Abstract
Objective(s):
To the best of our knowledge, this is the first report on the contributions of GST genetic variants to the risk of diabetic retinopathy in an Iranian population. Therefore, the objective of this study was to determine whether sequence variation in glutathione S-transferase gene (GSTM1 and GSTT1) is associated with development of diabetic retinopathy in type 2 diabetes mellitus (T2DM) Iranian patients.
Materials and Methods:
A total of 605 subjects were investigated in this case-control study; Study groups consisted of 201 patients with diabetic retinopathy (DR), 203 subjects with no clinically significant signs of DR and a group of 201 cases of healthy volunteers with no clinical evidence of diabetes mellitus or any other diseases. The GSTM1 and GSTT1 were genotyped by multiplex-polymerase chain reaction (multiplex-PCR) analysis in all 404 T2DM patients and 201 healthy individuals served as control.
Results:
Increased odds ratio showed that GSTM1-null genotype had a moderately higher occurrence in T2DM patients (OR=1.43, 95% CI=1.01–2.04; P=0.03) than in healthy individuals. However, the frequency of GSTT1 genotype (OR=1.41; 95% CI=0.92-2.18; P=0.09) was not significantly different comparing both groups. Although, regression analysis in T2DM patients showed that GSTM1 and GSTT1 genotypes are not associated with T2DM retinopathy development.
Conclusion:
Our findings suggest that GSTM1 and GSTT1 genotypes might not be involved in the pathogenesis of type 2 diabetes mellitus retinopathy in the Southern Iranian population. However, further investigations are needed to confirm these results in other larger populations.
Keywords: Diabetic retinopathy, Gene polymorphisms, Glutathione S-Transferase, Iranian population
Introduction
Chronic hyperglycemia in diabetes stimulates reactive oxygen species (ROS) production with development of microangiopathic complications such as diabetic retinopathy (DR) and diabetic nephropathy (DN) (1). DR is the major cause of blindness in adults over 40 years of age and is associated with endothelial cell proliferation (1). Duration of diabetes and also poor glycemic control are the most important factors in prevalence of DR (2). During the first two decades of disease, almost 29–78% of patients with type 2 diabetes show signs of DR; however, some people who have a long duration of diabetes do not show any symptoms of DR. Therefore, genetic risk factors are thought to play a role in development of DR (3-5). Identifying the candidate genes which contribute to diabetic retinopathy pathogenesis is more challenging due to the complexity of the disease (3, 5). Antioxidant enzymes such as manganese superoxide dismutase (MnSOD), catalase (CAT) and glutathione-S-transferases enzyme family (GSTs) detoxify ROS (6-8).
GSTM1 (GST-mu) and GSTT1 (GST-theta) are two classes of multi-functional GST enzymes involved in the detoxification of a wide variety of toxic and carcinogenic compounds (9-11). Gluthatione and GSTs take part in the antioxidant defense mechanisms against an extensive array of free radicals that are formed during oxidative stress in diabetes especially in ocular tissues (12). Glutathione S-transferases (GSTs) are a group of candidate genes whose polymorphisms have been associated with the development of chronic diseases like type 2 diabetes mellitus (T2DM) and malignancies (13–16). One important polymorphism accompanied by lacking enzyme activity is GSTM1 and GSTT1 null mutation (17, 18). Homozygous deletions of either GSTM1 or GSTT1 have no functional enzyme activity. The GSTM1and GSTT1 gene loci have been mapped on 1p13.3 and 22q11.2 chromosomes, respectively (17, 18).
Many studies have evaluated the association between GST polymorphism and macrovascular complications of diabetes; yet few published articles have investigated the possible correlation of these polymorphisms with DR and DN as microvascular diabetic complications in Caucasian population. Cilenšek et al, found that GSTT1-null genotype was associated with greater risk of DR (19). Another study by Hovnik et al reported that GSTM1-present genotype was more frequent in patients with DR (20).
The objective of the current study was to determine the frequency of GST genotypes in T2DM patients with DR and find the possible relation between GSTs gene polymorphism and diabetic retinopathy in an Iranian population.
Materials and Methods
Subjects
Studied individuals consisted of 404 Iranian patients (T2DM without DR; n=203 and T2DM with DR; n=201) of more than 10 years’ duration from Nemazi Hospital, affiliated to Shiraz University of Medical Sciences. Out of 201 controls, none had a history of retinopathy or diabetic ailments. The clinical and demographic data, including body mass index (BMI), age, gender, duration of diabetes, blood glucose, and HbA1c were obtained from the study subjects during the time of blood collection. The American Diabetes Association Guidelines (21) were followed to identify the T2DM patients.
Exclusion criteria included: age less than 20 years, history of hematological diseases, hepatic disorders and malignancy. Patients with secondary diabetes such as chronic pancreatitis, Cushing's disease, polycystic ovary disease, and drug induced diabetes were also excluded from study.
The patients underwent a complete ocular examination including visual field testing, slit indirect ophthalmoscopy and lamp biomicroscopy. The findings were recorded by an ophthalmologist experienced in diagnosis of diabetic retinopathy (22). Prior to the commencement of the research, informed consents were obtained from participants according to the ethics committee approval.
GST genotyping
Genomic DNA was extracted from whole blood by Cinnagen Kit DNP™ protocol (DNG plus DNA Extraction Kit, Sinagene Company, Tehran, Iran). The multiplex PCR was performed for detection of presence or absence of GSTM1 and GSTT1 genotypes and a part of exon-7 CYP1A1 gene was amplified and used as an internal control in this method. GSTM1, T1 and exon-7 CYP1A1 fragments were amplified by using the following primers (7, 8):
GSTM1: forward: 5’-GAACTCCCTGAAAAGCTAAAGC-3’, reverse: 5’-GTTGGGCTCAAATATACGGTGG -3’.
GSTT1: forward: 5’-TTCCTTACTGGTCCTCACATCTC-3’, reverse: 5’-TCCCAGGTCACCGGATCAT-3’.
Exon7-CYP1A1:forward:5’-GAACTGCCACTTCAGCTGTCT-3’, reverse: 5’-CAGCTGCATTTGGAAGTGCTC-3’.
In brief, PCR was carried out using 10 pmol of each primer, 200 μM dNTPs, 1.5 mM MgCl2, and 1U Taq polymerase enzyme in a 10 mM PCR buffer, and 300–500 ng genomic DNA in a total volume of 25 μl. The PCR protocol consisted of 2 min at 94°C, 35 cycles of 2 min at 94°C, 1 min at 64°C, 1 min at 72°C, and then 10 min at 72°C. Finally, the co-amplified products (GSTM1: 215 bp, GSTT1: 466 bp and exon-7 CYP1A1: 312 bp) were analyzed by electrophoresis on 1.5% agarose gel and GSTM1 and GSTT1 genotypes were determined (Figure 1).
Figure 1.
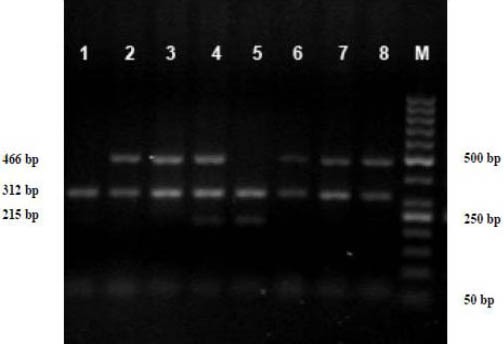
A multiplex-PCR analysis of GSTM1 and GSTT1 gene polymorphism. GSTM1 and GSTT1 PCR products were analyzed directly by electrophoresis on a 1.5% agaros gel. GSTT1(466 bp), GSTM1(215 bp) and exon 7-CYP1A1(312 bp) genes. Lane 1 GSM1-null/GSTT1-null, Lane 2,3,6,7,8 GSTM1-null/GSTT1-present, Lane 4 GSTM1-present/GSTT1-present, Lane 5 GSTM1-present/GSTT1-null sample. Lane M is a 50-bp DNA ladder
Statistical analysis
Comparisons between continuous variables were made by t-test. Also, Chi-Square (χ2) test was used for comparisons among categorical variables. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for the genetic variants and their risk for developing the disease by logistic regression analysis. All of our statistical analysis was performed with SPSS software (Statistical Package for the Social Sciences, version 16, SSPS Inc., Chicago, IL, USA). Statistically significant differences were accepted for P≤0.05.
Results
To investigate the association of GSTM1 and T1 gene polymorphism with diabetic retinopathy, 404 patients (T2DM-DR=201 and T2DM=203) and 201 controls were involved in this study which were matched for their gender and age. The results of basic demographic data and clinical laboratory tests showed no significant differences between the three populations in mean age (51.3±8.6 vs. 52.7±10.1 vs. 51.1±9.2), gender (74.12% vs. 72.41% vs. 71.14%), and BMI (26.7±3.8 vs. 27.6±5.1 vs. 26.6±4.9) respectively. There was no statistically significant difference in the distribution of FBS, HbA1c, and duration of diabetes between the two diabetic groups (P>0.05) (Table 1). However, in patients (both T2DM-DR and T2DM) the established risk factors (HbA1c and high level of fasting blood glucose) for diabetes were more elevated than in control (P<0.05). The distribution of GST genotypes of the study populations are given in Table 2.
Table 1.
Clinical and biochemical characteristics of diabetic studied subjects
| 2DM without DR (n=203) | T2DM with DR (n=201) | P-value a | |
|---|---|---|---|
| Age (years) | 51.3 ± 8.6 | 52.7 ± 10.1 | 0.14 |
| Gender (male/female) | 54/149 | 54/147 | 0.86 |
| Fasting blood glucose (mg/dl) | 187.5 ± 75.2 | 194.9 ± 78.7 | 0.52 |
| HbA1c (%) | 8.0 ± 2.0 | 8.4 ± 2.2 | 0.30 |
| BMI (kg/m2) | 26.7 ± 3.8 | 27.6 ± 5.1 | 0.19 |
| Diabetic duration (years) | 8.3 ± 6.0 | 9.9 ± 5.5 | 0.10 |
Significant differences between groups (P < 0.05). Data are reported as means ± SD
Table 2.
Comparison of GST genotypes among three populations
| Controls (n = 201) n (%) | T2DM without DR (n=203) n (%) | T2DM with DR n=201) n (%) | Association (df=2)a | P- valueb | |
|---|---|---|---|---|---|
| GSTM1 | |||||
| Present | 103(51.2) | 93 (45.8) | 78 (38.8) | χ2 = 6.31 | 0.043 |
| null | 98(48.8) | 110 (54.2) | 23 (61.2) | ||
| GSTT1 | |||||
| Present | 161 (72.9) | 148 (72.9) | 151 (75.1) | χ2 = 3.00 | 0.223 |
| null | 40 (27.1) | 55 (27.1) | 50 (24.9) |
Pearson Chi-Square test (χ2)
Significant differences between groups (P < 0.05)
The GSTM1-null genotype was significantly more frequent in T2DM and T2DM-DR patients than in controls (54.2%, 61.2% and 48.8%, χ2=6.31, P=0.043) and associated with a 1.43 fold increase in development of T2DM (OR=1.43; 95% CI=1.01-2.04; P=0.03). However, GSTT1-null genotype was not frequent in these three population (27.1%, 24.9% and 27.1%, χ2=3.00, P=0.223) and did not change the risk for T2DM as compared with controls (OR=1.41; 95% CI=0.92-2.18; P=0.09) (Table 2, 3). Table 4 represents the frequency distributions of GST genotypes and their relationship with the risk of T2DM associated DR risk. There was no evidence for GSTM1-null and GSTT1-null genotypes to affect the DR risk as compared with T2DM patients without DR.
Table 3.
Comparison of GST genotypes frequencies between T2DM and control groups
| Controls (n=201) n% | T2DM (n=404) n (%) | OR a | 95% CI b | P value c | |
|---|---|---|---|---|---|
| GSTM1 | |||||
| Present | 103(51.2) | 171 (42.3) | 1 (reference) | --- | --- |
| null | 98(48.8) | 233 (57.7) | 1.43 | 1.01-2.04 | 0.03 |
| GSTT1 | |||||
| Present | 161 (72.9) | 299 (75.1) | 1 (reference) | --- | --- |
| null | 40 (27.1) | 105 (24.9) | 1.41 | 0.92-2.18 | 0.09 |
| GSTM1-present/GSTT1-present | 86 (42.8) | 133 (32.9) | 1 (reference) | --- | --- |
| GSTM1-present/GSTT1-null | 17 (8.5) | 38 (9.4) | 1.45 | 0.74-2.86 | 0.25 |
| GSTM1-null/GSTT1-present | 75 (37.3) | 166 (41.1) | 1.43 | 0.96-2.14 | 0.07 |
| GSTM1-null/GSTT1-null | 23 (11.4) | 67 (16.6) | 1.88 | 1.06-3.38 | 0.02 |
Odds ratio
Confidence interval from conditional logistic regression analysis
Significant differences between groups (P < 0.05)
Table 4.
Frequency distributions of GST genotypes and their relationship with the risk of T2DM
| T2DM without DR (n=203) n (%) | T2DM with DR (n=201) n (%) | OR a | 95% CI b | P value c | ||
|---|---|---|---|---|---|---|
| GSTM1 | ||||||
| Present | 93 (45.8) | 78 (38.8) | 1 (reference) | --- | 0.15 | |
| null | 110 (54.2) | 123 (61.2) | 1.33 | 0.88-2.02 | ||
| GSTT1 | ||||||
| Present | 148 (72.9) | 1 (reference) | --- | 0.61 | ||
| null | 55 (27.1) | 50 (24.9) | 0.89 | 0.56-1.42 | ||
Odds ratio
Confidence interval from conditional logistic regression analysis
Significant differences between groups (P < 0.05)
Discussion
Diabetes is associated with a high endogenous inflammatory load and oxidative stress with an increased susceptibility to microvascular angiopathy such as DR. It is believed that ROS activates the STAT3 pathway and up regulates retinal vascular endothelial growth factor (VEGF), an angiogenic factor that enhances abnormal blood vessel growth. Oxidative stress also induces apoptosis of vascular cells under hyperglycemic conditions (23, 24). The GST enzymes catalyze the conjugation of glutathione to a wide variety of electrophiles and represent a protective mechanism against oxidative stress (25- 27).
In different ethnic groups, polymorphisms in GST encoding genes were associated with increased risk of diabetes and macrovascular or microvascular complication development. In Egypt, the GSTT1- and GSTM1-null genotypes were significantly more prevalent in diabetic individuals, and patients carrying both null polymorphisms had a 3.17-fold increased risk of having type-2 diabetes mellitus (28). Another study from India, reported a significant association of GSTM1 null with T2DM with no significant association with GSTT1 null genotype (8).
However, in a study on Indian patients, significant differences were observed in the distribution of both GSTM1 and GSTT1 polymorphisms between T2DM patients and asymp-tomatic carriers (29). In our population, we recently found that the frequency of GSTM1-null genotype in the diabetic group was significantly higher than that in control. The combination of GSTT1-null and GSTM1-null genotypes was significantly higher in patients compared to controls (30). GSTT1-present and GSTT1-present/GSTM1-null represented a risk factor for cardiovascular autonomic neuropathy development in Slovak adolescents with type 1 diabetes mellitus (31). Homozygous deletion of the GSTT1 gene is a risk factor for developing end-stage renal disease (ESRD) in diabetic patients from Taiwan. No association between homozygous deletion of GSTM1 and development of ESRD was found in either diabetic patient (32). Fujita et al, suggested that GSTM1 null genotype was not contributive to the development of diabetic nephropathy in Japanese type 2 diabetic patients (33). A study carried out by Ramprasath et al, demonstrated that GSTT1-null genotype had a moderately higher occurrence in T2DM with coronary artery disease (CAD) patients than T2DM patients without CAD (29).
The present study was carried out to investigate the relationship between genetic polymorphism of GSTM1, GSTT1, and the risk of DR development. We found a statistically significant association between GSTM1-null genotype and T2DM development. Also, the synergistic effect of GSTM1 and GSTT1 polymorphisms was evaluated and a 1.88 fold increase in development of T2DM in individuals carrying the GSTM1-null/GSTT1-null genotype was observed. In T2DM patients, no significant correlation was found between GSTM1-null and GSTT1-null genotypes and the risk of DR development.
A few studies with different conclusions have been conducted in different populations in order to find any association between GSTM1 and GSTT1 polymorphisms and DR. A study carried out by Doney et al, demonstrated that GSTT1-null individuals have a more generalized vasculopathy with an increased risk of progression of both retinopathy and nephropathy (34).
Cilenšek et al, also confirmed Doney’s findings and found that GSTT1-null genotype was associated with greater risk of DR (19). In this study, comparison of patients with DR and subjects who had no clinical signs of DR indicated that GSTT1 null genotype is a risk factor for DR in Caucasians whereas GSTM1-null genotype might protect against retinopathy (19). Another study which was conducted by Hovnik et al, reported that GSTM1-present genotype was more frequent in patients with DR (20).
The results of our study were not in accordance with the results of above mentioned studies. The conflicting results obtained by research in different populations might be attributed to the interaction between different genetic and environmental factors. DR has a complex pathobiologic network and chronic inflammation with cytokines like leukotrienes has been implicated in this process. Poor glycemic control is one of the most important non-genetic risk factors for diabetic retinopathy (35–38).
Other genetic polymorphisms in antioxidant enzymes such as manganese superoxide dismutase (MnSOD) and catalase (CAT), which directly eliminated ROS, may influence our results (20). Moreover, differences in ethnic background of each population influences the risk of T2DM and DR. To the best of our knowledge, this study is the first to report on the contributions of GST genetic variants to the risk of diabetic retinopathy in an Iranian population.
Conclusion
Our findings suggest that GST allelic variants are not associated with individual susceptibility to diabetic retinopathy. However, our study sample was relatively small and further studies with larger samples are warranted to confirm our results.
Acknowledgment
The authors would like to gratefully thank the staff of clinical diagnostic laboratory of Shahid Motahhari outpatients and Nemazi Hospital of Shiraz University of Medical Sciences, Shiraz, Iran. The results reported in this paper were part of a student thesis supported by Shiraz University of Medical Sciences.
Footnotes
Conflict of interest
The authors declare no conflict of interest for the present research outcome.
References
- 1.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82. [Google Scholar]
- 2.Klein R, Klein BE, Moss SE, Cruickshanks KJ. Relationship of hyperglycemia to the long-term incidence and progression of diabetic retinopathy. Arch Intern Med. 1994;154:2169–2178. [PubMed] [Google Scholar]
- 3.Looker HC, Nelson RG, Chew E. Genome-wide linkage analyses to identify loci for diabetic retinopathy. Diabetes. 2007;56:1160–1166. doi: 10.2337/db06-1299. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102:527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 5.Liew G, Klein R, Wong TY. The role of genetics in susceptibility of diabetic retinopathy. Int Ophthalmol Clin. 2009;49:35–52. doi: 10.1097/IIO.0b013e31819fd5d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorbin L, Green T, Sim X, Jensen RA, Shyong-Tai E, Ting-Tay W. Candidate gene association study for diabetic retinopathy in persons with type 2 diabetes: The candidate gene association resource (CARe) Invest Ophthalmol Vis Sci. 2011;52:7593–7602. doi: 10.1167/iovs.11-7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yalin S, Hatungil R, Preek A. Glutathione S-transferase gene polymorphisms in Turkish patients with diabetes mellitus. Cell Biochem Funct. 2007;25:509–513. doi: 10.1002/cbf.1339. [DOI] [PubMed] [Google Scholar]
- 8.Bid HK, Konwar R, Saxena M, Chaudhari P, Agrawal CG, Banerjee M. Association of glutathione S-transferase (GSTM1, T1 and P1) gene polymorphisms with type 2 diabetes mellitus in North Indian population. J Postgrad Med. 2010;56:176–181. doi: 10.4103/0022-3859.68633. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong RN. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem Res Toxicol. 1997;10:2–18. doi: 10.1021/tx960072x. [DOI] [PubMed] [Google Scholar]
- 10.Josephy PD. Genetic variation in human glutathione s-transferase enzymes: significance for pharmacology and toxicology. Hum Genomics Proteomics 2010. 2010 doi: 10.4061/2010/876940. 876490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakoby WB, Ziegler DM. The enzymes of detoxification. J Biol Chem. 1990;265:20715–20718. [PubMed] [Google Scholar]
- 12.Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res 2007. 2007 doi: 10.1155/2007/43603. 43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Economopoulos KP, Sergentanis TN. GSTM1, GSTT1, GSTP1, GSTA1 and colorectal cancer risk: a comprehensive meta-analysis. Eur J Cancer. 2010;46:1617–1631. doi: 10.1016/j.ejca.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Tripathi S, Ghoshal U, Ghoshal UC, Mittal B, Krishnani N, Chourasia D, et al. Gastric carcinogenesis: Possible role of polymorphisms of GSTM1, GSTT1, and GSTP1 genes. Scand J Gastroenterol. 2008;43:431–439. doi: 10.1080/00365520701742930. [DOI] [PubMed] [Google Scholar]
- 15.Franco RL, Schenka NG, Schenka AA, Rezende LF, Gurgel MS. Glutathione S-transferase Pi expression in invasive breast cancer and its relation with the clinical outcome. J BUON. 2012;17:259–264. [PubMed] [Google Scholar]
- 16.Wang G, Zhang L, Li Q. Genetic polymorphisms of GSTT1, GSTM1, and NQo1 genes and diabetes mellitus risk in Chinese population. Biochem Biophys Res commun. 2006;341:310–313. doi: 10.1016/j.bbrc.2005.12.195. [DOI] [PubMed] [Google Scholar]
- 17.Xu SJ, Wang YP, Roa B, Pearson WR. Characterization of the human class mu Glutathione S-transferase gene cluster and the GSTM1 deletion. J Biol Chem. 1998;273:3517–3527. doi: 10.1074/jbc.273.6.3517. [DOI] [PubMed] [Google Scholar]
- 18.Landi S. Mammalian class theta GST and differential susceptibility to car cinogenesis: a review. Mutat Res. 2000;463:247–283. doi: 10.1016/s1383-5742(00)00050-8. [DOI] [PubMed] [Google Scholar]
- 19.Cilenšek I, Mankoč S, Petrovič MG, Petrovič D. GSTT1 null genotype is a risk factor for diabetic retinopathy in Caucasians with type 2 diabetes, whereas, GSTM1 null genotype might confer protection against retinopathy. Dis Markers. 2012;32:93–99. doi: 10.3233/DMA-2011-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hovnik T, Dolzan V, Bratina NU, Podkrajsek KT, Battelino T. Genetic polymorphisms in genes encoding antioxidant enzymes are associated with diabetic retinopathy in type 1 diabetes. Diabetes Care. 2009;32:2258–2262. doi: 10.2337/dc09-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care. 2011;34:S12–S47. doi: 10.2337/dc11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 23.Busik JV, Mohr S, Grant MB. Hyperglycemiainduced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57:1952–1965. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 25.Soliman GZA. Blood lipid per oxidation (superoxide dismutase, malondialdehyde, glutathione) levels in Egyptian type 2 diabetic patients. Singapore Med J. 2008;49:129–136. [PubMed] [Google Scholar]
- 26.Vidya D, Shekhar R, Prabodh S, Chowdary MC. Oxidative stress in diabetic retinopathy. J Clin Diagn Res. 2011;5:994–997. [Google Scholar]
- 27.Ketterer B. Glutathione S-transferases and prevention of cellular free radical damage. Free Radic Res. 1998;28:647–658. doi: 10.3109/10715769809065820. [DOI] [PubMed] [Google Scholar]
- 28.Amer MA, Ghattas MH, Abo-Elmatty DM, Abou-El-Ela SH. Influence of glutathione S-transferase polymorphisms on type-2 diabetes mellitus risk. Genet Mol Res. 2011;10:3722–3730. doi: 10.4238/2011.October.31.14. [DOI] [PubMed] [Google Scholar]
- 29.Ramprasath T, Murugan PS, Prabakaran AD, Gomathi P, Rathinavel A, Selvam GS. Potential risk modifications of GSTT1, GSTM1 and GSTP1 (glutathione-S-transferases) variants and their association to CAD in patients with type-2 diabetes. Biochem Biophys Res Commun. 2011;407:49–53. doi: 10.1016/j.bbrc.2011.02.097. [DOI] [PubMed] [Google Scholar]
- 30.Moasser E, Kazemi-Nezhad SR, Saadat M, Azarpira N. Study of the association between glutathione S-transferase (GSTM1, GSTT1, GSTP1) polymorphisms with type II diabetes mellitus in southern of Iran. Mol Biol Rep. 2012;39:10187–10192. doi: 10.1007/s11033-012-1893-4. [DOI] [PubMed] [Google Scholar]
- 31.Vojtková J, Durdík P, Ciljaková M, Michnová Z, Turčan T, Babušíková E. The association between glutathione S-transferase T1 and M1 gene polymorphisms and cardiovascular autonomic neuropathy in Slovak adolescents with type 1 diabetes mellitus. J Diabetes Complications. 2013;27:44–48. doi: 10.1016/j.jdiacomp.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Kao MT, Chang CC, Chung SY, Chen CM, Tsai JJ, et al. Glutathione S-transferase T1 deletion is a risk factor for developing end-stage renal disease in diabetic patients. Int J Mol Med. 2004;14:855–859. [PubMed] [Google Scholar]
- 33.Fujita H, Narita T, Meguro H, Shimotomai T, Kitazato H, Kagaya E, et al. No association of glutathione S-transferase M1 gene polymorphism with diabetic nephropathy in Japanese type 2 diabetic patients. Ren Fail. 2000;22:479–486. doi: 10.1081/jdi-100100889. [DOI] [PubMed] [Google Scholar]
- 34.Doney AS, Lee S, Leese GP, Morris AD, Palmer CN. Increased cardiovascular morbidity and mortality with the glutathione S-transferaset theta-null genotype. Circulation. 2005;111:2927–2934. doi: 10.1161/CIRCULATIONAHA.104.509224. [DOI] [PubMed] [Google Scholar]
- 35.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 36.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 37.Emanuele N, Sacks J, Klein R. Ethnicity, race, and baseline retinopathy correlates in veterans affairs diabetes trail. Diabetes Care. 2005;28:1954–1958. doi: 10.2337/diacare.28.8.1954. [DOI] [PubMed] [Google Scholar]
- 38.Kazemi Arababadi M, Pourfathollah AA, Daneshmandi S, Hassanshahi G, Rezazadeh Zrandi E, Shamsizadeh A, et al. Evaluation of relation between IL-4 and IFN-γ polymorphisms and type 2 diabetes. Iran J Basic Med Sci. 2009;12:100–104. [Google Scholar]


