34,35,38,42,43,48 contributed to the model that estimated the OR for high nodal disease burden in patients with a positive UNB versus those with a negative UNB: study-specific and pooled estimates are shown in Figure 2. Based on the model, the OR for high nodal disease burden for a positive UNB (versus negative UNB) was 4.38 (95% CI: 3.13, 6.13), P<0.001. The estimated proportion of patients with high nodal disease burden (>3 nodes) from this model was 58.9% (95% CI: 50.2%, 67.0%) for a positive UNB result, whereas the estimated proportion with high nodal disease burden was 24.6% (95% CI: 17.7%, 33.2%) if UNB was negative.
Figure 2.
Estimated OR for high nodal disease burden in patients with a positive UNB vs. those with a negative UNB amongst patients with axillary node metastases. Study-specific and pooled estimates shown in plot; pooled OR=4.38 (95% CI: 3.13, 6.13); a positive UNB refers to positive ultrasound/positive needle biopsy, a negative UNB refers to positive ultrasound/negative needle biopsy amongst patients subsequently shown to have nodal metastases on SNB and/or ALND; high nodal disease burden refers to >3 nodes (relative to 1-3 nodes) however the study from Garcia-Fernandez 2011 used ≥2 nodes to classify higher node disease burden. UNB, ultrasound-guided needle biopsy; SNB, sentinel node biopsy; OR, odds ratio; ALND, axillary lymph-node dissection.
Discussion
In this review, we summarize the clinical utility of axillary UNB as a median 19.8% (IQR 11.6%-26.7%) across 35 primary studies, based on 10,934 patients with breast cancer in whom a positive UNB result allowed triage to axillary surgical treatment (and hence avoidance of two-stage axillary surgery) for those harbouring nodal metastases. This median proportion has been calculated from all subjects included in these studies, therefore it also includes all those who had axillary ultrasound but did not proceed to needle biopsy. The UNB utility % is essentially unchanged from our earlier meta-analysis which reported a utility ranging between 17.7% and 19.8% (based on data for 4,941 patients) representing the proportion of all subjects who are triaged, or could be triaged, directly to ALND5 thus avoiding SNB. The UNB utility proportions we describe represent good clinical utility in the context of a management framework whereby patients with UNB-confirmed axillary node metastases can proceed to ALND given the high specificity of the test (and assuming at least moderate sensitivity as estimated in meta-analyses5,6). However, when interpreting UNB utility, it should be noted that many of the studies had moderate to high underlying prevalence of node metastases (Figure 1) implying that published studies may have selected patients at relatively higher risk of harbouring metastatic nodes, which could over-estimate UNB utility.
Another utility for preoperative ultrasound and UNB, described in several studies, relates to axillary staging in patients who are to receive neoadjuvant therapy9,13,22,24,26,31,47,51,52. For example, one study52 reported that UNB for axillary staging can support planning of neoadjuvant therapy in breast cancer patients, and other studies included in our analysis reported use of this axillary staging strategy to affect neoadjuvant therapy followed by axillary surgery9,31,41,47.
In this review, we considered studies that provided data on ultrasound-guided FNAB or CNB of axillary nodes, which we collectively referred to as UNB; however, clinicians may want to know whether there are differences in the accuracy between FNAB and CNB for preoperative axillary staging. In our earlier work we compared sensitivity for studies using FNAB with those using CNB but we did not find statistically significant differences5—this is most likely because the vast majority of published studies used FNAB, or used FNAB or CNB in the same study but most subjects had FNAB, which limits meaningful statistical comparisons. Of note, when we examined data in our previous meta-analysis and included insufficient UNB (as negative test result), we found some difference in sensitivity of FNAB (72.2%; 95% CI: 63.9%, 79.3%) and CNB (83.3%; 95% CI: 70.0%, 91.4%) than estimates that excluded insufficient data from the analysis, but this difference between FNAC and CNB sensitivity was not statistically significant (P=0.13)5. Therefore, either FNAB or CNB of the axilla provide good accuracy in this clinical context and can be used to manage patients. In the updated literature review, we identified one study that uniquely performed both FNAB and CNB on the (same) suspicious node in each of 66 patients, meaning that each suspicious axillary node was sampled within-patient using both FNAB and CNB. In this study, Rautiainen et al.30 reported that the sensitivities of FNAB and CNB were 72.5% and 88.2% respectively (P=0.008), and specificity was 100% for FNAB and for CNB (as was PPV). Although this was a relatively small study, it showed that CNB is more sensitive than FNAB, however, each of these tests are highly specific in the preoperative axillary staging setting and hence either of these axillary UNB tests would confer good utility for triaging to axillary surgery.
Because the accuracy of UNB has been comprehensively reported in our meta-analysis5 and also in another recent meta-analysis from Diepstraten et al.6, we did not repeat analyses of UNB accuracy in this paper, instead we summarized this information (Table 1). However, it was noted through our updated literature search that some studies reported data showing that UNB is much more likely to be correctly positive(more sensitive) if macro metastases are present in the axillary nodes than if only micrometastases (<2 mm) are present16,31,43,49. Britton et al.16 reported UNB sensitivity as 60.3% for macro metastases and as <30% for micrometastases; and Garcia-Ortega31 reported a sensitivity of UNB of 71% for macro metastases whereas none of 12 cases (0%) with micrometastases were correctly diagnosed using UNB.
We extended our previous work to describe UNB utility as the proportion of women (from all subjects) triaged to ALND, based on published studies at January 2014. It is noteworthy that we had also examined a related measure of UNB utility in our earlier review5, defined as the median proportion of women with metastatic axillary nodes triaged or potentially triaged directly to ALND if UNB is used routinely in patients with invasive breast cancer: the previous analysis was based on 2,162 UNBs in 4,451 subjects, and showed that UNB utility measure differed by (study-level) median tumor size5. We reported that the median proportion of women with metastatic axillary nodes triaged was 42.2% (IQR 30.6%-49.2%) for studies with a median tumor size <21 mm, and 65.6% (IQR 48.9%-69.7%) for studies with median tumor size ≥21 mm, and the OR for the proportion triaged in median tumor size ≥21 mm relative to <21 mm studies was 2.57 (95% CI: 1.29, 5.09), P=0.0095. This indicates that axillary UNB has significantly higher utility in women with larger cancers, which is not surprising given that they have a relatively higher likelihood of having nodal metastases than women with smaller cancers.
While the above data and discussion to this point highlight the clinical utility of axillary UNB, given the evidence from the Z0011 trial3,53 and its apparent impact on practice54, raises the issue of whether UNB remains useful at present and whether it will have utility in breast cancer staging in the future. In the subgroup of patients defined by the Z0011 criteria (clinical stage T1-2 and N0 patients having breast conservation and whole-breast radiation)3,53 there may be relatively less utility for UNB because patients with node metastases in only 1-2 sentinel nodes would not necessarily be managed with ALND: hence the utility of axillary ultrasound with UNB will depend on whether or not the surgeon has adopted omission of ALND in patients with minimal sentinel node disease. Surgeons who have modified their practice according to the Z0011 trial3,53 may find preoperative axillary ultrasound with UNB of limited or questionable utility, because there is little evidence that axillary UNB can differentiate between minimal and more advanced nodal disease.
We therefore interrogated existing data on UNB in an attempt to gain further insights regarding UNB outcome and nodal disease burden. However, only seven studies identified in our review20,34,35,38,42,43,48 provided relevant information allowing us to model data in a subgroup analysis—we estimated that amongst patients with axillary nodal metastases, the OR for high nodal disease burden in those with a positive UNB versus those with a negative UNB was 4.38 (95% CI: 3.13, 6.13), P<0.001. This means that the odds of harbouring high nodal disease burden are significantly increased in patients who have a positive UNB relative to those who have a negative UNB. From this model, the estimated proportion of patients with high nodal disease burden was 58.9% for a positive UNB, whereas the estimated proportion with high nodal disease burden was 24.6% if UNB was negative. These findings indicate that amongst those with a positive UNB there is a relatively higher proportion of patients with high nodal disease burden but still a substantial proportion (approximately 40%) will have low nodal disease burden. So overall, axillary UNB has good clinical utility and a positive UNB can effectively triage to ALND. However, the changing landscape of axillary surgical treatment means that a positive UNB may have relatively less utility where surgeons have modified their practice to omission of ALND for minimal nodal metastatic disease. Also, our model is limited by the paucity of studies contributing data to this subgroup analysis.
Although the utility of UNB appears questionable if there is broader and progressive adoption of SNB-only for minimal axillary node metastases, it is possible that the reverse may occur. Because the algorithm for axillary surgical management in invasive breast cancer is evolving, this could result in a potentially more pragmatic approach to the application of axillary ultrasound with UNB. For example, axillary ultrasound might be used to look for multiple abnormal nodes, and to triage those with multiple metastatic nodes to ALND. Other more novel possibilities include enhanced application of ultrasound, either through refined systematic scanning of the axilla (as shown by Britton et al.55), or through technologic developments, for example contrast-enhanced ultrasound56,57, mayallow precise UNB-sampling of the sentinel node(s). Britton and colleagues have described systematic scanning of the axilla, with emphasis on level I nodes and with particular attention to identifying the lowest 1-2 nodes, and have reported that use of that approach can lead to UNB of sentinel nodes in 64% of patients55. Because false negative ultrasound and UNB may be due to failure to find and sample the sentinel node, or may be due to failure to adequately sample metastatic disease within correctly identified diseased sentinel node(s), Sever et al. have investigated contrast-enhanced ultrasound with microbubbles (injected intradermally in the periareolar region)56,57: this research showed the potential to improve identification as well as sampling of the sentinel node(s) through targeted UNB of the microbubble enhancing axillary lymph node57. Such novel approaches to preoperative axillary staging, that support identification and/or sampling of sentinel nodes, could mean that many patients may not require any axillary intervention in future (in the context of the results of Z0011 trial3,53). So further research to develop and evaluate these staging strategies would be worthy and could contribute to future practice.
An important study in this field is a trial currently in progress in Europe: a prospective randomized controlled trial using axillary ultrasound to decide surgical management of the axilla [Sentinel node vs. Observation after axillary Ultra-Sound (SOUND) trial] in patients with early breast cancer (tumors ≤2 cm and clinically node-negative axillae) who are candidates for breast-conserving surgery58. Patients will have axillary ultrasound to assess whether or not they have suspicious nodal involvement, and those shown to have a negative ultrasound or (for a single abnormal node) negative UNB will be randomized to SNB or no further axillary surgery. The SOUND trial represents yet another possibility for a potential shift in axillary management towards less intervention and may see an extended role for axillary ultrasound with selective UNB in breast cancer staging in future58.
Conclusion
Preoperative ultrasound-based staging of the axilla using ultrasound with selective UNB is moderately sensitive but highly specific and provides a staging strategy that allows patients to be triaged to ALND (based on a positive result); this helps avoid unnecessary two-stage axillary surgery, whereas those with a negative UNB proceed to standard SNB for staging. A large number of non-randomised studies report that UNB provides good clinical utility for axillary surgical management, quantified in this paper as a median utility of 19.8% (IQR 11.6%-26.7%) of breast cancer patients (across 35 studies) who can be triaged to axillary surgery based on a positive UNB, and without SNB. However, ongoing evolution of axillary surgical treatment may render preoperative axillary UNB less useful depending on local surgical practice, and specifically on whether omission of ALND in patients with minimal nodal metastatic burden has been adopted into practice. Future research that allows enhanced application of ultrasound with UNB to identify and target sentinel node(s) and/or to discriminate between minimal versus advanced axillary nodal metastatic involvement is likely to contribute substantially towards management of the axilla in invasive breast cancer.
Acknowledgements
This work is partly funded by National Health and Medical Research Council (NHMRC) program (Grant No. 633003) to the Screening & Test Evaluation Program, Australia.
Footnotes
No potential conflicts of interest are disclosed.
References
- 1.Kell MR, Burke JP, Barry M, Morrow M. Outcome of axillary staging in early breast cancer: a meta-analysis. Breast Cancer Res Treat 2010;120:441-447 [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Wu LC, Chen JQ. Sentinel lymph node biopsy compared with axillary lymph node dissection in early breast cancer: a meta-analysis. Breast Cancer Res Treat 2011;129:675-689 [DOI] [PubMed] [Google Scholar]
- 3.Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossuyt PM, Reitsma JB, Linnet K, Moons KG. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem 2012;58:1636-1643 [DOI] [PubMed] [Google Scholar]
- 5.Houssami N, Ciatto S, Turner RM, Cody HS, 3rd, Macaskill P. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann Surg 2011;254:243-251 [DOI] [PubMed] [Google Scholar]
- 6.Diepstraten SC, Sever AR, Buckens CF, Veldhuis WB, van Dalen T, van den Bosch MA, et al. Value of preoperative ultrasound-guided axillary lymph node biopsy for preventing completion axillary lymph node dissection in breast cancer: a systematic review and meta-analysis. Ann Surg Oncol 2014;21:51-59 [DOI] [PubMed] [Google Scholar]
- 7.Verbanck J, Vandewiele I, De Winter H, Tytgat J, Van Aelst F, Tanghe W.Value of axillary ultrasonography and sonographically guided puncture of axillary nodes: a prospective study in 144 consecutive patients. J Clin Ultrasound 1997;25:53-56 [DOI] [PubMed] [Google Scholar]
- 8.Bonnema J, van Geel AN, van Ooijen B, Mali SP, Tjiam SL, Henzen-Logmans SC, et al. Ultrasound-guided aspiration biopsy for detection of nonpalpable axillary node metastases in breast cancer patients: new diagnostic method. World J Surg 1997;21:270-274 [DOI] [PubMed] [Google Scholar]
- 9.Holwitt DM, Swatske ME, Gillanders WE, Monsees BS, Gao F, Aft RL, et al. Scientific Presentation Award: The combination of axillary ultrasound and ultrasound-guided biopsy is an accurate predictor of axillary stage in clinically node-negative breast cancer patients. Am J Surg 2008;196:477-482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popli MB, Sahoo M, Mehrotra N, Choudhury M, Kumar A, Pathania OP, et al. Preoperative ultrasound-guided fine-needle aspiration cytology for axillary staging in breast carcinoma. Australas Radiol 2006;50:122-126 [DOI] [PubMed] [Google Scholar]
- 11.Davis JT, Brill YM, Simmons S, Sachleben BC, Cibull ML, McGrath P, et al. Ultrasound-guided fine-needle aspiration of clinically negative lymph nodes versus sentinel node mapping in patients at high risk for axillary metastasis. Ann Surg Oncol 2006;13:1545-1552 [DOI] [PubMed] [Google Scholar]
- 12.Deurloo EE, Tanis PJ, Gilhuijs KG, Muller SH, Kröger R, Peterse JL, et al. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur J Cancer 2003;39:1068-1073 [DOI] [PubMed] [Google Scholar]
- 13.Abe H, Schmidt RA, Kulkarni K, Sennett CA, Mueller JS, Newstead GM. Axillary lymph nodes suspicious for breast cancer metastasis: sampling with US-guided 14-gauge core-needle biopsy--clinical experience in 100 patients. Radiology 2009;250:41-49 [DOI] [PubMed] [Google Scholar]
- 14.Sapino A, Cassoni P, Zanon E, Fraire F, Croce S, Coluccia C, et al. Ultrasonographically-guided fine-needle aspiration of axillary lymph nodes: role in breast cancer management. Br J Cancer 2003;88:702-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brancato B, Zappa M, Bricolo D, Catarzi S, Risso G, Bonardi R, et al. Role of ultrasound-guided fine needle cytology of axillary lymph nodes in breast carcinoma staging. Radiol Med 2004;108:345-355 [PubMed] [Google Scholar]
- 16.Britton PD, Goud A, Godward S, Barter S, Freeman A, Gaskarth M, et al. Use of ultrasound-guided axillary node core biopsy in staging of early breast cancer. Eur Radiol 2009;19:561-569 [DOI] [PubMed] [Google Scholar]
- 17.Podkrajsek M, Music MM, Kadivec M, Zgajnar J, Besic N, Pogacnik A, et al. Role of ultrasound in the preoperative staging of patients with breast cancer. Eur Radiol 2005;15:1044-1050 [DOI] [PubMed] [Google Scholar]
- 18.Bedrosian I, Bedi D, Kuerer HM, Fornage BD, Harker L, Ross MI, et al. Impact of clinicopathological factors on sensitivity of axillary ultrasonography in the detection of axillary nodal metastases in patients with breast cancer. Ann Surg Oncol 2003;10:1025-1030 [DOI] [PubMed] [Google Scholar]
- 19.Cowher MS, Erb KM, Poller W, Julian TB. Correlation of the use of axillary ultrasound and lymph node needle biopsy with surgical lymph node pathology in patients with invasive breast cancer. Am J Surg 2008;196:756-759 [DOI] [PubMed] [Google Scholar]
- 20.Damera A, Evans AJ, Cornford EJ, Wilson AR, Burrell HC, James JJ, et al. Diagnosis of axillary nodal metastases by ultrasound-guided core biopsy in primary operable breast cancer. Br J Cancer 2003;89:1310-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Rijk MC, Deurloo EE, Nieweg OE, Gilhuijs KG, Peterse JL, Rutgers EJ, et al. Ultrasonography and fine-needle aspiration cytology can spare breast cancer patients unnecessary sentinel lymph node biopsy. Ann Surg Oncol 2006;13:31-35 [DOI] [PubMed] [Google Scholar]
- 22.Boughey JC, Middleton LP, Harker L, Garrett B, Fornage B, Hunt KK, et al. Utility of ultrasound and fine-needle aspiration biopsy of the axilla in the assessment of invasive lobular carcinoma of the breast. Am J Surg 2007;194:450-455 [DOI] [PubMed] [Google Scholar]
- 23.Genta F, Zanon E, Camanni M, Deltetto F, Drogo M, Gallo R, et al. Cost/accuracy ratio analysis in breast cancer patients undergoing ultrasound-guided fine-needle aspiration cytology, sentinel node biopsy, and frozen section of node. World J Surg 2007;31:1155-1163 [DOI] [PubMed] [Google Scholar]
- 24.Koelliker SL, Chung MA, Mainiero MB, Steinhoff MM, Cady B. Axillary lymph nodes: US-guided fine-needle aspiration for initial staging of breast cancer--correlation with primary tumor size. Radiology 2008;246:81-89 [DOI] [PubMed] [Google Scholar]
- 25.Altomare V, Guerriero G, Carino R, Battista C, Primavera A, Altomare A, et al. Axillary lymph node echo-guided fine-needle aspiration cytology enables breast cancer patients to avoid a sentinel lymph node biopsy. Preliminary experience and a review of the literature. Surg Today 2007;37:735-739 [DOI] [PubMed] [Google Scholar]
- 26.Jain A, Haisfield-Wolfe ME, Lange J, Ahuja N, Khouri N, Tsangaris T, et al. The role of ultrasound-guided fine-needle aspiration of axillary nodes in the staging of breast cancer. Ann Surg Oncol 2008;15:462-471 [DOI] [PubMed] [Google Scholar]
- 27.Gilissen F, Oostenbroek R, Storm R, Westenend P, Plaisier P.Prevention of futile sentinel node procedures in breast cancer: ultrasonography of the axilla and fine-needle aspiration cytology are obligatory. Eur J Surg Oncol 2008;34:497-500 [DOI] [PubMed] [Google Scholar]
- 28.Luparia A, Campanino P, Cotti R, Lucarelli D, Durando M, Mariscotti G, et al. Role of axillary ultrasound in the preoperative diagnosis of lymph node metastases in patients affected by breast carcinoma. Radiol Med 2010;115:225-237 [DOI] [PubMed] [Google Scholar]
- 29.Duchesne N, Jaffey J, Florack P, Duchesne S.Redefining ultrasound appearance criteria of positive axillary lymph nodes. Can Assoc Radiol J 2005;56:289-296 [PubMed] [Google Scholar]
- 30.Rautiainen S, Masarwah A, Sudah M, Sutela A, Pelkonen O, Joukainen S, et al. Axillary lymph node biopsy in newly diagnosed invasive breast cancer: comparative accuracy of fine-needle aspiration biopsy versus core-needle biopsy. Radiology 2013;269:54-60 [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Ortega MJ, Benito MA, Vahamonde EF, Torres PR, Velasco AB, Paredes MM. Pretreatment axillary ultrasonography and core biopsy in patients with suspected breast cancer: diagnostic accuracy and impact on management. Eur J Radiol 2011;79:64-72 [DOI] [PubMed] [Google Scholar]
- 32.Hackney L, Williams S, Bajwa S, Morley-Davies AJ, Kirby RM, Britton I. Influence of tumor histology on preoperative staging accuracy of breast metastases to the axilla. Breast J 2013;19:49-55 [DOI] [PubMed] [Google Scholar]
- 33.de Kanter AY, van Eijck CH, van Geel AN, Kruijt RH, Henzen SC, Paul MA, et al. Multicentre study of ultrasonographically guided axillary node biopsy in patients with breast cancer. Br J Surg 1999;86:1459-1462 [DOI] [PubMed] [Google Scholar]
- 34.Kuenen-Boumeester V, Menke-Pluymers M, de Kanter AY, Obdeijn IM, Urich D, Van Der Kwast TH. Ultrasound-guided fine needle aspiration cytology of axillary lymph nodes in breast cancer patients. A preoperative staging procedure. Eur J Cancer 2003;39:170-174 [DOI] [PubMed] [Google Scholar]
- 35.Ciatto S, Brancato B, Risso G, Ambrogetti D, Bulgaresi P, Maddau C, et al. Accuracy of fine needle aspiration cytology (FNAC) of axillary lymph nodes as a triage test in breast cancer staging. Breast Cancer Res Treat 2007;103:85-91 [DOI] [PubMed] [Google Scholar]
- 36.Sahoo S, Sanders MA, Roland L, Pile N, Chagpar AB. A strategic approach to the evaluation of axillary lymph nodes in breast cancer patients: analysis of 168 patients at a single institution. Am J Surg 2007;194:524-526 [DOI] [PubMed] [Google Scholar]
- 37.Tahir M, Osman KA, Shabbir J, Rogers C, Suarez R, Reynolds T, et al. Preoperative axillary staging in breast cancer-saving time and resources. Breast J 2008;14:369-371 [DOI] [PubMed] [Google Scholar]
- 38.Hinson JL, McGrath P, Moore A, Davis JT, Brill YM, Samoilova E, et al. The critical role of axillary ultrasound and aspiration biopsy in the management of breast cancer patients with clinically negative axilla. Ann Surg Oncol 2008;15:250-255 [DOI] [PubMed] [Google Scholar]
- 39.Baruah BP, Goyal A, Young P, Douglas-Jones AG, Mansel RE. Axillary node staging by ultrasonography and fine-needle aspiration cytology in patients with breast cancer. Br J Surg 2010;97:680-683 [DOI] [PubMed] [Google Scholar]
- 40.Lee MC, Eatrides J, Chau A, Han G, Kiluk JV, Khakpour N, et al. Consequences of axillary ultrasound in patients with T2 or greater invasive breast cancers. Ann Surg Oncol 2011;18:72-77 [DOI] [PubMed] [Google Scholar]
- 41.Chang MC, Crystal P, Colgan TJ. The evolving role of axillary lymph node fine-needle aspiration in the management of carcinoma of the breast. Cancer Cytopathol 2011;119:328-334 [DOI] [PubMed] [Google Scholar]
- 42.García Fernández A, Fraile M, Giménez N, Reñe A, Torras M, Canales L, et al. Use of axillary ultrasound, ultrasound-fine needle aspiration biopsy and magnetic resonance imaging in the preoperative triage of breast cancer patients considered for sentinel node biopsy. Ultrasound Med Biol 2011;37:16-22 [DOI] [PubMed] [Google Scholar]
- 43.Schiettecatte A, Bourgain C, Breucq C, Buls N, De Wilde V, de Mey J.Initial axillary staging of breast cancer using ultrasound-guided fine needle aspiration: a liquid-based cytology study. Cytopathology 2011;22:30-35 [DOI] [PubMed] [Google Scholar]
- 44.Solon JG, Power C, Al-Azawi D, Duke D, Hill AD. Ultrasound-guided core biopsy: an effective method of detecting axillary nodal metastases. J Am Coll Surg 2012;214:12-17 [DOI] [PubMed] [Google Scholar]
- 45.Rattay T, Muttalib M, Khalifa E, Duncan A, Parker SJ. Clinical utility of routine pre-operative axillary ultrasound and fine needle aspiration cytology in patient selection for sentinel lymph node biopsy. Breast 2012;21:210-214 [DOI] [PubMed] [Google Scholar]
- 46.Leenders MW, Broeders M, Croese C, Richir MC, Go HL, Langenhorst BL, et al. Ultrasound and fine needle aspiration cytology of axillary lymph nodes in breast cancer. To do or not to do? Breast 2012;21:578-583 [DOI] [PubMed] [Google Scholar]
- 47.Caretta-Weyer H, Sisney GA, Beckman C, Burnside ES, Salkowsi LR, Strigel RM, et al. Impact of axillary ultrasound and core needle biopsy on the utility of intraoperative frozen section analysis and treatment decision making in women with invasive breast cancer. Am J Surg 2012;204:308-314 [DOI] [PubMed] [Google Scholar]
- 48.van Wely BJ, de Wilt JH, Schout PJ, Kooistra B, Wauters CA, Venderinck D, et al. Ultrasound-guided fine-needle aspiration of suspicious nodes in breast cancer patients; selecting patients with extensive nodal involvement. Breast Cancer Res Treat 2013;140:113-118 [DOI] [PubMed] [Google Scholar]
- 49.Cools-Lartigue J, Sinclair A, Trabulsi N, Meguerditchian A, Mesurolle B, Fuhrer R, et al. Preoperative axillary ultrasound and fine-needle aspiration biopsy in the diagnosis of axillary metastases in patients with breast cancer: predictors of accuracy and future implications. Ann Surg Oncol 2013;20:819-827 [DOI] [PubMed] [Google Scholar]
- 50.Amonkar SJ, Oates E, McLean L, Nicholson S. Pre-operative staging of the axilla in primary breast cancer. Byredefining the abnormal appearing node can we reduce investigations without affecting overall treatment? Breast 2013;22:1114-1118 [DOI] [PubMed] [Google Scholar]
- 51.Topal U, Punar S, Tasdelen I, Adim SB. Role of ultrasound-guided core needle biopsy of axillary lymph nodes in the initial staging of breast carcinoma. Eur J Radiol 2005;56:382-385 [DOI] [PubMed] [Google Scholar]
- 52.Joh JE, Han G, Kiluk JV, Laronga C, Khakpour N, Lee MC. Indications for axillary ultrasound use in breast cancer patients. Clin Breast Cancer 2012;12:433-437 [DOI] [PubMed] [Google Scholar]
- 53.Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 2010;252: 426-32; discussion 432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dengel LT, Van Zee KJ, King TA, Stempel M, Cody HS, El-Tamer M, et al. Axillary dissection can be avoided in the majority of clinically node-negative patients undergoing breast-conserving therapy. Ann Surg Oncol 2014;21:22-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Britton P, Moyle P, Benson JR, Goud A, Sinnatamby R, Barter S, et al. Ultrasound of the axilla: where to look for the sentinel lymph node. Clin Radiol 2010;65:373-376 [DOI] [PubMed] [Google Scholar]
- 56.Sever A, Jones S, Cox K, Weeks J, Mills P, Jones P.Preoperative localization of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasonography in patients with breast cancer. Br J Surg 2009;96:1295-1299 [DOI] [PubMed] [Google Scholar]
- 57.Sever AR, Mills P, Jones SE, Cox K, Weeks J, Fish D, et al. Preoperative sentinel node identification with ultrasound using microbubbles in patients with breast cancer. AJR Am J Roentgenol 2011;196:251-256 [DOI] [PubMed] [Google Scholar]
- 58.Gentilini O, Veronesi U.Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation after axillary UltraSouND). Breast 2012;21:678-681 [DOI] [PubMed] [Google Scholar]